Abstract
Bcr-Abl (break-point cluster region-abelson), the oncogenic trigger of chronic myelogenous leukemia (CML), has previously been shown to up-regulate the expression and activity of sphingomyelin synthase 1 (SMS1), which contributes to the proliferation of CML cells; however, the mechanism by which this increased expression of SMS1 is mediated remains unknown. In the current study, we show that Bcr-Abl enhances the expression of SMS1 via a 30-fold up-regulation of its transcription. Of most interest, the Bcr-Abl–regulated transcription of SMS1 is initiated from a novel transcription start site (TSS) that is just upstream of the open reading frame. This shift in TSS utilization generates an SMS1 mRNA with a substantially shorter 5′ UTR compared with its canonical mRNA. This shorter 5′ UTR imparts a 20-fold greater translational efficiency to SMS1 mRNA, which further contributes to the increase of its expression in CML cells. Therefore, our study demonstrates that Bcr-Abl increases SMS1 protein levels via 2 concerted mechanisms: up-regulation of transcription and enhanced translation as a result of the shift in TSS utilization. Remarkably, this is the first time that an oncogene—Bcr-Abl—has been demonstrated to drive such a mechanism that up-regulates the expression of a functionally important target gene, SMS1.—Moorthi, S., Burns, T. A., Yu, G.-Q., Luberto, C. Bcr-Abl regulation of sphingomyelin synthase 1 reveals a novel oncogenic-driven mechanism of protein up-regulation.
Keywords: transcription, alternative TSS, cancer, translation, translation efficiency
Sphingomyelin synthase (SMS/SGMS) is a class of mammalian transferases encoded by 2 distinct genes, SGMS1 and SGMS2, and is responsible for the synthesis of the phosphosphingolipid, sphingomyelin (SM) (1, 2). SMS catalyzes the transfer of the phosphorylcholine moiety from phosphatidylcholine onto ceramide, which produces both SM and diacylglycerol (DAG) (3–10). As such, SMS can affect important cell functions by directly regulating the abundance of SM, a critical structural component of plasma membranes and lipid microdomains (11), as well as the levels of ceramide and DAG, 2 important bioactive lipids (12–20). The ability of SMS to affect the integrity of lipid microdomains at the plasma membrane has been demonstrated to have biological implications in cell survival and inflammation as a result of the regulation of receptor-mediated signaling by FasL and TNF-α and engagement of T-cell receptor and TLR4 (21–26). Moreover, regulation of plasma membrane microdomains by SMSs contributes to the cytotoxic activity of alkyl lysophospholipids (27).
In contrast, SMS activity has been also demonstrated to support cell survival and cell proliferation (28–30) independently of changes in SM levels and, rather, because of the regulation of ceramide, which is generally associated with negative effects on cell proliferation and/or DAG, a well-established mitogenic lipid (20). For example, in HeLa cells, the down-regulation of either SGMS1 or SGMS2 caused the accumulation of ceramide, reduced levels of SM, and impaired cell growth. As cell growth was not restored upon the addition of exogenous SM, this suggests that the effects on cell growth were triggered by changes in the bioactive lipids, ceramide, and/or DAG (31). In Neuro-2a cells, inhibition of SGMS1 caused a G0/G1 cell-cycle arrest, which resulted in decreased proliferation (32), whereas up-regulation of SMS1 in response to H2O2 counteracted the cytotoxic effect of H2O2, possibly by keeping the levels of ceramide in check (33). In line with a prosurvival function, silencing of SMS1 enhanced the accumulation of ceramide in response to photodamage and exacerbated cell death (34).
In cancer, increased SMS activity has been associated with both pro- and antitumorigenic effects. In fact, whereas activation of SMS and enhanced production of SM has been demonstrated to mediate, in part, the anticancer activity of 2-hydroxyoleic acid in glioma cells (35), increased SMS activity correlated with drug resistance in samples that were derived from patients with acute myelogenous leukemia and chronic myelogenous leukemia (CML) (36). Furthermore, a study from our laboratory showed that increased SMS activity contributed to the proliferation of the CML cell line, K562 (37).
CML is a myeloproliferative disorder of hematopoietic stem cells and accounts for 20% of all leukemias affecting adults (38, 39). CML is caused by reciprocal translocation between the long arms of chromosome 22 and 9 that results in the formation of the chimeric Bcr-Abl (break-point cluster region-abelson) oncogene. The resulting Bcr-Abl oncogene generates a constitutively active Abl tyrosine kinase, which is responsible for initiating CML (40–42). CML progresses through 3 clinical stages: chronic phase (hyperexpansion of mature myeloid cells), accelerated phase (acquisition of additional cytogenetic aberrations), and blast phase (proliferation of immature cells), each of which is identified by specific hematologic and molecular features. Treatment of patients in the chronic phase with small-molecule tyrosine kinase inhibitors (TKIs), such as imatinib (Gleevec/STI571) (43), has greatly increased the progression-free survival of patients (44, 45). Conversely, as treatment with TKIs does not eradicate the disease—it does not kill leukemic stem cells (46) —patients with CML are on TKI therapy for life. The need for long-term therapy favors the insurgence in 20–30% of all patients with CML with TKI resistance, which currently represents the foremost challenge in the treatment of CML (47, 48). Of interest, sphingolipids have been implicated in some of the mechanisms of TKI resistance, such as stabilization of the Bcr-Abl protein (49, 50).
Previous work from our laboratory has discovered Bcr-Abl as the first-known upstream regulator of SGMS1 (37). Bcr-Abl increased the expression of SGMS1 mRNA and protein, which we demonstrated contributed to the proliferation of the CML cell line, K562, via modulation of ceramide and DAG; however, the precise molecular mechanism involved in the regulation of SGMS1 expression by Bcr-Abl remains to be elucidated.
In the current study, we first demonstrate that Bcr-Abl increases SGMS1 mRNA expression by increasing its transcription. Second, we show that Bcr-Abl shifts the transcription start site (TSS) of SGMS1 just upstream of the translation start site. Third, we demonstrate that this Bcr-Abl–induced shift in TSS generates an SGMS1 mRNA, which has a shorter 5′ UTR compared with Bcr-Abl–negative cells. Finally, we demonstrate that this shorter SGMS1 mRNA isoform has an increased translational efficiency, which further drives up the expression of SMS1.
MATERIALS AND METHODS
Cell lines and media
K562, HL-60, and HeLa cell lines were purchased from American Type Culture Collection (Manassas, VA, USA). Human acute myeloid leukemia HL-60 cells that stably express p185 Bcr-Abl (HL-60/Bcr-Abl) were a generous gift from Dr. K. Bhalla (The University of Texas MD Anderson Cancer Center, Houston, TX, USA). LAMA-84 and JURL-MK-1 cell lines were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (Brunswick, Germany). K562, LAMA-84, and JURL-MK-1 cells were grown in RPMI 1640 (Thermo Fisher Scientific, Waltham, MA, USA) that was supplemented with 10% fetal bovine serum (FBS; heat inactivated; Thermo Fisher Scientific) or 20% FBS for HL-60 and HL-60-Bcr-Abl, and 100 U/ml penicillin and 100 μg/ml streptomycin (Thermo Fisher Scientific). HeLa cells were grown in DMEM that was supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Thermo Fisher Scientific). Cells were maintained at 37°C and 5% CO2 in a humidified incubator at supplier-suggested cell concentrations.
RNA isolation and cDNA synthesis
To analyze RNA expression [heteronuclear RNA (hnRNA) and mRNA], total RNA was isolated from 1–4 × 106 cells that were collected by centrifugation at 800 g for 5 min at 4°C. Cells were immediately resuspended in lysis buffer with 2-ME (Sigma-Aldrich, St. Louis, MO, USA), and total RNA was isolated by using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol, including the on-column DNase digestion. An additional round of DNase digestion was performed by using Turbo DNA Free Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol to ensure complete elimination of genomic DNA. cDNA was prepared by using a 2-step method. First, an RNA mix was prepared with 1 μg of total RNA, 1 μl of Oligo (dT)20 primer (50 μM; Thermo Fisher Scientific), and 1 μl of 10 mM dNTP (Thermo Fisher Scientific) mixed to a final volume of 12 μl with sterile water and incubated at 65°C for 5 min. Next, a second master mix of 4 μl of 5× first strand buffer, 2 μl of 0.1 M DTT, and 1 μl each of RNase Out and Superscript III (Thermo Fisher Scientific) was added to the RNA mix. The 20-μl reaction was then incubated at 42°C for 52 min and then at 70°C for 15 min.
Real-time quantitative RT-PCR
To evaluate mRNA and hnRNA expression, we performed real-time quantitative RT-PCR (qRT-PCR) using the SYBR green mixture (Bio-Rad, Hercules, CA, USA) with primers within exons for mRNA, or within introns or at intron–exon junctions for hnRNA (primer sequences are reported in Supplemental Table 1). All experiments were performed on an Applied Biosystems 7500 Real-time PCR system (Thermo Fisher Scientific). The following cycling conditions were used: 1 cycle of 3 min at 94°C, 40 cycles of 20 s at 94°C and 30 s at 60°C. Amplification efficiency for each primer pair was assessed as being between 90 and 100% across a range of cDNA concentrations. Results were normalized to an internal control gene—β-actin—and amplification efficiency. qRT-PCR results were analyzed by using Q-Gene software (51) as the mean of normalized expression (MNE). To assess genomic DNA contamination, cDNA was prepared without reverse transcriptase. These controls did not amplify during qRT-PCR, thus demonstrating the absence of genomic DNA.
mRNA stability
Cells were resuspended in complete medium (without antibiotics) at a concentration of 0.3 × 106 cells/ml and allowed to rest for 2 h. Cells were treated with 5 μg/ml actinomycin D or H2O (control) for 30 min, 1, 2, and 4 h, collected by centrifugation at 800 g for 5 min, and resuspended in RLT buffer that contained 2-ME. RNA and cDNA were prepared as previously described. mRNA expression was plotted against time to calculate the half-life by using an exponential 1-phase decay model.
5′ RNA ligase-mediated rapid amplification of cDNA ends
RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) was used to identify the TSS of SGMS1 in the Bcr-Abl–positive K562 cells according to the manufacturer’s protocol for Ambion FirstChoice RLM-RACE (Thermo Fisher Scientific). For the following enzymatic reactions, enzymes activities were terminated by the addition of phenol/chloroform, and RNA was subsequently extracted as suggested by the manufacturer. In summary, 1 μg RNA from K562 cells was either dephosphorylated with calf intestine phosphatase or left untreated at 37°C for 1 h. To remove the mRNA Cap structure, calf intestine phosphatase treated and nontreated RNA was either incubated with tobacco acid pyrophosphatase or left untreated at 37°C for 1 h. All control reactions were treated in a manner similar to the experimental samples in the subsequent RLM-RACE. Dephosphorylated and uncapped mRNA was added to the GeneRacer RNA Oligo (provided in the kit) and incubated with T4 RNA ligase at 37°C for 1 h. The uncapped, full-length mRNA that was ligated to the GeneRacer RNA oligo (provided in the kit) was used for reverse transcription of mRNA into cDNA using Superscript III reverse transcriptase and random primers according to the standard protocol (Thermo Fisher Scientific). This cDNA was used as a template for an initial outer PCR to amplify cDNA ends. Outer PCR was performed by using 0.25 U of platinum high-fidelity Taq polymerase, and all primers were used at 0.4 μM for a standard 50 µl PCR reaction as suggested by the manufacturer (Thermo Fisher Scientific). Primers used for the outer PCR included the GeneRacer 5′ primer (kit component) and the following gene-specific primer 2R (GSP2R): 5′-CAAGAACGGCCATGCCAATGG-3′. The following negative controls were added: PCR reaction without template cDNA, PCR reaction without GSP2R, and a PCR reaction without the GeneRacer 5′ primer. In addition, to ensure the fidelity of the kit and all its enzymes, 1 µl HeLa cDNA was used as a template with the GeneRacer 5′ primer and control primer B.1 that was provided with the kit. Two additional PCR reactions were used as positive controls for the verification of the presence of the target RNA and for testing the RLM-RACE procedure. PCR reactions utilized primers that were synthesized upstream to GSP2 and far enough downstream to produce a resolvable PCR product as visualized by agarose gel electrophoresis. The combination of primers used for these positive control reactions were as follows: GSP/outer1F with GSPR (5′-TGCCAAACAAGTCTCTGCTC-3′), or GSP/outer1F with GSP3R (5′-AAGTCCTGGCCTGTGAAATG-3′). PCR amplification conditions for the outer PCR were as follows: 94°C for 3 min and 35 cycles of 94°C for 30 s, 60°C for 30 s, 72°C for 30 s, followed by an extension at 72°C for 7 min. The product from the outer PCR was used as a template for the inner 5′ RLM-RACE PCR with the primers GSP3R and 5′-RACE inner primer provided with the kit. Negative controls were added as above for inner PCR, and PCR application was performed by using the same PCR amplification conditions as above. After both outer and inner PCR reactions, 10 µl of the reactions and all above-mentioned controls were added to a 2% agarose gel that contained 100 μg/ml ethidium bromide for the visualization of PCR products. PCR products were excised from the gel and purified under standard conditions (Qiagen). Gel-purified products were then ligated into the pCR-2.1-TOPO cloning vector, transformed into DH5α cells according to standard protocol (Thermo Fisher Scientific), and grown overnight on LB plates that contained 100 μg/ml ampicillin for selection. Bacterial colonies were selected, and plasmid DNA was purified from these colonies according to the manufacturer’s recommended protocol (Qiagen) and used for sequencing with both M13F and M13R primers. We repeated the entire 5′ RLM-RACE protocol twice.
First-exon profiling
First-exon profiling (FEP) is a method that was developed in our laboratory to measure the abundance of mRNA generated from alternative TSSs by qRT-PCR. Primers are designed within the first exon of each identified mRNA isoform generated from the different TSSs as identified by 5′ RLM-RACE. As the first exon is never spliced out, this method allows for the quantitation of the transcriptional abundance of specific mRNAs without the interference of possible splicing. For SGMS1, 4 TSSs were identified by 5′ RLM-RACE in K562 cells. Each TSS had a unique alternative first exon residing within introns I, II, and VI, and exon 7, respectively. Primer sequences used for each mRNA isoform are reported in Supplemental Table 1. Amplification efficiency was determined to be between 90 and 100% for each primer pair before abundance measurement in Bcr-Abl–positive and –negative cell lines.
Generation of SGMS1 promoter-7 luciferase construct
Genomic DNA was isolated from exponentially growing K562 cells. Fifteen million cells were centrifuged at 800 g for 5 min at 4°C and resuspended in 500 μl of freshly prepared lysis buffer that contained 50 mM EDTA (pH 8), 1% SDS, 50 mM Tris (pH 7.4), 100 mM NaCl2, and 750 μg/ml proteinase K (Roche, Indianapolis, IN, USA). K562 cell lysate was then incubated for 1 h at 60°C, vortexed briefly, and incubated at 60°C overnight. After overnight incubation, 200 μl of ice-cold 5 M potassium acetate was added; the lysate was vortexed for 30 s, incubated on ice for 1.5 h, and centrifuged at 1300 g for 15 min; and 500 μl of the supernatant was removed. To precipitate DNA, 1 ml of ice-cold ethanol was added to the top of the supernatant and the mixture was gently rocked a few times at 90°C. DNA was spooled from the interface of the supernatant and washed first with 70% ethanol and then with 100% ethanol. DNA precipitate was air dried for 10 min and resuspended in 100 μl of sterile double-distilled H2O. Promoter 7 was amplified from K562 genomic DNA by PCR using primers as indicated in Supplemental Table 1 in a 100-μl volume reaction that contained ∼40 ng of DNA and the following PCR conditions: 0.2 mM dNTP, 1.5 mM MgCl2, 0.75 μM of primers, and 2.5 U Taq DNA polymerase (Thermo Fisher Scientific). PCR amplification conditions for promoter 7 isolation were as follows: 95°C for 5 min and 35 cycles of 95°C for 50 s, 52°C for 50 s, and 72°C for 2 min, followed by an extension at 72°C for 10 min. PCR amplicons were cloned into the pCR 2.1 TOPO-TA subcloning vector and transformed into DHA5α bacterial cells using standard conditions (Thermo Fisher Scientific). Plasmids were extracted from ampicillin-resistant bacterial colonies, and inserts were excised from the pCR 2.1 TOPO-TA vector by using KpnI and ZhoI (Thermo Fisher Scientific) and cloned into the final pGL3-basic vector (Promega, Madison, WI, USA). All sequences were verified (Genewiz, South Plainfield, NJ, USA). We performed sequence analysis and verification by using the Jellyfish software (sequence in Supplemental Table 3). DNA was prepared for transfections by using the Endofree Plasmid Maxi Kit (Qiagen).
Transient transfection and promoter 7 luciferase activity assay
Two million cells were transfected with 5 μg each of pGL3 basic vector or pGL3 basic promoter 7 construct and pCMV β-galactosidase reporter construct. Plasmids were transfected into cells by using the Neon Electroporation system (Thermo Fisher Scientific). K562 cells were electroporated at 1000 V, 50 ms, 1 pulse, whereas HL-60 and HL-60-Bcr-Abl cells were electroporated at 1000 V, 35 ms, 2 pulses and resuspended in 2 ml of growth medium. Cells were harvested after an overnight incubation, then lysed with 1× reporter lysis buffer, and luciferase activity was measured by using a Promega kit per the manufacturer’s protocol (Promega). We measured luminescence by using a Sirius Luminometer (Bethold Technologies, Bad Wildbad, Germany) that was programmed with a 2-s measurement delay, followed by a 10-s measurement read. β-Galactosidase activity was used to normalize cell transfection efficiency by using a colorimetric method as suggested by the manufacturer (Promega). Promoter activity from cell lysates was calculated after normalization with the amount of cell lysate used for the luciferase activity assay to the β-galactosidase activity of each sample and by subtraction of background values as determined from pGL3 vector-only controls.
Imatinib treatment
For measurement of SGMS1 hnRNA levels, K562 cells were seeded at 0.1 × 106 cells/ml and treated for 6 h with 1 μM imatinib (Santa Cruz Biotechnology, Dallas, TX, USA) that was prepared in DMSO. Cells were collected for both SGMS1 hnRNA and Western blot analysis of phospho-signal transducer and activator of transcription 5 (STAT5; Cell Signaling Technology, Danvers, MA, USA) vs. total STAT5 (Santa Cruz Biotechnology). For measurement of promoter 7 activity, K562 cells were transfected with 1.5 μg of pGL3 basic vector or pGL3 basic promoter 7 constructs and 5 μg of pCMV β-galactosidase reporter construct as previously described. After 1 h in the incubator, cells were diluted to 0.1 × 106 cells/ml, treated for 8 h with 1 μM imatinib, and luciferase activity was determined as previously indicated.
Translational efficiency of TSS II and TSS 7 mRNA
Transcript IIb and transcript 7 expression constructs, including full SGMS1 open reading frame, were synthesized by GenScript (Piscataway, NJ, USA) and cloned into pcDNA3.1 expression vector (for HeLa) and pEF6/V5-TOPO Vector (for K562; sequence in Supplemental Table 4). DNA was prepared for transfection by using the Endofree Plasmid Maxi Kit (Qiagen). HeLa cells (1.5 × 105) were plated in 6-well plates 24 h before transfection. Two hundred microliters of Opti-MEM (Thermo Fisher Scientific) was mixed with 6 μl of Xtreme Gene (Roche) and incubated with 2 μg of vector, transcript IIb, or transcript 7 for 20 min at room temperature. Cell medium in wells was replaced with 2.8 ml of fresh growth medium without antibiotics, and the transfection mix was then added drop by drop. Cells were collected after 18 and 26 h for mRNA and Western blot analysis. Isolation of mRNA, cDNA synthesis, and qRT-PCR were performed as described above. FEP primers were used for real-time experiments. For Western blot analysis, cell pellets were resuspended in 100 μl of 0.5% SDS on ice. Samples were sonicated for 20 s. Cell lysates were then resuspended in loading buffer and boiled for 8 min. Proteins (10 μg) were run and blotted with primary Abs against SMS1 (Exalpha, Shirley, MA, USA) and β-actin (Santa Cruz Biotechnology). Western blot band intensity was measured by using ImageJ (National Institutes of Health, Bethesda, MD, USA) (52), and values were recorded after subtracting background. For K562 cells, transcripts IIb and 7 were subcloned into the pEF6/V5-His-TOPO vector (Thermo Fisher Scientific). Plasmids (5 μg) were then transfected into K562 cells via electroporation as previously described. Cells were collected at 8 and 10 h to process for mRNA expression and Western blot analysis as described for HeLa cells. Translation efficiency was calculated by dividing protein band intensity (amount of protein) by the MNE of mRNA expression.
Promoter prediction software
Genomatix (Munich, Germany; http://www.genomatix.de/) was used to help identify the TSS 7 promoter as an authentic TSS.
RESULTS
Bcr-Abl up-regulates the transcription of SGMS1
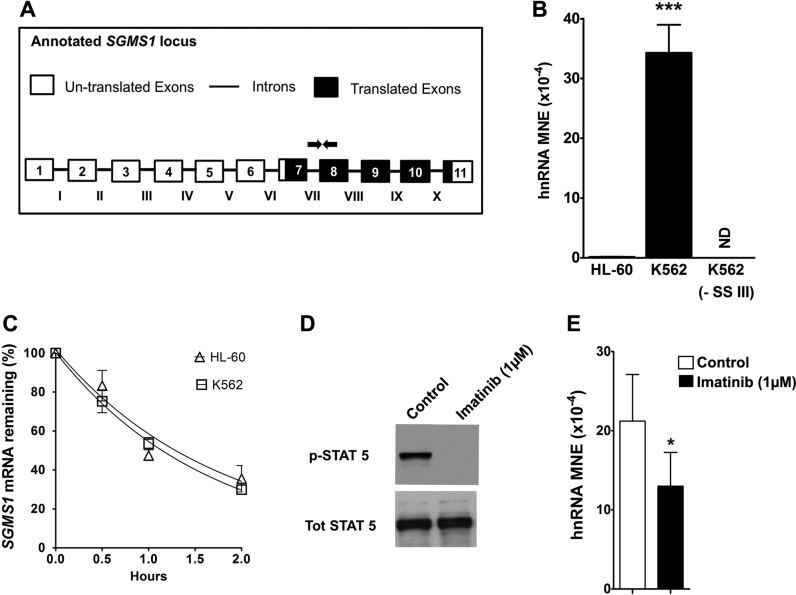
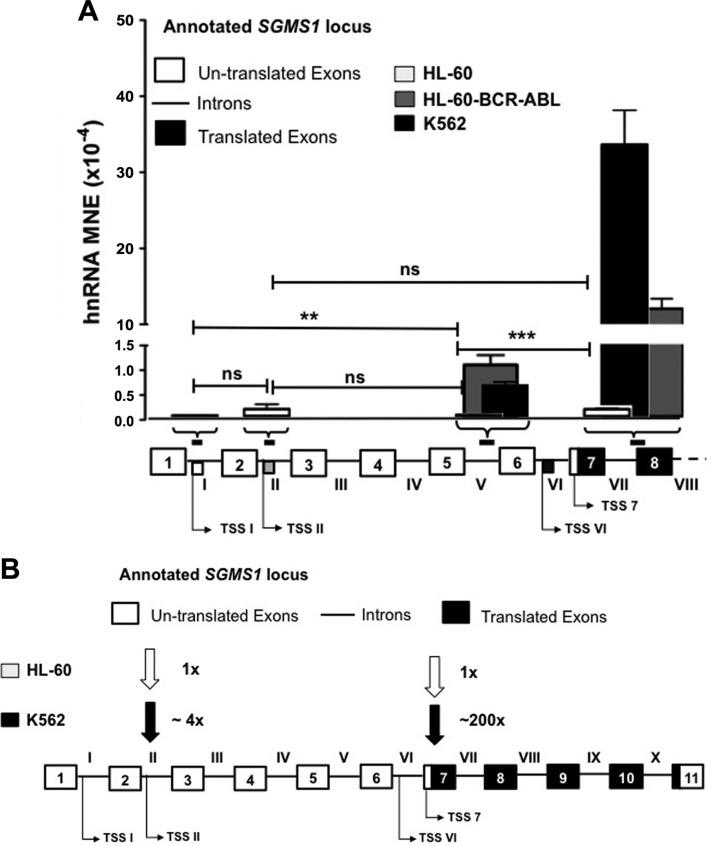
Previous work from our laboratory has demonstrated that Bcr-Abl increased SMS1 protein and activity by greatly elevating SGMS1 mRNA (37). To investigate the mechanism that is responsible for the elevation of SGMS1 mRNA, we first determined the effect of Bcr-Abl on SGMS1 transcription. We measured transcription by quantifying hnRNA—the newly transcribed and unspliced mRNA (53–55)—by using qRT-PCR in Bcr-Abl–positive (K562) and Bcr-Abl–negative (HL-60) cells (Fig. 1A). Specific quantification of hnRNA—without contamination from mRNA—was obtained by using primers that spanned the junction of intron VII and exon 8, downstream of the translation start site (all utilized primer sequences are reported in Supplemental Table 1). As shown in Fig. 1B, SGMS1 hnRNA abundance in K562 cells was ∼200-fold higher than that of HL-60 cells. Of importance, when reverse transcriptase (Superscript III) was not added to the cDNA preparation, no qRT-PCR signal was detected, which demonstrated that samples were devoid of contamination from genomic DNA. These results suggest enhanced transcription of SGMS1 in K562 cells.
Figure 1.
SGMS1 is transcriptionally up-regulated in K562 cells. A, B) SGMS1 transcription was assessed by quantifying hnRNA by qRT-PCR. Primers that targeted intron VII and exon 8 (primer sequences provided in Supplemental Table 1) were used to quantify hnRNA expression, normalized to β-actin and expressed as MNE. Primer locations are indicated on the SGMS1 locus (white boxes represent untranslated exons, black boxes represent translated exons, and lines represent introns). Negative control for cDNA synthesis (without superscript III) in K562 showed no amplification, thus verifying the absence of genomic DNA. Results from 3 independent experiments are shown. C) K562 and HL-60 cells were treated with vehicle (H2O) or actinomycin D (5 μg/ml) over a 2-h time course. SGMS1 mRNA abundance was measured at different times by qRT-PCR using primers within exon 7 (primer sequences provided in Supplemental Table 1) to quantify the percent of remaining mRNA. D) K562 cells were treated with imatinib (1 μM) for 8 h. Cells were harvested and lysates were prepared for Western blot analysis. Total STAT5 and pSTAT5 levels were measured in control and treated cells. E) In control and imatinib-treated cells, RNA was extracted to measure hnRNA levels of SGMS1. Intron VII and exon 8–specific RT-PCR primers were used. Results represent 3 independent experiments. ND, not detectable; SSIII, superscript III. *P < 0.05, ***P < 0.0005.
We next examined the contribution of mRNA stability to the elevated SGMS1 mRNA expression in Bcr-Abl–positive cells. K562 and HL-60 cells were treated with either vehicle (H2O) or actinomycin D (5 μg/ml) to block transcription, and mRNA degradation was measured over a 2-h time course (Fig. 1C). Primers used to measure SGMS1 mRNA levels were designed within exon 7 (downstream of the translation start site) and expression was quantified by qRT-PCR. As shown in Fig. 1C, there was no significant difference in the rate of mRNA degradation between the 2 cell lines. Taken together, these results indicate that, in K562 cells, SGMS1 mRNA expression was likely up-regulated by increased transcription and not via altered mRNA stability.
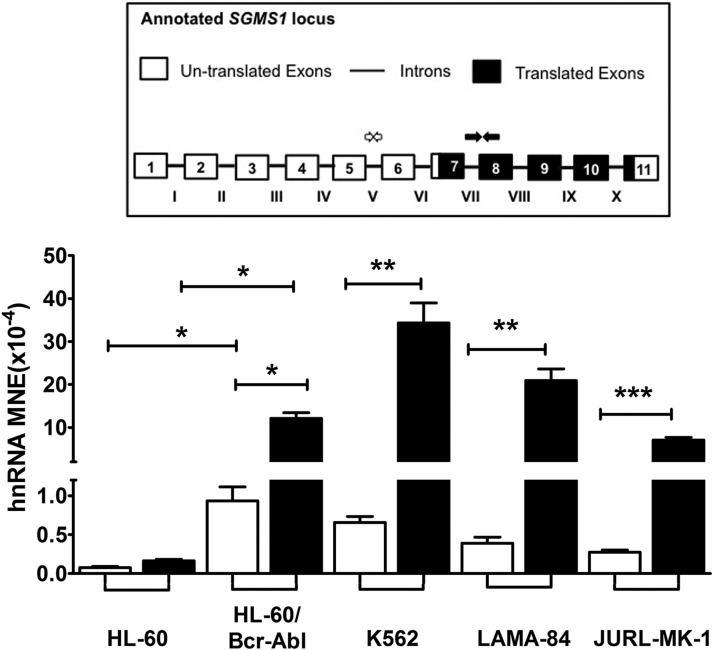
To determine whether Bcr-Abl mediated the observed increase of SGMS1 transcription in K562 cells, we assessed the effect of inhibition of Bcr-Abl kinase activity on hnRNA levels. To this end, K562 cells were treated with 1 μM of the Bcr-Abl kinase inhibitor, imatinib (STI571), for 8 h. To confirm the inhibition of Bcr-Abl, phosphorylation of STAT5, a well-established downstream target of Bcr-Abl signaling, was determined by Western blot analysis (56–58). As shown in Fig. 1D, 8 h of imatinib treatment achieved nearly complete dephosphorylation of STAT5, which confirmed the effectiveness of the treatment. In these conditions, imatinib decreased the levels of SGMS1 hnRNA by 40–50% (Fig. 1E). No greater decrease in the SGMS1 hnRNA level was detected at different times of incubation (data not shown). To further confirm the role of Bcr-Abl in SGMS1 transcripts, we measured hnRNA levels in HL-60 cells that stably overexpress Bcr-Abl by using primers at the junction of intron VII and exon 8 (Fig. 2, black bars). Similar to K562 cells, HL-60-Bcr-Abl cells demonstrated a 70-fold-higher SGMS1 hnRNA level than that of HL-60 cells (Fig. 2, black bars). Of importance, increased SGMS1 hnRNA abundance was also observed in the other CML cell lines, LAMA-84 and JURL-MK-1 (Fig. 2).
Figure 2.
Bcr-Abl up-regulates SGMS1 transcription and shifts transcription initiation. Transcriptional up-regulation of SGMS1 was verified by qRT-PCR of hnRNA (normalized to β-actin) using 2 primer pairs, 1 designed to span within intron V (white arrows and white bars), the other pair across the junction of intron VII and exon 8 (black arrows and black bars; primer sequences are provided in Supplemental Table 1) in HL-60, HL-60-Bcr-Abl, K562, LAMA-84, and JURL-MK-1 cells. Approximate primer locations are indicated on the SGMS1 locus. Results represent 3 independent experiments. *P < 0.05, **P < 0.005, ***P < 0.0005.
Taken together, these results suggest that, in CML cells, elevated SGMS1 mRNA expression is likely caused by a Bcr-Abl–mediated increase in transcription and that the increase is dependent, in part, on Bcr-Abl kinase activity.
Bcr-Abl initiates SGMS1 transcription from alternative start sites
Of interest, when SGMS1 hnRNA was measured in K562 cells by using a second set of primers that targeted intron V (Fig. 2, white arrows and bars), the hnRNA level was found to be ∼50-fold-lower than that of the hnRNA level measured at the junction of intron VII and exon 8 (Fig. 2, black bars vs. white bars). The difference in SGMS1 hnRNA levels between the 2 regions was also observed in other CML cell lines and in HL-60-Bcr-Abl cells (Fig. 2, black bars vs. white bars). Conversely, in HL-60 cells, hnRNA levels upstream (intron V) and downstream of the open reading frame (intron VII and exon 8) were comparable. Consequently, there was an increase in SGMS1 hnRNA of ∼200-fold in K562 vs. HL-60 cells when using primers downstream of the open reading frame (intron VII and exon 8; black bars), whereas the increase was only ∼4-fold when hnRNA was probed by using primers within intron V (white bars). These differences in hnRNA were also observed for the other CML cells.
Thus, our results indicate first that, in Bcr-Abl–positive cells, transcription of SGMS1 is initiated from at least 2 different start sites—one upstream and one downstream of intron V—and, second, that Bcr-Abl increases transcription primarily from an initiation site that is downstream of intron V.
In addition, we determined whether the differences that were observed in hnRNA levels were reflected in the mRNA expression pattern as well. Primer sets were designed to target mRNA by probing exon–exon junctions upstream (exon 5–exon 6) and downstream (within exon 7) of exon 6. As shown in Supplemental Fig. 1, mRNA data followed a pattern that was similar to hnRNA, which indicated that the specific transcriptional pattern induced by Bcr-Abl is also maintained at the mRNA level.
Identification of SGMS1 TSSs in K562 by 5′ RLM-RACE
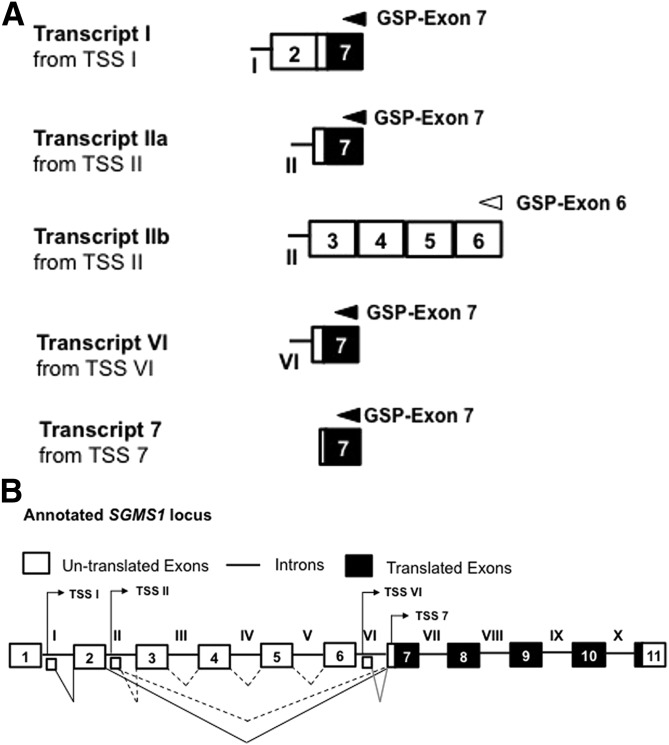
To identify the distinct TSSs, a 5′ RLM-RACE was performed on total RNA from the Bcr-Abl–positive cell line, K562. The 5′ end of SGMS1 transcripts was targeted by using 2 different reverse primers, one within exon 6 (GSP–exon 6) and the second within exon 7 (GSP–exon 7). The 5′ RLM-RACE identified 4 different TSSs, which resulted in 5 different mRNA transcript variants (Fig. 3A). Three of these TSSs resided within introns (I, II, and VI), and the fourth TSS within exon 7, upstream of the translation start site (Fig. 3B; sequences of the different 5′ RLM-RACE products are reported in Supplemental Table 2). Each TSS was annotated by the intron or exon in which it resided—TSS I, TSS II, TSS VI, and TSS 7—and they each generated transcripts with alternative first exons. Thus, consistent with results in Fig. 2, transcription of SGMS1 in K562 is initiated from 2 TSSs that are upstream of intron V (TSS I and TSS II) and 2 TSSs that are downstream of exon 6 (TSS VI and TSS 7).
Figure 3.
Identification of SGMS1 TSSs in K562 cells. A) Representation of the 5′ RLM-RACE products of SGMS1. Five different transcript variants were identified and annotated on the basis of the location of transcription initiation site—that is, TSS I, TSS II, TSS VI, and TSS 7. Gene-specific primers within exon 7 (black arrow) and exon 6 (white arrow) that were used to perform the 5′ RLM-RACE in K562 cells are indicated above the respective exons. Results are representative of 2 independent 5′ RLM-RACE experiments (sequences of clones are given in Supplemental Table 2). B) Graphical representation of the 4 TSSs that were identified by 5′ RLM-RACE positioned on the SGMS1 locus. Large white boxes with α-numerals represent annotated exons, straight connecting solid lines with Roman numerals represent annotated introns, and small white boxes represent new first exons identified by 5′ RLM-RACE. Solid/dashed connector lines correspond to potential splicing events that result in mature transcripts.
Bcr-Abl transforms the transcriptional landscape of SGMS1
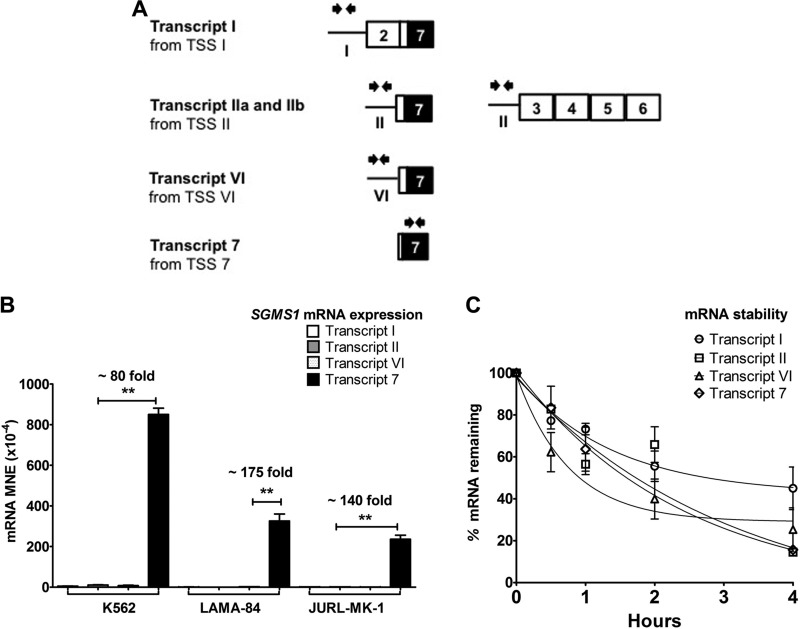
To identify which of these TSSs accounted for the increased SGMS1 transcription in Bcr-Abl–positive cells, we developed the FEP method by real-time PCR. The FEP method takes advantage of the fact that the first exon of a transcript is never spliced out and therefore represents an accurate measure of the total mRNA generated from individual TSSs, regardless of downstream splicing. Thus, we designed primers that targeted each SGMS1 alternative first exon and measured their abundance by RT-PCR (for primer sequences, refer to Supplemental Table 1; Fig. 4A). FEP results in Fig. 4B clearly demonstrate that, in all CML cell lines tested, TSS 7 is responsible for SGMS1 mRNA expression. In K562 cells and other CML cell lines, mRNA levels generated from TSS 7 were ∼80-fold higher than that generated from TSS II, the second most abundant SGMS1 mRNA isoform. To evaluate whether mRNA stability contributed to the differences observed in the mRNA levels of the different transcripts, mRNA degradation was assessed in K562 cells and no significant differences were observed among the half-lives of the different transcripts (Fig. 4C). Taken together, these results indicate that, in Bcr-Abl–positive cells, transcription of SGMS1 primarily occurred via TSS 7.
Figure 4.
SGMS1 is transcriptionally up-regulated via TSS 7 in CML cells. A) Representation of primers used for FEP analysis to quantify the transcriptional abundance from each TSS. Black arrows (facing each other, above each new first exon) represent the location of primers at each new first exon (primer sequences are provided in Supplemental Table 1). B) Abundance of mRNA from each TSS was measured by using the FEP method in Bcr-Abl–positive cells (K562, LAMA-84, and JURL-MK-1). Expression of mRNA was normalized to β-actin and expressed as MNE. C) Stability of mRNA of the different transcripts in K562 cells. Cells were treated with vehicle (H2O) or actinomycin D (5 μg/ml) for different intervals over a 4-h time course (described in Materials and Methods). Abundance of mRNA was measured by FEP to quantify the percent of remaining mRNA. Results represent 3 independent qRT-PCR experiments. There is no significant difference in the stability of different SGMS1 transcripts. The SGMS1 locus is graphically represented (white boxes represent untranslated exons, black boxes represent translated exons, and lines represent introns). *P < 0.05, **P < 0.005.
To determine whether the preferential utilization of TSS 7 is dependent on Bcr-Abl, we assessed the transcriptional landscape of SGMS1, comparing HL-60 with K562 and HL-60-Bcr-Abl cells (Fig. 5A). hnRNA abundance was plotted in the context of the SGMS1 locus. First, FEP primers that targeted TSS I and TSS II were used to quantify transcriptional abundance in HL-60 cells (white bars). As shown in Fig. 5A, both primer sets picked up signals, the intensity of which was in the range of hnRNA levels of SGMS1 in HL-60 cells (MNE values in HL-60 cells are ∼0.1 × 10−4 for hnRNA vs. ∼10–20 × 10−4 for mRNA), thus indicating that, in these cells, the portions of the transcript were not alternative first exons retained in the mRNA, as in CML cells, but part of hnRNAs. Furthermore, in HL-60 cells, SGMS1 hnRNA levels at TSS I and TSS II were comparable with those within intron V and at the junction of intron VII and exon 8 (Fig. 5A), which suggests the existence of a single transcript that starts upstream of TSS I. In contrast, in K562 and HL-60-Bcr-Abl cells, transcripts that originate from TSS II and TSS 7 are both elevated compared with HL-60 cells; however, whereas transcripts that originate from TSS II in K562 cells are ∼4- to 5-fold higher than the single, long transcript in HL-60 cells (Fig. 5B), transcripts that originate from TSS 7 are 200-fold higher, which indeed indicates that Bcr-Abl is shifting transcription initiation to TSS 7.
Figure 5.
Bcr-Abl transforms the transcriptional landscape of SGMS1. A) Bar graph displays qRT-PCR data of hnRNA abundance with primers at intron I, II, and V and intron VII–exon 8 in Bcr-Abl–positive (K562 and HL-60-Bcr-Abl) and negative (HL-60) cells. Shown below the bar graph are the (approximate) regions on the SGMS1 locus targeted by primers used (primer sequences are provided in Supplemental Table 1) and the position of the different TSSs that were identified in K562 cells. Results represent 3 independent qRT-PCR experiments. B) Graphical representation of the transcriptional landscape of SGMS1 in Bcr-Abl–positive vs. –negative cells. Arrows indicate the utilization of different TSSs in Bcr-Abl–positive and –negative cells with fold abundance compared with TSS II in HL-60 cells, as quantified from qRT-PCR data. The SGMS1 locus is graphically represented (white boxes represent untranslated exons, black boxes represent translated exons, and lines represent introns). **P < 0.005, ***P < 0.0005.
Identification of the TSS 7 promoter and its regulation by Bcr-Abl
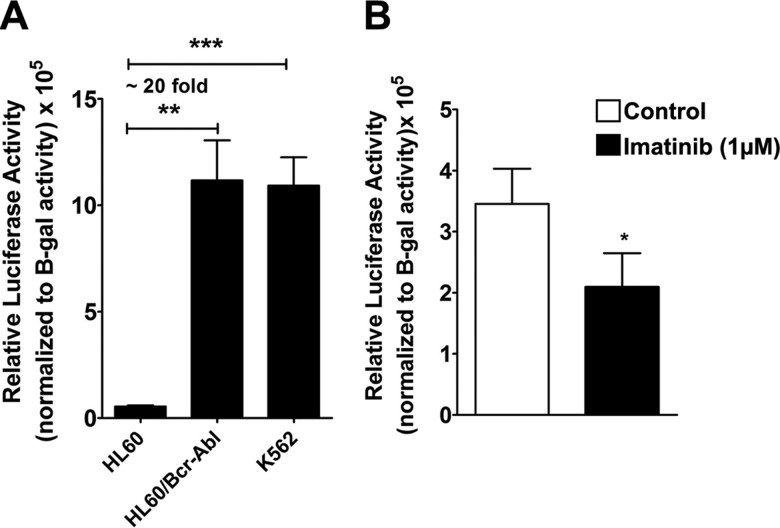
To further validate TSS 7 as an authentic TSS, we set out to identify, isolate, and assess the activity of its putative promoter. On the basis of the promoter prediction software, Genomatix, a stretch of 832 bp upstream of TSS 7 was isolated from genomic DNA of K562 cells and cloned into the pGL3-basic luciferase reporter vector and named promoter 7 (the sequence of promoter 7 is reported in Supplemental Table 3). Promoter 7-pGL3 and pCMV β-galactosidase (transfection control) constructs (5 μg of DNA each) were cotransfected into Bcr-Abl–positive (HL-60-Bcr-Abl and K562) and –negative (HL-60) cells, and luciferase activity was measured and normalized to β-galactosidase activity (Fig. 6A). As shown in the figure, the isolated genomic region that corresponded to the putative promoter 7 induced transcription as indicated by the high luciferase activity obtained in K562 cells. Of importance, the promoter activity in both K562 and HL-60-Bcr-Abl cells was ∼20-fold higher than in Bcr-Abl–negative HL-60 cells.
Figure 6.
Identification of a promoter upstream of TSS 7 and its regulation by Bcr-Abl. A) A stretch of 832 bp upstream of TSS 7 was isolated from K562 genomic DNA and cloned into the pGL3-basic vector to assess promoter activity (construct sequence is provided in Supplemental Table 3). HL-60, HL-60-Bcr-Abl, and K562 cells were transfected with 5 μg each of the promoter 7 pGL3 construct or pGL3-basic vector and 5 μg pCMV β-galactosidase plasmid vectors (transfection control). Promoter activity was measured by luciferase activity normalized to β-galactosidase (β-gal) activity, subtracted from the vector activity, and expressed as relative luciferase units (RLU). Results represent 3 independent experiments. B) K562 cells were cotransfected with 1.5 μg of the promoter 7 pGL3 or 1.5 μg of pGL3-basic vector and 5 μg of pCMV β-galactosidase plasmid, then treated with 1 μM imatinib for 8 h. Cells were then analyzed for promoter activity. *P < 0.05, **P < 0.005, ***P < 0.0005.
We next assessed the effect of the inhibition of Bcr-Abl kinase activity by imatinib on promoter 7. To this end, K562 cells were transfected with promoter 7-pGL3 (1.5 μg) and pCMV β-galactosidase (5 μg) and treated with 1 μM imatinib after 1 h. After 8 h of treatment with imatinib, cells were collected and relative luciferase activity of promoter 7 was measured. As shown in Fig. 6B, the inhibition of Bcr-Abl kinase activity resulted in a 40% decrease in promoter activity, which was similar to the effect of imatinib on SGMS1 hnRNA (Fig. 1E).
These results suggest that promoter 7 is a bona fide promoter and is activated by Bcr-Abl, thus validating the increased utilization of TSS 7 in CML cells shown in Fig. 2.
Increased translational efficiency of SGMS1 Transcript-7
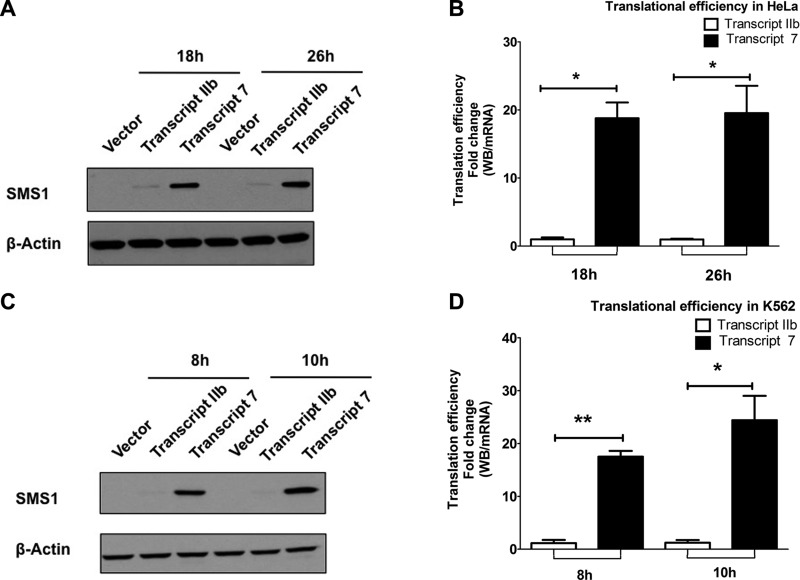
On the basis of the results from FEP and mRNA (Fig. 4B and Supplemental Fig. 1), transcript IIb and transcript 7 are the 2 most abundant SGMS1 mRNA transcripts in CML cells, with overwhelming preference for transcript 7. Of interest, transcript 7 is characterized by a shorter 5′ UTR (∼135 bp), whereas transcript IIb has the longest 5′ UTR (574 bp) among all the mRNA variants present in K562 cells (Figs. 3A and 5B). Thus, we wondered whether the preferential utilization of TSS 7 had any functional consequence as a result of the shorter 5′ UTR. It has been reported that the length and structure of the 5′ UTR can affect the translation efficiency of an mRNA (59, 60); therefore, we set out to test whether there was a difference in the efficiency of the translation between the 2 transcripts. Mammalian expression plasmids were generated with the 5′ UTR from either transcript 7 or transcript IIb and the full SGMS1 coding sequence with a Flag tag at the C terminus (sequences of vectors are reported in Supplemental Table 4). To determine whether the intrinsic features of the 5′ UTRs directly affected the efficiency of translation—independently of any effect mediated by Bcr-Abl—we first transfected the constructs in HeLa cells. After transfection, samples were collected at 10 and 26 h—expression was not saturated at these time points—and assessed by Western blot analysis to measure protein levels (Fig. 7A and Supplemental Fig. 2A) and by qRT-PCR to measure mRNA levels (Supplemental Fig. 2B). To obtain translational efficiency, Western blot bands that corresponded to SMS1 were quantified (Supplemental Fig. S2A) and divided by SGMS1 mRNA levels as measured by qRT-PCR using FEP primers (Supplemental Fig. 2B). As shown in the figure, the translational efficiency of transcript 7 was ∼20-fold higher than that of transcript IIb (Fig. 7B).
Figure 7.
SGMS1 mRNA from TSS 7 is translated more efficiently. A) Representative Western blot of SMS1 protein in HeLa cells that overexpress either transcript IIb or transcript 7 (upper; sequences are provided in Supplemental Table 4) and β-actin (lower). B) Translational efficiency of the 2 transcripts was calculated by dividing the intensity of the Western blot band over mRNA at 10 and 26 h post-transfection. mRNA was quantified by using transcript-specific primers as in FEP. C) Representative Western blot of SMS1 protein in K562 cells that overexpress either transcript IIb or transcript 7 (upper) and β-actin (lower). D) Translational efficiency of the 2 transcripts was calculated by dividing the intensity of the Western blot band over mRNA at 8 and 10 h post-transfection. mRNA was quantified by using transcript-specific primers. All results represent 3 independent experiments. *P < 0.05, **P < 0.005, ***P < 0.0005.
To assess whether Bcr-Abl exerted an additional effect on the regulation of translation of these transcripts, constructs were transfected in K562 cells and we measured proteins and mRNA levels at 8 and 10 h post-transfection. Similar to HeLa cells, transcript 7 was translated ∼20-fold more efficiently than transcript IIb in K562 cells (Fig. 7C, D and Supplemental Fig. 3), with no additional effect as a result of the presence of Bcr-Abl.
Altogether these results demonstrate that the intrinsic features in the longer 5′ UTR of transcript IIb exert an inhibitory effect on the translation of SGMS1 compared with the shorter 5′ UTR in transcript 7.
DISCUSSION
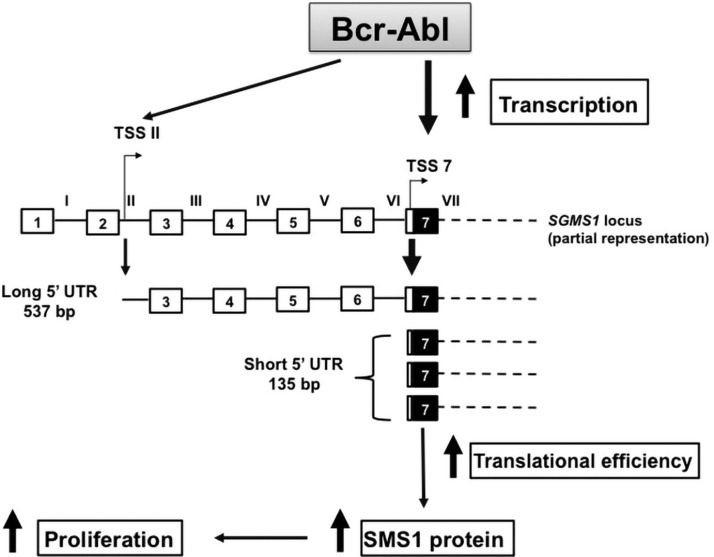
The current study demonstrates that Bcr-Abl increases SMS1 abundance by regulating its transcription—qualitatively and quantitatively—and thus functionally affects its translation. First, Bcr-Abl stimulates the transcription of SGMS1 and shifts its TSS close to the translation start site. Second, the shift in TSS generates an mRNA that is translated with greater efficiency (Fig. 8). This coordinated action, which is mediated by Bcr-Abl, leads to an exponential increase in SMS1 abundance in CML cells.
Figure 8.
Model for the regulation of SMS1 expression by Bcr-Abl. Bcr-Abl up-regulates SGMS1 mRNA by promoting transcription mostly from 2 alternative TSSs, TSS II and TSS 7; however, in Bcr-Abl–positive cells, the maximum transcription of SGMS1 occurs from TSS 7. mRNA from TSS 7 is characterized by a comparatively shorter 5′ UTR (135 bp), which is devoid of translational inhibitory features that are present in the longer SGMS1 5′ UTR found in Bcr-Abl–negative HL-60 cells. The Bcr-Abl–induced TSS 7 transcript is therefore translated more efficiently, which results in an additional increase of the SMS1 protein. Overall, Bcr-Abl exponentially enhances the expression of SGSM1 by up-regulating the transcription of a specific SGMS1 mRNA isoform that is translated more efficiently. Increased SGMS1 expression supports Bcr-Abl–mediated proliferation (1). Of note, the SGMS1 locus is represented up to exon 7, beyond which the mRNA structure is represented as dashed lines and is assumed to be identical for both transcripts shown. The SGMS1 locus is graphically represented (white boxes represent untranslated exons, black boxes represent translated exons, and lines represent introns).
Several observations in our study support the transcriptional regulation of SGMS1 by Bcr-Abl. First, we have shown elevated SGMS1 hnRNA levels in CML cells compared with Bcr-Abl–negative HL-60 cells. Second, we have demonstrated that Bcr-Abl is sufficient to elevate SGMS1 hnRNA, as the increase in SGMS1 hnRNA is also observed in HL-60 cells that overexpress Bcr-Abl. In addition, we identified 4 SGMS1 TSSs in the CML cell line, K562, and through our newly developed method, FEP, we determined that, in CML cells, SGMS1 mRNA is primarily generated from TSS 7. The preferential usage of TSS 7 in CML cells was also confirmed by up-regulation of its promoter activity in Bcr-Abl–positive cells. Finally, we found that the regulation of SGMS1 transcription by Bcr-Abl is dependent, in part, on its kinase activity. In fact, the inhibition of Bcr-Abl kinase activity by imatinib inhibits transcription from the alternative start site (TSS 7) as demonstrated by lower SGMS1 hnRNA levels and reduced promoter 7 activity. Taken together, these results unveil, for the first time to our knowledge, Bcr-Abl to be an upstream transcriptional regulator of SGMS1 and characterize promoter 7 as the first bona fide SGMS1 promoter.
Since its identification (1, 2), the regulation of SGMS1 has been largely unexplored, primarily because of a lack of studies that could directly implicate the regulation of its expression in physiologic or pathologic conditions. SGMS1 is a large gene that spans 320 kb of human chromosome 10 and contains 11 annotated exons. The SGMS1 transcript is characterized by its exceptionally long predicted 5′ and 3′ UTRs of 954 and 1538 bp, respectively (61). Dergunova et al. (62) reported that the lengthy 3′ UTR of SGMS1 contains alternative 3′ polyadenylation sites that could possibly affect mRNA stability and, therefore, its abundance; however, our assessment of SGMS1 mRNA stability demonstrated no differences between Bcr-Abl–positive and –negative cells, thus validating the concept that the increased abundance of SGMS1 in CML cells was likely a result of increased transcription.
Regulation of transcription initiation of the SGMS1 is complex. Indeed, preliminary experiments from our laboratory that have investigated the use of the alternative TSSs identified in this study suggest that, even within the hematopoietic lineage, SGMS1 uses different transcription initiation sites (data not shown). Moreover, a BLAST of our 5′ RACE sequences against the expressed sequence tag databases for cells and tissues revealed that these sequences might also be expressed in other cancer cell lines, such as Ramos and Jurkat, and potentially also in normal adult tissues, such as the uterus, trachea, and testis. As entries in the expressed sequence tag databases can represent partial sequences, this type of analysis is only suggestive and not conclusive, although it supports heterogeneity in the SGMS1 5′ UTRs (Supplemental Table 5). This diversity is not unique to humans, and, in fact, Mus musculus SGMS1 has 4 different TSSs, which results in 3 different protein isoforms with varied tissue distribution (63). In humans, the existence of a vast repertoire of SGMS1 5′ UTRs is corroborated by studies conducted by Rozhkova et al. (64) and Sudarkina et al. (65) on mRNA from different human tissues, although the corresponding TSSs could not be identified, with the exception of TSS VI.
Our study, in contrast, offers an exhaustive investigation of transcriptional regulation from diverse TSSs that have been previously unreported for human SGMS1. Indeed, hnRNA data and results from 5′-RACE, FEP, and promoter studies all support the preferential utilization of TSS 7 in CML cells compared with HL-60 cells. Although the conclusive identification of promoter 7 would ultimately require the genomic deletion of its regulatory regions, this is the first time that a mechanism of upstream regulation for SGMS1 has been identified, and it points to the up-regulation of SGMS1 transcription by the Bcr-Abl oncogene from a newly identified and confirmed alternative start site, TSS 7.
The molecular mechanism that leads to the activation of promoter 7 remains to be understood. Of interest, preliminary analysis of promoter 7 revealed several potential binding sites for transcription factors that are known to be increased and/or activated in the context of CML, such as STAT5 and YY1 (66, 67), and they thus represent viable candidate regulators of SGMS1 transcription. Of interest, inhibition of the kinase activity of Bcr-Abl partially decreased SGMS1 hnRNA levels and promoter 7 activity. These results suggest that the promoter activity of SGMS1 is still partially under the acute control of Bcr-Abl; however, there is also a component of this regulation that cannot be reverted by the inhibition of Bcr-Abl kinase activity, which indicates the possible involvement of either Bcr-Abl–initiated, but irreversible, epigenetic modifications of the promoter or, potentially, a kinase-independent effect of Bcr-Abl (68–70). We are currently in the process of investigating these possibilities, as the elucidation of such a mechanism would also allow us to potentially uncover other conditions in which SGMS1 is similarly regulated.
The current study also established that the observed Bcr-Abl–mediated shift in the transcription initiation of SGMS1 functionally enhances SGMS1 translation independently and in addition to the observed increase in transcription (Fig. 7). In fact, we clearly have demonstrated in 2 different cell types that transcripts from TSS 7 display a 20-fold-higher translation efficiency compared with transcript IIb, the second most abundant transcript type in CML cells.
It is well accepted that the structural characteristics of 5′ UTRs, such as their length, presence of upstream open reading frames (uORFs), secondary structures, and terminal oligopyrimidine tracts, can have repressive effects on translation (60, 71–74) —and this seems to be the case for SGMS1. Most of the alternative transcripts that have we identified in K562 cells have uORFs—transcript I has 2, transcript IIb has 1, and transcript VI has 1 uORF, whereas transcript IIa and transcript 7 have no uORFs. However, total expression of transcript IIa is extremely low compared with transcript IIb; therefore, it does not significantly contribute to SMS1 expression. Furthermore, our own analysis of the 5′ UTR of annotated SGMS1—the longest transcript of SGMS1, starting at canonical exon 1—revealed 2 uORFs on the mRNA that is derived from the minus strand of genomic DNA, where SGMS1 resides. Furthermore, the 5′ UTR of transcript 7 is shorter than the average length of 5′ UTRs of human genes (200 bp), it has minimal secondary structures, low comparative G + C content, and low total free energy (Supplemental Table 6). The lack of all these translational inhibitory features in the 5′ UTR of transcript 7 supports and provides a mechanism for the increased translational efficiency of TSS 7 compared with TSS IIb.
The preferential use of TSS 7 in Bcr-Abl–positive cells is driven by a shift in promoter activation. Classically, in normal human tissues, alternative promoters are used to allow the spatiotemporal expression of specific proteins via differential expression of specific transcription factor regulatory programs (75–77). In cancer, the use of alternative TSSs has been primarily associated with the production of different isoforms of relevant proteins, which causes either loss or gain of function; this is the case, for instance, in LEF1, DLC1, and p73 (78–83). In the current study, the shift in promoter utilization induced by Bcr-Abl increased the translation of the full-length SMS1 protein, as detected by Western blot analysis and increased SMS activity (ref. 37 and Fig. 7). A limited number of studies have described the alteration in levels of cancer-associated full-length proteins as a consequence of a promoter shift. Similar to SGMS1, increased expression of Axin2 and mdm2 has been associated with a shift in promoter use, which resulted in increased translational efficiency (84–89), whereas, in contrast to SMS1, lower BRCA1 (breast cancer gene 1) levels in a subset of breast cancers may be a consequence of inefficient translation as a result of a shift in promoter use toward a longer 5′ UTR (90, 91). However, in reported cases, the trigger for the shift in promoter use is yet to be identified. Remarkably, our study not only demonstrated an association of the shift in promoter use with increased translation efficiency of SMS1—a protein that is important for the proliferation of Bcr-Abl–positive cells (37)—but, for the first time to our knowledge, we have established a causative link between an oncogene (Bcr-Abl) and such a mechanism of up-regulation.
Important questions arise from these observations, some that are specific to the transforming ability of Bcr-Abl and some of a more general nature. For instance, is this mechanism a general way by which Bcr-Abl up- or down-regulates important molecular targets? In other words, is SMS1 the only target of such a regulatory mechanism by Bcr-Abl? A preliminary analysis identified 2 other genes, TLE3 and Rab 10, that are regulated in a manner that is similar to SMS1 by Bcr-Abl. As the analysis is only partial, the discovery of other genes that are regulated like SMS1 may suggest the existence of a novel Bcr-Abl–mediated regulatory mechanism to collectively affect the expression of several downstream targets. If so, does such regulation take place via the coordinated modulation of specific transcription factors and/or epigenetic events? Is this mechanism also an underappreciated way of coordinating protein expression patterns (translatome) in other types of cancers? Furthermore, in a noncancer context, such regulation may also explain the disconnect between mRNA and protein levels that is often observed but vastly unexplained.
To begin to address some of these questions, we are investigating the role of Bcr-Abl in genome-wide promoter shifting and its functional consequence in CML cells. We are also dissecting the molecular mechanism by which Bcr-Abl activates promoter 7 to then evaluate the involvement of such a mechanism in the regulation of other functionally relevant genes in CML.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Paola Signorelli (Department of Health Sciences, University of Milan, Milan, Italy) and Dr. Daniella Ishimaru (Medical University of South Carolina) for expert advice. The authors also thank Dr. Can Senkal and Dr. Yusuf Hannun (both of the Department of Medicine and Cancer Center at Stony Brook University) for critical input when writing the manuscript. The authors thank the Stony Brook DNA Sequencing facility for timely assistance. This work was supported by U.S. National Institutes of Health, National Cancer Institute Grant P01-CA097132 (to C. L. for Project #4) and the Stony Brook Scholars in Biomedical Sciences Award (to S. M.). The authors declare no conflicts of interest.
Glossary
- Bcr-Abl
break-point cluster region-abelson
- CML
chronic myelogenous leukemia
- DAG
diacylglycerol
- FBS
fetal bovine serum
- FEP
first-exon profiling
- GSP
gene-specific primer
- hnRNA
heteronuclear RNA
- MNE
mean normalized expression
- qRT-PCR
quantitative RT-PCR
- RLM-RACE
RNA ligase-mediated rapid amplification of cDNA ends
- SGMS
sphingomyelin synthase
- SM
sphingomyelin
- SMS
sphingomyelin synthase
- STAT5
signal transducer and activator of transcription 5
- TKI
tyrosine kinase inhibitor
- TSS
transcription start site
- uORF
upstream open reading frame
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Moorthi designed and performed research, analyzed data, and wrote the paper; T. A. Burns performed research and analyzed data; G.-Q. Yu performed research; and C. Luberto designed and performed research and assisted in data analysis and the writing of the paper.
REFERENCES
- 1.Huitema K., van den Dikkenberg J., Brouwers J. F., Holthuis J. C. (2004) Identification of a family of animal sphingomyelin synthases. EMBO J. 23, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaoka S., Miyaji M., Kitano T., Umehara H., Okazaki T. (2004) Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J. Biol. Chem. 279, 18688–18693 [DOI] [PubMed] [Google Scholar]
- 3.Bernert J. T., Jr., Ullman M. D. (1981) Biosynthesis of sphingomyelin from erythro-ceramides and phosphatidylcholine by a microsomal cholinephosphotransferase. Biochim. Biophys. Acta 666, 99–109 [DOI] [PubMed] [Google Scholar]
- 4.Hatch G. M., Vance D. E. (1992) Stimulation of sphingomyelin biosynthesis by brefeldin A and sphingomyelin breakdown by okadaic acid treatment of rat hepatocytes. J. Biol. Chem. 267, 12443–12451 [PubMed] [Google Scholar]
- 5.Marggraf W. D., Anderer F. A., Kanfer J. N. (1981) The formation of sphingomyelin from phosphatidylcholine in plasma membrane preparations from mouse fibroblasts. Biochim. Biophys. Acta 664, 61–73 [DOI] [PubMed] [Google Scholar]
- 6.Marggraf W. D., Zertani R., Anderer F. A., Kanfer J. N. (1982) The role of endogenous phosphatidylcholine and ceramide in the biosynthesis of sphingomyelin in mouse fibroblasts. Biochim. Biophys. Acta 710, 314–323 [DOI] [PubMed] [Google Scholar]
- 7.Merrill A. H., Jr., Jones D. D. (1990) An update of the enzymology and regulation of sphingomyelin metabolism. Biochim. Biophys. Acta 1044, 1–12 [DOI] [PubMed] [Google Scholar]
- 8.Ullman M. D., Radin N. S. (1974) The enzymatic formation of sphingomyelin from ceramide and lecithin in mouse liver. J. Biol. Chem. 249, 1506–1512 [PubMed] [Google Scholar]
- 9.Voelker D. R. K., Kennedy E. P. (1982) Cellular and enzymic synthesis of sphingomyelin. Biochemistry 21, 2753–2759 [DOI] [PubMed] [Google Scholar]
- 10.Diringer H., Marggraf W. D., Koch M. A., Anderer F. A. (1972) Evidence for a new biosynthetic pathway of sphingomyelin in SV 40 transformed mouse cells. Biochem. Biophys. Res. Commun. 47, 1345–1352 [DOI] [PubMed] [Google Scholar]
- 11.Slotte J. P. (2013) Biological functions of sphingomyelins. Prog. Lipid Res. 52, 424–437 [DOI] [PubMed] [Google Scholar]
- 12.Hannun Y. A. (1996) Functions of ceramide in coordinating cellular responses to stress. Science 274, 1855–1859 [DOI] [PubMed] [Google Scholar]
- 13.Hannun Y. A., Luberto C. (2000) Ceramide in the eukaryotic stress response. Trends Cell Biol. 10, 73–80 [DOI] [PubMed] [Google Scholar]
- 14.Lavieu G., Scarlatti F., Sala G., Levade T., Ghidoni R., Botti J., Codogno P. (2007) Is autophagy the key mechanism by which the sphingolipid rheostat controls the cell fate decision? Autophagy 3, 45–47 [DOI] [PubMed] [Google Scholar]
- 15.Obeid L. M., Hannun Y. A. (1995) Ceramide: a stress signal and mediator of growth suppression and apoptosis. J. Cell. Biochem. 58, 191–198 [DOI] [PubMed] [Google Scholar]
- 16.Pettus B. J., Chalfant C. E., Hannun Y. A. (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim. Biophys. Acta 1585, 114–125 [DOI] [PubMed] [Google Scholar]
- 17.Ruvolo P. P. (2003) Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol. Res. 47, 383–392 [DOI] [PubMed] [Google Scholar]
- 18.Saba J. D., Obeid L. M., Hannun Y. A. (1996) Ceramide: an intracellular mediator of apoptosis and growth suppression. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 351, 233–240; discussion 240–231 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Li X., Becker K. A., Gulbins E. (2009) Ceramide-enriched membrane domains—structure and function. Biochim. Biophys. Acta 1788, 178–183 [DOI] [PubMed] [Google Scholar]
- 20.Luberto C., Hannun Y. A. (1998) Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J. Biol. Chem. 273, 14550–14559 [DOI] [PubMed] [Google Scholar]
- 21.Lafont E., Milhas D., Carpentier S., Garcia V., Jin Z. X., Umehara H., Okazaki T., Schulze-Osthoff K., Levade T., Benoist H., Ségui B. (2010) Caspase-mediated inhibition of sphingomyelin synthesis is involved in FasL-triggered cell death. Cell Death Differ. 17, 642–654 [DOI] [PubMed] [Google Scholar]
- 22.Hailemariam T. K., Huan C., Liu J., Li Z., Roman C., Kalbfeisch M., Bui H. H., Peake D. A., Kuo M. S., Cao G., Wadgaonkar R., Jiang X. C. (2008) Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscler. Thromb. Vasc. Biol. 28, 1519–1526 [DOI] [PubMed] [Google Scholar]
- 23.Luberto C., Yoo D. S., Suidan H. S., Bartoli G. M., Hannun Y. A. (2000) Differential effects of sphingomyelin hydrolysis and resynthesis on the activation of NF-kappa B in normal and SV40-transformed human fibroblasts. J. Biol. Chem. 275, 14760–14766 [DOI] [PubMed] [Google Scholar]
- 24.Jin Z. X., Huang C. R., Dong L., Goda S., Kawanami T., Sawaki T., Sakai T., Tong X. P., Masaki Y., Fukushima T., Tanaka M., Mimori T., Tojo H., Bloom E. T., Okazaki T., Umehara H. (2008) Impaired TCR signaling through dysfunction of lipid rafts in sphingomyelin synthase 1 (SMS1)-knockdown T cells. Int. Immunol. 20, 1427–1437 [DOI] [PubMed] [Google Scholar]
- 25.Bourteele S., Hausser A., Döppler H., Horn-Müller J., Röpke C., Schwarzmann G., Pfizenmaier K., Müller G. (1998) Tumor necrosis factor induces ceramide oscillations and negatively controls sphingolipid synthases by caspases in apoptotic Kym-1 cells. J. Biol. Chem. 273, 31245–31251 [DOI] [PubMed] [Google Scholar]
- 26.Watanabe M., Kitano T., Kondo T., Yabu T., Taguchi Y., Tashima M., Umehara H., Domae N., Uchiyama T., Okazaki T. (2004) Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. Cancer Res. 64, 1000–1007 [DOI] [PubMed] [Google Scholar]
- 27.Van der Luit A. H., Budde M., Zerp S., Caan W., Klarenbeek J. B., Verheij M., Van Blitterswijk W. J. (2007) Resistance to alkyl-lysophospholipid-induced apoptosis due to downregulated sphingomyelin synthase 1 expression with consequent sphingomyelin- and cholesterol-deficiency in lipid rafts. Biochem. J. 401, 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding T., Li Z., Hailemariam T., Mukherjee S., Maxfield F. R., Wu M. P., Jiang X. C. (2008) SMS overexpression and knockdown: impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis. J. Lipid Res. 49, 376–385 [DOI] [PubMed] [Google Scholar]
- 29.Miró-Obradors M. J., Osada J., Aylagas H., Sánchez-Vegazo I., Palacios-Alaiz E. (1993) Microsomal sphingomyelin accumulation in thioacetamide-injured regenerating rat liver: involvement of sphingomyelin synthase activity. Carcinogenesis 14, 941–946 [DOI] [PubMed] [Google Scholar]
- 30.Riboni L., Viani P., Bassi R., Giussani P., Tettamanti G. (2001) Basic fibroblast growth factor-induced proliferation of primary astrocytes. evidence for the involvement of sphingomyelin biosynthesis. J. Biol. Chem. 276, 12797–12804 [DOI] [PubMed] [Google Scholar]
- 31.Tafesse F. G., Huitema K., Hermansson M., van der Poel S., van den Dikkenberg J., Uphoff A., Somerharju P., Holthuis J. C. (2007) Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J. Biol. Chem. 282, 17537–17547 [DOI] [PubMed] [Google Scholar]
- 32.Wesley U. V., Hatcher J. F., Dempsey R. J. (2015) Sphingomyelin synthase 1 regulates Neuro-2a cell proliferation and cell cycle progression through modulation of p27 expression and Akt signaling. Mol. Neurobiol. 51, 1530–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu R., Yang W., Hu Z. (2016) Inhibition of sphingomyelin synthase 1 affects ceramide accumulation and hydrogen peroxide-induced apoptosis in Neuro-2a cells. Neuroreport 27, 967–973 [DOI] [PubMed] [Google Scholar]
- 34.Separovic D., Hanada K., Maitah M. Y., Nagy B., Hang I., Tainsky M. A., Kraniak J. M., Bielawski J. (2007) Sphingomyelin synthase 1 suppresses ceramide production and apoptosis post-photodamage. Biochem. Biophys. Res. Commun. 358, 196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barceló-Coblijn G., Martin M. L., de Almeida R. F., Noguera-Salvà M. A., Marcilla-Etxenike A., Guardiola-Serrano F., Lüth A., Kleuser B., Halver J. E., Escribá P. V. (2011) Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc. Natl. Acad. Sci. USA 108, 19569–19574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itoh M., Kitano T., Watanabe M., Kondo T., Yabu T., Taguchi Y., Iwai K., Tashima M., Uchiyama T., Okazaki T. (2003) Possible role of ceramide as an indicator of chemoresistance: decrease of the ceramide content via activation of glucosylceramide synthase and sphingomyelin synthase in chemoresistant leukemia. Clin. Cancer Res. 9, 415–423 [PubMed] [Google Scholar]
- 37.Burns T. A., Subathra M., Signorelli P., Choi Y., Yang X., Wang Y., Villani M., Bhalla K., Zhou D., Luberto C. (2013) Sphingomyelin synthase 1 activity is regulated by the BCR-ABL oncogene. J. Lipid Res. 54, 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter B. Z., Mak D. H., Cortes J., Andreeff M. (2010) The elusive chronic myeloid leukemia stem cell: does it matter and how do we eliminate it? Semin. Hematol. 47, 362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrotti D., Silvestri G., Stramucci L., Yu J., Trotta R. (2017) Cellular and molecular networks in chronic myeloid leukemia: the leukemic stem, progenitor and stromal cell interplay. Curr. Drug Targets 18, 377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowell P. C., Hungerford D. A. (1960) A minute chromosome in human chronic granulocytic leukemia. Science 132, 1497. [DOI] [PubMed] [Google Scholar]
- 41.Nowell P. C., Hungerford D. A. (1960) Chromosome studies on normal and leukemic human leukocytes. J. Natl. Cancer Inst. 25, 85–109 [PubMed] [Google Scholar]
- 42.Rowley J. D. (1973) Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243, 290–293 [DOI] [PubMed] [Google Scholar]
- 43.Druker B. J., Tamura S., Buchdunger E., Ohno S., Segal G. M., Fanning S., Zimmermann J., Lydon N. B. (1996) Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 2, 561–566 [DOI] [PubMed] [Google Scholar]
- 44.Shah N. P., Tran C., Lee F. Y., Chen P., Norris D., Sawyers C. L. (2004) Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305, 399–401 [DOI] [PubMed] [Google Scholar]
- 45.Weisberg E., Manley P. W., Breitenstein W., Brüggen J., Cowan-Jacob S. W., Ray A., Huntly B., Fabbro D., Fendrich G., Hall-Meyers E., Kung A. L., Mestan J., Daley G. Q., Callahan L., Catley L., Cavazza C., Azam M., Neuberg D., Wright R. D., Gilliland D. G., Griffin J. D. (2005) Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 7, 129–141 [DOI] [PubMed] [Google Scholar]
- 46.Corbin A. S., Agarwal A., Loriaux M., Cortes J., Deininger M. W., Druker B. J. (2011) Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J. Clin. Invest. 121, 396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nardi V., Azam M., Daley G. Q. (2004) Mechanisms and implications of imatinib resistance mutations in BCR-ABL. Curr. Opin. Hematol. 11, 35–43 [DOI] [PubMed] [Google Scholar]
- 48.Azam M., Latek R. R., Daley G. Q. (2003) Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell 112, 831–843 [DOI] [PubMed] [Google Scholar]
- 49.Salas A., Ponnusamy S., Senkal C. E., Meyers-Needham M., Selvam S. P., Saddoughi S. A., Apohan E., Sentelle R. D., Smith C., Gault C. R., Obeid L. M., El-Shewy H. M., Oaks J., Santhanam R., Marcucci G., Baran Y., Mahajan S., Fernandes D., Stuart R., Perrotti D., Ogretmen B. (2011) Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood 117, 5941–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moorthi S., Luberto C. (2015) Role of sphingolipids in hematological malignancies: myeloproliferative disorders. In Bioactive Sphingolipids in Cancer Biology and Therapy (Hannun, Y. A., Luberato, C., Mao, C., Obeid, and L. M., eds.) pp. 53–79, Springer International Publishing, Cham, Switzerland
- 51.Simon P. (2003) Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19, 1439–40 [DOI] [PubMed] [Google Scholar]
- 52.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J. Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elferink C. J., Reiners J. J., Jr. (1996) Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20, 470–477 [DOI] [PubMed] [Google Scholar]
- 54.Goldberg S., Schwartz H., Darnell J. E., Jr. (1977) Evidence from UV transcription mapping in HeLa cells that heterogeneous nuclear RNA is the messenger RNA precursor. Proc. Natl. Acad. Sci. USA 74, 4520–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipson K. E., Baserga R. (1989) Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc. Natl. Acad. Sci. USA 86, 9774–9777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlesso N., Frank D. A., Griffin J. D. (1996) Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J. Exp. Med. 183, 811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ilaria R. L., Jr., Van Etten R. A. (1996) P210 and P190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J. Biol. Chem. 271, 31704–31710 [DOI] [PubMed] [Google Scholar]
- 58.Shuai K., Halpern J., ten Hoeve J., Rao X., Sawyers C. L. (1996) Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene 13, 247–254 [PubMed] [Google Scholar]
- 59.Van der Velden A. W., Thomas A. A. (1999) The role of the 5′ untranslated region of an mRNA in translation regulation during development. Int. J. Biochem. Cell Biol. 31, 87–106 [DOI] [PubMed] [Google Scholar]
- 60.Johnstone T. G., Bazzini A. A., Giraldez A. J. (2016) Upstream ORFs are prevalent translational repressors in vertebrates. EMBO J. 35, 706–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vladychenskaya I. P., Dergunova L. V., Dmitrieva V. G., Limborska S. A. (2004) Human gene MOB: structure specification and aspects of transcriptional activity. Gene 338, 257–265 [DOI] [PubMed] [Google Scholar]
- 62.Dergunova L. V., Rozhkova A. V., Sudarkina O. Y., Limborska S. A. (2013) The use of alternative polyadenylation in the tissue-specific regulation of human SMS1 gene expression. Mol. Biol. Rep. 40, 6685–6690 [DOI] [PubMed] [Google Scholar]
- 63.Yang Z., Jean-Baptiste G., Khoury C., Greenwood M. T. (2005) The mouse sphingomyelin synthase 1 (SMS1) gene is alternatively spliced to yield multiple transcripts and proteins. Gene 363, 123–132: [DOI] [PubMed] [Google Scholar]
- 64.Rozhkova A. V., Dmitrieva V. G., Zhapparova O. N., Sudarkina O. Y., Nadezhdina E. S., Limborska S. A., Dergunova L. V. (2011) Human sphingomyelin synthase 1 gene (SMS1): organization, multiple mRNA splice variants and expression in adult tissues. Gene 481, 65–75 [DOI] [PubMed] [Google Scholar]
- 65.Sudarkina O. Y., Filippenkov I. B., Brodsky I. B., Limborska S. A., Dergunova L. V. (2015) Comparative analysis of sphingomyelin synthase 1 gene expression at the transcriptional and translational levels in human tissues. Mol. Cell. Biochem. 406, 91–99 [DOI] [PubMed] [Google Scholar]
- 66.Berger A., Sexl V., Valent P., Moriggl R. (2014) Inhibition of STAT5: a therapeutic option in BCR-ABL1-driven leukemia. Oncotarget 5, 9564–9576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atchison M., Basu A., Zaprazna K., Papasani M. (2011) Mechanisms of Yin Yang 1 in oncogenesis: the importance of indirect effects. Crit. Rev. Oncog. 16, 143–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maru Y. (2012) Molecular biology of chronic myeloid leukemia. Cancer Sci. 103, 1601–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wertheim J. A., Forsythe K., Druker B. J., Hammer D., Boettiger D., Pear W. S. (2002) BCR-ABL-induced adhesion defects are tyrosine kinase-independent. Blood 99, 4122–4130 [DOI] [PubMed] [Google Scholar]
- 70.Warmuth M., Bergmann M., Priess A., Häuslmann K., Emmerich B., Hallek M. (1997) The Src family kinase Hck interacts with Bcr-Abl by a kinase-independent mechanism and phosphorylates the Grb2-binding site of Bcr. J. Biol. Chem. 272, 33260–33270 [DOI] [PubMed] [Google Scholar]
- 71.Machova Polakova K., Koblihova J., Stopka T. (2013) Role of epigenetics in chronic myeloid leukemia. Curr. Hematol. Malig. Rep. 8, 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davuluri R. V., Suzuki Y., Sugano S., Zhang M. Q. (2000) CART classification of human 5′ UTR sequences. Genome Res. 10, 1807–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Araujo P. R., Yoon K., Ko D., Smith A. D., Qiao M., Suresh U., Burns S. C., Penalva L. O. (2012) Before it gets started: regulating translation at the 5′ UTR. Comp. Funct. Genomics 2012, 475731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hughes T. A. (2006) Regulation of gene expression by alternative untranslated regions. Trends Genet. 22, 119–122 [DOI] [PubMed] [Google Scholar]
- 75.Maire P., Gautron S., Hakim V., Gregori C., Mennecier F., Kahn A. (1987) Characterization of three optional promoters in the 5′ region of the human aldolase A gene. J. Mol. Biol. 197, 425–438 [DOI] [PubMed] [Google Scholar]
- 76.Costanzo P., Lupo A., Rippa E., Grosso M., Salvatore F., Izzo P. (1993) Multiple control elements regulate transcription from the most distal promoter of human aldolase A gene. Biochem. Biophys. Res. Commun. 195, 935–944 [DOI] [PubMed] [Google Scholar]
- 77.Lupo A., Costanzo P., Medugno L., Salvatore F., Izzo P. (1995) Characterization of a silencer that modulates transcription of the human distal aldolase A promoter. Biochem. Biophys. Res. Commun. 216, 69–77 [DOI] [PubMed] [Google Scholar]
- 78.Asally M., Yoneda Y. (2005) Beta-catenin can act as a nuclear import receptor for its partner transcription factor, lymphocyte enhancer factor-1 (lef-1). Exp. Cell Res. 308, 357–363 [DOI] [PubMed] [Google Scholar]
- 79.Bujko M., Kober P., Rusetska N., Wakuła M., Goryca K., Grecka E., Matyja E., Neska J., Mandat T., Bonicki W., Siedlecki J. A. (2016) Aberrant DNA methylation of alternative promoter of DLC1 isoform 1 in meningiomas. J. Neurooncol. 130, 473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ko F. C., Yeung Y. S., Wong C. M., Chan L. K., Poon R. T., Ng I. O., Yam J. W. (2010) Deleted in liver cancer 1 isoforms are distinctly expressed in human tissues, functionally different and under differential transcriptional regulation in hepatocellular carcinoma. Liver Int. 30, 139–148 [DOI] [PubMed] [Google Scholar]
- 81.Li T. W., Ting J. H., Yokoyama N. N., Bernstein A., van de Wetering M., Waterman M. L. (2006) Wnt activation and alternative promoter repression of LEF1 in colon cancer. Mol. Cell. Biol. 26, 5284–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yokoyama N. N., Pate K. T., Sprowl S., Waterman M. L. (2010) A role for YY1 in repression of dominant negative LEF-1 expression in colon cancer. Nucleic Acids Res. 38, 6375–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaika A. I., Slade N., Erster S. H., Sansome C., Joseph T. W., Pearl M., Chalas E., Moll U. M. (2002) DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J. Exp. Med. 196, 765–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landers J. E., Cassel S. L., George D. L. (1997) Translational enhancement of mdm2 oncogene expression in human tumor cells containing a stabilized wild-type p53 protein. Cancer Res. 57, 3562–3568 [PubMed] [Google Scholar]
- 85.Hughes T. A., Brady H. J. (2005) Expression of axin2 is regulated by the alternative 5′-untranslated regions of its mRNA. J. Biol. Chem. 280, 8581–8588 [DOI] [PubMed] [Google Scholar]
- 86.Hughes T. A., Brady H. J. (2006) Regulation of axin2 expression at the levels of transcription, translation and protein stability in lung and colon cancer. Cancer Lett. 233, 338–347 [DOI] [PubMed] [Google Scholar]
- 87.Clemens M. J., Bommer U. A. (1999) Translational control: the cancer connection. Int. J. Biochem. Cell Biol. 31, 1–23 [DOI] [PubMed] [Google Scholar]
- 88.Davuluri R. V., Suzuki Y., Sugano S., Plass C., Huang T. H. (2008) The functional consequences of alternative promoter use in mammalian genomes. Trends Genet. 24, 167–177 [DOI] [PubMed] [Google Scholar]
- 89.Dieudonné F.-X., O’Connor P. B. F., Gubler-Jaquier P., Yasrebi H., Conne B., Nikolaev S., Antonarakis S., Baranov P. V., Curran J. (2015) The effect of heterogeneous transcription start sites (TSS) on the translatome: implications for the mammalian cellular phenotype. BMC Genomics 16, 986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu C. F., Brown M. A., Chambers J. A., Griffiths B., Nicolai H., Solomon E. (1995) Distinct transcription start sites generate two forms of BRCA1 mRNA. Hum. Mol. Genet. 4, 2259–2264 [DOI] [PubMed] [Google Scholar]
- 91.Sobczak K., Krzyzosiak W. J. (2002) Structural determinants of BRCA1 translational regulation. J. Biol. Chem. 277, 17349–17358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.