Abstract
Hibernating animals show resistance to hypothermia-induced cardiac arrhythmias. However, it is not clear whether and how mammalian hibernators are resistant to ischemia-induced arrhythmias. The goal of this investigation was to determine the susceptibility of woodchucks (Marmota monax) to arrhythmias and their mechanisms after coronary artery occlusion at the same room temperature in both winter, the time for hibernation, and summer, when they do not hibernate. By monitoring telemetric electrocardiograms, we found significantly higher arrhythmia scores, calculated as the severity of arrhythmias, with incidence of ventricular tachycardia, ventricular fibrillation, and thus sudden cardiac death (SCD) in woodchucks in summer than they had in winter. The level of catalase expression in woodchuck hearts was significantly higher, whereas the level of oxidized Ca2+/calmodulin-dependent protein kinase II (CaMKII) was lower in winter than it was in summer. Ventricular myocytes isolated from woodchucks in winter were more resistant to H2O2-induced early afterdepolarizations (EADs) compared with myocytes isolated from woodchucks in summer. The EADs were eliminated by inhibiting CaMKII (with KN-93), l-type Ca current (with nifedipine), or late Na+ current (with ranolazine). In woodchucks, in the summer, the arrhythmia score was significantly reduced by overexpression of catalase (via adenoviral vectors) or the inhibition of CaMKII (with KN-93) in the heart. This study suggests that the heart of the mammalian hibernator is more resistant to ischemia-induced arrhythmias and SCD in winter. Increased antioxidative capacity and reduced CaMKII activity may confer resistance in woodchuck hearts against EADs and arrhythmias during winter. The profound protection conferred by catalase overexpression or CaMKII inhibition in this novel natural animal model may provide insights into clinical directions for therapy of arrhythmias.—Zhao, Z., Kudej, R. K., Wen, H., Fefelova, N., Yan, L., Vatner, D. E., Vatner, S. F., Xie, L.-H. Antioxidant defense and protection against cardiac arrhythmias: lessons from a mammalian hibernator (the woodchuck).
Keywords: hibernating animals, ischemia, oxidative stress, CaMKII, triggered activity
Sudden cardiac death (SCD), which is caused by severe ventricular arrhythmias, such as ventricular tachycardia (VT) and ventricular fibrillation (VF), often occurs after a heart attack or myocardial infarction (1). In patients who have had heart attacks, VFs may occur within minutes or hours after the onset of chest pain. Despite recent advances in both mechanistic study and clinical treatment of arrhythmias, mortality from SCD remains unacceptably high. Thus, new strategies to prevent SCD necessitate a comprehensive understanding of the complex mechanisms underlying initiation and maintenance of ischemia-induced arrhythmias.
Natural hibernation is a way of preserving energy in mammals during cold seasons when there is a shortage of food. It is characterized by marked reductions in oxygen consumption, metabolism, and heart rate (2). When deep hibernation is reached, the heart rate can decrease to 2–10 beats/min. Preliminary studies by Johansson (3, 4) suggest that hibernators are resistant to severe cardiac arrhythmias induced by hypothermia or other proarrhythmic factors. However, detailed mechanisms for the resistance to arrhythmias and SCD in hibernators are not yet fully clarified. It has been reported that hibernator’s hearts undergo adaptation of many aspects during hibernation (5). Those factors include connexins (6), Ca2+ handling proteins (7), and other ion channels and transporters (7, 8). Interestingly, mRNA levels of the antioxidant system (9, 10), calmodulin (CaM), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) (11) have been reported to be remodeled in hibernating animals during hibernation. In addition, our group has previously shown that external application of the reactive oxygen species (ROS), such as H2O2, promotes the generation of early afterdepolarization (EAD) and delayed afterdepolarization (DAD) via the activation (oxidation) of CaMKII in rabbit myocytes (12). Therefore, we hypothesized that the adaptation/remodeling in myocardial antioxidative capacity may account for seasonal difference in arrhythmia-resistance in the hibernators’ hearts.
A recent study from our group has demonstrated that the hibernating woodchuck heart is a novel model for studying cardioprotection because it resembles ischemic preconditioning in the absence of any preconditioning stimuli (13). However, the potential antiarrhythmic property has not been studied in this valuable animal model. In this study, we used the natural hibernator woodchuck as a unique model for studying the underlying molecular and cellular mechanisms that account for its resistance to ischemia-induced arrhythmias. We believe that provision of insights into the natural antiarrhythmic adaptation in hibernating mammals may offer novel approaches toward developing effective therapeutic strategies to prevent/treat ischemia-related arrhythmias in humans.
MATERIALS AND METHODS
Animals
Experiments were performed in wild-caught woodchucks (Marmota monax; ∼1–2 yr old; both genders) either in summer (June–July) or winter (December–January). The woodchucks were purchased from Northeastern Wildlife (Harrison, ID, USA). After being caught in the wild and shipped to the animal facility in Rutgers-New Jersey Medical School (Newark, NJ, USA), the woodchucks were housed in individual cages with free access to food and water and were exposed to 12/12-h light/dark cycles under room temperature (24–26°C) in both summer and winter seasons. The animals were normally kept in this captive housing condition for 2–4 wk before the terminal studies. This investigation conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (Bethesda, MD, USA; NIH publication 85-23, revised 2011). All animal experimental procedure were reviewed and approved by the Institutional Animal Care and Use Committee at the Rutgers University-New Jersey Medical School.
Coronary artery occlusion procedure
General anesthesia was induced with ketamine (37.5 mg/kg, i.m.) and xylazine (2.5 mg/kg, i.m.), and was maintained with isoflurane (0.5–1.5 vol%) following tracheal intubation. A left thoracotomy was performed at the fifth intercostal space. Coronary artery occlusion (CAO) was performed by ligating the obtuse marginal branch of the left circumflex coronary artery at about the one-third point between the atrioventricular groove and the apex. Electrocardiogram (ECG) signals were monitored with telemetry techniques (see the next section) for 24 h. Ventricular tissues were collected 24 h after CAO for biochemical analyses.
ECG telemetry monitoring
A telemetric transmitter (model d70-pctp; Data Sciences International, St. Paul, MN, USA) was implanted in the peritoneal cavity with paired wire electrodes placed subcutaneously over the thorax (chest bipolar ECG lead). ECGs were recorded from the untethered woodchucks immediately after CAO procedures were conducted and were returned to housing. The ECG data were analyzed off-line for ventricular arrhythmias. An arrhythmia scoring system modified from several articles (14–17) was used to evaluate susceptibility to arrhythmogenesis. Arrhythmias were categorized into 5 groups and assigned the following point values: 0 points, no arrhythmia; 1 point, 1–3 premature ventricular complexes (PVCs); 2 points, nonsustained VT (4–10 consecutive PVCs, including bigeminal/trigeminal PVCs); 3 points, sustained VT (≥10 consecutive PVCs); and 4 points, VF/SCD.
Monophasic action potentials recording
Monophasic action potentials (MAPs) were recorded with an epicardial contact electrode (Harvard Apparatus, Holliston, MA, USA) and a differential amplifier (Warner Instruments, Hamden, CT, USA), as previously reported (18–20). Alternation of action potential duration (APD) and occurrence of early afterdepolarizations (EADs) or delayed afterdepolarizations (DADs) were monitored in 4 woodchucks before and after acute CAO procedures in the summer.
Western blot analysis
Total protein extract from heart tissues (or isolated myocytes) were prepared in a lysis buffer containing protease, kinase, and phosphatase inhibitors. See online Supplemental Materials for details.
Myocyte isolation
Ventricular myocytes were enzymatically isolated from woodchuck hearts as previously described (with modifications) (12). Briefly, woodchucks (1–2 yr old; 2–3 kg) of either gender were injected with 1000 U of heparin and then euthanized by overdose of Euthasol (pentobarbital 390 mg/ml + phenytoin 50 mg/ml; Virbac, Fort Worth, TX, USA) via a vein in the right front leg. The heart was removed via a left thoracotomy and retrograde-perfused in a Langendorff fashion. After perfusion with oxygenated, nominally Ca2+-free Tyrode’s solution for 4–5 min, the solution was changed to the Ca2+-free Tyrode’s solution containing ∼1.4 mg/ml collagenase (type II; Worthington Biochemical, Lakewood, NJ, USA) and 0.1 mg/ml protease (type XIV; MilliporeSigma, Billerica, MA, USA) for 25–30 min at 37°C. After washing the enzyme solution with a 0.2-mM Ca2+-containing Tyrode’s solution, the heart was removed from the perfusion apparatus. Myocytes were exclusively isolated from the free wall of the left ventricle. The cells were triturated gently with a Pasteur pipette and swirled in a culture dish. The Ca2+ concentration was increased to 1.0 mM, and the cells were stored at room temperature and were used within 8 h.
Patch-clamp experiments
Myocytes were patch-clamped using the whole-cell configuration of the patch-clamp technique. Action potentials (APs) and whole cell currents were recorded under a current clamp and voltage clamp configuration, respectively. For AP recordings, patch pipettes (resistance, 2–4 MΩ) were filled with pipette solution containing (in millimolars): 110 K-aspartate, 30 KCl, 5 NaCl, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 0.1 EGTA, 5 MgATP, 5 Na2-creatine phosphate, and 0.05 cAMP, pH 7.2, adjusted with KOH. The cells were superfused with a modified Tyrode’s solution containing (in millimolars): 136 NaCl, 5.4 KCl, 0.33 Na2PO4, 1.0 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.4, adjusted with NaOH. To record the l-type calcium current (ICa,L), patch pipettes (resistance 2–4 MΩ) were filled with pipette solution containing (in millimolars): 110 Cs-aspartate, 30 CsCl, 5 NaCl, 10 HEPES, 0.1 EGTA, 5 MgATP, 5 Na2–creatine phosphate, and 0.05 cAMP, pH 7.2, adjusted with CsOH, and the cells were superperfused with a modified Tyrode’s solution in which KCl was replaced by CsCl. Sodium current (INa) was inactivated with a prepulse step (−80 to −40 mV for 40 ms). Membrane current and voltage were measured with a MultiClamp 700 A patch-clamp amplifier controlled with a personal computer and a DigiData 1322 acquisition board (Molecular Devices, Sunnyvale, CA, USA) driven by pCLAMP 10 software (Molecular Devices). All patch-clamp experiments were performed at 34–36°C.
Intracellular calcium measurement
Myocytes were loaded with the Ca indicator Fluo-4 AM (Thermo Fisher Scientific, Waltham, MA, USA). Fluo-4 fluorescence was excited at 485 nm, and the emission was measured at 515 nm. The fluorescence was monitored with an Eclipse TE200 inverted microscope (Nikon, Tokyo, Japan) with a Fluor ×40 oil objective lens (numerical aperture 1.3) and recorded with an Ixon charge-coupled device camera (Andor Technology, Belfast, United Kingdom) operating at ∼50 frames/s with a spatial resolution of 500 × 400 pixels. Two-dimensional Ca2+ imaging of isolated myocytes allowed visualization of the intracellular calcium (Cai) transient dynamics in different regions of the myocyte. Fluorescence intensity was recorded as the ratio F/F0 of the fluorescence (F) to the basal diastolic fluorescence (F0).
Minipump delivery of drugs (KN-93 and KN-92)
To maintain a stable plasma concentration, the drugs were continuously delivered via osmotic minipumps (model 2 ML1; Alza Corporation, Johnson & Johnson, Vacaville, CA, USA) as previously described in rabbits (21). The pump was inserted into an s.c. pocket and secured to the abdominal wall with sutures on the left flank. Right femoral arteries of woodchucks were ligated with 2 ligatures about 1.5 cm apart, and the pump was connected to the proximal stump of the ligated femoral artery by a catheter pointing upstream. One group of woodchucks (n = 4) received the membrane-permeable CaMKII inhibitor KN-93 (125 μg/kg per hour) after an initial bolus (300 μg/kg) was injected via the femoral vein before CAO. Another group (n = 4) received KN-92 as a control. After implantation of the osmotic minipumps, animals underwent a CAO protocol (for 24 h) as described above.
Intramyocardial injection of catalase adenovirus
Animals were anesthetized and underwent a thoracotomy for intramyocardial injection of adenovirus vector harboring catalase (Ad-catalase) or green fluorescent protein (Ad-GFP, control; Vector Biolabs, Malvern, PA, USA). A total of 0.5 ∼ 1 × 109 plaque-forming units per heart Ad-catalase or Ad-GFP was delivered via direct myocardial injection using a 31-gauge needle attached to an insulin syringe at 4 sites in the ischemia risk area of the left ventricle. After adenovirus administration, the chest was closed, and the animals recovered for 48 h. The targeted proteins were allowed to express for 48 h. Then, the woodchucks underwent the CAO procedure, and telemetric ECGs were monitored for 24 h before the animals were sacrificed and the left ventricular tissues were collected.
Statistical analysis
All data were shown as means ± sem. Statistical differences were evaluated with Student’s paired or unpaired t tests. Values of P < 0.05 were considered statistically significant.
RESULTS
Woodchuck hearts exhibited intrinsic resistance to ischemia-induced arrhythmias and SCD in winter
Woodchuck, the largest true hibernator, has been used in our laboratory for cardiac-protection studies (13). One of our projects was designed to evaluate remodeling changes in woodchuck hearts subjected to permanent (2 mo) coronary occlusion (and subsequent myocardial infarction). We performed the same permanent CAO surgeries in 33 woodchucks in summer and 20 woodchucks in winter. (The long-term remodeling results will be reported in a different article). Interestingly, we observed a striking difference in mortality rates between woodchucks in the summer and those in the winter during the first 24 h after the CAO. In summer, 42.4% of the woodchucks died within the first 24 h after CAO. In contrast, a significantly lower mortality rate (5%) was found in woodchucks during winter (P < 0.05, with Fisher’s exact test), indicating that woodchucks exhibit greater resistance to ischemia-induced sudden death in winter than they do in summer.
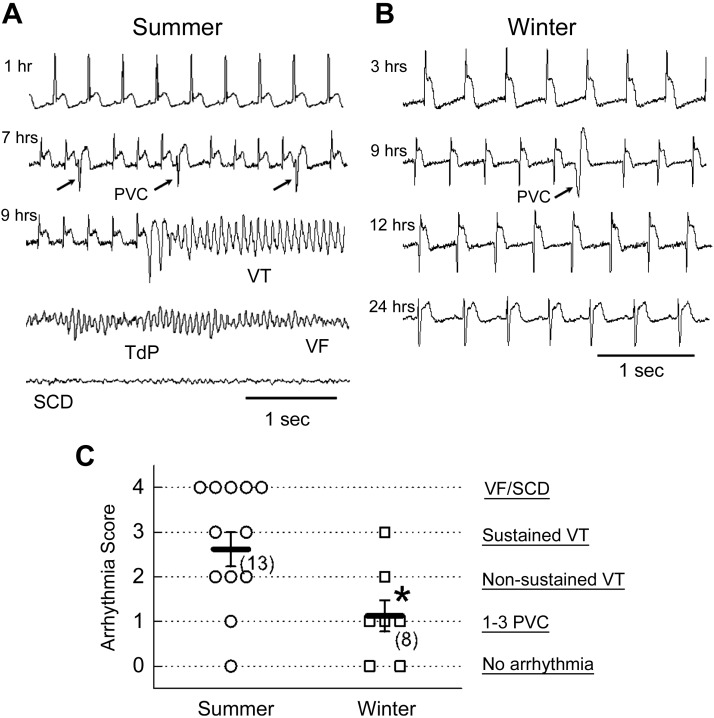
To determine the cause of death in the woodchucks after CAO, we monitored telemetric ECGs in 13 woodchucks in winter and 8 woodchucks in summer. Representative ECG traces recorded from woodchucks after CAO surgery either in summer (Fig. 1A) or in winter (Fig. 1B) are depicted. The ST-segments were elevated in both cases, indicating the apparent cardiac ischemia induced by CAO. In summer (Fig. 1A), frequent PVCs (as indicated by the arrows) were observed at 7 h after CAO and an episode of VT at 9 h after CAO. Eventually the VT transformed to VF and caused SCD (the bottom trace in Fig. 1A). We observed the same ECG appearances in 5 (of 13) woodchucks that died within 24 h after CAO in summer, indicating the deaths were due to lethal arrhythmias, such as VTs/VFs in summer. In winter, however, only sporadically occurring PVCs were observed (as indicated by the arrow at 9 h after CAO), without VTs/VFs occurring in this representative recording (Fig. 1B). The same experiments were performed in 8 woodchucks in winter, but only 1 benign monomorphic VT episode was detected in 1 woodchuck in winter. As shown in the summarized results (Fig. 1C), the arrhythmia score was significantly lower in woodchucks in winter than it was in summer, suggesting greater resistance to ischemia-induced lethal arrhythmias in the hearts of mammalian hibernators in winter.
Figure 1.
Lower incidence of arrhythmias and SCD induced by CAO in woodchucks in winter than in summer. A) Representative ECG traces recorded from a woodchuck in summer at 1, 7, and 9 h after CAO. PVCs (arrows) VT, Torsade de Points (TdP), and VF were observed before SCD occurred. B) Representative ECG traces recorded in a woodchuck in winter. Time after CAO is indicated to the left of each trace. Only scarcely occurring PVCs (arrow) were observed. C) Summarized data of arrhythmia scores measured in woodchucks in summer and in winter. Both individual data points and means ± sem values are presented. *P < 0.05 compared with the summer group by unpaired Student’s t test.
Afterdepolarizations and triggered arrhythmias occurred during acute ischemia in woodchucks in summer
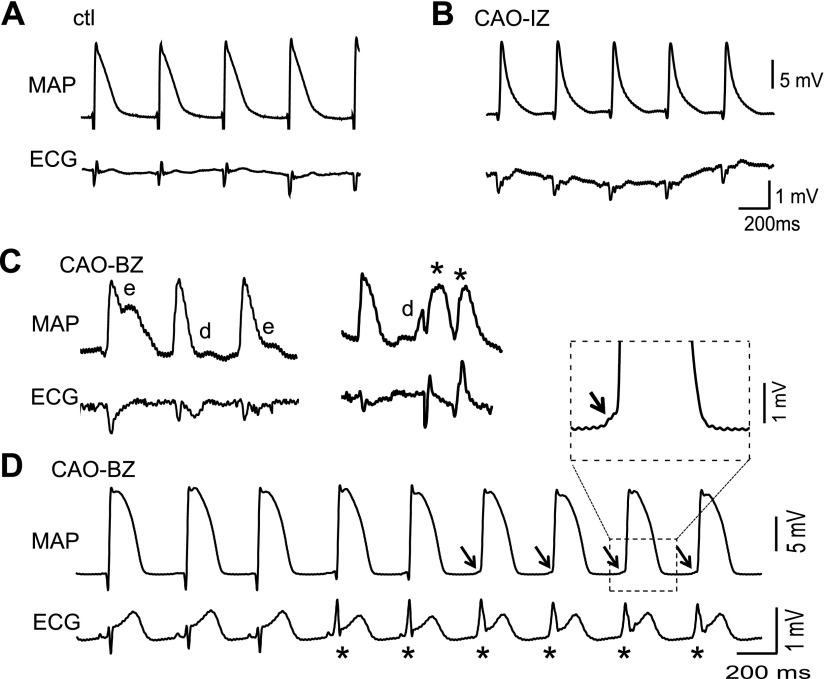
Both afterdepolarizations (i.e., EADs and DADs) and reentry may have pivotal roles in initiation or maintenance of arrhythmias in acute ischemia (22). To obtain evidence for afterdepolarizations occurring in this woodchuck ischemic model, we recorded MAPs simultaneously with ECG signals from woodchucks after the CAO procedure in summer. Representative episodes of MAP and ECG recordings are shown in Fig. 2. Although normal AP and ECG were obtained under control condition (Fig. 2A, before CAO), typical ischemic alterations, i.e., shortening of APD and triangulation of AP morphology were observed in the ischemic zone immediately after CAO (Fig. 2B). At 1 h after CAO, EADs, DADs, and triggered PVCs were recorded at border zone (Fig. 2C) in 2 of 4 hearts. A sustained VT (as indicated by asterisks in the ECG, with altered QRS morphology and disappearance of P waves) were observed from 1 woodchuck (Fig. 2D). The smooth, spontaneously depolarizing phases at the beginning of the MAPs (a sample as expanded in the inset) implicated DADs as the likely trigger for the VT. These results, obtained from intact animal hearts, suggest that afterdepolarizations and triggered activities are involved in the genesis of ischemia-induced arrhythmias in woodchucks in summer.
Figure 2.
Ischemia-induced afterdepolarizations and triggered activities in intact hearts of woodchucks in summer. A) Representative, normal MAP and ECG signals recorded from the left ventricular epicardium before CAO. ctl, control B) Shortened MAP duration in ischemic zone (IZ) at 1 h after CAO. C) MAP and ECG traces recorded from border zone (BZ) during CAO (1 h), showing EADs (e), DADs (d), and PVCs (*). D) Ventricular tachycardia (*) observed during CAO. Arrows show smooth depolarizing phase, suggesting DADs are the triggers. Note the disappearance of the P waves and alteration of QRS complexes in the ECG signal.
Expression levels of intrinsic antioxidant enzymes were increased in woodchuck hearts in winter compared with summer
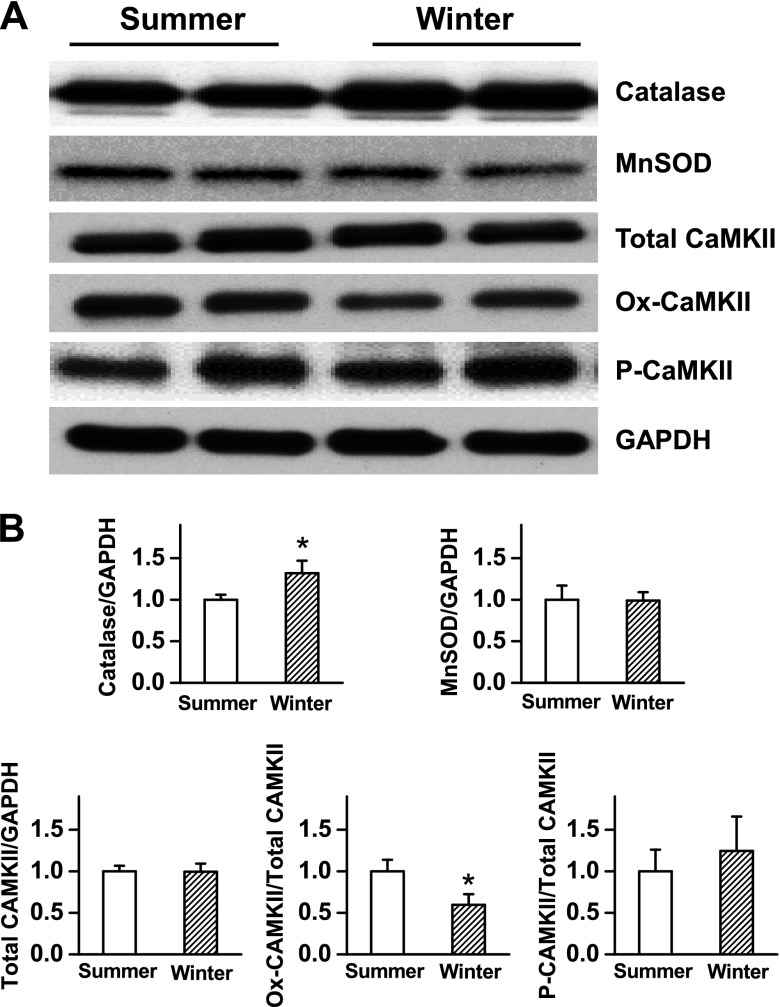
Disruption of the reduction–oxidation balance may contribute mechanistically to disorders of cardiac electrophysiology and the risk of arrhythmias. Recent studies, including ours, have suggested a close link among oxidant stress, activation of CaMKII, and cardiac arrhythmogenesis (12, 23, 24), probably in ischemic or failing hearts. To determine the capacity of cardiac tissues to tolerate oxidative stress by the intrinsic antioxidant defense system, we next conducted Western blotting analyses and compared the expression levels of key antioxidant enzymes. The heart tissues were collected from the left ventricles from control woodchucks in summer and in winter at baseline. As shown in Fig. 3, the expression level of catalase was significantly greater in woodchuck hearts in winter than it was in summer, whereas the magnesium superoxide dismutase (MnSOD) levels remained the same. These results suggest that the woodchuck hearts seem to be prepared with greater antioxidant capacity in winter in comparison with summer.
Figure 3.
Western blot analysis of the antioxidant enzymes and activation of CaMKII in woodchuck hearts in summer vs. winter. A) Representative blot bands of catalase, MnSOD, total CaMKII, Ox-CaMKII, and P-CaMKII in the left ventricular tissue of woodchucks in summer vs. winter. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B) Summarized bar graph showing the levels of catalase, MnSOD total CaMKII, Ox-CaMKII ratio, and P-CaMKII ratio in woodchucks in summer vs. winter. Data are presented as means ± sem. *P < 0.05 compared with the normalized summer group (n = 4–6).
Oxidized CaMKII levels were lower in woodchuck hearts in winter than in summer
Previous studies have suggested that extensive activation (oxidation) of CaMKII links to arrhythmogenesis under oxidative stress condition (12, 17, 25, 26). To determine whether CaMKII activation is involved in different arrhythmic susceptibilities in woodchucks in different seasons, we also compared the levels of oxidized CaMKII (Ox-CaMKII) and phosphorylated CaMKII (P-CaMKII) in woodchuck hearts in winter vs. summer at baseline. As shown in Fig. 3, although the total CaMKII expression level remained the same in the hearts in winter and summer, the level of Ox-CaMKII (ratio Ox-CaMKII to CaMKII) was significantly less in woodchuck hearts in winter, consistent with a higher level of antioxidant in winter than in summer. No significant difference in the level of P-CaMKII (P-CaMKII/CaMKII) was found in woodchuck hearts between winter and summer. Taken together, these results indicate that the activities of CaMKII are lessened in woodchuck heart tissue in winter compared with that in summer, likely because of stronger antioxidative ability (i.e., greater catalase expression levels).
Woodchuck ventricular myocytes exhibited greater resistance to H2O2-induced EADs in winter than in summer
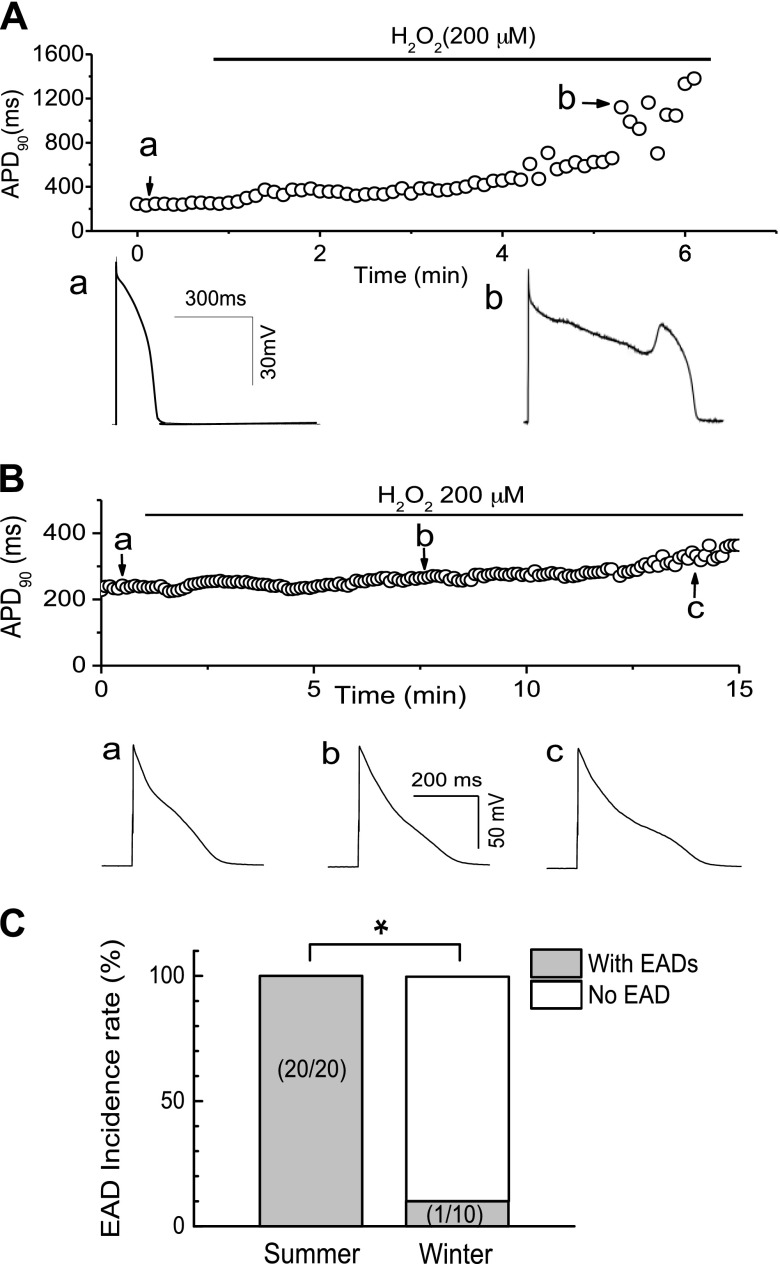
Our previous study showed that ROS lead to EAD generation via the activation of ICa,L and INa, mediated by CaMKII in rabbit myocytes (12). To test whether the same cellular mechanism is involved in woodchuck myocytes and may account for different susceptibilities to arrhythmogenesis, we performed patch-clamp experiments in isolated ventricular myocytes. As shown in Fig. 4A, we examined the effect of H2O2 on APs recorded in ventricular myocytes isolated from woodchucks in summer. After APD and morphology reached a steady state (control), the cells were superfused with 200 µM H2O2. EADs were induced at ∼4 min after H2O2 perfusion in this representative cell. The summarized results (Fig. 4C) showed that EADs were consistently induced with H2O2 (200 µM) at an average perfusion period (3.5 ± 0.3 min) in all 20 myocytes isolated from 3 woodchucks in summer.
Figure 4.
Lower incidence of H2O2-induced EADs in woodchuck myocytes in winter compared with those in summer. A) Time course of APD at 90% repolarization (APD90) in a ventricular myocyte isolated from woodchuck in the summer group after it was superfused with 200 µM H2O2. EADs were induced 4 min after H2O2 perfusion. Representative APs at points a and b are shown below. B) No EADs were observed in a ventricular myocyte isolated from woodchuck in the winter group even after >14 min H2O2 perfusion. Representative APs at points a–c are shown below. C) Summarized data showing lower incidence rate of EADs in myocytes from woodchucks in the winter group compared with those in the summer group. Numbers of myocytes from 3 woodchucks in each group are indicated. *P < 0.05 compared with the summer group using Fisher’s exact test.
To test our hypothesis that myocytes from woodchucks in winter are more resistant to ROS-induced EADs and triggered activities (thus to cardiac arrhythmias), we carried out the same experiments in single ventricular myocytes isolated from woodchucks in winter. As shown in a representative recording (Fig. 4B), no EAD was induced by H2O2 (200 µM) perfusion for up to 14 min in this case (although APD was slightly prolonged). EADs were induced only in 1 cell (of 10 total cells) at 16 min after H2O2 (200 µM) perfusion in woodchuck cells in winter, which is a much longer perfusion time than needed in woodchuck myocytes in summer. Taken together, these results demonstrate that myocytes from woodchucks in winter exhibit lessened susceptibility to oxidative stress–induced EADs.
Cellular and ionic mechanisms of EADs
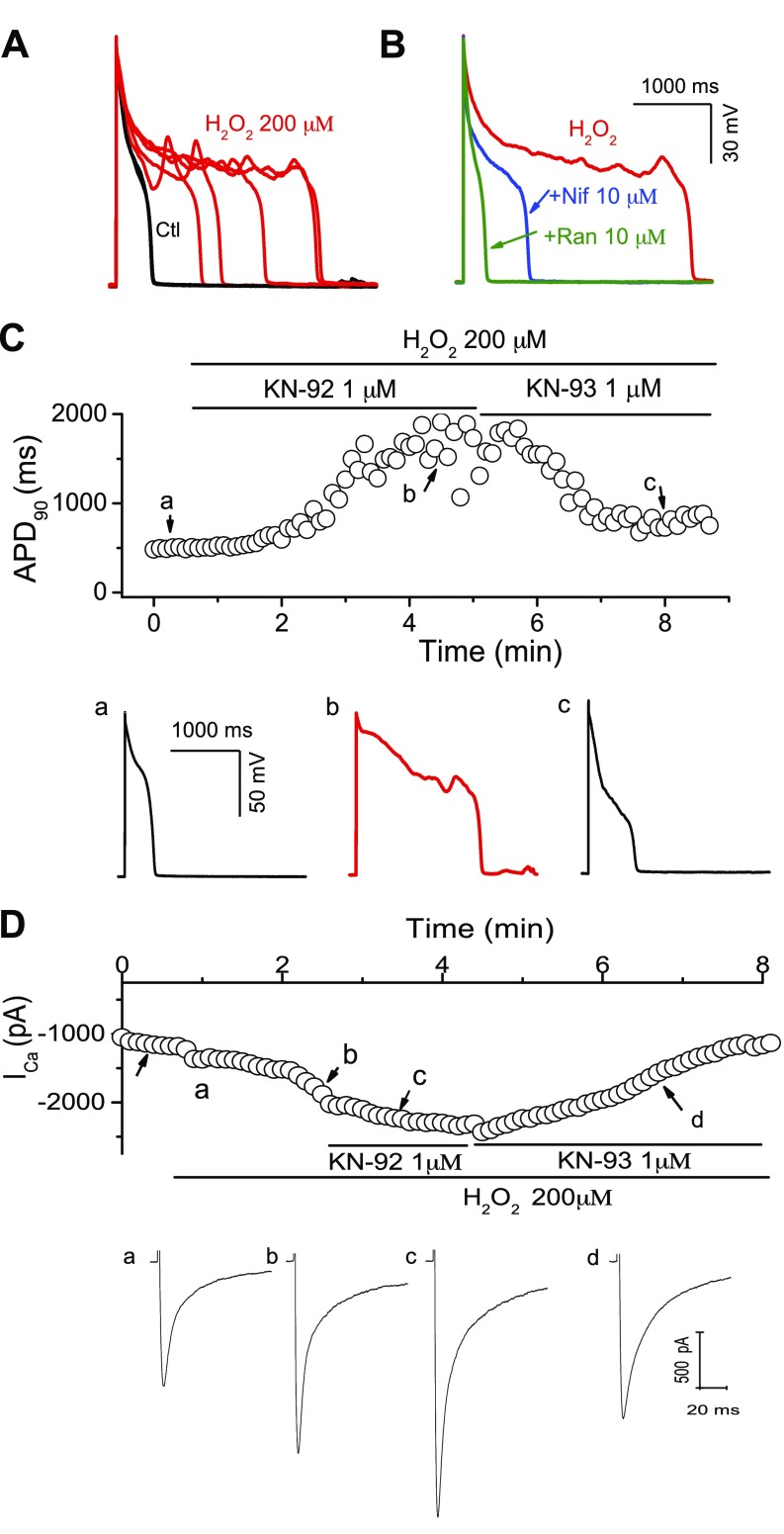
Because EADs were only readily induced in woodchuck myocytes in summer, we next analyzed the potential ionic mechanisms for H2O2-induced EADs in ventricular myocytes isolated from woodchucks in summer. As shown in Fig. 5A, B, H2O2-induced EADs were reversibly suppressed by ranolazine (10 µM), a selective late-phase Na+ current blocker. In addition, the selective ICa,L blocker nifedipine (10 µM) also eliminated EADs. These results were consistent with previous reports implicating both late INa and ICa,L in H2O2-induced EADs (12, 27, 28).
Figure 5.
Ionic mechanisms of H2O2-induced EADs in woodchuck myocytes in summer. A) Examples of EADs during exposure to H2O2. B) Suppression of EADs by the ICa,L blocker nifedipine (Nif) and the late INa blocker ranolazine (Ran). C) Suppression of H2O2-induced EADs by the CaMKII inhibitor KN-93, but not by KN-92, an inactive analog. Time course of APD90 (upper panel), and representative APs at time points a–c are shown. D) Suppression of H2O2-activated ICa,L by the CaMKII inhibitor KN-93. Time course of peak ICa,L (upper panel) and representative ICa,L traces at points a–c are shown.
Next, we tested the hypothesis that H2O2 induces EADs via activation of the intracellular CaMKII signaling pathway. The selective CaMKII inhibitor KN-93 (1 µM) attenuated the incidence of EADs, whereas KN-92 (1 µM), an inactive analog of KN-93, was ineffective at preventing EADs induced by treatment with 200 µM H2O2 (Fig. 5C). In agreement with EAD elimination, inhibition of CaMKII by KN-93 was also able to attenuate ICa,L enhanced by H2O2 treatment (Fig. 5D). The same results were observed in 4 cells. These results resemble those we previously obtained in rabbit cells (12), suggesting that elevated ROS (H2O2) levels consistently induce EADs in ventricular myocytes of woodchucks in summer, most likely via CaMKII activation.
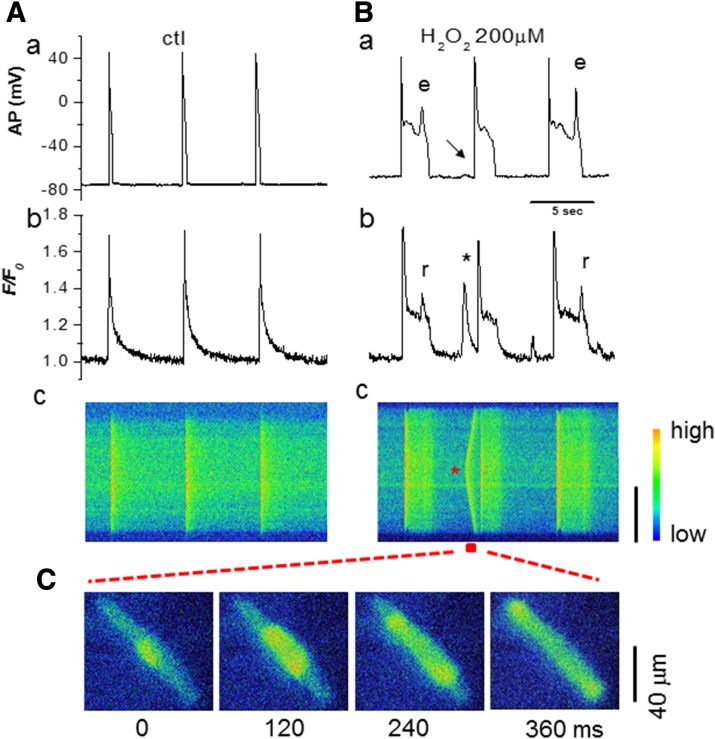
The involvement of Cai mishandling was examined by the simultaneous recordings of AP and Cai transients. Regular APs and Cai transients were observed under control condition (Fig. 6A). During H2O2-induced EADs (Fig. 6Ba, indicated as point e), Cai2+ remained elevated or increased even further (Fig. 6Bb), consistent with additional sarcoplasmic reticulum (SR) Ca2+ release because of reactivation of ICa,L. The pseudo line scan (Fig. 6Bc) and snapshots (Fig. 6C) clearly showed the elevation in intracellular Ca levels and propagation of a Ca2+ wave. These results suggest spontaneous SR Ca2+ release, in addition to ICa,L and late INa enhancement, contributes to EAD formation, similar to other animal species (12, 29–31). A DAD (the arrow in Fig. 6Ba), corresponding to a spontaneous Ca2+ wave [Fig. 6Bb indicated by an asterisk (*)], was also observed after prolonged perfusion of H2O2 in this case.
Figure 6.
Cai mishandling induced by H2O2 in woodchuck myocytes in summer. A) AP (a), Cai transient (b), and pseudoline-scan image (c) before H2O2. B) AP (a), Cai transient (b), and pseudoline-scan image (c) after H2O2 exposure for 5 min. APD prolongation and EADs occurred, leading to persistent elevation in Cai and additional Ca2+ release (r). Spontaneous Ca release (* in b) during the diastolic period correlated with a DAD (arrow in a). C) Snap shots from the time window indicated by the red horizontal bar in Bc, when the Cai wave propagated from the center to the 2 ends of the cell.
Overexpression of catalase suppressed CAO-induced arrhythmias in woodchucks in summer
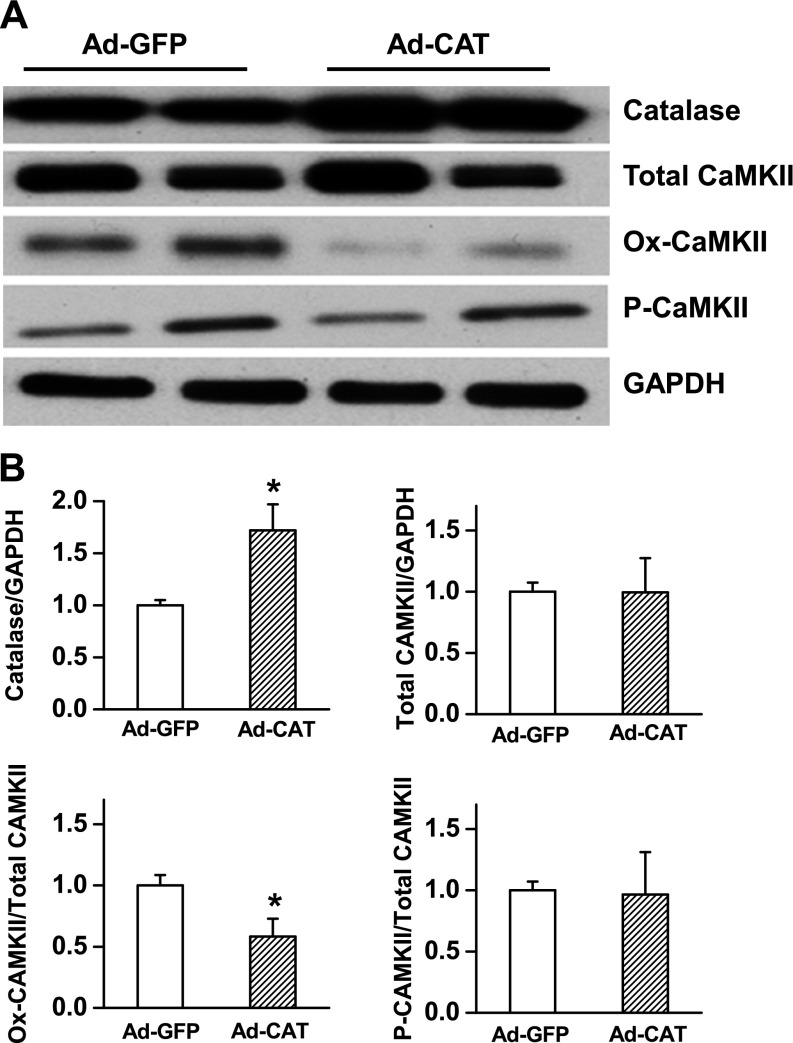
Catalase is a major antioxidant enzyme that reduces ROS levels. To examine the potential role of catalase in conferring antiarrhythmic ability in woodchuck hearts, we employed a proof-of-concept approach to overexpress catalase, with adenovirus vectors coding for catalase (Ad-catalase). As shown in Fig. 7, the level of catalase expression was significantly up-regulated in the Ad-catalase injection group compared with the Ad-GFP control group. Consistent with more catalase, the level of Ox-CaMKII (ratio Ox-CaMKII/CaMKII) was significantly lower in woodchuck hearts injected with Ad-catalase, whereas the P-CaMKII level was not altered.
Figure 7.
Western blot analysis of the antioxidant enzymes and activation of CaMKII in woodchuck hearts in summer with or without catalase overexpression. A) Representative blot bands of catalase, total CaMKII, Ox-CaMKII, and P-CaMKII in the left ventricular tissue of woodchucks in the summer group with Ad-GFP expression (control group) or Ad-catalase expression. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B) Summarized bar graph showing the levels of catalase, total CaMKII, Ox-CaMKII ratio, and P-CaMKII ratio in woodchucks in the 2 group. Data are presented as means ± sem. *P < 0.05 compared with normalized control group (n = 5–7).
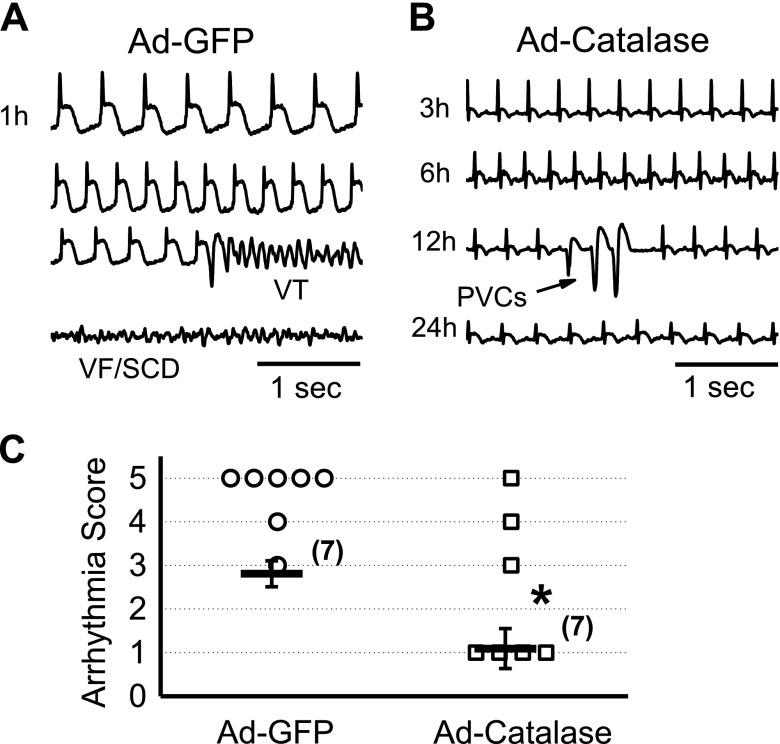
To determine the effect of catalase overexpression on arrhythmia propensity, we performed the same CAO experiments and monitored ECG telemetry for 24 h in woodchucks in summer. A representative recording from a woodchuck in Ad-GFP (control) group is shown in Fig. 8A. An episode of VTs occurred at 1 h after CAO in this case. Eventually the VT transformed to VF and caused SCD. In contrast, in a representative recording from a woodchuck Ad-catalase group (Fig. 8B), only PVCs (indicated by the arrow), but no severe arrhythmia events, such as VT/VF, were observed. As summarized in Fig. 8C, the average arrhythmia score was significantly lower in the Ad-catalase group than it was in the Ad-GFP group, which correlates well with the elevated level of catalase in the Ad-catalase group.
Figure 8.
Overexpression of catalase suppresses CAO-induced arrhythmias in woodchucks during summer. A) Representative ECG traces recorded in woodchucks in the summer group, with Ad-GFP overexpression (control). VT and VF/SCD were observed 1 h after CAO. B) Representative ECG recording in summer woodchucks, with Ad-catalase overexpression. Only scarcely occurring PVCs (arrow) were observed. C) Comparison of arrhythmia scores in woodchucks from the summer group between the Ad-GFP and Ad-catalase groups. Both individual data points and means ± sem values are presented. *P < 0.05 compared with the normalized control group by unpaired Student’s t test.
Inhibition of CaMKII attenuates CAO-induced arrhythmias in woodchucks in summer
Our results have revealed potential involvement of CaMKII in arrhythmogenesis at a single-cell level (Fig. 5). We, therefore, also evaluated the effect of the CaMKII inhibitor KN-93 on CAO-induced arrhythmias in summer. We performed CAO surgeries and monitored ECG by telemetry for 24 h. KN-93 significantly decreased the arrhythmia score in woodchucks in summer to 1.3 ± 0.6 (n = 4), compared with the summer control group (2.6 ± 0.4, n = 13) (P < 0.05). This result suggests that increased antioxidant enzyme levels and reduced CaMKII activity may account for the adaptation making the woodchuck heart resistance to CAO-induced arrhythmias during winter.
DISCUSSIONS
SCD claims the lives of 300,000–400,000 people in the United States each year (32). In many cases, SCD follows a VT/VF, which occurs after myocardial ischemia (22). In humans, VF may occur within minutes or hours after the onset of chest pain. Despite recent advances in the study of SCD caused by cardiac arrhythmias, their incidences in the population are still unacceptably high. The prevention and treatment of ischemia/myocardial ischemia–induced arrhythmias and SCDs remain a challenge clinically. New strategies to prevent SCDs necessitate a comprehensive understanding of the complex mechanism underlying initiation and maintenance of ischemia-induced arrhythmias. For that purpose, relevant models are needed to provide new insights and/or to better identify treatments for the ever-increasing clinical incidence. In the present study, we have shown, for the first time, to our knowledge, that the mammalian hibernator woodchuck exhibits seasonal susceptibility dependence to ischemia-induced arrhythmias and SCDs; that is, they exhibit significantly greater resistance to ischemia-induced arrhythmias in winter than they do in summer. These results suggest the woodchuck represents a natural model of resistance to fatal arrhythmias caused by ischemia, undergoing seasonal adaptation that protects the hibernating species against lethal cardiac arrhythmias during winter. We demonstrated that an enhanced capability for reducing oxidative stress and lowering CaMKII activities accounts for the woodchuck antiarrhythmic mechanism in winter. Furthermore, the transition of the woodchuck heart from the less-protective “summer mode” to greater-protective “winter mode” can be achieved by overexpressing catalase or inhibiting CaMKII. Therefore, induction of a winter mode, resembling that found in hibernating species, is likely to be beneficial for preventing arrhythmias in patients with heart attacks.
Differing antioxidative status in different seasons
ROS, including O2–, HO, and H2O2, are derivatives of oxygen characterized by their high reactivity (33). Under normal conditions, the myocardium reduces 95% of the oxygen to water via the mitochondrial electron transport chain, whereas the remaining 5% forms ROS. However, in some pathologic conditions, such as aging, heart failure, and ischemia/reperfusion, ROS percentages become elevated (34–37). For example, the generation of ROS is potentiated by an ischemia-induced shift in anaerobic metabolism after myocardial infarction (38, 39).
Because hibernators are confronted with oxidative stress over torpor–arousal cycles, they seem to have adapted by developing an increased antioxidant system. For example, the following findings have been reported: 1) increased superoxide dismutase (SOD) activities and glutathione peroxidase in the arousing hibernator (9), 2) elevated extracellular catalase during arousal (10), 3) high levels of metabolite antioxidant (ascorbate), and 4) up-regulated peroxiredoxins (Prdx1-3) during hibernation (H2O2 detoxification) (40). This study demonstrated that the adaptation in the heart of woodchuck during winter (winter mode) involves enhancing its antioxidative capability (reducing ROS level) and, consequently, reducing CaMKII activity, compared with the heart in the summer season (summer mode). The enhanced antioxidant status in the heart of the woodchuck during winter likely serves as a mechanism protecting against ischemia-induced arrhythmias.
The capacity of cardiac tissues to tolerate ROS is determined by the intrinsic antioxidant defense system that scavenges/degrades ROS to nontoxic molecules. This antioxidant defense system is composed of antioxidant enzymes, such as SOD, and catalase. Our group has shown that the woodchuck heart in winter exhibits intrinsic cardioprotection against ischemia/reperfusion injury. Myocardial infarction was significantly less in woodchucks in winter compared with that in summer. Resistance to oxidative stress is involved in ischemic preconditioning in the hearts of woodchucks in winter, as reflected by increased levels of both catalase and MnSOD in winters, modeled with a low temperature (10°C) for 7–10 d in a hibernaculum (1 group even entered into hibernation). The woodchucks used in our present study were all kept under room temperature after they were caught in the wild in either season (winter vs. summer). Although only catalase expression significantly increased during winter (but not MnSOD) in the present experimental setting (room temperature), it seems that woodchucks in winter still exhibit greater antioxidative capability and lower CaMKII activity, which is sufficient to prevent the occurrence of lethal arrhythmias. Consistent with that notion, we have shown that the woodchucks in the summer may be transformed from being in a summer mode to being in a winter mode by the modification of the ROS-CaMKII signaling pathway (i.e., overexpression of catalase and inhibition of CaMKII) and become arrhythmia resistant.
Previous studies have shown that hypothermia itself may exert confounding effects on cardioprotection (41, 42), heart rate (bradycardia), contractility, and energy/oxygen consumption (reduction). Our present experiments were performed at normal body temperature because the woodchucks were housed at room temperature in both summer and winter after being caught, thereby ruling out those confounding effects of hypothermia. Thus, our findings suggest a clinical relevance for modulating antioxidative capability and its antiarrhythmic potential.
Role of CaMKII activation
CaMKII is a serine/threonine kinase. Autophosphorylation of threonine 286 in the presence of Ca2+ and CaM activates CaMKII and has an essential role in cardiac physiologic function (26, 43). In addition to self-phosphorylation, CaMKII can also be activated by oxidation at M281/282 by ROS (23), and the ratio of oxidized CaMKII to total CaMKII (i.e., Ox-CaMKII/CaMKII) is correlated with the extent of CaMKII activation. Multiple lines of evidence have strongly suggested that excessive activation of CaMKII is linked to maladaptive cardiac remodeling, heart failure, arrhythmias, and SCD (26, 44–47). For example, ventricular myocytes with up-regulated CaMKII activity isolated from transgenic mouse (or structural heart disease) models exhibit EADs and tricuspid atresias (14, 43), whereas CaMKII inhibition suppresses EADs and triggered activities and decreases mortality (14). Alterations in ICa,L properties have been implicated as major factors by which CaMKII activation promotes EADs (14, 45). In addition, a recent study demonstrated that CaMKII activation also increased the late INa (48), which also facilitates EAD (27, 28). Furthermore, CaMKII activation via oxidation is responsible for apoptosis and impaired heart function after myocardial infarction (23). Interestingly, acute responses of CaMKII activation have also been reported in animal models for ischemia, ischemia/reperfusion, and acidosis (49, 50). Our previous studies in rabbit myocytes demonstrated that activation of CaMKII by oxidation is an important factor in the arrhythmogenic effects of H2O2, supporting a direct link between oxidative stress, CaMKII activation, and the genesis of afterdepolarizations and triggered activities (12). A study using a digital gene-expression assay revealed that the mRNA levels of CaM and CaMKII are down-regulated in ground squirrels during hibernation (11). Our study adds more evidence supporting the notion that reduced ROS level and decreased CaMKII activation potentially suppress afterdepolarizations and arrhythmias under ischemic conditions. In addition, KN-93 effectively reduces the phosphorylation level of CaMKII (P-CaMKII) (51). Therefore, inhibition of CaMKII activity by lessening its oxidized and/or phosphorylated levels may mediate its antiarrhythmic effects.
The data obtained from this novel animal model suggest that CaMKII inhibition may provide additional benefits for people susceptible to arrhythmias and SCD. However, this idea remains to be tested in patients.
Ionic mechanisms for ROS-induced afterdepolarizations in woodchuck ventricular myocytes
The mechanisms responsible for cardiac tachyarrhythmias can be divided into 2 broad groups: 1) abnormalities of impulse conduction, and 2) abnormalities of impulse generation (52–54). The former is usually associated with disruption of electrical conduction within the heart resulting in reentry, whereas the most important concept in the latter category is afterdepolarization and triggered activity (55). We do not exclude the involvement of reentry in our current model of ischemia-induced arrhythmia. For example, Fedorov et al. (6) demonstrated that Citellus undulatus (the Siberian ground squirrel) up-regulation of both Cx43 and Cx45 may help maintain regular conduction pattern during hibernation. Nevertheless, simultaneous recording of MAP and ECG (Fig. 2) demonstrated that afterdepolarizations and triggered activities are likely to be involved in the genesis of ischemia-induced arrhythmias in woodchucks in summer. Further electrophysiologic experiments at the single-cell level suggest that ionic mechanisms for EAD generation in woodchuck myocytes in summer resemble those in rabbit ventricular myocytes. The susceptibility to ROS-induced EADs is blunted in winter, which is likely to confer greater resistance against ischemia-induced arrhythmias and SCD in winter. We suggest that an enhanced ox-CaMKII level (thus CaMKII activity) may mediate H2O2-induced EADs/DADs and intracellular Ca2+ mishandling. However, we do not exclude other possible molecular/ionic mechanisms. For example, thiol oxidation of l-type calcium channels (56) or ryanodine receptors (57) may enhance their activities, which, in turn, lead to Ca2+ overload and arrhythmogenic SR Ca2+ leakage.
In addition, other adaptive alterations have been reported in hibernating animals, which may contribute to cardiac protection during winter (7, 58). For example, the Cai handling by myocytes is modified in winter by 1) reduced channel density and faster inactivation of ICa,L, 2) up-regulated SR Ca ATPase, and 3) down-regulated phospholamban. These adaptations in Cai handling may provide a mechanism for protection against Cai overload and arrhythmias because lower Cai levels (from decreased ICa,L and increased SR Ca ATPase functions) reduce Na+-Ca2+ exchanger activity and, therefore, prevent generation of DADs and EADs (31).
A unique animal model for cardiac protection research and clinical relevance
Woodchucks (M. monax) are the largest true-hibernating animals, and their body temperature can drop close to the freezing point during the winter season. Nonhibernating mammals suffer severe arrhythmias (heart arrests) when their body temperature decreases to 10–15°C, whereas hibernators are extremely resistant to hypothermia-induced arrhythmias (59, 60). In this study, we observed that this mammalian hibernator also exhibited powerful, intrinsic protective ability against ischemia-induced arrhythmias in winter. Elevated antioxidative capability (overexpression of MnSOD and catalase) and reduced CaMKII activity seem to be critical intrinsic adaptations, which may ensure the animals prepare for hibernation and, at the same time, confer resistance to arrhythmias and acute ischemia from low body temperature (hypothermia). These experiments in a novel animal model reveal a link between oxidant stress, CaMKII activation, and cardiac arrhythmias, which may, therefore, provide clues for future studies in human patients. The manipulation of ROS-CaMKII signaling holds therapeutic potential for treating cardiac arrhythmias. As suggested by Tomaselli and Barth (24), “an ideal antiarrhythmic agent may be one that rebalances oxidant-regulated processes rather than one whose efficacy is based on the inhibition of individual ion channels.” Therefore, the hibernating mammal represents a novel model, which may provide insights into the mechanism and prevention of ischemia-related arrhythmias and SCD. The examination of mediating genes and/or proteins in the woodchuck heart in winter is novel and sheds new light on the mechanisms mediating arrhythmogenesis. Understanding these intrinsic antiarrhythmic mechanisms existing in hibernating species may help to develop novel treatments for severe arrhythmias in other species including human.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Mark Anderson (Johns Hopkins, Baltimore, MD, USA) for kindly providing oxidized CaMKII antibodies. This work was supported by the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (Grants R01HL97979 and R01HL133294 to L.-H.X and R01HL130848 to S.F.V.), the American Heart Association (Grant 16GRNT31100022 to L.-H.X.), and the National Natural Science Foundation of China (Grants 81470510 to Z.Z. and 81503068 to H.W.). The authors declare no conflicts of interest.
Glossary
- AP
action potential
- APD
action potential duration
- Cai
intracellular calcium
- CaM
calmodulin
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CAO
coronary artery occlusion
- DAD
delayed afterdepolarization
- EAD
early afterdepolarization
- ECG
electrocardiogram
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- ICa,L
l-type calcium current
- INa
sodium current
- MAP
monophasic action potential
- MnSOD
magnesium superoxide dismutase
- Ox-CaMKII
oxidized CaMKII
- P-CaMKII
phosphorylated CaMKII
- PVC
premature ventricular complex
- ROS
reactive oxygen species
- SCD
sudden cardiac death
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Z. Zhao, R. K. Kudej, H. Wen, N. Fefelova, L. Yan, and L.-H, Xie performed the experiments; Z. Zhao, H. Wen, N. Fefelova, L. Yan, and L.-H. Xie analyzed the data; Z. Zhao and L.-H. Xie wrote the paper; and D. E. Vatner, S. F. Vatner, and L.-H. Xie conceived of and designed the research.
REFERENCES
- 1.Mehta D., Curwin J., Gomes J. A., Fuster V. (1997) Sudden death in coronary artery disease: acute ischemia versus myocardial substrate. Circulation 96, 3215–3223 10.1161/01.CIR.96.9.3215 [DOI] [PubMed] [Google Scholar]
- 2.Carey H. V., Andrews M. T., Martin S. L. (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153–1181 10.1152/physrev.00008.2003 [DOI] [PubMed] [Google Scholar]
- 3.Johansson B. W. (1996) The hibernator heart—nature’s model of resistance to ventricular fibrillation. Cardiovasc. Res. 31, 826–832 [DOI] [PubMed] [Google Scholar]
- 4.Johansson B. W. (1985) Ventricular repolarization and fibrillation threshold in hibernating species. Eur. Heart J. 6(Suppl D), 53–62 [DOI] [PubMed] [Google Scholar]
- 5.Andrews M. T. (2007) Advances in molecular biology of hibernation in mammals. BioEssays 29, 431–440 10.1002/bies.20560 [DOI] [PubMed] [Google Scholar]
- 6.Fedorov V. V., Li L., Glukhov A., Shishkina I., Aliev R. R., Mikheeva T., Nikolski V. P., Rosenshtraukh L. V., Efimov I. R. (2005) Hibernator Citellus undulatus maintains safe cardiac conduction and is protected against tachyarrhythmias during extreme hypothermia: possible role of Cx43 and Cx45 up-regulation. Heart Rhythm 2, 966–975 10.1016/j.hrthm.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 7.Yatani A., Kim S. J., Kudej R. K., Wang Q., Depre C., Irie K., Kranias E. G., Vatner S. F., Vatner D. E. (2004) Insights into cardioprotection obtained from study of cellular Ca2+ handling in myocardium of true hibernating mammals. Am. J. Physiol. Heart Circ. Physiol. 286, H2219–H2228 10.1152/ajpheart.01096.2003 [DOI] [PubMed] [Google Scholar]
- 8.Hampton M., Melvin R. G., Kendall A. H., Kirkpatrick B. R., Peterson N., Andrews M. T. (2011) Deep sequencing the transcriptome reveals seasonal adaptive mechanisms in a hibernating mammal. PLoS One 6, e27021 10.1371/journal.pone.0027021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzadzić B., Spasić M., Saicić Z. S., Radojicić R., Petrović V. M., Halliwell B. (1990) Antioxidant defenses in the ground squirrel Citellus citellus, 2: the effect of hibernation. Free Radic. Biol. Med. 9, 407–413 10.1016/0891-5849(90)90017-D [DOI] [PubMed] [Google Scholar]
- 10.Ohta H., Okamoto I., Hanaya T., Arai S., Ohta T., Fukuda S. (2006) Enhanced antioxidant defense due to extracellular catalase activity in Syrian hamster during arousal from hibernation. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 143, 484–491 10.1016/j.cbpc.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 11.Brauch K. M., Dhruv N. D., Hanse E. A., Andrews M. T. (2005) Digital transcriptome analysis indicates adaptive mechanisms in the heart of a hibernating mammal. Physiol. Genomics 23, 227–234 10.1152/physiolgenomics.00076.2005 [DOI] [PubMed] [Google Scholar]
- 12.Xie L. H., Chen F., Karagueuzian H. S., Weiss J. N. (2009) Oxidative-stress–induced afterdepolarizations and calmodulin kinase II signaling. Circ. Res. 104, 79–86 10.1161/CIRCRESAHA.108.183475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan L., Kudej R. K., Vatner D. E., Vatner S. F. (2015) Myocardial ischemic protection in natural mammalian hibernation. Basic Res. Cardiol. 110, 9 10.1007/s00395-015-0462-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y., Temple J., Zhang R., Dzhura I., Zhang W., Trimble R., Roden D. M., Passier R., Olson E. N., Colbran R. J., Anderson M. E. (2002) Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation 106, 1288–1293 10.1161/01.CIR.0000027583.73268.E7 [DOI] [PubMed] [Google Scholar]
- 15.Curtis M. J., Walker M. J. (1988) Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc. Res. 22, 656–665 10.1093/cvr/22.9.656 [DOI] [PubMed] [Google Scholar]
- 16.Gao J., Zhang L., Wang Y., Lu B., Cui H., Fu W., Wang H., Yu Y., Yu X. (2008) Antiarrhythmic effect of acupuncture pretreatment in rats subjected to simulative global ischemia and reperfusion--involvement of adenylate cyclase, protein kinase A, and l-type Ca2+ channel. J. Physiol. Sci. 58, 389–396 10.2170/physiolsci.RP007108 [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z., Babu G. J., Wen H., Fefelova N., Gordan R., Sui X., Yan L., Vatner D. E., Vatner S. F., Xie L. H. (2015) Overexpression of adenylyl cyclase type 5 (AC5) confers a proarrhythmic substrate to the heart. Am. J. Physiol. Heart Circ. Physiol. 308, H240–H249 10.1152/ajpheart.00630.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franz M. R. (1999) Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc. Res. 41, 25–40 10.1016/S0008-6363(98)00268-5 [DOI] [PubMed] [Google Scholar]
- 19.Franz M. R., Flaherty J. T., Platia E. V., Bulkley B. H., Weisfeldt M. L. (1984) Localization of regional myocardial ischemia by recording of monophasic action potentials. Circulation 69, 593–604 10.1161/01.CIR.69.3.593 [DOI] [PubMed] [Google Scholar]
- 20.Xie J. T., Xie L. H. (1990) Can the MAP technique be applied to study triggered activities of the heart? Intracellular evidence in vivo. Methods Find. Exp. Clin. Pharmacol. 12, 419–424 [PubMed] [Google Scholar]
- 21.Boengler K., Pipp F., Fernandez B., Ziegelhoeffer T., Schaper W., Deindl E. (2003) Arteriogenesis is associated with an induction of the cardiac ankyrin repeat protein (carp). Cardiovasc. Res. 59, 573–581 10.1016/S0008-6363(03)00511-X [DOI] [PubMed] [Google Scholar]
- 22.Zipes D. P., Wellens H. J. (1998) Sudden cardiac death. Circulation 98, 2334–2351 10.1161/01.CIR.98.21.2334 [DOI] [PubMed] [Google Scholar]
- 23.Erickson J. R., Joiner M. L., Guan X., Kutschke W., Yang J., Oddis C. V., Bartlett R. K., Lowe J. S., O’Donnell S. E., Aykin-Burns N., Zimmerman M. C., Zimmerman K., Ham A. J., Weiss R. M., Spitz D. R., Shea M. A., Colbran R. J., Mohler P. J., Anderson M. E. (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133, 462–474 10.1016/j.cell.2008.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomaselli G. F., Barth A. S. (2010) Sudden cardio arrest: oxidative stress irritates the heart. Nat. Med. 16, 648–649 10.1038/nm0610-648 [DOI] [PubMed] [Google Scholar]
- 25.Foteinou P. T., Greenstein J. L., Winslow R. L. (2015) Mechanistic investigation of the arrhythmogenic role of oxidized CaMKII in the heart. Biophys. J. 109, 838–849 10.1016/j.bpj.2015.06.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luczak E. D., Anderson M. E. (2014) CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell. Cardiol. 73, 112–116 10.1016/j.yjmcc.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y., Shryock J. C., Wagner S., Maier L. S., Belardinelli L. (2006) Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J. Pharmacol. Exp. Ther. 318, 214–222 10.1124/jpet.106.101832 [DOI] [PubMed] [Google Scholar]
- 28.Ward C. A., Giles W. R. (1997) Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J. Physiol. 500, 631–642 10.1113/jphysiol.1997.sp022048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldhaber J. I. (1996) Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am. J. Physiol. 271, H823–H833 [DOI] [PubMed] [Google Scholar]
- 30.Hinata M., Matsuoka I., Iwamoto T., Watanabe Y., Kimura J. (2007) Mechanism of Na+/Ca2+ exchanger activation by hydrogen peroxide in guinea-pig ventricular myocytes. J. Pharmacol. Sci. 103, 283–292 10.1254/jphs.FP0060015 [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z., Wen H., Fefelova N., Allen C., Baba A., Matsuda T., Xie L. H. (2012) Revisiting the ionic mechanisms of early afterdepolarizations in cardiomyocytes: predominant by Ca waves or Ca currents? Am. J. Physiol. Heart Circ. Physiol. 302, H1636–H1644 10.1152/ajpheart.00742.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubart M., Zipes D. P. (2005) Mechanisms of sudden cardiac death. J. Clin. Invest. 115, 2305–2315 10.1172/JCI26381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordberg J., Arnér E. S. (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 31, 1287–1312 10.1016/S0891-5849(01)00724-9 [DOI] [PubMed] [Google Scholar]
- 34.Lakatta E. G. (2002) Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail. Rev. 7, 29–49 10.1023/A:1013797722156 [DOI] [PubMed] [Google Scholar]
- 35.Slezak J., Tribulova N., Pristacova J., Uhrik B., Thomas T., Khaper N., Kaul N., Singal P. K. (1995) Hydrogen peroxide changes in ischemic and reperfused heart: cytochemistry and biochemical and X-ray microanalysis. Am. J. Pathol. 147, 772–781 [PMC free article] [PubMed] [Google Scholar]
- 36.Neuman R. B., Bloom H. L., Shukrullah I., Darrow L. A., Kleinbaum D., Jones D. P., Dudley S. C., Jr (2007) Oxidative stress markers are associated with persistent atrial fibrillation. Clin. Chem. 53, 1652–1657 10.1373/clinchem.2006.083923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigo R., Cereceda M., Castillo R., Asenjo R., Zamorano J., Araya J., Castillo-Koch R., Espinoza J., Larraín E. (2008) Prevention of atrial fibrillation following cardiac surgery: basis for a novel therapeutic strategy based on non-hypoxic myocardial preconditioning. Pharmacol. Ther. 118, 104–127 10.1016/j.pharmthera.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 38.Kinugawa S., Tsutsui H., Hayashidani S., Ide T., Suematsu N., Satoh S., Utsumi H., Takeshita A. (2000) Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ. Res. 87, 392–398 10.1161/01.RES.87.5.392 [DOI] [PubMed] [Google Scholar]
- 39.Marczin N., El-Habashi N., Hoare G. S., Bundy R. E., Yacoub M. (2003) Antioxidants in myocardial ischemia-reperfusion injury: therapeutic potential and basic mechanisms. Arch. Biochem. Biophys. 420, 222–236 10.1016/j.abb.2003.08.037 [DOI] [PubMed] [Google Scholar]
- 40.Morin P., Jr., Storey K. B. (2007) Antioxidant defense in hibernation: cloning and expression of peroxiredoxins from hibernating ground squirrels, Spermophilus tridecemlineatus. Arch. Biochem. Biophys. 461, 59–65 10.1016/j.abb.2007.01.035 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz B. G., Kloner R. A., Thomas J. L., Bui Q., Mayeda G. S., Burstein S., Hale S. L., Economides C., French W. J. (2012) Therapeutic hypothermia for acute myocardial infarction and cardiac arrest. Am. J. Cardiol. 110, 461–466 10.1016/j.amjcard.2012.03.048 [DOI] [PubMed] [Google Scholar]
- 42.Yang X., Liu Y., Yang X. M., Hu F., Cui L., Swingle M. R., Honkanen R. E., Soltani P., Tissier R., Cohen M. V., Downey J. M. (2011) Cardioprotection by mild hypothermia during ischemia involves preservation of ERK activity. Basic Res. Cardiol. 106, 421–430 10.1007/s00395-011-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Couchonnal L. F., Anderson M. E. (2008) The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 23, 151–159 [DOI] [PubMed] [Google Scholar]
- 44.Kirchhof P., Fabritz L., Kilić A., Begrow F., Breithardt G., Kuhn M. (2004) Ventricular arrhythmias, increased cardiac calmodulin kinase II expression, and altered repolarization kinetics in ANP receptor deficient mice. J. Mol. Cell. Cardiol. 36, 691–700 10.1016/j.yjmcc.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 45.Wu Y., MacMillan L. B., McNeill R. B., Colbran R. J., Anderson M. E. (1999) CaM kinase augments cardiac l-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am. J. Physiol. 276, H2168–H2178 [DOI] [PubMed] [Google Scholar]
- 46.Gbadebo T. D., Trimble R. W., Khoo M. S., Temple J., Roden D. M., Anderson M. E. (2002) Calmodulin inhibitor W-7 unmasks a novel electrocardiographic parameter that predicts initiation of torsade de pointes. Circulation 105, 770–774 10.1161/hc0602.103724 [DOI] [PubMed] [Google Scholar]
- 47.Anderson M. E., Braun A. P., Wu Y., Lu T., Wu Y., Schulman H., Sung R. J. (1998) KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J. Pharmacol. Exp. Ther. 287, 996–1006 [PubMed] [Google Scholar]
- 48.Wagner S., Dybkova N., Rasenack E. C., Jacobshagen C., Fabritz L., Kirchhof P., Maier S. K., Zhang T., Hasenfuss G., Brown J. H., Bers D. M., Maier L. S. (2006) Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 116, 3127–3138 10.1172/JCI26620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Said M., Becerra R., Palomeque J., Rinaldi G., Kaetzel M. A., Diaz-Sylvester P. L., Copello J. A., Dedman J. R., Mundiña-Weilenmann C., Vittone L., Mattiazzi A. (2008) Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart: role of Ca2+/calmodulin-dependent protein kinase II. Am. J. Physiol. Heart Circ. Physiol. 295, H1669–H1683 10.1152/ajpheart.00010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vittone L., Mundiña-Weilenmann C., Said M., Ferrero P., Mattiazzi A. (2002) Time course and mechanisms of phosphorylation of phospholamban residues in ischemia-reperfused rat hearts: dissociation of phospholamban phosphorylation pathways. J. Mol. Cell. Cardiol. 34, 39–50 10.1006/jmcc.2001.1488 [DOI] [PubMed] [Google Scholar]
- 51.Sossalla S., Fluschnik N., Schotola H., Ort K. R., Neef S., Schulte T., Wittköpper K., Renner A., Schmitto J. D., Gummert J., El-Armouche A., Hasenfuss G., Maier L. S. (2010) Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ. Res. 107, 1150–1161 10.1161/CIRCRESAHA.110.220418 [DOI] [PubMed] [Google Scholar]
- 52.Zipes D. P., Wellens H. J. (2000) What have we learned about cardiac arrhythmias? Circulation 102 (20, Suppl 4), IV52–IV57 [DOI] [PubMed] [Google Scholar]
- 53.Zipes D. P. (1999) 50th anniversary historical article: a century of cardiac arrhythmias: in search of Jason’s golden fleece. J. Am. Coll. Cardiol. 34, 959–965 [DOI] [PubMed] [Google Scholar]
- 54.Vaquero M., Calvo D., Jalife J. (2008) Cardiac fibrillation: from ion channels to rotors in the human heart. Heart Rhythm 5, 872–879 10.1016/j.hrthm.2008.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosen M. R., Moak J. P., Damiano B. (1984) The clinical relevance of afterdepolarizations. Ann. N. Y. Acad. Sci. 427, 84–93 10.1111/j.1749-6632.1984.tb20776.x [DOI] [PubMed] [Google Scholar]
- 56.Hool L. C. (2008) Evidence for the regulation of l-type Ca2+ channels in the heart by reactive oxygen species: mechanism for mediating pathology. Clin. Exp. Pharmacol. Physiol. 35, 229–234 [DOI] [PubMed] [Google Scholar]
- 57.Terentyev D., Györke I., Belevych A. E., Terentyeva R., Sridhar A., Nishijima Y., de Blanco E. C., Khanna S., Sen C. K., Cardounel A. J., Carnes C. A., Györke S. (2008) Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 103, 1466–1472 10.1161/CIRCRESAHA.108.184457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S. Q., Lakatta E. G., Cheng H., Zhou Z. Q. (2002) Adaptive mechanisms of intracellular calcium homeostasis in mammalian hibernators. J. Exp. Biol. 205, 2957–2962 [DOI] [PubMed] [Google Scholar]
- 59.Caprette D. R., Senturia J. B. (1984) Isovolumetric performance of isolated ground squirrel and rat hearts at low temperature. Am. J. Physiol. 247, R722–R727 [DOI] [PubMed] [Google Scholar]
- 60.Burlington R. F., Darvish A. (1988) Low-temperature performance of isolated working hearts from a hibernator and a nonhibernator. Physiol. Biochem. Zool. 61, 387–395 10.1086/physzool.61.5.30161260 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.