Abstract
Background:
The King-Devick (K-D) test is a rapid number-naming task that has been well validated as a sensitive sideline performance measure for concussion detection. Patients with concussion take significantly longer to complete the K-D test than healthy controls. Previous research suggests that ocular motor deficits, specifically saccadic abnormalities, may be an underlying factor for the prolonged time. However, these findings have not been studied at length.
Hypothesis:
K-D testing time of concussed adolescents at the initial clinical concussion visit will positively correlate with vestibular/ocular motor screening (VOMS) total scores.
Study Design:
Case series.
Level of Evidence:
Level 3.
Methods:
A total of 71 patient charts were retrospectively analyzed between October 1, 2016, and January 31, 2017. Included charts consisted of patients between the ages of 10 and 18 years with a diagnosis of concussion and who had completed K-D testing and VOMS assessment at the initial physician visit. Univariate correlation between K-D testing time and the 7 VOMS items was assessed using Pearson correlation coefficients.
Results:
K-D testing time strongly correlated with all 7 VOMS items (r(69) = 0.325-0.585, P < 0.01). In a linear regression model that accounted for each VOMS item, the convergence (near point) item and the visual motion sensitivity item significantly predicted K-D testing time (β = 0.387, t(63) = 2.81, P < 0.01 and β = 0.375, t(63) = 2.35, P = 0.02, respectively). Additionally, 37.5% of the 24 patients with worsening symptoms after K-D testing freely reported increased visual problems.
Conclusion:
Our study suggests that prolonged K-D testing times in adolescents with concussion may be related to subtypes of vestibular/ocular motor impairment that extend beyond saccadic abnormalities.
Clinical Relevance:
Poor K-D testing performance of adolescents with concussion may indicate a range of vestibular/ocular motor deficits that need to be further identified and addressed to maximize recovery.
Keywords: King-Devick test, vestibular/ocular motor screening, concussion
Concussion is a major public health problem resulting from a change in brain physiology after a blow to the head or body.3 Concussions often produce symptoms such as blurred vision, dizziness, imbalance, and headaches, as well as measurable neurologic impairments in cognition, reaction time, balance, and behavior.15 More often, concussion symptoms occur immediately after the impact and resolve spontaneously; however, sometimes symptoms will develop over a longer period of time.15 Since concussion diagnosis and prognosis are determined by functional impairment rather than structural brain injury,15 it is challenging to capture a comprehensive picture of the various physical and behavioral elements that may have been impaired by concussion.18
Recently, researchers have studied eye movement abnormalities as an indication for impaired function in concussed patients.4,11 Although eye movement abnormalities are often combined with or interpreted as other related symptoms such as balance and/or dizziness, the vestibular/ocular motor system is also uniquely affected by concussion.23 A pediatric sports study showed that 81% of the adolescents diagnosed with concussion demonstrated vestibular deficits that were separate from other symptoms.5 Additionally, studies have shown that concussed adolescents with indicated visual impairments have worse neurocognitive performance,6,10,22 and concussed adolescents with vestibular abnormalities take longer to return to school and play.5 Therefore, it is important to evaluate the vestibular and ocular motor systems after concussion using standardized clinical assessments.
A well-validated tool for concussion detection is the King-Devick (K-D) test, a rapid number-naming task that requires a participant to correctly identify single digits that are variably spaced on 3 handheld cards.6,7 The K-D test broadly captures visual function and saccadic eye movements as well as attention and language function.6,7,9,10,13 While patients with concussion take a significantly longer time to complete the K-D test than healthy controls,8 the underlying reason for this deficit has largely been unknown.20 To this goal, a recent study observing 25 patients with concussion history who completed a computerized version of the K-D test under infrared-based video-oculography showed that the prolonged K-D test time may be due to saccadic abnormalities, specifically, prolonged intersaccadic intervals, greater number of saccades, and larger deviations of saccadic endpoints.19 Additionally, a study of the handheld version of the K-D test administered on rugby players showed its ability to assess saccadic eye movements to better identify concussion.12 However, the K-D test does not assess other areas of ocular motor function such as pursuit, convergence, or accommodation, all of which have shown to be valuable indicators of impairment from mild traumatic brain injury.2,4
A more recent assessment performed during concussion visits is vestibular/ocular motor screening (VOMS).16 The VOMS is a comprehensive examination of various saccadic eye movements with the intention of provoking symptoms after each assessment.16 The VOMS accurately differentiates between controls and athletes with concussion in the evaluation of smooth-pursuit eye movements, saccadic eye movements, near point of convergence (NPC), vestibular ocular reflex, and visual motion sensitivity.17 Abnormal VOMS results delay recovery time of concussed adolescents, indicating that the VOMS may assist in more effective prognosis of concussion.1 The VOMS captures the interaction of the vestibular and ocular motor systems in a comprehensive evaluation that involves both patient and clinician reporting.17 A previous study indicated that the VOMS does not provoke symptoms in healthy controls, and with a mean score of zero, there was no significant correlation between VOMS items and K-D test scores.23 However, correlation between the VOMS and K-D test scores of concussed patients has not been explored. Since the VOMS only provokes symptoms in impaired populations, it is important to determine whether a relationship exists between the VOMS and K-D test scores of concussed individuals. Comparing the VOMS scores of concussed patients with their K-D test performance will illuminate the potential range of vestibular and ocular motor deficits that may be associated with prolonged K-D testing time. To this aim, this study explores the relationship between K-D and VOMS testing and observes patient-reported visual symptoms before and after K-D testing to gain a more comprehensive understanding of the underlying vestibular/ocular motor impairments affecting the K-D test performance of concussed adolescents.
Methods
This study was approved by the institutional review board at Saint Barnabas Medical Center, RWJBarnabas Health. A total of 71 patient charts were retrospectively analyzed between October 1, 2016, and January 31, 2017 (mean patient age, 14 ± 2.1 years; mean symptom score at initial visit, 5.7/22 ± 5.7). A majority of patients (70.4%) sustained their concussion from sports, and 70.4% of patients had experienced first-time concussion at the time of testing. Included charts consisted of patients with a diagnosis of concussion who had completed K-D testing and VOMS assessment at their initial physician visit after their concussion (Table 1). The time from concussion to initial physician visit ranged from 1 to 49 days (mean, 11 days). Univariate correlation between K-D testing time and the 7 VOMS items was assessed using Pearson correlation coefficients. Multiple linear regression was performed with each VOMS item as an independent variable and the overall K-D testing time as the dependent variable.
Table 1.
Mean K-D and VOMS scores at initial physician visit (N = 71) a
| Mean | SD | |
|---|---|---|
| K-D total time, s | 64.75 | 33.88 |
| VOMS | ||
| Smooth pursuit score | 0.63 | 1.08 |
| Saccades (horizontal) score | 0.96 | 1.38 |
| Saccades (vertical) score | 1.08 | 1.55 |
| Convergence (near point) score | 0.52 | 1.24 |
| Vestibular-ocular reflect (horizontal) score | 1.3 | 1.57 |
| Vestibular-ocular reflect (vertical) score | 1.25 | 1.49 |
| Visual motion sensitivity score | 1.79 | 1.82 |
K-D, King-Devick; VOMS, vestibular/ocular motor screening.
VOMS scores are measured on a self-reported scale from 0 to 6, where 0 = no impairment and 6 = complete impairment.
Results
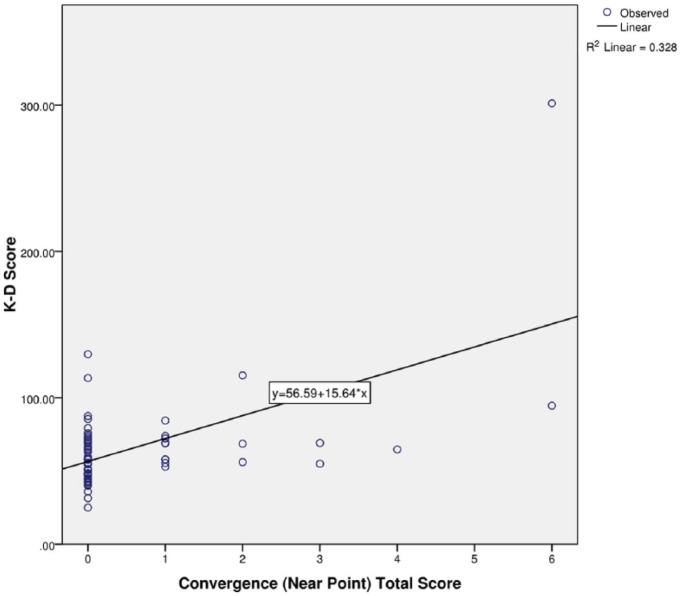
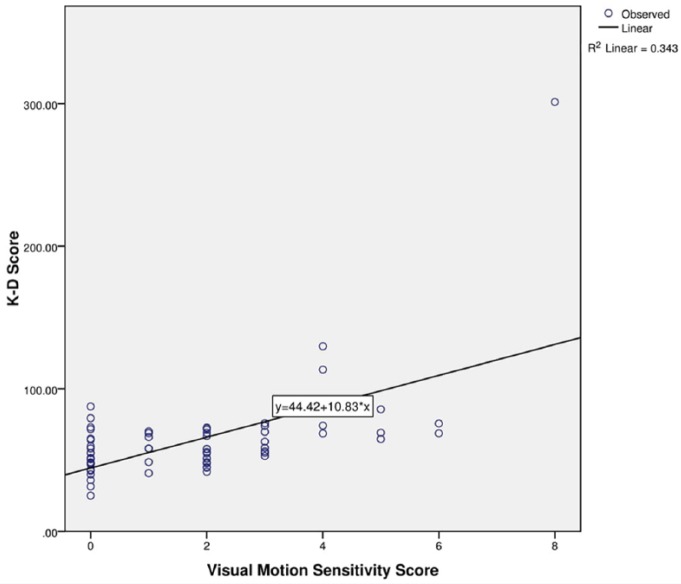
K-D testing time strongly correlated with all 7 VOMS items (r(69) = 0.325-0.585, P < 0.01) (Table 2). In a multiple linear regression model that accounted for each VOMS item, the NPC item and the visual motion sensitivity (VMS) item significantly predicted K-D testing time (β = 0.387, t(63) = 2.81, P < 0.01 and β = 0.375, t(63) = 2.35, P = 0.02, respectively) (Figures 1 and 2). Additionally, it is worth noting that 75.7% of patients reported positive on the VOMS test, and 37.5% of the 24 patients with worsening symptoms after the K-D testing freely reported increased visual problems.
Table 2.
Correlation of VOMS item total scores and K-D testing time scores of patients with a 1- to 49-day time span from concussion to initial testing a
| VOMS Item | K-D Score | |
|---|---|---|
| Smooth pursuits total score | Pearson correlation | 0.422 |
| Significance (2-tailed) | 0.000 | |
| N | 70 | |
| Saccades—horizontal total score | Pearson correlation | 0.325 |
| Significance (2-tailed) | 0.006 | |
| N | 70 | |
| Saccades—vertical total score | Pearson correlation | 0.464 |
| Significance (2-tailed) | 0.000 | |
| N | 71 | |
| Convergence (near point) total score | Pearson correlation | 0.573 |
| Significance (2-tailed) | 0.000 | |
| N | 71 | |
| Vestibular-ocular reflex—horizontal total score | Pearson correlation | 0.481 |
| Significance (2-tailed) | 0.000 | |
| N | 70 | |
| Vestibular-ocular reflex—vertical total score | Pearson correlation | 0.515 |
| Significance (2-tailed) | 0.000 | |
| N | 71 | |
| Visual motion sensitivity total score | Pearson correlation | 0.585 |
| Significance (2-tailed) | 0.000 | |
| N | 68 | |
| King-Devick score | Pearson correlation | 1 |
| N | 71 | |
K-D, King-Devick; VOMS, vestibular/ocular motor screening.
One participant did not complete smooth pursuits and horizontal saccades, so N = 70 for those items. Three participants did not complete visual motion sensitivity, so N = 68 for that item.
Figure 1.
Regression model of convergence and King-Devick (K-D) test scores.
Figure 2.
Regression model of visual motion sensitivity scores and King-Devick (K-D) test scores.
To strengthen the validity of the relationship between K-D and VOMS scores, we limited our data set to consist of the patients with a time span of 1 to 5 days from concussion to initial physician visit (N = 22). Again, K-D testing time correlated with all 7 VOMS items (r(20) = 0.425-0.760, P < 0.01-0.049) (Table 3).
Table 3.
Correlation of VOMS item total scores and K-D testing time scores of patients with a 1- to 5-day time span from concussion to initial testing a
| VOMS Item | K-D Score | |
|---|---|---|
| Smooth pursuits total score | Pearson correlation | 0.521 |
| Significance (2-tailed) | 0.013 | |
| N | 22 | |
| Saccades—horizontal total score | Pearson correlation | 0.425 |
| Significance (2-tailed) | 0.049 | |
| N | 22 | |
| Saccades—vertical total score | Pearson correlation | 0.718 |
| Significance (2-tailed) | 0.000 | |
| N | 22 | |
| Convergence (near point) total score | Pearson correlation | 0.652 |
| Significance (2-tailed) | 0.001 | |
| N | 22 | |
| Vestibular-ocular reflex—horizontal total score | Pearson correlation | 0.76 |
| Significance (2-tailed) | 0.000 | |
| N | 22 | |
| Vestibular-ocular reflex—vertical total score | Pearson correlation | 0.743 |
| Significance (2-tailed) | 0.000 | |
| N | 22 | |
| Visual motion sensitivity total score | Pearson correlation | 0.727 |
| Significance (2-tailed) | 0.000 | |
| N | 20 | |
| King-Devick score | Pearson correlation | 1 |
| N | 22 | |
K-D, King-Devick; VOMS, vestibular/ocular motor screening.
Two participants did not complete visual motion sensitivity, so N = 20 for that item.
Discussion
This pilot study found a significant relationship between the total scores of each VOMS item and overall K-D testing time in adolescents with concussion, supporting previous research that showed saccadic abnormalities affect K-D performance. As expected, the vertical and horizontal saccade items of the VOMS significantly correlated with prolonged K-D time. However, this study also suggests that prolonged K-D testing times may be related to vestibular/ocular motor impairment beyond saccadic abnormalities alone, as all the VOMS items correlated with K-D.
King-Devick Test
Specifically, this study showed that NPC and VMS uniquely predicted K-D performance. Additionally, while ocular motor deficits may be a contributor to the delay of completion in concussed patients, it is unknown whether attentional and/or cognitive deficits interact with ocular motor deficits during K-D performance.
Near Point of Convergence
While VOMS is becoming a more popular assessment for concussion, VOMS is rarely performed at baseline. This becomes particularly important when assessing NPC. Convergence insufficiency is present in 1 of every 20 children suggesting that, in a typical classroom, 1 to 2 children may have this condition.21 NPC is often undiagnosed in school-aged children and could therefore mislead a clinician in a concussive setting to believe that the NPC insufficiency is a direct result of concussion rather than acknowledge the possibility of a preexisting condition.14
Limitations
It is important to note that this is only a pilot study using retrospective data. While significant correlations emerged, this study is limited because it does not describe a causal relationship.
Conclusion
This pilot study supports the previous research that prolonged K-D testing times in adolescents with concussion may be related to vestibular/ocular motor impairment, particularly saccadic abnormalities. However, this study also suggests that vestibular/ocular motor impairment affecting K-D testing may extend beyond saccadic abnormalities alone.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Anzalone AJ, Blueitt D, Case T, et al. A positive vestibular/ocular motor screening (VOMS) is associated with increased recovery time after sports-related concussion in youth and adolescent athletes. Am J Sports Med. 2017;45:474-479. [DOI] [PubMed] [Google Scholar]
- 2. Capo-Aponte JE, Urosevich TG, Temme LA, Tarbett AK, Sanghera NK. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med. 2012;177:804-813. [DOI] [PubMed] [Google Scholar]
- 3. Carney N, Ghajar J, Jagoda A, et al. Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery. 2014;75(suppl 1):S3-S15. [DOI] [PubMed] [Google Scholar]
- 4. Ciuffreda KJ, Kapoor N, Rutner D, Suchoff IB, Han ME, Craig S. Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. J Am Optom Assoc. 2007;78:155-161. [DOI] [PubMed] [Google Scholar]
- 5. Corwin DJ, Wiebe DJ, Zonfrillo MR, et al. Vestibular deficits following youth concussion. J Pediatr. 2015;166:1221-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galetta KM, Barrett J, Allen M, et al. The King-Devick test as a determinant of head trauma and concussion in boxers and MMA fighters. Neurology. 2011;76:1456-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galetta KM, Brandes LE, Maki K, et al. The King-Devick test and sports-related concussion: study of a rapid visual screening tool in a collegiate cohort. J Neurol Sci. 2011;309:34-39. [DOI] [PubMed] [Google Scholar]
- 8. Galetta KM, Liu M, Leong DF, Ventura RE, Galetta SL, Balcer LJ. The King-Devick test of rapid number naming for concussion detection: meta-analysis and systematic review of the literature. Concussion. 2016;1:CNC8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galetta KM, Morganroth J, Moehringer N, et al. Adding vision to concussion testing: a prospective study of sideline testing in youth and collegiate athletes. J Neuroophthalmol. 2015;35:235-241. [DOI] [PubMed] [Google Scholar]
- 10. Galetta MS, Galetta KM, McCrossin J, et al. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;328:28-31. [DOI] [PubMed] [Google Scholar]
- 11. Heitger MH, Jones RD, Anderson TJ. A new approach to predicting postconcussion syndrome after mild traumatic brain injury based upon eye movement function. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3570-3573. [DOI] [PubMed] [Google Scholar]
- 12. King D, Brughelli M, Hume P, Gissane C. Concussions in amateur rugby union identified with the use of a rapid visual screening tool. J Neurol Sci. 2013;326:59-63. [DOI] [PubMed] [Google Scholar]
- 13. Leong DF, Balcer LJ, Galetta SL, Liu Z, Master CL. The King-Devick test as a concussion screening tool administered by sports parents. J Sports Med Phys Fitness. 2014;54:70-77. [PubMed] [Google Scholar]
- 14. Master CL, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55:260-267. [DOI] [PubMed] [Google Scholar]
- 15. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: The 4th international conference on concussion in sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250-258. [DOI] [PubMed] [Google Scholar]
- 16. Mucha A, Collins MW, Elbin RJ, et al. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42:2479-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulligan IJ, Boland MA, McIlhenny CV. The Balance Error Scoring System learned response among young adults. Sports Health. 2013;5:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Putukian M. Clinical evaluation of the concussed athlete: a view from the sideline. J Athl Train. 2017;52:236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rizzo JR, Hudson TE, Dai W, et al. Objectifying eye movements during rapid number naming: methodology for assessment of normative data for the King-Devick test. J Neurol Sci. 2016;362:232-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizzo JR, Hudson TE, Dai W, et al. Rapid number naming in chronic concussion: eye movements in the King-Devick test. Ann Clin Transl Neurol. 2016;3:801-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Storey EP, Master SR, Lockyer JE, Podolak OE, Grady MF, Master CL. Near point of convergence after concussion in children. Optom Vision Sci. 2017;94:96-100. [DOI] [PubMed] [Google Scholar]
- 22. Tjarks BJ, Dorman JC, Valentine VD, et al. Comparison and utility of King-Devick and ImPACT® composite scores in adolescent concussion patients. J Neurol Sci. 2013;334:148-153. [DOI] [PubMed] [Google Scholar]
- 23. Yorke AM, Smith L, Babcock M, Alsalaheen B. Validity and reliability of the vestibular/ocular motor screening and associations with common concussion screening tools. Sports Health. 2017;9:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]