Abstract
OBJECTIVE:
The objective of the study was to characterize the mechanism associated with metabolic syndrome (MetS)-associated cognitive decline and determine the effect of minocycline on the above condition in mice.
MATERIALS AND METHODS:
We developed a HFHC diet-induced MetS model in mice. Diagnostic characteristics of MetS including waist circumference, lipid levels, blood pressure, and fasting blood glucose were measured in these Swiss albino mice. Cognitive parameters were measured using passive avoidance and elevated plus maze test. Hippocampal acetylcholine esterase (AchE), reduced glutathione (GSH), and cytokine levels were measured and histopathological evaluation conducted. The MetS animals were administered minocycline (50 mg/kg; 10 days) and the above parameters were measured.
RESULTS:
We successfully induced MetS using HFHC diet in mice. Animals showed significantly higher fasting blood glucose levels (P < 0.001), systolic blood pressure (P < 0.01), waist circumference (P < 0.001), low-density lipoprotein (P < 0.001), and triglyceride (P < 0.01) and reduced high density lipoprotein levels (P < 0.05) compared to control animals. Both scopolamine and MetS significantly lowered (P < 0.01) step-down latency and increased transfer latency (P < 0.001). MetS animals showed significantly higher AchE (P < 0.001) and tumor necrosis factor-α (P < 0.001) and Interleukin-1 β (P < 0.01) and lower GSH (P < 0.001) levels and reduced both CA1 (P < 0.001) and CA3 (P < 0.01) neuronal density compared to controls. Minocycline treatment partially reversed the above neurobehavioral and biochemical changes and improved hippocampal neuronal density in MetS animals.
CONCLUSION:
MetS led to hippocampal oxidative stress and neuroinflammatory changes with a corresponding loss of hippocampal neuronal density and cognitive decline. Anti-inflammatory and antioxidant property of minocycline may be responsible for its neuroprotective actions in these animals.
Keywords: Cognition, learning and memory metabolic syndrome, minocycline, neuroinflammation
Introduction
Metabolic syndrome (MetS) is characterized by obesity, hyperlipidemia insulin resistance, and hypertension.[1] Almost 25% of the adults worldwide suffer from MetS. This disorder primarily increases the risk of heart attack, stroke, and type 2 diabetes (T2D).[2] Among Indians, MetS is predominantly common in women in Asian Indian phenotype.[3]
MetS is associated with the late complications that include depressive disorders, neuropathy, and cognition impairment.[4] In MetS, obesity and hypertension have a direct effect on cognitive performance.[5] High-fat diet-induced obesity leads to cognitive derangements along with brain inflammation and causes insulin resistance associated with an elevation in corticosterone levels and impairs hippocampal synaptic plasticity.[6] On the other side, in obesity, peripheral inflammation was observed where local inflammation within the hypothalamus accelerates the loss of metabolic control and synaptic plasticity.[7]
Minocycline is a well-established tetracycline having excellent bioavailability in central nervous system (CNS) with prominent action on obsessive–compulsive disorder and unipolar psychotic depression in combination with antidepressants.[8,9] Moreover, minocycline suppresses neuropathic pain by reducing cortical microglial gene expression in rats.[10] There are evidence that minocycline has neuroprotective activity in autoimmune-induced CNS inflammation.[11] Interestingly, minocycline has been shown to modulate cytokine levels (tumor necrosis factor [TNF]-α and Interleukin [IL]-1 β) in the hippocampus of hypothalamic–pituitary–adrenal-axis-hyperactivity-associated anxiety and depression in rodents.[12] In diabetic retinopathy, the migration and activation have been arrested by minocycline treatment.[13] Minocycline may also reduce cyclooxygenase (COX)-2, caspase 1, 3, and IL-1 β expression in focal cerebral ischemia brain in rats.[14] Coexistence of obesity, hyperlipidemia, insulin resistance, and hypertension and cognitive derangement has been observed in MetS in several population studies. The possible mechanisms of MetS-associated cognitive decline include insulin resistance due to metabolic derangements, and neurodegeneration due to circulating free fatty acids.[15] However, signaling pathways associating cognitive impairments to MetS are less well characterized.
In the present work, we evaluate the mechanisms associating HFHC diet-induced MetS with cognitive decline in Swiss albino mice. We also determine the therapeutic potential of minocycline in reversing the MetS-associated cognitive decline in these animals.
Materials and Methods
Chemicals
Normal pellet, maize starch, lard, casein, vitamin and mineral mixture, and sodium chloride were purchased from VRK nutritional solutions, Pune, and casein and fructose were purchased from Charotar Casein Company, Baroda, and Loba chem Lab, respectively. Triglyceride (TG), total cholesterol (TC), and high-density lipid measuring kit were purchased from coral diagnostics. Lowry's protein estimation kit from Bio-Rad Laboratories, Inc., Cytokine ELISA kits from Sigma-Aldrich, St Louis, MO, USA.
Experimental animals
Swiss albino male outbred mice (18–22 g) were used. Animals were housed under standard animal house conditions; 25°C, 12:12 h light:dark cycle, and 50%–55% relative humidity. Animals were allowed free access to food and water. Animal experiments were carried out as per CPCSEA guideline and protocol of the experiments was approved by the Institutional Animal Ethical Committee (GCTS/IAEC/March 02, 2017).
Experimental groups
Experimental groups (n = 10/group).
Group I – Mice got access to normal food and water for the entire experimental period
Group II – Mice got access to normal food and water and single dose of scopolamine ip (0.4 mg/kg)
Group III – Mice got access to 20% fructose water and fed with high-fat diet (HFHC diet) for 4 weeks
Group IV – Mice were fed with normal food and water for 4 weeks. Minocycline (Gift samples from Sun Pharmaceutical Industries Ltd.) 50 mg/kg by oral route was given during the last 10 days
Group V – Animals fed with HFHC diet for 4 weeks and minocycline 50 mg/kg by oral route during the last 10 days.
High-fat diet constituted maize starch 367 g/kg, lard 316 g/kg, casein 255 g/kg, vitamin-mineral mix 61 g/kg, and Sodium chloride 1 g/kg. Drinking water was supplemented with 20% fructose solution as a source of high carbohydrate in the diet.[16]
Assessment of metabolic syndrome
Changes in food intake, body weight, waist circumference, blood glucose and lipid levels, and systolic blood pressure were assessed in mice.
Fasting blood glucose
Animals fasted overnight. Fasting blood glucose was measured by tail-prick method using one touch glucose strips (Bayer India Ltd).
Lipid profile
After 4 weeks of HFHC diet, the mice were fasted overnight and anesthetized using ketamine, and cardiac puncture collected blood samples were used to measure TC, high-density lipoprotein (HDL), and TG in plasma.
Blood pressure
Systolic and diastolic blood pressure was measured by the noninvasive method (IITC Life Sciences, USA).
Behavioral evaluation
Elevated plus maze test
The elevated plus maze test was used to measure changes in learning and memory. The plus maze consists of two open arms and two enclosed arms (open roof), with the two open arms opposite to each other to form a square open area between two closed arms; hence, the open area continues between two closed arms. Height of the maze from the ground is 50 cm. Before training, animals in each group were allowed to move across the maze freely for 2 m and after the training period, each animal was kept on the edge of one of the open arms facing away from the closed arms. The time taken to move from open to closed arm is measured as transfer latency (TL) for the particular animal. Animals which displayed an initial TL of over 90 s were excluded from the investigation. TL of the animals of each group was estimated at day 1, 2, and 7. Scopolamine was used as the amnestic agent.[17]
Passive avoidance test
Passive avoidance test was done utilizing apparatus which comprised of two chambers associated with an entryway. One compartment was sufficiently bright, while the other one was made dark. The two compartments were fitted with metal grid floor which might be provided with electric current (Medicraft Electro Medicals Pvt. Ltd., Lucknow, India). Animals of each group were trained to enter the dark chamber by at first locating them in the lit-up chamber, while the minute they entered the dark chamber, the entryway was closed. After this underlying habituation, animals were made to enter from lit up to the dark chamber, but this time metal framework floor was provided with an electric current of 1 mA for 5 s. 60 s was considered as cutoff time to go through the dark chamber. Animals taking longer time were excluded from further studies. Following 24 h of training, retention time was measured on days 1, 2, and 7. The time taken by the animals to travel from lit up to the dark chamber with all the four paws in dark compartment, i.e., step through latency was measured. During the observation time frame, no current was provided to the metal grid floor. Three successive readings were taken at an interval of 30 m, while the cutoff time for the experiment was 5 min.[17] Scopolamine was used as an amnestic agent in this study.
Biochemical assays using isolated brain (hippocampal) homogenates
After performing the behavioral evaluation, animals were sacrificed by cervical dislocation and brains were isolated. Mouse brain was collected and kept in a container in phosphate-buffered saline (PBS) and the container was kept in dry ice for 15 m. The hippocampal parts of mouse brain were isolated at 0°C–4°C and stored at −20°C. Brain homogenates were prepared by homogenization at 9500 rpm in PBS. The homogenates were centrifuged 15,000 rpm for 15 m at 4°C and supernatants were used for measuring acetylcholinesterase (AchE) and reduced glutathione (GSH) and cytokine levels.[18]
Estimation of acetylcholinesterase activity
Acetylcholinesterase was assessed by utilizing Ellman's technique with minor modifications.[17] Brain tissue (hippocampal) homogenate in phosphate buffer was added to DTNB (5,5'-Dithiobis (2-nitrobenzoic acid)) solution. The absorbance of the above solution was added estimated at 412 nm. Acetylthiocholine iodide to the above solution and blended thoroughly, again absorbance was estimated at 412 nm at 30 s interim for 10 m. The change in absorbance per meter was measured to determine AchE activity.
Estimation of glutathione
Reduced GSH levels were evaluated as described by Moron et al., 1979, with minor modifications.[18] To the supernatant of brain homogenate, 4 ml DTNB and 1.5 ml phosphate buffer were added and absorbance was estimated at 412 nm. GSH level was measured against GSH standard curve. Amount of protein in the homogenates was estimated using Lowry's protein estimation kit (Bio-Rad Laboratories, Inc).
Cytokine estimation
Alterations in the hippocampal IL-1 β and TNF-α levels were evaluated. Hippocampal homogenates were used to measure TNF-α and IL-1 β levels utilizing corresponding cytokine ELISA kit based on manufacturer's instructions (Sigma-Aldrich, St Louis, MO, USA).
Histopathology
Animals were sacrificed and the full brain separated out. Buffered formalin (pH 7.6) was used to keep isolated brain for fixation. Tissue was taken through dehydration process in increasing concentration of alcohol (70%–100%). The tissue was then inserted in liquefied wax, keeping the temperature of the wax 60°C–70°C. On solidification of the wax, tissue was segmented into 5 μm thin segments. Sections were mounted on the slide and deparaffinized applying reducing concentrations of alcohol to water so that water-soluble dye can penetrate into the tissue sections. Slides were then treated with hematoxylin and eosin stain. CA1 and CA3 neurons were seen under the light microscope for any basic structural modifications at ×10 and ×40 magnification. Neurons were counted using Camera Lucida (The Western Electric and Scientific Works, Haryana, India) under a light microscope in ×40 amplification.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). For the data containing more than two experimental groups, we used one-way ANOVA and Tukey's multiple comparison test as post hoc test. P < 0.05 was considered to be statistically significant. The data were expressed as mean ± standard error of the mean (SEM)
Results
Development of metabolic syndrome in mice
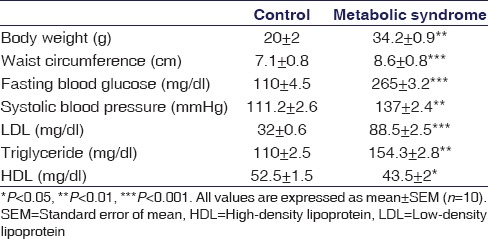
HFHC diet led to significant increase (P < 0.01) in the body weight as well as waist circumference (P < 0.001) compared to normal pellet diet animals. Other characteristics of MetS including increase in fasting blood glucose levels (P < 0.001) and systolic blood pressure (P < 0.01) was also observed. When it comes to cholesterol levels, there was significant increase in low-density lipoprotein (P < 0.001) and TG (P < 0.01) and a significant reduction in HDL levels (P < 0.05) were observed after 4 weeks in HFHC fed animals compared to their control counterparts, thus suggesting that mice on HFHC diet developed signs of MetS [Table 1].
Table 1.
Characterization of high-fat high-carbohydrate diet-induced metabolic syndrome
Neurobehavioral evaluation
In passive avoidance test, increase in memory is correlative of prolongation or increase in step-down latency (SDL). Scopolamine, an amnestic agent (0.4 mg/kg), was found to reduce SDL significantly (P < 0.01) compared to control animals. HFHC diet-induced MetS animals showed a significant decline in SDL (P < 0.01) compared to controls suggesting a cognitive decline. MetS-associated memory loss was significantly reversed after 10 days of minocycline treatment. This was evident by a pronounced increase in SDL (P < 0.01) over MetS mice [Figure 1a]. Improvement in memory is correlative with a decrease in TL in elevated plus maze test. Upon administration, scopolamine increased TL significantly (P < 0.001) a reflection that the animals forgot their task because of memory decline. MetS led to significant increase in TL (P < 0.001) in mice when compared to animals on normal diet indicating cognitive decline. Minocycline treatment showed a significant reduction in TL (P < 0.001) as compared to MetS animals, thus suggesting reversal of the cognitive decline in these animals [Figure 1b].
Figure 1.
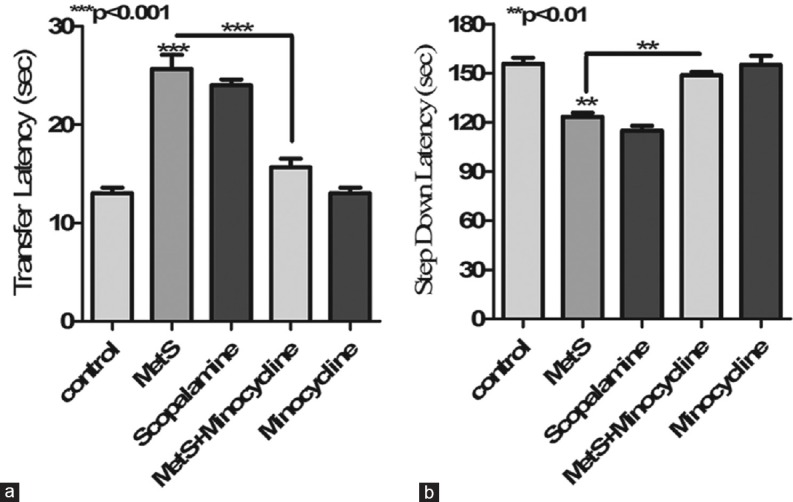
Effect of metabolic syndrome on learning and memory. (a) Metabolic syndrome was associated with a significant decrease in step-down latency in passive avoidance test which was significantly increased upon minocycline treatment. (b) Metabolic syndrome was associated with a significant increase in transfer latency in elevated plus maze test which was significantly decreased upon minocycline treatment. ***P < 0.001, **P < 0.01 all values are expressed as mean ± standard error of the mean for n = 10
Biochemical estimation of hippocampal homogenates
Reduction in hippocampal GSH suggests hippocampal oxidative and nitrosative stress.[17] MetS animals showed significantly reduced hippocampal GSH levels (P < 0.001) compared to normal animals suggesting increased hippocampal oxidative stress. However, 10 days of minocycline treatment significantly (P < 0.001) normalized the GSH levels [Figure 2b].
Figure 2.
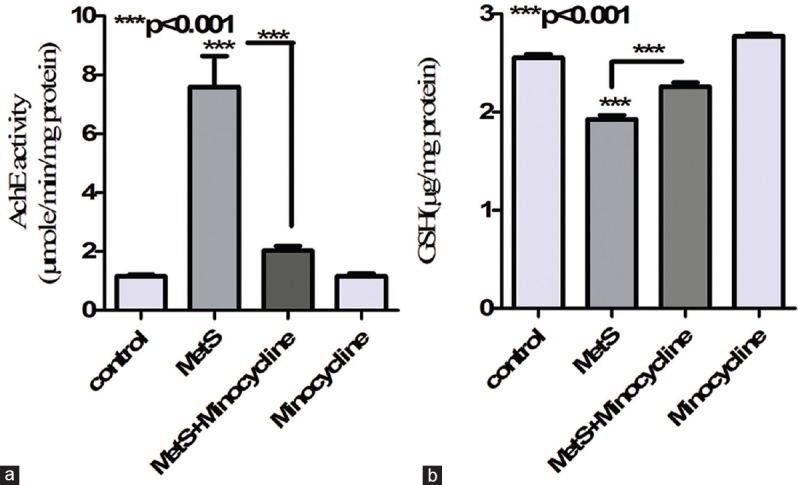
Effect of metabolic syndrome on hippocampal acetylcholine esterase activity and glutathione levels. (a) Metabolic syndrome led in significant increase in hippocampal acetylcholine esterase activity compared to control. While treatment with minocycline partially reversed metabolic syndrome-associated increase in hippocampal acetylcholine esterase activity (b) metabolic syndrome led to significant decrease in hippocampal glutathione levels compared to control. While treatment with minocycline successfully reversed metabolic syndrome associated decrease in glutathione levels. ***P < 0.001, **P < 0.01 all values are expressed as mean ± standard error of the mean for n = 10
Increased hippocampal AchE levels correlate well with reduced hippocampal acetylcholine levels and a corresponding memory decline. MetS animals showed a significant increase (P < 0.001) in hippocampal AchE levels which were successfully reversed (P < 0.001) upon minocycline treatment [Figure 2a].
Pro-inflammatory cytokine levels in the brain have long been associated with inflammation of the CNS. Hippocampal homogenates from MetS animals showed an increase in TNF-α (P < 0.001) and IL-1 β levels (P < 0.01) compared to controls. The anti-inflammatory antibiotic, minocycline treatment of MetS animals resulted in a pronounced decline in hippocampal TNF-α (P < 0.01) and IL-1 β levels (P < 0.01) [Figure 3].
Figure 3.
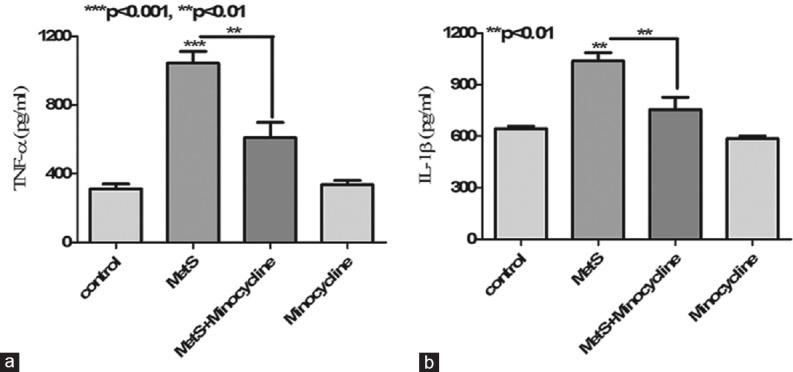
Effect of metabolic syndrome on hippocampal cytokine profile. (a) Metabolic syndrome led in a significant increase in hippocampal tumor necrosis factor-α levels compared to control. While treatment with minocycline partially reversed metabolic syndrome-associated increase in hippocampal tumor necrosis factor-α. (b) Metabolic syndrome led in significant increase in hippocampal Interleukin-1 β levels compared to control. While treatment with minocycline partially reversed metabolic syndrome-associated increase in hippocampal interleukin-1 β levels. ***P < 0.001, **P < 0.01 all values are expressed as mean ± standard error of the mean for n = 10
Histopathological evaluation
Histopathological studies on hippocampal sections from MetS animals confirmed the MetS-associated neuronal damage as revealed by a significant decline in the number of CA1 (P < 0.001) and CA3 neurons (P < 0.01). Interestingly, 10 days of minocycline treatment led to the significant rescue of the neurons both at CA1 (P < 0.01) and CA3 (P < 0.05) regions, thus confirming the neuroprotective actions of minocycline against MetS-associated damage [Figure 4].
Figure 4.
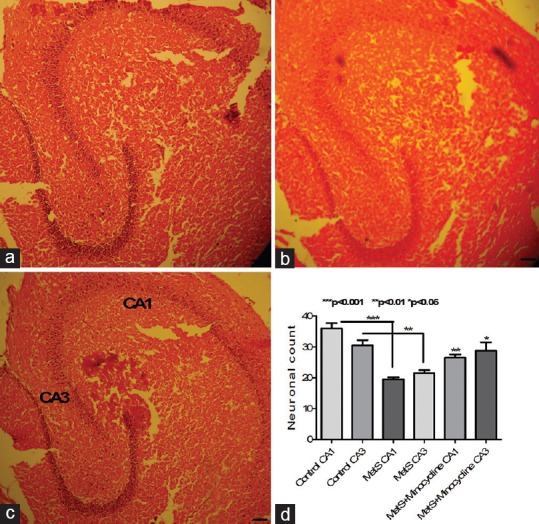
Photomicrographs of hippocampal region of mice brain. (a) Control showing healthy neurons; (b) metabolic syndrome animals showing loss of hippocampal neurons at CA1 and CA3 regions when compared to control; (c) minocycline-treated metabolic syndrome group showing partial reversal of metabolic syndrome-induced neuronal damage; (d) metabolic syndrome animals showing significant loss of CA1 and CA3 neurons compared to control animals. This loss was partially reversed upon minocycline treatment. Representative image from each group (n = 5) stained with H and E (10 × 10). ***P < 0.001, **P < 0.01, *P < 0.05 all values are expressed as mean ± standard error of the mean
Discussion
MetS has long been associated with inflammation with increased plasma levels of inflammatory mediators.[19] Obesity, a predominant feature of MetS, is also associated with increased circulating cytokine levels and alterations in cytokine profile.[20] Similarly, T2D which usually coexists with MetS disorder may also potentiate peripheral inflammation.[21] MetS is categorized as both metabolic and inflammatory disease which may also affect the immune system.[22] Peripheral and central inflammation has been shown to mediate the pathogenesis of various neuropsychiatric and neurodegenerative disorders.[23] A transgenic model of MetS, db/db mouse has shown reduced special memory with a corresponding increase in hippocampal pro-inflammatory cytokines.[24] Inflammation may also mediate oxidative stress. Oxidative stress has been strongly associated with neuronal injury in several neurodegenerative disorders.[25] While high-fat high fructose diet-induced MetS animals have been reported to show impaired novel object recognition as well as a reduced special memory with a corresponding decrease in cerebral blood volume and hippocampal brain-derived neurotrophic factor level.[26,27] Here, we show reduced special memory and fear-motivated memory in HFHC diet-induced MetS animals with a corresponding increase in hippocampal AchE activity. The MetS animals also showed increased hippocampal IL-1 β and TNF-α and reduced GSH levels. The above results suggest that MetS may result in hippocampal oxidative stress and inflammatory milieu which may prove to be neurotoxic to the hippocampal neurons in these animals. Histopathological studies confirmed the loss of CA1 and CA3 neurons in MetS animals which may be responsible for the reduction in special memory in these animals. Effect of minocycline on MetS brain was also studied.
Minocycline is a second-generation tetracycline antibiotic known for its high lipophilicity and blood–brain barrier permeability. It has been shown to be highly effective against various neuroinflammatory neurodegenerative disorders. Central prostaglandins are involved in neuroinflammation,[28] while minocycline has also been shown to reduce Prostaglandin E2 (PGE2) production and COX-2 levels by microglial cells.[14] It has also been shown to reduce PGE2 synthase and 8-iso-PGF2α production in lipopolysaccharide-activated primary rat microglial cells.[29] Thus, minocycline may potentiate the effects of nonsteroidal anti-inflammatory drug (NSAID) in reducing neuroinflammatory neurodegenerative disorders. However, although MetS increases PGE2 levels in the adipocytes,[30] it does not alter the brain eicosanoid (PGE2, thromboxane B2, and leukotriene B4) levels.[31] Thus, minocycline NSAID combination may not affectively alter MetS-associated inflammatory changes of the CNS. Minocycline may also improve cognitive impairment in the animal model of cerebral ischemia. It has also been shown to promote neurogenesis and has neuroprotective and antiviral potential.[32] Minocycline may also reduce hippocampal astrogliosis in vascular dementia model in rats, while our previous work also showed astrogliosis and microglial activation in diabetic rat brain.[14,33] We also saw a reduction in hippocampal chemokine, monocyte chemoattractant protein-1 levels by minocycline suggesting reduced astrogliosis in MetS brain (preliminary observation in our laboratory). While microglia-associated inflammation, leading to amyloidopathy and tauopathy, which has been reported in diabetic animal brain, minocycline, principally, acts on microglial cells [34] and may alleviate this Alzheimer's disease-like pathology through inhibition of nuclear factor-kappaB.[35] In the present work, we show minocycline-mediated reversal of MetS-associated increase in hippocampal inflammation as indicated by normalization of two cytokines primarily released by microglial cells.
We also report that minocycline may partially reverse MetS-associated cognitive decline with a corresponding increase in hippocampal GSH and decrease in AchE levels, suggesting reduced oxidative stress and improved hippocampal Ach levels, respectively. Histopathological studies confirmed improved CA1 and CA3 hippocampal neuronal density in MetS animals upon minocycline treatment. Thus, reduced oxidative stress and neuroinflammation may be responsible for minocycline-mediated protection of hippocampal cholinergic neurons in MetS animals.
Conclusion
MetS may lead to spontaneous cognitive decline in mice. While antioxidant and anti-inflammatory actions of minocycline may be responsible for its neuroprotective activity in MetS mice brain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Thanks are due to Professor Late Debesh Chandra Majumdar, Chairman, Trinity Trust, Gupta College of Technological Sciences, Asansol, for his support and encouragement. We are also thankful to Dr. S Samanta Professor and Head Department of Pharmaceutical Sciences and Technology, BIT, Mesra, for his support.
References
- 1.Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome – A new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents – An IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 3.Ravikiran M, Bhansali A, Ravikumar P, Bhansali S, Dutta P, Thakur JS, et al. Prevalence and risk factors of metabolic syndrome among Asian Indians: A community survey. Diabetes Res Clin Pract. 2010;89:181–8. doi: 10.1016/j.diabres.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Nerkar D, Mukherjee A, Mehta BK, Banerjee S. Metabolic syndrome associated complications. Int J Pharm Pharm Sci. 2015;7:22–5. [Google Scholar]
- 5.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of hypertension on cognitive function: A scientific statement from the American Heart Association. Hypertension. 2016;68:e67–94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller AA, Spencer SJ. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, Kawakami K, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: An open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:222–6. doi: 10.1016/j.pnpbp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez CI, Bender J, Jr, Marcus SM, Snape M, Rynn M, Simpson HB, et al. Minocycline augmentation of pharmacotherapy in obsessive-compulsive disorder: An open-label trial. J Clin Psychiatry. 2010;71:1247–9. doi: 10.4088/JCP.09l05805blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M. Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun. 2014;42:147–56. doi: 10.1016/j.bbi.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Maier K, Merkler D, Gerber J, Taheri N, Kuhnert AV, Williams SK, et al. Multiple neuroprotective mechanisms of minocycline in autoimmune CNS inflammation. Neurobiol Dis. 2007;25:514–25. doi: 10.1016/j.nbd.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Majidi J, Kosari-Nasab M, Salari AA. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and HPA axis activity in adult mice. Brain Res Bull. 2016;120:1–3. doi: 10.1016/j.brainresbull.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–65. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 14.Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J, et al. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbaraly TN, Kivimaki M, Shipley MJ, Tabak AG, Jokela M, Virtanen M, et al. Metabolic syndrome over 10 years and cognitive functioning in late midlife: The Whitehall II study. Diabetes Care. 2010;33:84–9. doi: 10.2337/dc09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee A, Sen KK, Banerjee S. Effect of metabolic syndrome on depression in mice. Indian J Pharm Educ. 2018;52:S46–53. [Google Scholar]
- 17.Mukherjee D, Banerjee S. Learning and memory promoting effects of crude garlic extract. Indian J Exp Biol. 2013;51:1094–100. [PubMed] [Google Scholar]
- 18.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 19.Marsland AL, McCaffery JM, Muldoon MF, Manuck SB. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism. 2010;59:1801–8. doi: 10.1016/j.metabol.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancello R, Clément K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 2006;113:1141–7. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 21.Donath MY, Böni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA. Cytokine production by islets in health and diabetes: Cellular origin, regulation and function. Trends Endocrinol Metab. 2010;21:261–7. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt MI, Duncan BB. Diabesity: An inflammatory metabolic condition. Clin Chem Lab Med. 2003;41:1120–30. doi: 10.1515/CCLM.2003.174. [DOI] [PubMed] [Google Scholar]
- 23.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinel AL, André C, Aubert A, Ferreira G, Layé S, Castanon N, et al. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 2011;6:e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–14. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 26.Gancheva SM, Zhelyazkova-Savova MD. Vitamin K2 improves anxiety and depression but not cognition in rats with metabolic syndrome: A role of blood glucose? Folia Med (Plovdiv) 2016;58:264–72. doi: 10.1515/folmed-2016-0032. [DOI] [PubMed] [Google Scholar]
- 27.Johnson LA, Zuloaga KL, Kugelman TL, Mader KS, Morré JT, Zuloaga DG, et al. Amelioration of metabolic syndrome-associated cognitive impairments in mice via a reduction in dietary fat content or infusion of non-diabetic plasma. EBioMedicine. 2016;3:26–42. doi: 10.1016/j.ebiom.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hein AM, O'Banion MK. Neuroinflammation and memory: The role of prostaglandins. Mol Neurobiol. 2009;40:15–32. doi: 10.1007/s12035-009-8066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva Bastos LF, Pinheiro de Oliveira AC, Magnus Schlachetzki JC, Fiebich BL. Minocycline reduces prostaglandin E synthase expression and 8-isoprostane formation in LPS-activated primary rat microglia. Immunopharmacol Immunotoxicol. 2011;33:576–80. doi: 10.3109/08923973.2010.544659. [DOI] [PubMed] [Google Scholar]
- 30.Iyer A, Lim J, Poudyal H, Reid RC, Suen JY, Webster J, et al. An inhibitor of phospholipase A2 group IIA modulates adipocyte signaling and protects against diet-induced metabolic syndrome in rats. Diabetes. 2012;61:2320–9. doi: 10.2337/db11-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taha AY, Gao F, Ramadan E, Cheon Y, Rapoport SI, Kim HW, et al. Upregulated expression of brain enzymatic markers of arachidonic and docosahexaenoic acid metabolism in a rat model of the metabolic syndrome. BMC Neurosci. 2012;13:131. doi: 10.1186/1471-2202-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: Far beyond an antibiotic. Br J Pharmacol. 2013;169:337–52. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta B, Banerjee S. Characterization of cognitive impairment in type 2 diabetic rats. Indian J Pharm Sci. 2017;79:785–93. [Google Scholar]
- 34.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai Z, Zhao Y, Yao S, Bin Zhao B. Increases in β-amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF-κB pathway activation. Pharmacol Rep. 2011;63:381–91. doi: 10.1016/s1734-1140(11)70504-7. [DOI] [PubMed] [Google Scholar]


