Abstract
OBJECTIVES:
Urinary tract infection (UTI) is a frequent disorder and depends on age and gender. Ineffective empiric treatment of UTI is common when associated with extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae. The aim of the study was to investigate the prevalence of Gram-negative uropathogens of E. coli and K. pneumoniae in different age groups along with the identification of ESBL-producing uropathogens and antimicrobial susceptibility profiles.
MATERIALS AND METHODS:
A total of 247 uropathogens of E. coli and K. pneumoniae were collected over a period of 1 year (January–December 2015) from various diagnostic centers of Karachi city (Pakistan). Antimicrobial susceptibility analysis was performed by disc diffusion method, and identification of ESBL was performed by double disc synergy test. Categorical data of ESBL and non-ESBL uropathogens were analyzed by Pearson's Chi-square test.
RESULTS:
The study of 247 patients with community-acquired UTI comprised 72% females and 28% males, illustrating an increased prevalence of UTIs among females. It was also revealed that 90% belonged to the age group of 16–30 years whereas 78% related to the age group of 46–60 years. ESBL was found positive in 33.5% (63/188) of E. coli and 15.25% (9/59) in K. pneumonia, with a significant association i.e., (p =0.007). Amikacin, fosfomycin, imipenem, and tazobactam/piperacillin were found to be the effective treatment options. A significant association was found between ESBL-producing uropathogens against ciprofloxacin, enoxacin, and amoxicillin/clavulanic acid resistance (P < 0.05).
CONCLUSIONS:
It was concluded that for effective treatment of UTIs, appropriate screening of ESBL and culture sensitivity must be employed instead of empiric treatment.
Keywords: Empiric treatment, extended-spectrum beta-lactamase, multidrug resistance, urinary tract infections
Introduction
It is very critical to isolate the specific pathogen, especially in case of life-threatening infections. It becomes more difficult when antimicrobial therapy is chosen based on the clinical investigation without knowing the etiology of the agents. The diagnosis of infectious disease also depends on history of a patient. Among humans, the utmost type of bacterial infection is urinary tract infection (UTI).[1] UTIs are more dominant in females in comparison with males after 1 year of age. However, UTIs are common in boys under the age of 1 year; the reason is short urethra and ascending infections in females and structural abnormalities found in males.[2] As per the investigation in Aga Khan University (Karachi, Pakistan), under the age of fertility, the ratio of UTI found in male and female is 1:50.[3]
Extended-spectrum beta-lactamases (ESBLs) have emerged as a challenge with higher incidence of resistance for the treatment of UTIs, more specifically in community onset.[4] The common pathogen associated with most of the acute uncomplicated UTIs is Escherichia coli, and several reports revealed accumulation of community-onset UTIs associated with ESBL-positive E. coli.[5,6,7]
Various published reports indicated that risk factors such as elderly, female sex, history of diabetes, recurrent episodes of UTI, urological surgical intervention, and previous utilization of antibiotics such as aminopenicillins, cephalosporins, or fluoroquinolones are associated with the development of community onset of UTI associated with ESBL-positive E. coli.[6,8,9,10,11,12]
In common practice for the treatment of community-related UTIs, trimethoprim/sulphamethoxazole (SXT), ciprofloxacin (CIP), cephalosporins, semi-synthetic penicillin with or without inhibitors, nitrofurantoin, and fosfomycin (FOS) are used as antibacterial drugs.[13] Currently, uropathogens with ESBL production have increased in patients both from hospital and community settings [14,15] and increased rate of resistance in most of E. coli-associated UTIs against oral antibiotics.[14,16] In these situations, with limited treatment options, the task for physicians to make an empirical antibiotic choice becomes more challenging.[17]
The main objective of this study was to investigate the prevalence of Gram-negative uropathogens of E. coli and Klebsiella pneumoniae in different age groups along with the identification of antimicrobial susceptibilities and multidrug resistance (MDR), to suggest an empiric treatment against these uropathogens. The study also focused on the identification of ESBL-producing uropathogens and their statistical significance with various age groups and antibacterial susceptibility profiles.
Materials and Methods
Sample collection
A total number of 247 uropathogens of E. coli and K. pneumoniae were collected over 1 year (January–December 2015) from various diagnostic centers of Karachi city (Pakistan).
Inclusion criteria
The urine specimens of patients with suspected UTIs associated with E. coli and K. pneumoniae
Outpatients having UTIs
Patients belonged to any age group
Both male and female specimens were included.
Exclusion criteria
The urine specimens of patients with suspected UTIs associated with pathogens other than E. coli and K. pneumoniae
Inpatients were excluded from the study.
Antimicrobial susceptibility test
Antimicrobial susceptibilities were determined for the initial identification of ESBL by double disc synergy test (DDST). Along with this, associated risk factors were also calculated. For antimicrobial susceptibility pattern, disc diffusion method was used using Mueller-Hinton agar as per the CLSI guidelines. The antibiotics included in the study were amikacin (AKN), gentamicin (GEN), tobramycin (TOB), ampicillin (AMP), amoxicillin/clavulanic acid (AMC), imipenem (IPM), tazobactam/piperacillin (PTZ), cefuroxime (CXM), cefixime (CFM), cefotaxime (CTX), ceftriaxone (CRO), Fosfomycin (FOS), ofloxacin (OFL), ciprofloxacin (CIP), enoxacin (ENX), doxycycline (DOX), and trimethoprim/sulphamethoxazole (SXT). Resistance and susceptibilities were determined by zones of inhibition as recommended in the guidelines.[18]
Phenotypic identification of extended-spectrum beta-lactamase
The presence of ESBL was determined by DDST using three antibiotic discs, i.e., CTX (30 μg), ceftazidime (30 μg), and AMC (20/10 μg). The disc of AMC was placed at the center and the other two were placed at distance of 30 mm from center disc and incubated for 24 h. Improvement in the inhibited zones of either cephalosporin discs to AMC indicated synergism with clavulanic acid and existence of ESBL.[19] Literature illustrates that the microorganisms that carried ESBLs were E. coli and K. pneumoniae. Therefore, K. pneumoniae 700603 was positive and E. coli 25922 used as negative controls.
Statistical analysis
Potential risk factors for ESBL-producing uropathogens were identified using univariate analysis. The Pearson's Chi-square or Fisher's exact test was used for categorizing the variables in different age groups of patients. The antimicrobial susceptibilities at the P < 0.05 were considered as statistically significant. SPSS (version 20) software was used for the analysis (SPSS Inc., Chicago, IL, USA).
Results
Uropathogens in various age groups
During 1 year, a total of 247 (male = 77; female = 177) Gram-negative uropathogens were collected for ESBL production and antimicrobial susceptibility tests. It was identified that 76% (188/247) were E. coli and 24% (59/247) were K. pneumoniae. It was estimated that 72% uropathogens were identified in females and 28% in males [Table 1]. It was also found that different age groups of patients (age range, 3 days to 95 years) were affected with UTIs [Figure 1].
Table 1.
Pattern and gender of uropathogens isolated (n=247)
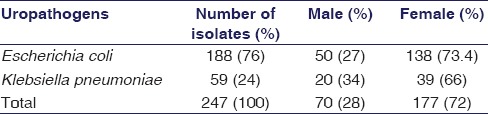
Figure 1.
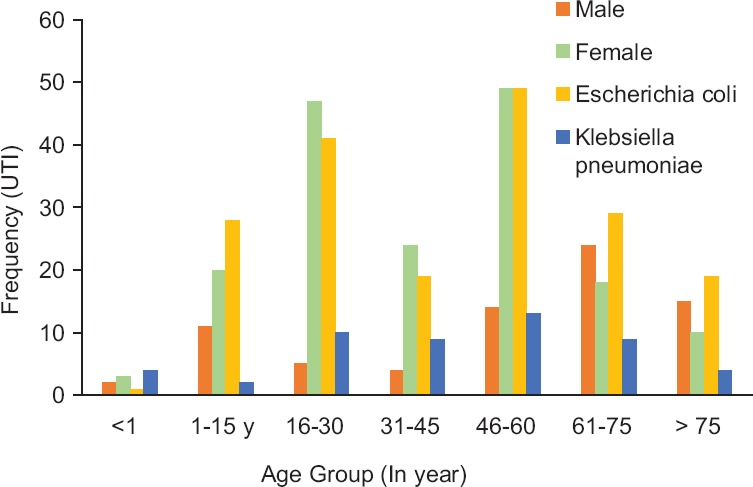
Distribution of uropathogens with gender in different age groups
In different age groups, frequencies of UTI were 5, 31, 52, 28, 63, 42, and 25, respectively. The results showed that K. penumoniae related UTIs were dominant in <1 year of age, whereas 90% and 78% of uropathogens were found in females related to 16-30 and 46-60 years of age groups.
Antimicrobial susceptibility and multidrug resistance
The susceptibility patterns of the antibiotics tested in uropathogens are listed in Table 2 and indicated the increase rate of resistance in all uropathogens. MDR was determined on the basis of antimicrobial susceptibility profiles under recommended guidelines [20] [Figure 2].
Table 2.
Antimicrobial susceptibility and resistance among uropathogens
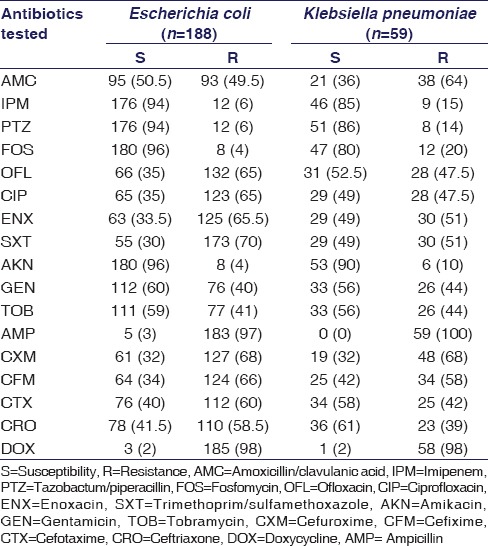
Figure 2.
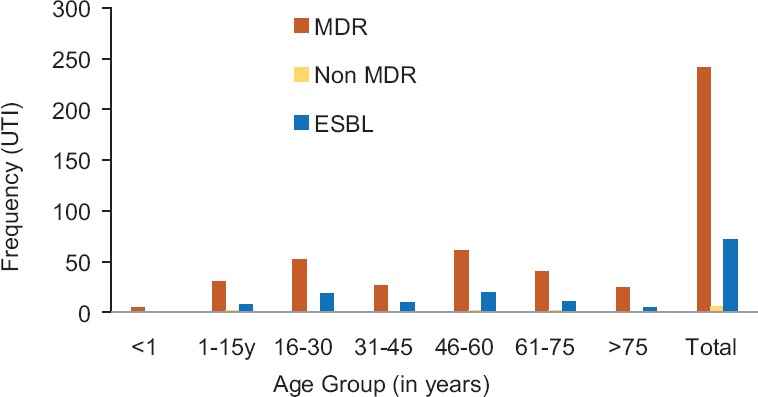
Multidrug-resistant and extended-spectrum beta-lactamase-positive uropathogens in different age groups
Extended-spectrum beta-lactamase-positive uropathogens
DDST [Figure 3] was used for the identification of ESBL. ESBL production was found in 29% (72/247) tested uropathogens [Figure 2]. MDR was found 100%, 94%, 100%, 100%, 97%, 95%, and 100%, respectively, in <1, 1-15, 16-30, 31-45, 46-60, 61-75 and >75 years of age groups. In various age groups, the percentages of ESBLs were 20%, 25.8%, 34.6%, 35.7%, 30%, 26%, and 20%, respectively.
Figure 3.

Estimation of extended-spectrum beta-lactamase production by double disc synergy test
Statistics
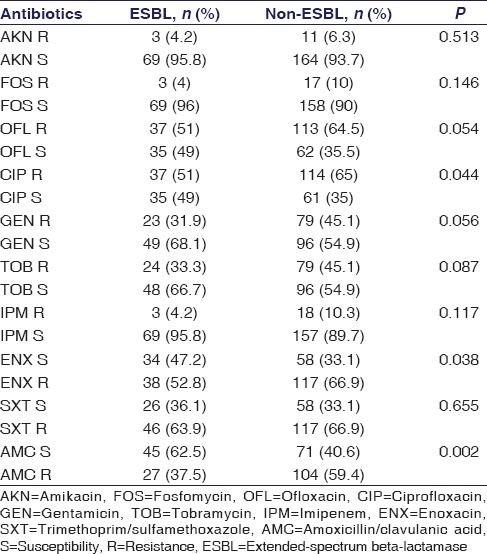
Statistical association was found between ESBL/non-ESBL uropathogens with high prevalence of E. coli as illustrated in Table 3 (P = 0.007), while gender and MDR were found nonsignificant. A nonsignificant association was found with ESBL-producing uropathogens in all age groups [Table 4]. ESBL production and antimicrobial resistance were found significant in CIP, ENX, and AMC [Table 5].
Table 3.
Statistical association of extended-spectrum beta-lactamase production with gender, multidrug resistant, and uropathogens
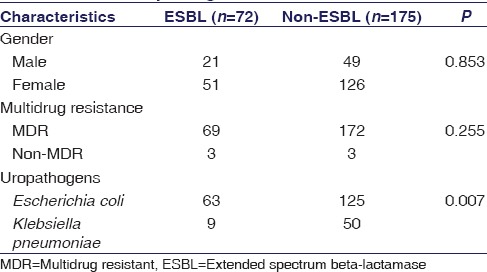
Table 4.
Association between extended-spectrum beta-lactamase and nonextended-spectrum beta-lactamase uropathogens in different age groups
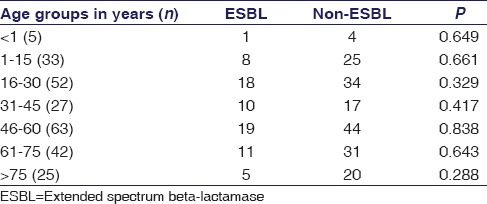
Table 5.
Association of extended-spectrum beta-lactamase/nonextended-spectrum beta-lactamase uropathogens with antimicrobial susceptibility
Discussion
Effective management for the treatment of uropathogens is commonly based on the identification of the type of organisms that cause the disease and the selection of an appropriate antibiotic for its treatment.[21] Uropathogens are the leading healthcare concern in all over the world.[22] As well in Pakistan, various studies have been attempted for the prevalence and resistance of various ESBL-associated uropathogens.[23,24]
In the present work, a total of 247 Gram-negative uropathogens of E. coli and K. pneumonia were studied. Among them, 177 (72%) UTIs have been isolated from females while 70 (28%) were from males, illustrating an increased prevalence of UTIs among females; the reasons may be illiteracy and inappropriate hygienic practices. The outcome of present study is supported by the previously published studies that have been done in Pakistan, reported 62.5%[25] and 67%[26] of uropathogens recovered from females. The study revealed high prevalence n = 47 (90.4%) of UTIs in females belonged to the age group of 16–30 years, whereas n = 49 (78%) uropathogens related to the age group of 46–60 years, in elderly women after menopause. UTI risks have increased due to hormonally induced changes in the vaginal flora [27] [Figure 1]. The same kind of data has been reported by Hooton et al. (1996) that young females with reproductive age of 18–30 years are at high risk of incidence of UTIs.[28] The study also proved that the most prevalent uropathogens were E. coli, 188 (76%) followed by K. pneumoniae, 59 (23.8%). Various data obtained from different parts of the world, such as 46.1% in Ukraine,[29] 50.1% from India,[30] and 90% from Ankara (Turkey),[10] demonstrated that E. coli is the most ubiquitous uropathogens. While the studies have been done in different cities of Pakistan, it was also verified that 84% cases of UTIs in Karachi [23] and 64% cases in Punjab [26] were associated with E. coli. In the present work, E. coli was found a very common uropathogen in each age group, as illustrated in Figure 1.
Culture yielded a total of 188 E. coli and 59 K. pneumoniae, out of these, 63 (33.5%) growths of ESBL are positive in E. coli and 9 (15.25%) in K. pneumoniae. This finding is supported by a recent study done in Karachi which reported that 29.5% of ESBL producers were uropathogenic E. coli.[23] In alliance with several other reports from Pakistan, it revealed high incidence rate of ESBL-related E. coli infections, i.e., 40%[31] and 56.9%,[24] and more preponderance in females. The present study also found that 51 (71%) ESBL-positive uropathogens recovered from females even though female gender was not found significantly associated with ESBL-producing uropathogens [Table 3]. ESBL production was statistically insignificant among age groups [Table 4], however, with high prevalence in 16–30, 31–45, and 46–60 years of age groups, i.e., 34.6%, 37.5%, and 30%, respectively. Ahmed et al. reported to some extent the similar results, i.e., 28.3% in the age range of 37–54 years.[23]
The antimicrobial resistance patterns in tested uropathogens of E. coli were found 6%, 6%, 4%, and 4% whereas of K. pneumonia showed 15%, 14%, 20%, and 10% resistant against IPM, PTZ, FOS, and AKN, respectively, as presented in Table 2. The present work also defined that a much higher resistance was observed in E. coli uropathogens isolated against commonly prescribed antibiotics, i.e., fluoroquinolones, cephalosporins, AMC, SXT, and AMP. Various studies done in Pakistan have also reported the similar results with increased rate of resistance.[24,31] In comparison to this, K. pneumoniae isolates showed less resistance against CRO and CTX, i.e., 39% and 42%, respectively [Table 2]. During the last 20 years, increased resistance against fluoroquinolones has been observed in Pakistan, especially in Karachi. It was 14% in 1997[32] and raised up to 35% in 2004.[33] In the proposed study, E. coli showed 65% resistance against CIP, OFL, and ENX, whereas K. pneumoniae showed a resistance of 47.5%, 47.5%, and 51%, respectively. This increased resistance is due to inappropriate and empiric use of antibiotics available for oral administration.
MDR is a natural phenomenon limiting the therapeutic choices because various microorganisms can survive by their ability to adapt to antimicrobial agents. In this study, an increased MDR in uropathogens was found, i.e., 96% (69/72) in ESBL-positive and 98% (172/175) in non-ESBL uropathogens [Figure 2]. This result is supported by a study done in Pakistan indicating 83% of MDR in tested uropathogens.[24] Hence, a nonsignificant association (P = 0.255) of MDR with ESBL production among uropathogens was found in the present study [Table 3].
The productivity of ESBL and antimicrobial resistance against three antibiotics, i.e., AMC, CIP, and ENX, were found significant [Table 5]. Hence, these findings are limiting the use of fluoroquinolones and AMC recommended as the first- and second-line choice in the treatment of ESBL-positive UTIs.[34] A study in 2005 done in Turkey explained that the CIP use has increased the risk of resistance in uropathogenic E. coli.[35] The study found FOS as a good choice to treat UTIs in association with other oral antibiotics [Table 2]. Previously published study suggested FOS as good choice to treat ESBL-positive UTIs with a resistance rate of 19.9% whereas 83.3% were resistant to fluoroquinolones.[17] Similarly, the present study also showed that FOS is a better choice over other oral antibiotics for the treatment of ESBL-positive UTIs with 96% of susceptibility rate.
Conclusions
The study concluded that a high rate of resistance has been developed in uropathogens with empiric antibiotic treatment, including fluoroquinolones, AMC, broad-spectrum cephalosporins, and SXT. This illustrates that the use of these antibiotics is not good as empiric treatment for community-onset UTIs. Therefore, the proper screening is needed for ESBLs detection in laboratories. ESBL-producing uropathogens are an emerging threat with few antibiotics such as FOS, IPM, PTZ, and AKN.
Financial support and sponsorship
We would like to thank the Department of Pharmaceutics, Faculty of Pharmacy, University of Karachi, for continuous support and encouragements.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Microbiology department, Dr. Essa laboratories for the collection of clinical isolates.
References
- 1.Nicolle LE. Epidemiology of urinary tract infections. Clin Microbiol Newsl. 2002;24:135–40. [Google Scholar]
- 2.Long SS, Klein JO. Infectious Diseases of the Fetus and Newborn. 7th ed. Philadelphia, USA: Elsevier, Saunders; 2011. Bacterial infections of the urinary tract; pp. 310–21. [Google Scholar]
- 3.Farooqui BJ, Alam M, Khurshid M. Urinary tract infection. JPMA. 1989;39:129. [PubMed] [Google Scholar]
- 4.Meier S, Weber R, Zbinden R, Ruef C, Hasse B. Extended-spectrum β-lactamase-producing gram-negative pathogens in community-acquired urinary tract infections: An increasing challenge for antimicrobial therapy. Infection. 2011;39:333–40. doi: 10.1007/s15010-011-0132-6. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Baño J, Navarro MD, Romero L, Martínez-Martínez L, Muniain MA, Perea EJ, et al. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J Clin Microbiol. 2004;42:1089–94. doi: 10.1128/JCM.42.3.1089-1094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897–902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 7.Laupland KB, Church DL, Vidakovich J, Mucenski M, Pitout JD. Community-onset extended-spectrum beta-lactamase (ESBL) producing Escherichia coli: Importance of international travel. J Infect. 2008;57:441–8. doi: 10.1016/j.jinf.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Calbo E, Romaní V, Xercavins M, Gómez L, Vidal CG, Quintana S, et al. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J Antimicrob Chemother. 2006;57:780–3. doi: 10.1093/jac/dkl035. [DOI] [PubMed] [Google Scholar]
- 9.Colodner R, Rock W, Chazan B, Keller N, Guy N, Sakran W, et al. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur J Clin Microbiol Infect Dis. 2004;23:163–7. doi: 10.1007/s10096-003-1084-2. [DOI] [PubMed] [Google Scholar]
- 10.Azap Ö, Arslan H, Serefhanoǧlu K, Colakoǧlu S, Erdoǧan H, Timurkaynak F, et al. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010;16:147–51. doi: 10.1111/j.1469-0691.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–90. doi: 10.1086/604713. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz E, Akalin H, Ozbey S, Kordan Y, Sinirtaş M, Gürcüoglu E, et al. Risk factors in community-acquired/onset urinary tract infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Chemother. 2008;20:581–5. doi: 10.1179/joc.2008.20.5.581. [DOI] [PubMed] [Google Scholar]
- 13.Hryniewicz K, Szczypa K, Sulikowska A, Jankowski K, Betlejewska K, Hryniewicz W, et al. Antibiotic susceptibility of bacterial strains isolated from urinary tract infections in Poland. J Antimicrob Chemother. 2001;47:773–80. doi: 10.1093/jac/47.6.773. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Sun JY, Liu QZ, Han LZ, Huang XH, Ni YX, et al. High prevalence of CTX-M β-lactamases in faecal Escherichia coli strains from healthy humans in Fuzhou, China. Scand J Infect Dis. 2011;43:170–4. doi: 10.3109/00365548.2010.538856. [DOI] [PubMed] [Google Scholar]
- 15.Gibold L, Robin F, Tan RN, Delmas J, Bonnet R. Four-year epidemiological study of extended-spectrum β-lactamase-producing Enterobacteriaceae in a French teaching hospital. Clin Microbiol Infect. 2014;20:O20–6. doi: 10.1111/1469-0691.12321. [DOI] [PubMed] [Google Scholar]
- 16.Gupta K, Trautner BW. Harrison's Principles of Internal Medicine. 18th ed. New Delhi: McGraw Hill; 2012. Urinary tract infections, pyelonephritis, and prostatitis; pp. 2387–91. [Google Scholar]
- 17.Linsenmeyer K, Strymish J, Weir S, Berg G, Brecher S, Gupta K, et al. Activity of fosfomycin against extended-spectrum-β-lactamase-producing uropathogens in patients in the community and hospitalized patients. Antimicrob Agents Chemother. 2016;60:1134–6. doi: 10.1128/AAC.02614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wayne P. CLSI Performance Standard of Antimicrobial Susceptibility Testing: Twenty-Fourth International Supplement. CLSI Document M100-S24. USA: Clinical and Laboratory Standard Institute; 2014. [Google Scholar]
- 19.Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: Hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–78. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 20.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 21.Foxman B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am J Med. 2002;113:5–13. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 22.Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med. 1993;329:1328–34. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed N, Kamal M, Kamran A, Zaidi AH. Frequency of extended spectrum beta lactamases in Enterobacteriaceae in urinary isolates related to age and gender. Med Channel. 2016;22:16–20. [Google Scholar]
- 24.Ullah F, Malik S, Ahmed J. Antibiotic susceptibility pattern and ESBL prevalence in nosocomial Escherichia coli from urinary tract infections in Pakistan. Afr J Biotechnol. 2009;8:3921–6. [Google Scholar]
- 25.Malik N, Ahmed M, ur Rehman M. Prevalence and antimicrobial susceptibility of uropathogens in patients reporting to a tertiary care facility in Peshawar, Pakistan. J Microbiol Antimicrob. 2015;7:6–12. [Google Scholar]
- 26.Sohail M, Khurshid M, Saleem HG, Javed H, Khan AA. Characteristics and antibiotic resistance of urinary tract pathogens isolated from Punjab, Pakistan. Jundishapur J Microbiol. 2015;8:e19272. doi: 10.5812/jjm.19272v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–6. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 28.Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton AE, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–74. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 29.Chub OI, Bilchenko AV, Khalin I. Extended spectrum beta-lactamase production in uropathogens isolated from hospitalized patients with chronic pyelonephritis. Open Urol Nephrol J. 2015;8:71–5. [Google Scholar]
- 30.Ranjini CY, Kasukurthi LR, Madhumati B, Rajendran R. Prevalence of multidrug resistance and extended spectrum beta-lactamases among uropathogenic Escherichia coli isolates in a tertiary care hospital in South India: An alarming trend. Community Acquir Infect. 2015;2:19–24. [Google Scholar]
- 31.Ali I, Rafaque Z, Ahmed S, Malik S, Dasti JI. Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan. Asian Pac J Trop Biomed. 2016;6:60–6. [Google Scholar]
- 32.Sturm AW, van der Pol R, Smits AJ, van Hellemondt FM, Mouton SW, Jamil B, et al. Over-the-counter availability of antimicrobial agents, self-medication and patterns of resistance in Karachi, Pakistan. J Antimicrob Chemother. 1997;39:543–7. doi: 10.1093/jac/39.4.543. [DOI] [PubMed] [Google Scholar]
- 33.Gul N, Mujahid TY, Ahmad S. Isolation, identification and antibiotic resistance profile of indigenous. Pak J Biol Sci. 2004;7:2051–4. [Google Scholar]
- 34.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: A clinical update. Clin Microbiol Rev. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arslan H, Azap OK, Ergönül O, Timurkaynak F. Urinary Tract Infection Study Group. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother. 2005;56:914–8. doi: 10.1093/jac/dki344. [DOI] [PubMed] [Google Scholar]


