Abstract
Colorectal cancer (CRC) is a complex and widespread disease, currently ranked as the third most frequent cancer worldwide. It is well known that the gut microbiota has an essential role in the initiation and promotion of different cancer types, particularly gastrointestinal tumors. In fact, bacteria can trigger chronic inflammation of the gastric mucosal, which can induce irreversible changes to intestinal epithelial cells, thus predisposing individuals to cancer. Some bacterial strains, such as Helicobacter pylori, Streptococcus bovis, Bacteroides fragilis, Clostridium septicum and Fusobacterium spp. have a well established role in CRC development. However, the role of Enterococcus faecalis still remains controversial. While part of the literature suggests a harmful role, other papers reported E. faecalis as an important probiotic microorganism, with great applicability in food products. In this review we have examined the vast majority of published data about E. faecalis either in CRC development or concerning its protective role. Our analysis should provide some answers regarding the controversial role of E. faecalis in CRC.
Keywords: chronic inflammation, colorectal cancer, Enterococcus faecalis, gastrointestinal cancer, gut microbiota
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers among both men and women worldwide, being the third most frequent in many developed countries, with an estimated 135,430 new cases expected in 2017 in the United States.1 The increasing incidence in developing countries seems to be closely related to changes in lifestyle.2,3 In fact, only 15% of patients with this disease present a genetic background of familiarity, whereas 85% of cases are represented by sporadic forms.4 Known environmental factors involved in CRC development include smoking, alcoholism, obesity, sedentary lifestyle, diabetes mellitus, consumption of red meat, a high-fat diet and inadequate intake of fibers.5 The gut microbial composition has also been reported as another important factor associated with CRC progress.
Recent findings show that gut microorganisms could modulate the mucosal immune system and change the expression of some host genes associated with important functions, such as nutrient uptake, metabolism, angiogenesis and mucosal barrier function.6,7 Accordingly, the imbalance of the symbiotic relationship that exists between the gut and its microbiota8 may disturb the intestinal epithelial integrity, resulting in multiple downstream consequences, including inflammation, oncogenesis and the progression of primary tumors into metastasis.9,10 However, even if some microorganisms constitute a fundamental part of natural gut composition, playing protective roles against cancer,11 the role of other strains, such as Enterococcus faecalis, still remains unclear.
E. faecalis is a Firmicutes member, sometimes used as a probiotic product.12,13 However, in some specific situations, E. faecalis can result in pathogenic and, as reported by some authors, a harmful microorganism on CRC development, due to its ability to damage colonic epithelial cell DNA.14 Because of these conflicting roles, in this review we examine the most relevant published data that correlate E. faecalis with CRC either in a harmful or a protective way.
Features of CRC
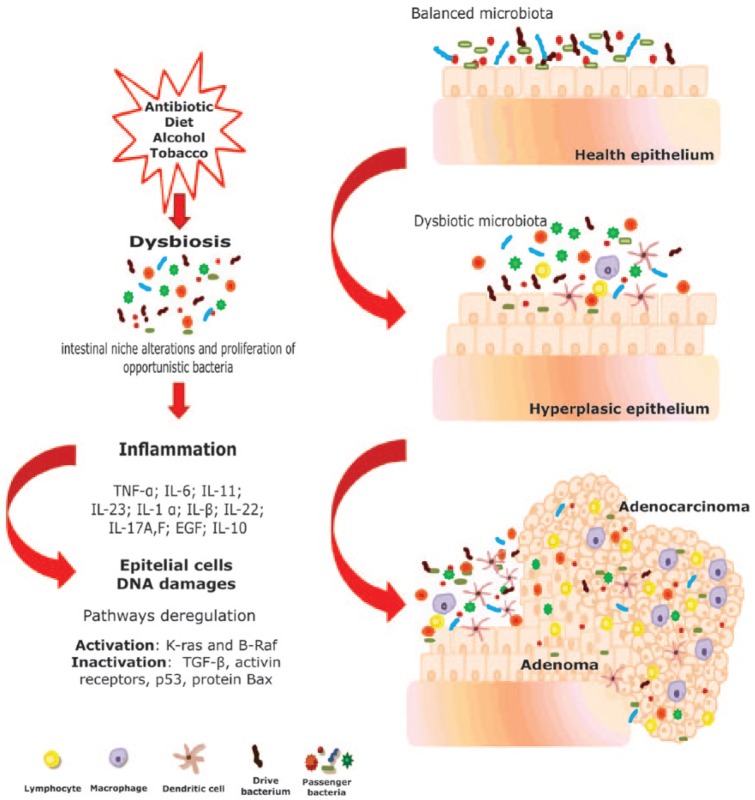
CRC is a multifactorial disease that occurs in a multistep process involving accumulating mutations in tumor suppressor genes and oncogenes. This means that the colorectal tumorigenesis includes several genetic and epigenetic changes required for tumor initiation and progression.15 CRC is one of the most genetically complex cancers that have been investigated, and its underlying genetic basis is described by the ‘adenoma–carcinoma sequence’ model (Figure 1), which posits that the genomic instability drives epithelial dysplasia and hyperplasia in the colon, resulting, eventually, in CRC.16
Figure 1.
‘Adenoma–carcinoma’ progression following the model proposed by Fearon and Vogelstein17 incorporating the ‘bacterial driver-passenger’ model of Tjalsma and colleagues. In this situation, the adenoma–carcinoma progression occurs because of the genomic instability (accumulating genetic and epigenetic mutations), caused by changes in gut microbiota. These changes initiate with the presence of a ‘drive bacteria’ which drives the epithelial DNA damage and contributes to colorectal cancer (CRC) promotion. Then the tumorigenesis induces intestinal niche alterations, which favor the proliferation of opportunistic bacteria (bacterial passengers). EGF, epidermal growth factor; IL, interleukin; K-ras, Kirsten rat sarcoma viral oncogene homolog; TGF, transforming growth factor; TNF, tumor necrosis factor.
Differently from the CRC molecular phenotype originating from genetic familiarity, that is characterized by high-frequency microsatellite instability phenotypes, and by germline mutations in the mismatch repair genes17 or the adenomatous polyposis coli (APC) gene,18 the sporadic cases of CRC phenotypes present chromosomal instability and allelic imbalance at several chromosomal loci (reviewed by Cunningham and colleagues).19 Besides the occurrence of genetic and epigenetic abnormalities, many aspects of CRC malignancy are affected by cancer-associated inflammation, such as proliferation and survival of malignant cells, angiogenesis and tumor metastasis. Finally, the presence of an inflammatory microenvironment also plays a crucial role in CRC development.20,21 In this scenario, cancer could be described not just as a concentration of malignant cells, but it is also composed of the stromal and infiltrating immunological or inflammatory cells.
It is well known that the diet is definitely the most important (and previously identified) exogenous factor in CRC etiology,22 since components, ingested through the diet, are the major source of mutagenic compounds that may promote both cancer initiation and progression.23 In addition, epidemiological studies show that diet–gene interactions are one of the leading causes explaining the wide variation in CRC developing risk among different individuals.24 For example, the excessive consumption of fats, animal proteins, processed meat, and heterocyclic amines (HCAs) has shown strong correlations with CRC incidence.25,26 However, a vegetarian diet helps to prevent CRC, since fruits and vegetables invariably contain antioxidants, which scavenge free radicals, inhibiting the DNA damage responsible for mutations and eventually cancer.27,28 The diet also influences the features of gut microbiota, as well as other factors, such as the host’s age, sex, geography and ethnicity.29
General aspects of gut microbiota
The gut microbiota, now fully recognized as a natural defensive barrier against infections, is involved in several physiological functions and plays a large role in maintaining the gut homeostasis.30 Almost immediately after birth, the human gastrointestinal tract (GI) is colonized by a large and diverse community of microorganisms which will compose the GI microbiota.31 The colonization pattern is influenced by the type of delivery (vaginal delivery or caesarean section)32 and the type of baby diet (breast or formula feeding).33 These pioneer microorganisms modulate the expression of some genes from host epithelial cells, creating a flattering habitat for themselves, and also preventing the growth of other microorganisms.34
The adult phylogenetic composition of gut microbiota could be influenced by a lot of factors like diet, antibiotic consumption, external environmental microorganisms, geographic/cultural traditions and age.35,36 Gut microbiota is normally compounded by autochthonous members, which occupy specific niches constituting the most stable populations over long periods, and by allochthonous members that may be found in any given habitat in significant numbers, but do not influence the gut ecosystem balance in the same way.37 Humans have a close relationship with these microorganisms, given that their health and wellbeing are closely interconnected with this complex mutualism.38 The microbiota plays a fundamental role in displaying some essential organismal functions such as helping food digestion, contributing to nutrition,39 modulating the immune system40,41 and defending against a lot of diseases caused by other pathological microbes.42
Over the gastric tract, there are variations in the concentration of microorganisms, species and specific functions. For example, in the proximal GI tract, the relatively low (108 cells/ml) microbial biomass is associated with the host amino acid requirements,43 and is composed of Bacteroidetes, members of the Clostridiales clusters (clusters XIV and I), and specifically in lumen by Enterobacteriaceae V. This restricted bacterial composition is determined by several factors such as acid pH, rapid lumen flow tendency, bile salts and the presence of immunoglobulin A (IgA). In contrast, the large intestine, which is associated, for example, with the production of short-chain fatty acids (SCFAs) by fermentation of dietary compounds (that escape from the digestion in the small intestine), is colonized by higher concentrations (1011 cells/g) of microorganisms, mainly by Firmicutes (clusters IX, XIV, and XVI), Bacteroidetes, Actinobacteria, Verrucomicrobia, Proteobacteria and Fusobacteria (reviewed by Walter and Ley).44
Even if these data are well known, there is no consensus about what is a ‘healthy’ or ‘average’ intestinal microbiota. For example, studies with rodents have demonstrated that a given bacterial species can have opposite effects in disease induction in one susceptible host, but can protect another rodent strain. Furthermore, different bacterial species can induce variable clinical phenotypes in a single genetically susceptible host.45 Nevertheless, it seems that beyond the organization of this complex and diverse bacterial ecosystem, the two most common phyla present in the gut, are the Firmicutes and the Bacteroidetes,46 with a documented hierarchy of dominant anaerobic bacteria, like Bacteroides, Eubacterium, Bifidobacterium, Peptostreptococcus, Ruminococcus, Clostridium and Propionibacterium, and a subdominant bacteria represented by the Enterobacteriaceae family, especially Escherichia coli and the genera Streptococcus, Enterococcus, Lactobacillus, Fusobacterium, Desulfovibrio and Methanobrevibacter.47 In fact, one of the most studied parameters regarding the ‘normal composition’ of gut microbiota is the Firmicutes/Bacteroidetes ratio, as well as its variations between individuals,42 and it seems to be closely linked to health problems such as obesity and metabolic disorders.48,49
Gut microbiota and CRC
As well as in different types of tumors such as skin, liver, lungs and breast cancers, CRC can be associated with host microbiome dysbiosis.50,51 In fact, microorganisms are suspected to be involved in approximately 20% of cancers,52 especially CRC,53 since the dynamic crosstalk between intestinal epithelial cells (IECs), the microbes that colonize their apical surface and the surrounding local immune cells, is necessary to maintain intestinal homeostasis.54 Changes in the Firmicutes/Bacteroidetes ratio55 as well as other microbiota imbalances56,57 are associated with the beginning, maintenance, and determination of the phenotype of human inflammatory bowel diseases (IBDs), especially Crohn’s disease and ulcerative colitis. These diseases, characterized by chronic inflammation of the GI tract (as a result of inappropriate activation of intestinal mucosal immunity), affect more than 0.4% of Europeans and North Americans,58 and are associated with an increased risk of CRC development.59,60
The relationship between the commensal microbiota and IBD could occur in different forms (reviewed by Sartor and Mazmanian),61 but all of them seem to be related to the increase in bacterial antigens’ exposure to effector T cells and innate immune cells (resident in the intestinal mucosa) or alteration of the host immune response to commensal bacteria.62 Nevertheless, different studies have also demonstrated that patients with IBD present a reduction of Firmicutes members, specifically Faecalibacterium and Roseburia, which play a role in protecting the intestine by producing SCFAs.63
However, an interesting way to explain the role of microorganisms in CRC development was proposed by Tjalsma and colleagues in the ‘bacterial driver-passenger’ model.64 According to this model, the DNA damage caused by distinct indigenous intestinal bacteria (driver bacteria) can drive genome instability, which starts the first phase of cancer progression. These microorganisms may be able to induce alterations in mucosal permeability, which favors the translocation of bacteria and bacterial toxins, causing a gut inflammatory response that contributes to the development and progression of cancer. The intestinal inflammation can result from an aberrant ratio of protective (tolerogenic) to aggressive (proinflammatory, damage-inducing, protumorigenic) microbiota, since GI bacteria are able to trigger production of both interleukin (IL)-10 (tolerogenic) and IL-17 (proinflammatory) cytokines.65 This CRC microenvironment could impact the microbial regulation, alter microbiota composition by selective pressure on the microbial community, and thus could support the outgrowth of specific opportunistic bacteria (passengers bacteria) that potentially have carcinogenic effects63,66 (Figure 1).
Furthermore, this inflammatory scenario has an important role in tumor development and maintenance. Once activated, inflammatory cells produce reactive oxygen species (ROS) and reactive nitrogen that can promote the accumulation of additional mutations and epigenetic changes. These mutations can activate oncogenes or inactive tumor suppressor genes, thus increasing the risk of cancer development.23,67,68 One of the important bacteria cited as an ‘inducer’ of genetic instability in colonic epithelial cells’ DNA, through the oxidative process, is E. faecalis.61,69
Properties of E. faecalis
Among the enterococci that colonize the GI tract, the most prevalent cultured strain found in human feces is E. faecalis (105–107 colony-forming units (CFU)/g) followed by E. faecium (104–105 CFU/g). However these numbers can change with the host’s geographical location, and especially, diet. For instance, Hill and colleagues demonstrated that feces samples of individuals from England, Scotland and the USA (who have a Western diet) present lower concentrations of Enterococcus spp. compared with subjects from India, Japan and Uganda, who adopt a mainly vegetarian diet.70 This Gram-positive commensal bacterium belongs to the lactic acid bacteria (LAB), it is a facultative anaerobic, resistant to extreme environmental challenges and is usually found in the human oral cavity, GI and vagina mucosa.71 However, it can emerge as a human pathogen of significant concern72 and so can be associated with various pathologies including urinary tract infections,73 endocarditis,74 persistent endodontic diseases,75 blood stream infections76 and chronic periodontitis.77
E. faecalis is the first colonizer of the human GI tract and has a major impact on intestinal immune development in the very early stages of life.78 In newborn babies, it plays a protective role regulating the colonic homeostasis during development by suppressing pathogen-mediated inflammatory responses in human IECs, inducing IL-10 expression,79 and attenuating the secretion of proinflammatory cytokines, especially IL-8.80 Moreover, because of this anti-inflammatory potential, E. faecalis is commonly adopted as a probiotic in the treatment of some diseases such as recurrent chronic sinusitis, bronchitis or infant acute diarrhea.13,14 In fact, it has been demonstrated that, compared with 70 different LABs isolated from healthy adults, E. faecalis showed the highest probiotic activity.81 Furthermore, due to its thermophilic fermentation potential (it can ferment different types of sugars, grows at 10°C, and survives at 60°C for 30 min), E. faecalis is commonly used in the production of some cheeses and fermented sausages.82,83
The ‘Gianus’ role of E. faecalis in cancer development
Data about the role of E. faecalis in CRC are discordant: some authors suggest a protective role or no role at all in CRC while others demonstrated harmful activity. For example, Viljoen and colleagues did not find any significant clinical association between this bacterium and colon adenocarcinoma.84 In another study, when cocultivated with HCT-116 (an aggressive CRC linage), E. faecalis was able to downregulate the expression of the FIAF gene (angiopoietin-like protein 4), normally associated with the development of some cancer types.85 In a mouse model of ulcerative colitis, the increase in E. faecalis colonization after a treatment with vinegar was associated with the inhibition of inflammation by suppressing T helper (Th)-1 and Th17 responses.86 On human peripheral blood mononuclear cells, the heat-killed E. faecalis YM-73 strain shows immunomodulatory ability, increasing Th1- and reducing Th2-associated cytokines.87
In a recent study, when murine primary colon epithelial cells were cocultured with M1 macrophages polarized by E. faecalis, their Wnt/β-catenin signaling was activated and pluripotent transcription factors associated with dedifferentiation were induced. Consequently, these cells were reprogrammed and transformed the primary colon epithelial cells, thus suggesting a role of the microbiome in inducing CRC.88 In contrast, Miyamoto and colleagues demonstrated that in Min mice (APC-mutant mice) which develop many intestinal polyps through activation of β-catenin signaling, administration of the heat-killed E. faecalis EC-12 strain tends to reduce polyp development in the proximal to middle portion of the small intestine, by suppressing β-catenin signaling.89 Nevertheless, the same probiotic strain EC-12 has been demonstrated to protect the host against pathogens by inducing B-cell activation in the gut.90 The other probiotic strain, CECT7121, can protect animals from lymphoma challenge and rechallenge by downregulating LBC cell – a poorly immunogenic cell line derived from a spontaneous murine T-cell lymphoma – proliferation, inducing apoptosis in these cells and enhancing the immune response.91 This strain is also able to enhance and skew the cytokine profile to the Th1 phenotype in different conditions such as vaccination, antitumor immunity, and allergic reactions.92,93 Furthermore, Hassan and colleagues suggested that E. faecalis strains, isolated from human breast milk, could be candidates for breast cancer prevention and treatment once they are able to inhibit the proliferative activity of breast cancer cells.94
The anticarcinogenic role of some LABs has already been described and related to their immunomodulatory activities, inducing changes in the cytokine profile.95 These changes are orchestrated by the activation of dendritic cells (DCs), which recognize and respond to microbial structures via PRRs (pattern-recognition receptors) such as toll-like receptors (TLRs).96 In addition, different stimuli can induce the production of specific cytokines that are responsible for the fine tuning of an adequate immune response in each pathogen.97 For example, in the case of the CECT7121 strain, it has been demonstrated that, whereas cell wall and soluble lysates are capable of activating DCs and induce dose-dependent secretion of IL-6 and IL-12, only the cell wall, but not the soluble lysates, can induce tumor necrosis factor α and IL-10 secretion.93
Despite the fact that commensal and clinical strains share the same evolutionary origin, behavioral differences may occur when harmless ones acquire antibiotic resistant or putative virulent genes from other bacteria, via horizontal gene transfer (HGT). HGT favors rapid changes occurring in bacterial structure, generating resistance and pathogenicity island (a dynamic component of their genome), which can affect or influence their virulence.98,99 Many putative virulence factors of E. faecalis have been described. Most of them are associated with aggregation substance, surface adhesins, sex pheromones, lipoteichoic acid, extracellular superoxide, gelatinase, hyaluronidase and cytolysin (hemolysin) (reviewed by Kayaoglu and Ørstavik).100
The harmful role of E. faecalis has been suggested to be mainly associated with its ability to generate ROS and extracellular superoxide that can cause genomic instability, damaging colonic DNA, and because of that, predisposing the host to mutations and thus cancer.16 Moreover, E. faecalis has been demonstrated to produce metalloprotease that can directly compromise the intestinal epithelial barrier and induce inflammation.101 It can also activate the macrophage matrix metalloprotease MMP-9102 and lead to disruption of monolayer integrity, which could be responsible for morphological transformation of progenitor cells that get a migrating phenotype, contributing to epithelial mesenchymal transition.103 Furthermore, some authors have observed that, compared with healthy controls, the fecal E. faecalis population was increased in Indian patients with CRC,11,104 which is also noted in oral cancerous lesions.105,106
These contrasting data suggest that the origin of the isolated strain is an important variable that should be considered when its role is analysed.107 This is also motivated by the rapid and continuous changes in the DNA of E. faecalis that could modify genes associated with important features of enterococcal virulence, including hemolytic, gelatinase activities,108 antibiotic resistance and biofilm production.109 Different dysbiotic scenarios, such as those promoted by the use of antibiotics, which perturb the normal commensal microbiota and set the stage for intestinal domination, could favor these gene changes between the strains, thus suggesting possible explanations for the different roles of this bacterium in IBD, dysplasia and carcinoma.110
Discussion
In recent years, crucial discoveries and findings about gut microbiota and its impact on host functions have increased the interest evaluating the microbiome role in human health. Finding microorganisms that can be used as probiotics, either to contrast the infections, or for antitumor treatments, has become a fundamental area of investigation in translational research, generating high expectations for science and medicine.
In this review, we report that E. faecalis has been associated with human IEC injury by ROS production. However, it has also been described as showing a protective role in the same cells by inducing IL-10 expression, attenuating the secretion of the proinflammatory cytokines (e.g. IL-8).
In order to provide an explanation for this scenario, we suggest that this conflicting role could be attributed to the different isolated and investigated strains of E. faecalis. In fact, this bacterium could come up against various mutations due to gene transfer. These mutations have shown the potential to make it more or less virulent,111 especially by changing the cytokine/functional profile of the respondents of APCs, like DCs and macrophages.
Furthermore, when we analyzed the studies associating E. faecalis with cancer development, we noted that data demonstrated only an increase in its concentration, but not the functional roles of its bacterium on CRC development. In the case of documented cell or tissue injuries, the conditions were extreme, such as immunosuppressed animals. Therefore, we propose that in CRC, E. faecalis could act as a ‘passenger’ bacterium, rather than a ‘driver’, as suggested by Tjalsma and colleagues. We also suggest that, due to its intrinsic resistance and ability to acquire it (by mutations or HGT), E. faecalis can emerge as pathogenic only when the major environment undergoes alterations, such as during the appearance of cytokines and mucins or during changes in oxygen tension (typically observed on CRC onset and progression conditions).112 By considering all environmental changes caused by tumorigenesis, E. faecalis can grow uncontrolled, thus increasing the possibility of new mutations that can modify its virulence and also change the final product of its metabolism, becoming potentially harmful to the epithelial tissue. This could be an explanation for the increased concentrations of E. faecalis that were found in some studies on CRC patients’ feces, as well as its harmful role in immunodeficient mice.
Conclusion
By considering all these contrasting data, we suggest that the role of E. faecalis should be investigated in more detail and under different experimental conditions since its adoption not only as a fermentative bacterium in food manufacturing, but also as a probiotic product. Our group is currently studying the role of this bacterium in Italian patients with CRC. Our recent data are showing interesting results about the presence and incidence of E. faecalis in patients with CRC, as well as the effects of different strains on tumor cells and inflammatory response (data forthcoming).
Acknowledgments
We want to thank Dr Karin Rodland, Pacific Northwest National Laboratory (Biological Sciences Division Richland, WA, USA) and Dr Federico Boem (Dipartimento di Oncologia ed Emato-Oncologia, Università degli Studi di Milano) for critical revision of the manuscript.
Footnotes
Funding: The research was founded with a grant from the regional contribution of The Programma Attuativo Regionale (Toscana) funded by FAS (now FSC) – MICpROBIMM, the Foundation ‘Ente Cassa di Risparmio di Firenze’.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Carolina Vieira de Almeida, Department of Surgery and Translational Medicine, University of Florence, Florence, Italy.
Antonio Taddei, Department of Surgery and Translational Medicine, University of Florence, Florence, Italy.
Amedeo Amedei, Department of Experimental and Clinical Medicine, University of Florence, Viale Pieraccini, 6, 50139 Florence, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. das Neves FJ, Mattos IE, Koifman RJ. Colon and rectal cancer mortality in Brazilian capitals, 1980-1997. Arq Gastroenterol 2005; 42: 63–70. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Shin H, Bray F, et al. GLOBOCAN 2008, cancer incidence and mortality worldwide. Lyon, France: International Agency for Research on Cancer, 2010. [Google Scholar]
- 4. MacFarlane AJ, Stover PJ. Convergence of genetic, nutritional and inflammatory factors in gastrointestinal cancers. Nutr Rev 2007; 65(12 Pt 2): S157–S166. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Colorectal cancer risk factors, http://www.cdc.gov/cancer/colorectal/basic_info/risk_factors.htm (2016, accessed 19 September 2016).
- 6. Umesaki Y, Okada Y, Matsumoto S, et al. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol 1995; 39: 555–562. [DOI] [PubMed] [Google Scholar]
- 7. Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci U S A. 2003; 100: 10452–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zur Hausen H. The search for infectious causes of human cancers: where and why. Virology 2009; 392: 1–10. [DOI] [PubMed] [Google Scholar]
- 9. Rieder F. The gut microbiome in intestinal fibrosis: environmental protector or provocateur? Sci Transl Med 2013; 5: 190ps10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balamurugan R, Rajendiran E, George S, et al. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J Gastroenterol Hepatol 2008; 23(8 Pt 1): 1298–1303. [DOI] [PubMed] [Google Scholar]
- 11. Prakash S, Rodes L, Coussa-Charley M, et al. Gut microbiota: next frontier in understanding human health and development of biotherapeutics. Biologics 2011; 5: 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Habermann W, Zimmermann K, Skabaris H, et al. The effect of a bacterial immunostimulant (human Enterococcus faecalis bacteria) on the occurrence of relapse in patients with chronic bronchitis. Arzneimittel-Forschung 2001; 51: 931–937. [DOI] [PubMed] [Google Scholar]
- 13. Gong J, Bai T, Zhang L, et al. Inhibition effect of Bifidobacterium longum, Lactobacillus acidophilus, Streptococcus thermophilus and Enterococcus faecalis and their related products on human colonic smooth muscle in vitro. PLoS One 2017; 12: e0189257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 2002; 23: 529–536. [DOI] [PubMed] [Google Scholar]
- 15. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–767. [DOI] [PubMed] [Google Scholar]
- 16. Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol 2011; 6: 479–507. [DOI] [PubMed] [Google Scholar]
- 17. Silva FC, Valentin MD, Ferreira FO, et al. Mismatch repair genes in Lynch syndrome: a review. São Paulo Med J 2009; 127: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Miranda NF, Nielsen M, Pereira D, et al. MUTYH associated polyposis carcinomas frequently lose HLA class I expression – a common event amongst DNA-repair-deficient colorectal cancers. J Pathol 2009; 219: 69–76. [DOI] [PubMed] [Google Scholar]
- 19. Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet 2010; 375: 1030–1047. [DOI] [PubMed] [Google Scholar]
- 20. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008; 454: 436–444. [DOI] [PubMed] [Google Scholar]
- 21. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7: 211–217. [DOI] [PubMed] [Google Scholar]
- 22. World Cancer Research Found/American Institute for Cancer Research. Food, nutrition, physical activity, and prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research, 2007. [Google Scholar]
- 23. Branca F, Hanley AB, Pool-Zobel BL, et al. Biomarkers in disease and health. Br J Nutr 2001; 86: S55–S92. [DOI] [PubMed] [Google Scholar]
- 24. Bingham SA. Diet and colorectal cancer prevention. Biochem Soc Trans 2000; 28: 12–16. [DOI] [PubMed] [Google Scholar]
- 25. Norat T, Lukanova A, Ferrari P, et al. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer 2002; 98: 241–256. [DOI] [PubMed] [Google Scholar]
- 26. Butler LM, Sinha R, Millikan RC, et al. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol 2003; 157: 434–445. [DOI] [PubMed] [Google Scholar]
- 27. McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: a review. Public Health Nutr 2012; 15: 2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Møller P, Loft S. Dietary antioxidants and beneficial effect on oxidatively damaged DNA. Free Radic Biol Med 2006; 41: 388–415. [DOI] [PubMed] [Google Scholar]
- 29. Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 30. Boleij A, Tjalsma H. Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer. Biol Rev Camb Philos Soc 2012; 87: 701–730. [DOI] [PubMed] [Google Scholar]
- 31. Koenig J, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 2011; 108: 4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biasucci G, Rubini M, Riboni S, et al. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev 2010; 86(Suppl. 1): 13–15. [DOI] [PubMed] [Google Scholar]
- 33. Collado MC, Delgado S, Maldonado A, et al. Assessment of the bacterial diversity of breast milk of healthy women by quantitative real-time PCR. Lett Appl Microbiol 2009; 48: 523–528. [DOI] [PubMed] [Google Scholar]
- 34. Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001; 291: 881–884. [DOI] [PubMed] [Google Scholar]
- 35. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology 2009; 136: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 1977; 31: 107–133. [DOI] [PubMed] [Google Scholar]
- 38. Costello EK, Lauber CL, Hamady M, et al. Bacterial community variation in human body habitats across space and time. Science 2009; 326: 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flint HJ, Bayer EA, Rincon MT, et al. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 2008; 6: 121–131. [DOI] [PubMed] [Google Scholar]
- 40. Kosiewicz MM, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol 2011; 2: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 2011; 8: 523–553. [DOI] [PubMed] [Google Scholar]
- 42. Corr SC, Hill C, Gahan CG. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res 2009; 56: 1–15. [DOI] [PubMed] [Google Scholar]
- 43. Raj T, Dileep U, Vaz M, et al. Intestinal microbial contribution to metabolic leucine input in adult men. J Nutr 2008; 138: 2217–2221. [DOI] [PubMed] [Google Scholar]
- 44. Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol 2011; 65: 411–429. [DOI] [PubMed] [Google Scholar]
- 45. Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005; 128: 891–906. [DOI] [PubMed] [Google Scholar]
- 46. Tap J, Mondot S, Levenez F, et al. Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol 2009; 11: 2574–2584. [DOI] [PubMed] [Google Scholar]
- 47. Harmsen HJ, Raangs GC, He T, et al. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl Environ Microbiol 2002; 68: 2982–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. DiBaise JK, Frank DN, Mathur R. Impact of the gut microbiota on the development of obesity: current concepts. Am J Gastroenterol Suppl 2012; 1: 22–27. [Google Scholar]
- 49. Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 50. Dapito D, Mencin A, Gwak G, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012; 21: 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dove W, Clipson L, Gould K, et al. Intestinal neoplasia in the ApcMin mouse: independence from the microbial and natural killer (beige locus) status. Cancer Res 1997; 57: 812–814. [PubMed] [Google Scholar]
- 52. Zur Hausen H. The search for infectious causes of human cancers: where and why. Virology 2009; 392: 1–10. [DOI] [PubMed] [Google Scholar]
- 53. Collins D, Hogan AM, Winter DC. Microbial and viral pathogens in colorectal cancer. Lancet Oncol 2011; 12: 504–512. [DOI] [PubMed] [Google Scholar]
- 54. Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008; 8: 411–420. [DOI] [PubMed] [Google Scholar]
- 55. Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002; 122: 44–54. [DOI] [PubMed] [Google Scholar]
- 56. Podolsky DK. The current future understanding of inflammatory bowel disease. Best Pract Res Clin Gastroenterol 2002; 16: 933–943. [DOI] [PubMed] [Google Scholar]
- 57. Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology 2010; 139: 1816–1819. [DOI] [PubMed] [Google Scholar]
- 58. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 59. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001; 48: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011; 140: 1807–1816. [DOI] [PubMed] [Google Scholar]
- 61. Huycke MM, Joyce W, Wack MF. Augmented production of extracellular superoxide by blood isolates of Enterococcus faecalis. J Infect Dis 1996; 173: 743–746. [DOI] [PubMed] [Google Scholar]
- 62. Sartor RB, Mazmanian SK. Intestinal microbes in inflammatory bowel diseases. Am J Gastroenterol Suppl 2012; 1: 15–21. [Google Scholar]
- 63. Kostic AD, Chun E, Meyerson M, et al. Microbes and inflammation in colorectal cancer. Cancer Immunol Res 2013; 1: 150–157. [DOI] [PubMed] [Google Scholar]
- 64. Tjalsma H, Boleij A, Marchesi J, et al. A bacterial driver–passenger model for colorectal cancer: beyond the usual suspects. Nat Ver Microbiol 2012; 10: 575–582. [DOI] [PubMed] [Google Scholar]
- 65. Scanlan PD, Shanahan F, Clune Y, et al. Culture-independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ Microbiol 2008; 10: 789–798. [DOI] [PubMed] [Google Scholar]
- 66. Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 2013; 13: 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meira LB, Bugni JM, Green SL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 2008; 118: 2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Westbrook AM, Wei B, Braun J, et al. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res 2009; 69: 4827–4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang X, Allen TD, May RJ, et al. Enterococcus faecalis induces aneuploidy and tetraploidy in colonic epithelial cells through a bystander effect. Cancer Res 2008; 68: 9909–9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hill MJ, Drasar BS, Aries V, et al. Bacteria and aetiology of cancer of large bowel. Lancet 1971; 1: 95–100. [DOI] [PubMed] [Google Scholar]
- 71. Jett BD, Huycke MM, Gilmore MS. Virulence of enterococci. Clin Microbiol Ver 1994; 7: 462–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pillar CM, Gilmore MS. Enterococcal virulence–pathogenicity island of E. Faecalis. Front Biosci 2004; 9: 2335–2346. [DOI] [PubMed] [Google Scholar]
- 73. Moellering RC., Jr. Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992; 14: 1173–1176. [DOI] [PubMed] [Google Scholar]
- 74. Ogihara S, Saito R, Sawabe E, et al. First Japanese case of infectious endocarditis due to Enterococcus faecalis small-colony variants. J Infect Chemother 2016; 22: 716–719. [DOI] [PubMed] [Google Scholar]
- 75. Lee SW, Shet UK, Park SW, et al. Identification of Enterococcus faecalis antigens specifically expressed in vivo. Restor Dent Endod 2015; 40: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with health care-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol 2013; 34: 1–14. [DOI] [PubMed] [Google Scholar]
- 77. Souto R, Colombo AP. Prevalence of Enterococcus faecalis in subgingival biofilm and saliva of subjects with chronic periodontal infection. Arch Oral Biol 2008; 53: 155–160. [DOI] [PubMed] [Google Scholar]
- 78. Fanaro S, Chierici R, Guerrini P, et al. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 2003; 91: 48–55. [DOI] [PubMed] [Google Scholar]
- 79. Are A, Aronsson L, Wang S, et al. Enterococcus faecalis from newborn babies regulate endogenous PPARgamma activity and IL-10 levels in colonic epithelial cells. Proc Natl Acad Sci U S A 2008; 105: 1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang S, Hibberd ML, Pettersson S, et al. Enterococcus faecalis from healthy infants modulates inflammation through MAPK signaling pathways. PLoS One 2014; 9: e97523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nueno-Palop C, Narbad A. Probiotic assessment of Enterococcus faecalis CP58 isolated from human gut. Int J Food Microbiol 2011; 145: 390–394. [DOI] [PubMed] [Google Scholar]
- 82. Devriese LA, Pot B, Collins MD. Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J Appl Bacteriol 1993; 75: 399–408. [DOI] [PubMed] [Google Scholar]
- 83. Franz CM, Huch M, Abriouel H, et al. Enterococci as probiotics and their implications in food safety. Int J Food Microbiol 2011; 151: 125–140. [DOI] [PubMed] [Google Scholar]
- 84. Viljoen KS, Dakshinamurthy A, Goldberg P, et al. Quantitative profiling of colorectal cancer-associated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PLoS One. 2015; 10: e0119462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grootaert C, Van de, Wiele T, Van Roosbroeck I, et al. Bacterial monocultures, propionate, butyrate and H2O2 modulate the expression, secretion and structure of the fasting-induced adipose factor in gut epithelial cell lines. Environ Microbiol 2011; 13: 1778–1789. [DOI] [PubMed] [Google Scholar]
- 86. Shen F, Feng J, Wang X. Vinegar treatment prevents the development of murine experimental colitis via inhibition of inflammation and apoptosis. J Agric Food Chem 2016; 10; 64: 1111–1121. [DOI] [PubMed] [Google Scholar]
- 87. Ou CC, Lin SL, Tsai, et al. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J Food Sci 2011; 76: M260–M267. [DOI] [PubMed] [Google Scholar]
- 88. Wang X, Yang Y, Huycke MM. Commensal-infected macrophages induce dedifferentiation and reprogramming of epithelial cells during colorectal carcinogenesis. Oncotarget 2017; 8: 102176–102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Miyamoto S, Komiya M, Fujii G, et al. Preventive effects of heat-killed Enterococcus faecalis strain EC-12 on mouse intestinal tumor development. Int J Mol Sci 2017; 18: pii: E826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vacheron F, Guenounou M, Nauciel C. Induction of interleukin 1 secretion by adjuvant-active peptidoglycans. Infect Immun 1983; 42: 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Castro MS, Molina MA, Di Sciullo P, et al. Beneficial activity of Enterococcus faecalis CECT7121 in the anti-lymphoma protective response. J Appl Microbiol 2010; 109: 1234–1243. [DOI] [PubMed] [Google Scholar]
- 92. Castro MS, Azpiroz MB, Molina MA, et al. Preliminary studies on the prevention of the ovalbumin-induced allergic response by Enterococcus faecalis CECT7121 in mice. Int Arch Allergy Immunol 2012; 157: 11–20. [DOI] [PubMed] [Google Scholar]
- 93. Molina MA, Díaz AM, Hesse C, et al. Immunostimulatory effects triggered by Enterococcus faecalis CECT7121 probiotic strain involve activation of dendritic cells and interferon-gamma production. PLoS One 2015; 10: e0127262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hassan Z, Mustafa S, Rahim RA, et al. Anti-breast cancer effects of live, heat-killed and cytoplasmic fractions of Enterococcus faecalis and Staphylococcus hominis isolated from human breast milk. In Vitro Cell Dev Biol Anim 2016; 52: 337–348. [DOI] [PubMed] [Google Scholar]
- 95. Zabala A, Martın MR, Haza AI, et al. Anti-proliferative effect of two lactic acid bacteria strains of human origin on the growth of a myeloma cell line. Lett Appl Microbiol 2001; 32: 287–292. [DOI] [PubMed] [Google Scholar]
- 96. van Vliet SJ, den Dunnen J, Gringhuis SI, et al. Innate signaling and regulation of dendritic cell immunity. Curr Opin Immunol 2007; 19: 435–440. [DOI] [PubMed] [Google Scholar]
- 97. Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 2010; 8: 171–184. [DOI] [PubMed] [Google Scholar]
- 98. Nallapareddy SR, Duh RW, Singh KV, et al. Molecular typing of selected Enterococcus faecalis isolates: pilot study using multilocus sequence typing and pulsed-field gel electrophoresis. J Clin Microbiol 2002; 40: 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McBride SM, Fischetti VA, Leblanc DJ, et al. Genetic diversity among Enterococcus faecalis. PLoS One 2007; 2: e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kayaoglu G, Ørstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med 2004; 15: 308–320. [DOI] [PubMed] [Google Scholar]
- 101. Steck N, Hoffmann M, Sava IG, et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 2011; 141: 959–971. [DOI] [PubMed] [Google Scholar]
- 102. Shogan BD, Belogortseva N, Luong PM, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med 2015; 7: 286ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hao L, Zhang C, Qiu Y, et al. Recombination of CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human breast cancer. Cancer Lett 2007; 253: 34–42. [DOI] [PubMed] [Google Scholar]
- 104. Zhou Y, He H, Xu H, et al. Association of oncogenic bacteria with colorectal cancer in South China. Oncotarget 2016; 7: 80794–80802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Boonanantanasarn K, Gill AL, Yap Y, et al. Enterococcus faecalis enhances cell proliferation through hydrogen peroxide-mediated epidermal growth factor receptor activation. Infect Immun 2012; 80: 3545–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Byakodi R, Krishnappa R, Keluskar V, et al. The microbial flora associated with oral carcinomas. Quintessence Int 2011; 42: e118-23. [PubMed] [Google Scholar]
- 107. Mohamed JA, Huang W, Nallapareddy SR, et al. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect Immun 2004; 72: 3658–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Banwo K, Sanni A, Tan H. Technological properties and probiotic potential of Enterococcus faecium strains isolated from cow milk. J Appl Microbiol 2013; 114: 229–241. [DOI] [PubMed] [Google Scholar]
- 109. Popović N, Dinić M, Tolinački M, et al. New Insight into biofilm formation ability, the presence of virulence genes and probiotic potential of Enterococcus sp. dairy isolates. Front Microbiol 2018; 9: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol 2002; 160: 2253–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbial Rev 2000; 13: 686–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Colgan SP, Curtis VF, Campbell EL. The inflammatory tissue microenvironment in IBD. Inflamm Bowel Dis 2013; 19: 2238–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]