Many musculoskeletal imaging advancements have been made within the past decade. Initial emphasis was placed on diagnosis, demonstrating that imaging can provide a noninvasive, accurate assessment of joint integrity, typically using arthroscopic surgery as a standard. More recent advances to improve both in-plane and through-plane resolution as well as tissue contrast now enable complete comprehensive assessment of the joint, providing detection of lesions that cannot be seen during arthroscopy, either because they are not accessible or because they are present within the deeper structures of the cartilage-bone interface. Magnetic resonance imaging (MRI) has evolved from a tool that demonstrates anatomy to one that noninvasively assesses tissue biochemistry by the use of parametric mapping techniques, including T2 mapping, T2* mapping, and T1 rho. These quantitative MRI (qMRI) techniques provide a more sensitive means to detecting tissue pathology before structural breakdown is apparent on high-resolution morphologic images, allowing for risk assessments in cohorts with predispositions toward musculoskeletal pathologies such as the early onset of osteoarthritis (OA) in patients with developmental hip dysplasia, femoroacetabular impingement (FAI), or after anterior cruciate ligament (ACL) injury in the knee. These techniques also provide a noninvasive assessment of cartilage repair surgeries, obviating the need for second-look arthroscopy. More recent advances have been to link parametric mapping techniques to structural capacity of tissue to bear load. Combined, these data can benefit the clinical management of musculoskeletal injuries, as they provide a quantitative metric by which to guide treatment plans, evaluate intervention efficacy, and better inform decisions regarding the appropriate timing for return to play. While many advances have been made in imaging, this current report will focus on 3 of the more recent advances, including an update on MRI parametric mapping, peripheral nerve imaging, and shear wave sonoelastography.
Ultrasound Elastography
Ultrasound elastography can detect changes in tissue stiffness, and shear wave elastography (SWE) has the ability to provide a quantitative assessment of such changes. Studies evaluating SWE in musculoskeletal soft tissue structures (ie, muscles and tendons) have shown sufficient repeatability56,58,67,80 and provide technical guidelines on measuring musculoskeletal tissue stiffness.39,78,90
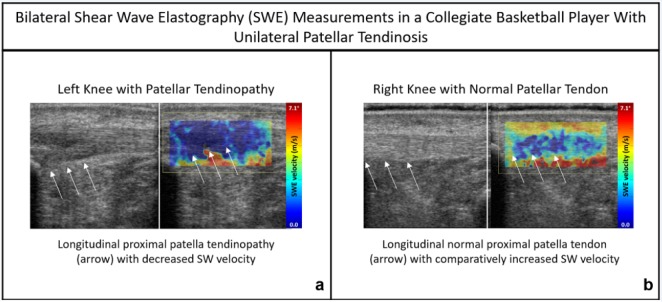
Although its musculoskeletal applications are only just emerging, there are some promising developments on the utility of SWE in sports medicine. Several studies have correlated SWE measurements in muscle and tendon to biomechanical tissue properties.49,69 There are numerous studies characterizing tendinopathy with SWE (Figure 1).5,15,19 Others have correlated symptom scores for tendinopathy to SWE measures, with some showing better correlation with SWE than conventional ultrasound techniques.23 SWE has also demonstrated the ability to detect changes in intrinsic tendon stiffness in asymptomatic athletes compared with age-matched healthy nonathletes.6 These and other studies have established the potential for SWE to inform on musculoskeletal tissue health beyond the capabilities of conventional ultrasound.
Figure 1.
(a) Left knee displays patellar tendinosis and comparatively lower (deep blue) SW velocity in comparison to the contralateral (right) knee. (b) Increased (lighter blue) patellar tendon SWE velocity (m/s) demonstrated within the normal, healthy right knee of the same subject.
qMRI And Parametric Mapping Techniques
Over the past decade, qMRI techniques have been developed as a noninvasive means by which to evaluate the biochemical status of tissues. These techniques work by exploiting inherent differences in MRI tissue relaxometry that reflect tissue integrity. Currently, the most commonly used qMRI sequences can largely be divided into 2 general categories: proteoglycan (PG)- or glycosaminoglycan (GAG)-sensitive techniques and collagen-sensitive techniques (Table 1).
Table 1.
Clinically useful qMRI techniques for assessment of the biochemical composition of tissues
| MRI Technique | Biostructural Property Evaluated | Strengths | Limitations |
|---|---|---|---|
| T1ρ mapping | PG/GAG content and distribution | Sensitive to early PG depletion; does not necessitate use of contrast agent | Optimal at 3T; not available at all institutions |
| dGEMRIC | PG/GAG content and distribution | Well validated as an indirect measurement of PG/GAG content (high sensitivity and specificity) | Requires use of Gd contrast agent (contraindicated in patients with renal impairments); long delay (≈1.5 hours) between Gd administration and postcontrast MRI |
| T2 mapping | Collagen orientation and water content | Well validated; does not necessitate use of contrast agent; compatible with most MRI systems and field strengths | Susceptible to magic angle effects; cannot evaluate deeper (calcified) cartilage layers or other short T2 species (tendon, bone, ligaments, etc) |
| T2* mapping | Collagen orientation and water content | Does not necessitate use of contrast agent; can be faster than T2 mapping; UTE sequences allow for evaluation of short T2 species (calcified cartilage layer, tendon, ligament, etc) | Susceptible to magnetic field inhomogeneities and magic angle effects |
dGEMRIC, delayed gadolinium-enhanced magnetic resonance imaging of cartilage; GAG, glycosaminoglycan; Gd, gadolinium; MRI, magnetic resonance imaging; PG, proteoglycan; qMRI, quantitative magnetic resonance imaging; UTE, ultrashort echo time.
Proteoglycan-Sensitive Techniques: T1ρ and T1 (dGEMRIC)
T1ρ Mapping
Quantification of T1ρ allows for mapping of the low-magnitude movements and interactions between constrained water protons and their local environment. Accordingly, quantitative measurements of T1ρ reflect the loss or gain of matrix constituents that work to retain water, such as the negatively charged GAG side chains on PG macromolecules, which impart resistance to compressive loads in articular cartilage. T1ρ values have demonstrated strong correlations with both histological PG content and fixed charge density in cartilage,2,84 with an elevation of T1ρ values indicative of a net loss of PG.20,36,61 Previous reports have found that T1ρ values are capable of differentiating OA patients from healthy controls,76 and strong correlations have been demonstrated between T1ρ values and in vivo measurements of disease severity within OA patients.44 T1ρ measurements of cartilage have also proven to be well correlated to arthroscopic findings in patients with posttraumatic cartilage injury and chondromalacia.48,89
T1 Mapping (dGEMRIC)
Alone, T1 relaxometry is not adequately sensitive to provide meaningful information about the biochemical composition of articular or meniscal cartilage. However, quantification of T1 relaxation can be useful in the presence of a negatively charged gadolinium (Gd) contrast agent, which is repelled by the negative charges of GAG and therefore diffuses toward areas of low GAG concentration. In this way, delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) allows for evaluation of the fixed charge density content and distribution of PG and associated GAG. Decreased dGEMRIC indices have been found in osteoarthritic cartilage as well as in the cartilage of patients with other joint conditions such as FAI, ACL injuries, and meniscal injuries.26,27,40,51,59,81,86 In addition, several studies have provided evidence that dGEMRIC indices afford clinically useful predictive information about progression of joint degeneration and outcomes of surgical intervention.10,21,52,65 Drawbacks of dGEMRIC techniques are both the required delay (approximately 90 minutes) between the administration of the Gd contrast agent and postcontrast MRI acquisition and the fact that Gd is contraindicated in patients with impaired renal function, limiting its more ubiquitous application.28
Collagen-Sensitive Techniques: T2 and T2*
T2 Mapping
T2 characteristics of a tissue reflect the interaction between free water protons (spins) within the tissue and how rapidly they diphase relative to one another after the application of a radio frequency pulse. Anisotropic and compact tissue structures force more interactions between the proton spins resulting in more rapid dephasing, which will manifest as a shorter T2 decay. In this way, T2 relaxation times are sensitive to both tissue hydration and orientation of collagen within a tissue matrix and can be comprehensively evaluated either on a voxel-by-voxel basis (voxel-based relaxometry) or through the analysis of T2 maps (texture). Injuries or degenerative processes that result in damage to a tissue’s matrix will manifest as a prolongation of T2 relaxation times, as spin-spin interactions within the tissue will be decreased.3,25,46,47,54,60,75,85 Recent studies have used T2 relaxometry as an image-based biomarker for the detection of early changes associated with cartilage degeneration as well as a quantitative metric by which to monitor disease progression and intervention efficacy.7,8,11,25,32,33,38,42,45,53,57,60,61,64,65,68,70,71,77,79,87 Within knee OA patients, mean T2 values have been shown to be significantly correlated with pain, function, and morphologic cartilage measurements.25 Notably, cartilage T2 measurements in patients without evidence of severe OA at baseline have also been found to be predictive of morphologic progression of OA at 2-year follow-up.35,61,79 Similarly, a recent prospective study by Williams et al87 demonstrated that early changes in articular cartilage T2 metrics after ACL injury correlated with later changes in both cartilage T2 and cartilage thickness. Prolonged cartilage T2 values have also been found in the talocrural and subtalar joints within patients who suffer from chronic lateral ankle instability.35,79 In a recent study of patients with FAI, Samaan et al70 reported that voxel-based relaxometry analysis of T2 radial heterogeneity was better able to detect cartilage delamination compared with global T2 mapping and that FAI patients with more severe cam impingements displayed increased T2 heterogeneity.
T2* Mapping
While T2 mapping is useful for the evaluation of cartilage, standard techniques are insensitive to short and ultra-short signal decays and are therefore limited in their ability to evaluate the deeper cartilage layers. Standard T2 mapping techniques are similarly inappropriate for the assessment of other short T2 species such as tendons, ligaments, and bone, as a majority of the signal will have decayed prior to echo formation. Therefore, specialized ultrashort echo time MRI techniques have been developed to effectively capture these rapidly decaying signals and allow for quantitative evaluation of T2*.13,14,18,24,43,55,63,66,82,85,88
Previous studies have established good correlations between T2* values, histopathology, microscopy, and biomechanical testing.38,50,72 As with T2 metrics, numerous studies have demonstrated that prolonged T2* values are indicative of the presence and severity of tissue pathology.12,34,38,50,85 Evaluation of meniscal samples obtained from patients undergoing total knee arthroplasty has established that regions with significantly prolonged T2* values also display corresponding histological degeneration.50 In a recent study, Titchenal et al82 reported that patients 2 years post–ACL reconstruction demonstrated elevated T2* values within the deep layers of the medial tibiofemoral cartilage compared with uninjured controls. Interestingly, the same study also reported that elevated T2* values correlated with higher knee adduction moment and a more varus mechanical axis, both of which have been previously implicated as factors contributing to risk of OA development.82 As degenerative changes often precede tendon rupture, evaluation of T2* values has also proven useful for the evaluation of tendinosis.14,34,63 Future applications of these techniques will be directed to correlating MRI assessment of collagen orientation to material properties, such as the patellar tendon in elite basketball players,4 as T2* values are also affected by direct tissue loads.37 Such quantitative assessments, like shear wave sonoelastography, may provide an objective noninvasive assessment of the ability of tissues such as tendons and ligaments to withstand repetitive loads, which has implications with regard to athletic performance and return to play.
Peripheral Nerve Imaging
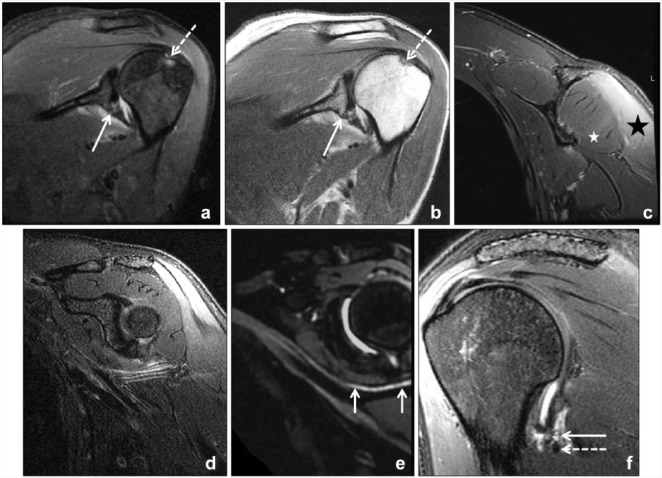
Peripheral nerve diagnostic imaging has rapidly evolved over the past 20 years with advances in both ultrasound and MRI. In 1992, Howe et al30 coined the term MR neurography to describe MRI sequences that combined fat suppression and diffusion techniques to optimize contrast in order to distinguish peripheral nerves from adjacent soft tissue. Only within the past 10 years, with the advent and availability of high-performance gradient 3.0-T magnets and multichannel surface coils that facilitate high–spatial resolution acquisition, has MRI become routinely effective in evaluating peripheral nerve pathology (Figure 2).16 Nonetheless, MR neurography is not ubiquitous in radiology practices today, as it requires careful planning and real-time monitoring by specialty-trained radiologists and experienced technologists to ensure success.
Figure 2.
A 33-year-old woman status post left shoulder dislocation. (a) Oblique coronal inversion recovery and (b) proton density magnetic resonance images of the left shoulder demonstrate a Hill-Sachs lesion (dashed arrow) and partial capsular detachment from the scapula (solid arrow). Dedicated magnetic resonance imaging of the left axillary nerve was acquired at 6-week follow-up to evaluate a dense axillary nerve palsy postdislocation. (c) Coronal T2-weighted Dixon fat-suppressed image confirms denervation edema of deltoid muscle (black star) and relative sparing of the teres minor (white star). (e) Axillary nerve (arrows) is better delineated from adjacent vessels on vascular-suppressed, 3-dimensional T2-weighted curved multiplanar reformatted image compared with (d) the 2-dimensional image without vascular suppression. (f) T2-weighted sagittal image confirms suspected stretch injury, with signal hyperintensity of the axillary nerve (solid arrow) adjacent to the capsule with the posterior circumflex humeral artery (dashed arrow).
MRI complements electrodiagnostic testing (EDX) for the evaluation of peripheral neuropathies and is comparatively noninvasive, painless, potentially less prone to interobserver reliability, and can often accurately localize the site of focal pathology.29,41 Precise localization can potentially save hours in the operating room and decrease patient morbidity associated with extensive nerve exploration. MRI also affords concomitant assessment of all regional muscles within a prescribed field of view when evaluating for denervation, whereas EDX involves direct needle electromyography of each individual muscle. One potential pitfall of EDX localization is when a “lesion” does not involve the entirety of a nerve but rather comprises partial insult to 1 or more of its fascicles. EDX may then erroneously pinpoint pathology within a more distal branch nerve rather than within the parent nerve. A prime example of this is anterior interosseous neuropathy, a subtype of Parsonage-Turner syndrome. In this scenario, recent MRI studies (with surgical confirmation) have demonstrated an idiopathic hourglass constriction of the anterior interosseous fascicular bundle of the median nerve proper, immediately proximal to the elbow joint.73,74 This finding directs surgical management in cases of refractory disease to neurolysis at the fascicular level rather than “decompression” of the anterior interosseous nerve proper within the forearm, as has been traditionally performed based on EDX localization.
Peripheral neuropathies commonly encountered by sports medicine specialists and routinely diagnosed with MR neurography include ulnar neuropathy in the throwing athlete, axillary neuropathy after shoulder dislocation, sciatic neuropathy following hamstring tear, and common peroneal neuropathy following knee dislocation. Even more common is iatrogenic injury following orthopaedic surgery (eg, direct impingement by orthopaedic hardware, stretch injury, compression from hematoma/seroma, and nerve transection). In these settings, MRI can pinpoint specific pathology and facilitate early intervention, or alternatively provide reassurance to both the surgeon and patient that no injury has occurred. Metal artifact reduction techniques, similar to those used for arthroplasty imaging, can be combined with MR neurography techniques to determine the precise relationship of hardware to the nerve in question.1,62 Ultrasound also nicely complements MR for the evaluation of nerves, particularly when metal artifact reduction techniques are overwhelmed by a susceptibility effect from the metal.
In addition to hardware advancements, the development of novel pulse sequences has also assisted the evolution of MR neurography. Heavily T2-weighted fat-suppressed sequences, critical for elucidating nerve pathology, have improved with the advent of Dixon fat-water suppression techniques that provide robust fat suppression in regions notoriously difficult to achieve adequate suppression and a high signal-to-noise ratio. These can be combined with 3-dimensional acquisitions and reconstructed into multiple arbitrary planes to follow the course of a nerve.17 Specialized vascular-suppression techniques have facilitated more reliable nerve identification and confidence in interpretation as they enable suppression of signal within the blood vessels that run alongside or in close proximity to the nerve of interest.9,73,83
One major limitation of MR neurography is its overall qualitative nature and inability to reliably quantify the extent of nerve injury and regeneration, particularly when a nerve remains in continuity (eg, stretch or compression injury). Diffusion tensor imaging, a qMRI technique developed for the brain that measures the degree of anisotropy, or preferential movement of water molecules in a particular direction, has been used for the assessment of peripheral nerve structural integrity.31 As healthy nerves are inherently anisotropic given their longitudinal organization, damage to a nerve is typically reflected as decreased anisotropy. Peripheral nerve diffusion tensor imaging, however, is still a research tool with numerous technical hurdles that will need to be addressed before becoming a mainstay in clinical practice.
Peripheral neuropathies, as previously mentioned, are encountered in sports medicine practice often as a secondary insult after sports injury or surgery. As peripheral nerves, even small sensory branches,22 can be visualized on routine MRI examinations (eg, of the knee or elbow), it is imperative that musculoskeletal radiologists familiarize themselves with the relevant anatomy and add these structures to their routine “search pattern.” It is our experience in partnering closely with peripheral nerve surgeons, neurologists, and physiatrists over the past 5 years that MR neurography provides a valuable element in patient care.
Each issue of Sports Health in 2018 will feature a Guest Editorial highlighting changes seen in both the journal and the disciplines of athletic training, orthopaedic surgery, primary care, and sports physical therapy, as well as this special update on imaging over the past 10 years. Be sure to look out for the continuation of this series in the September/October issue!
Contributor Information
O. Kenechi Nwawka, Department of Radiology and Imaging, Hospital for Special Surgery, New York, NY, USA.
Hollis G. Potter, Sports Health Associate Editor for Imaging, Department of Radiology and Imaging, Hospital for Special Surgery, New York, NY, USA.
References
- 1. Ahlawat S, Stern SE, Belzberg AJ, Fritz J. High-resolution metal artifact reduction MR imaging of the lumbosacral plexus in patients with metallic implants. Skeletal Radiol. 2017;46:897-908. [DOI] [PubMed] [Google Scholar]
- 2. Akella SV, Regatte RR, Wheaton AJ, Borthakur A, Reddy R. Reduction of residual dipolar interaction in cartilage by spin-lock technique. Magn Reson Med. 2004;52:1103-1109. [DOI] [PubMed] [Google Scholar]
- 3. Argentieri EC, Burge AJ, Potter HG. Magnetic resonance imaging of articular cartilage within the knee. J Knee Surg. 2018;31:155-165. [DOI] [PubMed] [Google Scholar]
- 4. Argentieri EC, Shah PH, Potter HG, Nwawka OK, Koff MF. Patellar tendon T2* MRI demonstrates longitudinal changes of tendon degeneration over a season in collegiate basketball players. Paper presented at: Orthopaedic Research Society Annual Meeting; March 10-13, 2018; New Orleans, LA. [Google Scholar]
- 5. Aubry S, Nueffer JP, Tanter M, Becce F, Vidal C, Michel F. Viscoelasticity in Achilles tendonopathy: quantitative assessment by using real-time shear-wave elastography. Radiology. 2015;274:821-829. [DOI] [PubMed] [Google Scholar]
- 6. Balaban M, Idilman IS, Ipek A, Ikiz SS, Bektaser B, Gumus M. Elastographic findings of Achilles tendons in asymptomatic professional male volleyball players. J Ultrasound Med. 2016;35:2623-2628. [DOI] [PubMed] [Google Scholar]
- 7. Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage. 2013;21:1474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65:1184-1194. [DOI] [PubMed] [Google Scholar]
- 9. Cervantes B, Kirschke JS, Klupp E, et al. Orthogonally combined motion- and diffusion-sensitized driven equilibrium (OC-MDSDE) preparation for vessel signal suppression in 3D turbo spin echo imaging of peripheral nerves in the extremities. Magn Reson Med. 2018;79:407-415. [DOI] [PubMed] [Google Scholar]
- 10. Chandrasekaran S, Vemula SP, Lindner D, Lodhia P, Suarez-Ahedo C, Domb BG. Preoperative delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) for patients undergoing hip arthroscopy: indices are predictive of magnitude of improvement in two-year patient-reported outcomes. J Bone Joint Surg Am. 2015;97:1305-1315. [DOI] [PubMed] [Google Scholar]
- 11. Chang AL, Yu HJ, von Borstel D, et al. Advanced imaging techniques of the wrist. AJR Am J Roentgenol. 2017;209:497-510. [DOI] [PubMed] [Google Scholar]
- 12. Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J Magn Reson Imaging. 2015;41:870-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaudhari AS, Sveinsson B, Moran CJ, et al. Imaging and T2 relaxometry of short-T2 connective tissues in the knee using ultrashort echo-time double-echo steady-state (UTEDESS). Magn Reson Med. 2017;78:2136-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen B, Zhao Y, Cheng X, et al. Three-dimensional ultrashort echo time cones (3D UTE-Cones) magnetic resonance imaging of entheses and tendons. Magn Reson Imaging. 2018;49:4-9. [DOI] [PubMed] [Google Scholar]
- 15. Chen XM, Cui LG, He P, Shen WW, Qian YJ, Wang JR. Shear wave elastographic characterization of normal and torn Achilles tendons: a pilot study. J Ultrasound Med. 2013;32:449-455. [DOI] [PubMed] [Google Scholar]
- 16. Chhabra A, Madhuranthakam AJ, Andreisek G. Magnetic resonance neurography: current perspectives and literature review. Eur Radiol. 2018;28:698-707. [DOI] [PubMed] [Google Scholar]
- 17. Chhabra A, Thawait GK, Soldatos T, et al. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol. 2013;34:486-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu CR, Williams AA, West RV, et al. Quantitative magnetic resonance imaging UTE-T2* mapping of cartilage and meniscus healing after anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42:1847-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coombes BK, Tucker K, Vicenzino B, et al. Achilles and patellar tendinopathy display opposite changes in elastic properties: a shear wave elastography study. Scand J Med Sci Sports. 2018;28:1201-1208. [DOI] [PubMed] [Google Scholar]
- 20. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31:37-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cunningham T, Jessel R, Zurakowski D, Millis MB, Kim YJ. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage to predict early failure of Bernese periacetabular osteotomy for hip dysplasia. J Bone Joint Surg Am. 2006;88:1540-1548. [DOI] [PubMed] [Google Scholar]
- 22. Deshmukh S, Carrino JA, Feinberg JH, Wolfe SW, Eagle S, Sneag DB. Pins and needles from fingers to toes: high-resolution MRI of peripheral sensory mononeuropathies. AJR Am J Roentgenol. 2017;208:W1-W10. [DOI] [PubMed] [Google Scholar]
- 23. Dirrichs T, Quack V, Gatz M, et al. Shear wave elastography (SWE) for monitoring of treatment of tendinopathies: a double-blinded, longitudinal clinical study. Acad Radiol. 2018;25:265-272. [DOI] [PubMed] [Google Scholar]
- 24. Du J, Bydder M, Takahashi AM, Carl M, Chung CB, Bydder GM. Short T2 contrast with three-dimensional ultrashort echo time imaging. Magn Reson Imaging. 2011;29:470-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ericsson YB, Tjornstrand J, Tiderius CJ, Dahlberg LE. Relationship between cartilage glycosaminoglycan content (assessed with dGEMRIC) and OA risk factors in meniscectomized patients. Osteoarthritis Cartilage. 2009;17:565-570. [DOI] [PubMed] [Google Scholar]
- 27. Fleming BC, Oksendahl HL, Mehan WA, et al. Delayed gadolinium-enhanced MR imaging of cartilage (dGEMRIC) following ACL injury. Osteoarthritis Cartilage. 2010;18:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108. [DOI] [PubMed] [Google Scholar]
- 29. Hilgenfeld T, Jende J, Schwarz D, et al. Somatotopic fascicular lesions of the brachial plexus demonstrated by high-resolution magnetic resonance neurography. Invest Radiol. 2017;52:741-746. [DOI] [PubMed] [Google Scholar]
- 30. Howe FA, Filler AG, Bell BA, Griffiths JR. Magnetic resonance neurography. Magn Reson Med. 1992;28:328-338. [DOI] [PubMed] [Google Scholar]
- 31. Jeon T, Fung MM, Koch KM, Tan ET, Sneag DB. Peripheral nerve diffusion tensor imaging: overview, pitfalls, and future directions. J Magn Reson Imaging. 2018;47:1171-1189. [DOI] [PubMed] [Google Scholar]
- 32. Jungmann PM, Brucker PU, Baum T, et al. Bilateral cartilage T2 mapping 9 years after Mega-OATS implantation at the knee: a quantitative 3T MRI study. Osteoarthritis Cartilage. 2015;23:2119-2128. [DOI] [PubMed] [Google Scholar]
- 33. Kaipel M, Schreiner M, Kellner R, et al. Beneficial clinical effects but limited tissue quality following osteochondral repair with a cell-free multilayered nano-composite scaffold in the talus. Foot Ankle Surg. 2017;23:302-306. [DOI] [PubMed] [Google Scholar]
- 34. Kijowski R, Wilson JJ, Liu F. Bicomponent ultrashort echo time T2* analysis for assessment of patients with patellar tendinopathy. J Magn Reson Imaging. 2017;46:1441-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim HS, Yoon YC, Sung KS, Kim MJ, Ahn S. Comparison of T2 relaxation values in subtalar cartilage between patients with lateral instability of the ankle joint and healthy volunteers [published online April 17, 2018]. Eur Radiol. doi: 10.1007/s00330-018-5390-6 [DOI] [PubMed] [Google Scholar]
- 36. Knox J, Pedoia V, Wang A, et al. Longitudinal changes in MR T1ρ/T2 signal of meniscus and its association with cartilage T1p/T2 in ACL-injured patients. Osteoarthritis Cartilage. 2018;26:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koff MF, Pownder SL, Shah PH, Yang LW, Potter HG. Ultrashort echo imaging of cyclically loaded rabbit patellar tendon. J Biomech. 2014;47:3428-3432. [DOI] [PubMed] [Google Scholar]
- 38. Koff MF, Shah P, Pownder S, et al. Correlation of meniscal T2* with multiphoton microscopy, and change of articular cartilage T2 in an ovine model of meniscal repair. Osteoarthritis Cartilage. 2013;21:1083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kot BC, Zhang ZJ, Lee AW, Leung VY, Fu SN. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS One. 2012;7:e44348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lattanzi R, Petchprapa C, Ascani D, et al. Detection of cartilage damage in femoroacetabular impingement with standardized dGEMRIC at 3 T. Osteoarthritis Cartilage. 2014;22:447-456. [DOI] [PubMed] [Google Scholar]
- 41. León Cejas L, Binaghi D, Socolovsky M, et al. Intraneural perineuriomas: diagnostic value of magnetic resonance neurography. J Peripher Nerv Syst. 2018;23:23-28. [DOI] [PubMed] [Google Scholar]
- 42. Li H, Chen S, Tao H, Chen S. Quantitative MRI T2 relaxation time evaluation of knee cartilage: comparison of meniscus-intact and -injured knees after anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43:865-872. [DOI] [PubMed] [Google Scholar]
- 43. Li Q, Ma K, Tao H, et al. Clinical and magnetic resonance imaging assessment of anatomical lateral ankle ligament reconstruction: comparison of tendon allograft and autograft. Int Orthop. 2018;42:551-557. [DOI] [PubMed] [Google Scholar]
- 44. Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929-936. [DOI] [PubMed] [Google Scholar]
- 45. Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1ρ and T2—initial experience with 1-year follow-up. Radiology. 2011;258:505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li X, Majumdar S. Quantitative MRI of articular cartilage and its clinical applications. J Magn Reson Imaging. 2013;38:991-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li X, Pai A, Blumenkrantz G, et al. Spatial distribution and relationship of T1ρ and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61:1310-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lozano J, Li X, Link TM, Safran M, Majumdar S, Ma CB. Detection of posttraumatic cartilage injury using quantitative T1ρ magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88:1349-1352. [DOI] [PubMed] [Google Scholar]
- 49. Martin JA, Biedrzycki AH, Lee KS, et al. In vivo measures of shear wave speed as a predictor of tendon elasticity and strength. Ultrasound Med Biol. 2015;41:2722-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nebelung S, Tingart M, Pufe T, Kuhl C, Jahr H, Truhn D. Ex vivo quantitative multiparametric MRI mapping of human meniscus degeneration. Skeletal Radiol. 2016;45:1649-1660. [DOI] [PubMed] [Google Scholar]
- 51. Neuman P, Tjörnstrand J, Svensson J, et al. Longitudinal assessment of femoral knee cartilage quality using contrast enhanced MRI (dGEMRIC) in patients with anterior cruciate ligament injury—comparison with asymptomatic volunteers. Osteoarthritis Cartilage. 2011;19:977-983. [DOI] [PubMed] [Google Scholar]
- 52. Owman H, Tiderius CJ, Neuman P, Nyquist F, Dahlberg LE. Association between findings on delayed gadolinium-enhanced magnetic resonance imaging of cartilage and future knee osteoarthritis. Arthritis Rheum. 2008;58:1727-1730. [DOI] [PubMed] [Google Scholar]
- 53. Pagliazzi G, Vannini F, Battaglia M, Ramponi L, Buda R. Autologous chondrocyte implantation for talar osteochondral lesions: comparison between 5-year follow-up magnetic resonance imaging findings and 7-year follow-up clinical results. J Foot Ankle Surg. 2018;57:221-225. [DOI] [PubMed] [Google Scholar]
- 54. Pan J, Pialat JB, Joseph T, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261:507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pauli C, Bae WC, Lee M, et al. Ultrashort-echo time MR imaging of the patella with bicomponent analysis: correlation with histopathologic and polarized light microscopic findings. Radiology. 2012;264:484-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Payne C, Watt P, Cercignani M, Webborn N. Reproducibility of shear wave elastography measures of the Achilles tendon. Skeletal Radiol. 2018;47:779-784. [DOI] [PubMed] [Google Scholar]
- 57. Pedoia V, Samaan MA, Inamdar G, Gallo MC, Souza RB, Majumdar S. Study of the interactions between proximal femur 3D bone shape, cartilage health, and biomechanics in patients with hip Osteoarthritis. J Orthop Res. 2018;36:330-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peltz CD, Haladik JA, Divine G, Siegal D, van Holsbeeck M, Bey MJ. Shear wave elastography: repeatability for measurement of tendon stiffness. Skeletal Radiol. 2013;42:1151-1156. [DOI] [PubMed] [Google Scholar]
- 59. Perets I, Chaharbakhshi EO, Hartigan DE, Ortiz-Declet V, Mu B, Domb BG. The correlation between arthroscopically defined acetabular cartilage defects and a proposed preoperative delayed gadolinium-enhanced magnetic resonance imaging of cartilage index in hips of patients with femoroacetabular impingement syndrome. Arthroscopy. 2018;34:1202-1212. [DOI] [PubMed] [Google Scholar]
- 60. Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40:276-285. [DOI] [PubMed] [Google Scholar]
- 61. Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T1ρ and T2 relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Probst M, Richter V, Weitz J, et al. Magnetic resonance imaging of the inferior alveolar nerve with special regard to metal artifact reduction. J Craniomaxillofac Surg. 2017;45:558-569. [DOI] [PubMed] [Google Scholar]
- 63. Qiao Y, Tao HY, Ma K, Wu ZY, Qu JX, Chen S. UTE-T2* analysis of diseased and healthy Achilles tendons and correlation with clinical score: an in vivo preliminary study. Biomed Res Int. 2017;2017:2729807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rehnitz C, Klaan B, Burkholder I, von Stillfried F, Kauczor HU, Weber MA. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 mapping at 3T MRI of the wrist: feasibility and clinical application. J Magn Reson Imaging. 2017;45:381-389. [DOI] [PubMed] [Google Scholar]
- 65. Rehnitz C, Kuni B, Wuennemann F, et al. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 mapping of talar osteochondral lesions: indicators of clinical outcomes. J Magn Reson Imaging. 2017;46:1601-1610. [DOI] [PubMed] [Google Scholar]
- 66. Robson MD, Gatehouse PD, So PW, Bell JD, Bydder GM. Contrast enhancement of short T2 tissues using ultrashort TE (UTE) pulse sequences. Clin Radiol. 2004;59:720-726. [DOI] [PubMed] [Google Scholar]
- 67. Rosskopf AB, Ehrmann C, Buck FM, Gerber C, Fluck M, Pfirrmann CW. Quantitative shear-wave US elastography of the supraspinatus muscle: reliability of the method and relation to tendon integrity and muscle quality. Radiology. 2016;278:465-474. [DOI] [PubMed] [Google Scholar]
- 68. Russell C, Pedoia V, Amano K, Potter H, Majumdar S; AF-ACL Consortium. Baseline cartilage quality is associated with voxel-based T1ρ and T2 following ACL reconstruction: a multicenter pilot study. J Orthop Res. 2017;35:688-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sahr M, Sturnick DR, Nwawka OK. Quantitative ultrasound assessment of the Achilles tendon under varied loads [published online March 8, 2018]. J Ultrasound Med. doi: 10.1002/jum.14589 [DOI] [PubMed] [Google Scholar]
- 70. Samaan MA, Pedoia V, Zhang AL, et al. A novel MR-based method for detection of cartilage delamination in femoroacetabular impingement patients. J Orthop Res. 2018;36:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schreiner MM, Mlynarik V, Zbýnˇ Š, et al. New technology in imaging cartilage of the ankle. Cartilage. 2017;8:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shao H, Chang EY, Pauli C, et al. UTE bi-component analysis of T2* relaxation in articular cartilage. Osteoarthritis Cartilage. 2016;24:364-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sneag DB, Rancy SK, Wolfe SW, et al. Brachial plexitis or neuritis? MRI features of lesion distribution in Parsonage-Turner syndrome [published online February 20, 2018]. Muscle Nerve. doi: 10.1002/mus.26108 [DOI] [PubMed] [Google Scholar]
- 74. Sneag DB, Saltzman EB, Meister DW, Feinberg JH, Lee SK, Wolfe SW. MRI bullseye sign: an indicator of peripheral nerve constriction in Parsonage-Turner syndrome. Muscle Nerve. 2017;56:99-106. [DOI] [PubMed] [Google Scholar]
- 75. Souza RB, Kumar D, Calixto N, et al. Response of knee cartilage T1ρ and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stahl R, Luke A, Li X, et al. T1ρ, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients—a 3.0-Tesla MRI study. Eur Radiol. 2009;19:132-143. [DOI] [PubMed] [Google Scholar]
- 77. Su F, Hilton JF, Nardo L, et al. Cartilage morphology and T1ρ and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage. 2013;21:1058-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Taljanovic MS, Gimber LH, Becker GW, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017;37:855-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tao H, Hu Y, Qiao Y, et al. T2-mapping evaluation of early cartilage alteration of talus for chronic lateral ankle instability with isolated anterior talofibular ligament tear or combined with calcaneofibular ligament tear. J Magn Reson Imaging. 2018;47:69-77. [DOI] [PubMed] [Google Scholar]
- 80. Tas S, Onur MR, Yilmaz S, Soylu AR, Korkusuz F. Shear wave elastography is a reliable and repeatable method for measuring the elastic modulus of the rectus femoris muscle and patellar tendon. J Ultrasound Med. 2017;36:565-570. [DOI] [PubMed] [Google Scholar]
- 81. Tiderius CJ, Olsson LE, Nyquist F, Dahlberg L. Cartilage glycosaminoglycan loss in the acute phase after an anterior cruciate ligament injury: delayed gadolinium-enhanced magnetic resonance imaging of cartilage and synovial fluid analysis. Arthritis Rheum. 2005;52:120-127. [DOI] [PubMed] [Google Scholar]
- 82. Titchenal MR, Williams AA, Chehab EF, et al. Cartilage subsurface changes to magnetic resonance imaging UTE-T2* 2 years after anterior cruciate ligament reconstruction correlate with walking mechanics associated with knee osteoarthritis. Am J Sports Med. 2018;46:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang X, Harrison C, Mariappan YK, et al. MR neurography of brachial plexus at 3.0 T with robust fat and blood suppression. Radiology. 2017;283:538-546. [DOI] [PubMed] [Google Scholar]
- 84. Wheaton AJ, Casey FL, Gougoutas AJ, et al. Correlation of T1ρ with fixed charge density in cartilage. J Magn Reson Imaging. 2004;20:519-525. [DOI] [PubMed] [Google Scholar]
- 85. Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis Cartilage. 2010;18:539-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Williams A, Sharma L, McKenzie CA, Prasad PV, Burstein D. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: findings at different radiographic stages of disease and relationship to malalignment. Arthritis Rheum. 2005;52:3528-3535. [DOI] [PubMed] [Google Scholar]
- 87. Williams A, Winalski CS, Chu CR. Early articular cartilage MRI T2 changes after anterior cruciate ligament reconstruction correlate with later changes in T2 and cartilage thickness. J Orthop Res. 2017;35:699-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Williams AA, Titchenal MR, Andriacchi TP, Chu CR. MRI UTE-T2* profile characteristics correlate to walking mechanics and patient reported outcomes 2 years after ACL reconstruction. Osteoarthritis Cartilage. 2018;26:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Witschey WR, Borthakur A, Fenty M, et al. T1ρ MRI quantification of arthroscopically confirmed cartilage degeneration. Magn Reson Med. 2010;63:1376-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yoshitake Y, Takai Y, Kanehisa H, Shinohara M. Muscle shear modulus measured with ultrasound shear-wave elastography across a wide range of contraction intensity. Muscle Nerve. 2014;50:103-113. [DOI] [PubMed] [Google Scholar]