Abstract
Background and Objectives:
The present study was designed to explore the utility of ultrasound-guided diaphragmatic thickness in the preoperative period in healthy controls scheduled for live-related donor hepatectomy and patients suffering from chronic liver disease scheduled for liver transplantation (LT) and its use as a predictor of postoperative weaning failure.
Materials and Methods:
This prospective observational study was conducted in a tertiary health care center and 65 adult (18–70 years) participants (30 healthy liver donors and 35 liver transplant recipients) were enrolled for this study. Right diaphragmatic thickness of both donors and recipients was measured by B-mode ultrasound using a 10 MHz linear array transducer in the supine position in the operation theater just before induction of anesthesia. For subgroup analysis of the recipients, we further divided them into two groups – Group 1 (diaphragmatic thickness < 2 mm) and Group 2 (diaphragmatic thickness > 2 mm), and comparison was done for duration of mechanical ventilation. Intergroup comparison was made for duration of mechanical ventilation and various other parameters.
Results:
The sonographic measurement of diaphragm revealed that its thickness is decreased in patients with chronic liver disease patients (2.12 ± 0.54 mm) as compared to healthy donors (3.70 ± 0. 58 mm). On multiple logistic regression, higher duration of mechanical ventilation was associated with diaphragmatic thickness < 2 mm (Group 1 of recipients) (adjusted odds ratio 0.86; 95% confidence interval: 0.75–0.99; P = 0.013) after adjusting for age, gender, and body mass index.
Conclusions:
Diaphragmatic thickness is decreased in patients with chronic liver disease as compared to healthy liver donors. Preoperative measurement of ultrasound-guided right hemidiaphragm thickness can be used to predict weaning failure in patients undergoing LT. Other studies are needed to confirm these finding on different group of patients.
Keywords: Diaphragmatic thickness, ultrasound, weaning failure
Introduction
Postoperative pulmonary complications (pleural effusion, atelectasis, pulmonary edema, acute respiratory distress syndrome, and pneumonia) following liver transplantation (LT) are common and have been associated with increased morbidity and mortality.[1,2,3,4,5,6]
Reduced diaphragmatic function has been reported to cause respiratory complications in different subsets of patients. Structurally, the diaphragm consists of four parts: transverse septum (anterior, central tendon of the diaphragm), pleuroperitoneal folds, esophageal mesentery, and muscular body wall laterally.[7]
Imaging or functions of the diaphragm can be studied by various modalities, i.e., chest X-ray, ultrasound, computed tomography, dynamic magnetic resonance imaging, electromyography, and pulmonary function tests (PFTs). Sonographic evaluation of diaphragm has gained wide acceptance to assess the presence of postoperative diaphragm dysfunction and other respiratory complications. Ultrasound of the diaphragm is noninvasive, radiation-free, relatively inexpensive, and it is readily accessible in the Intensive Care Units (ICUs).[8] It provides high-resolution images of diaphragmatic muscle movement, thickness, and echogenicity. Average thickness of the diaphragm <2.0 mm, measured by ultrasound at the end of expiration, has been proposed as the cutoff to define diaphragm atrophy.[7]
Sarcopenia or loss of skeletal muscle mass is well documented (prevalence 30%–70%) in cirrhotics, but whether it has a bearing on diaphragmatic thickness is not studied till now.[9] The etiology of sarcopenia in these group of patients involves three major factors: inadequate dietary intake, metabolic disturbances, and malabsorption. Modification of model for end-stage liver disease (MELD) score to include sarcopenia (MELD-sarcopenia) is associated with improved prediction of mortality in patients with cirrhosis.[9]
Although sonographic evaluation of diaphragm has been evaluated in critically ill patients[10,11,12] and patients with neuromuscular disease[7] and chronic obstructive pulmonary disease (COPD),[13,14,15] it has not been studied in cirrhotic patients.
We hypothesize that diaphragmatic thickness is decreased in chronic liver disease patients as compared to healthy donors. We also hypothesize that diaphragmatic thickness is further reduced in cirrhotic patients with increased severity of decompensation and can predict weaning failure from mechanical ventilation following LT.
Materials and Methods
This single-center, prospective, observational study was conducted at a tertiary care hospital in Northern India. After ethical clearance by the institutional ethical committee and registering it in the Clinical Trials Registry India (CTRI/2017/05/008631), we included adult (18–70 years) patients with chronic liver disease (recipients), with body mass index (BMI) >18 <30 kg/m2, MELD score ≥15 undergoing living donor LT, and voluntary American Society of Anesthesiologists (ASA) I or II eligible donors with BMI >18 <30 kg/m2, after obtaining informed written consent. We excluded subjects with age <18 and >70 years, history of COPD, asthma, BMI <18 or >30 kg/m2, previous major thoracic or abdominal surgery (both donors and recipients), acute liver failure, repeat transplant, and in subgroup analysis, recipients with primary graft nonfunction (PGNF) and who reintubated due to repeat surgery were excluded. To study 30% difference between two groups with power of 80 and type II error of 5%, we required to study 30 subjects in each group.
Pretransplant demographic parameters, clinical history, etiology of liver disease with its severity (Child-Turcotte-Pugh [CTP] and MELD score) and decompensation (ascites), medical comorbidities, and PFT were collected for each patient.
Right diaphragmatic thickness was measured before induction of anesthesia in the operation theater at end-expiration in a spontaneously breathing patient in supine position. A high-resolution portable ultrasound MyLab 30 Gold Vet® (Esaote, Italy) machine was used with a 10 MHz linear array transducer. The right hemidiaphragm was identified by placing the transducer perpendicular to the chest wall, in the seventh to ninth intercostal space, between the anterior axillary and the midaxillary lines, to observe the zone of apposition of the muscle 0.5–2 cm below the costophrenic sinus.
Right hemidiaphragm was imaged as a structure with three distinct layers, including two parallel echoic lines (the diaphragmatic pleura and the peritoneal membrane) and a hypoechoic structure between them (the muscle itself). Three images for each position at right side were collected and averaged to determine a thickness at resting end-expiration. On each frozen B-mode image, the diaphragm thickness was measured from center of the pleural line to center of the peritoneal line [Figure 1]. The images were measured on the ultrasound system itself, using the measurement tool. All images were captured by same investigator to eliminate interobserver bias. The anesthetist managing the patient in the perioperative period was blinded to the ultrasonographic evaluation of diaphragmatic thickness. Anesthesia was administered as per the institutional protocol. The decision to extubate the patient or to continue mechanical ventilation at the end of the surgery was left to the anesthetist's discretion.
Figure 1.
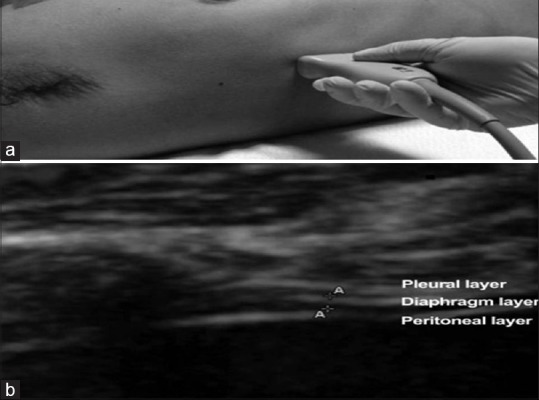
(a) Ultrasound probe position for B-mode diaphragmatic thickness measurement, (b) a diaphragmatic thickness image displaying three distinct layers from top to bottom
Intraoperative details such as surgical duration, cold ischemia time, warm ischemia time, graft-recipient weight ratio, and blood loss were recorded in recipients. In the postoperative period, time of extubation, duration of mechanical ventilation support, weaning failure, and ICU and hospital stay were recorded.
For subgroup analysis of cirrhotic recipients, we further divided them into two groups – Group 1 (diaphragmatic thickness <2 mm) and Group 2 (diaphragmatic thickness >2 mm) – as it has been proposed as the cutoff to define diaphragm atrophy.[7] Intergroup comparison was made for following variables: CTP, MELD, age, BMI, duration of mechanical ventilation, and ICU and hospital stay. Patients with initial poor graft function and primary nonfunction and patients who were reintubated for repeat surgery due to any other cause were excluded from the study. In the postoperative period, the presence of weaning failure was noted. Weaning failure was defined as failure to pass a spontaneous-breathing trial or the need for reintubation within 48 h, following extubation.
Statistical analysis was done using IBM SPSS statistical software (SPSS v. 21.0 for Mac OS X). Descriptive statistics was expressed as mean ± standard deviation for parametric continuous data and number (%) for categorical data. Comparison of continuous variables was done by the Student's t-test for parametric or Mann–Whitney test for nonparametric data. Categorical variables were compared by Fisher's exact test. All statistical tests were two-tailed, and a significance level (P) of 0.05 was used.
Results
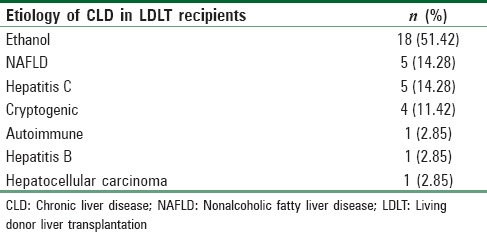
In this single-center prospective observational investigation, 30 healthy donors and 35 recipients with various etiology of chronic liver disease undergoing LT participated after scrutinizing inclusion and exclusion criteria. In the present study, the most common etiology [Table 1] for which a recipient underwent LT was ethanol-related CLD (N = 18, 51.42%). The other causes were nonalcoholic fatty liver disease (N = 5, 14.28%), hepatitis C (N = 5, 14.28%), cryptogenic (N = 4, 11.42%), autoimmune (N = 1, 2.85%), hepatitis B (N = 1, 2.85%), and hepatocellular carcinoma (N = 1, 2.85%). The sonographic measurement of diaphragm revealed that its thickness was decreased in patients with chronic liver disease patients (2.12 ± 0.54 mm) as compared to healthy donors (3.70 ± 0. 58 mm).
Table 1.
Various etiology of chronic liver diseases in recipients undergoing liver transplantation
Of 35 recipients, one patient was reintubated for repeat surgery and another one patient developed PGNF. These two patients were excluded from further subgroup analysis. In subgroup analysis of recipients, eight patients had diaphragmatic thickness <2 mm (Group 1) and 25 patients had diaphragmatic thickness >2 mm (Group 1) [Figure 2 and Table 2].
Figure 2.
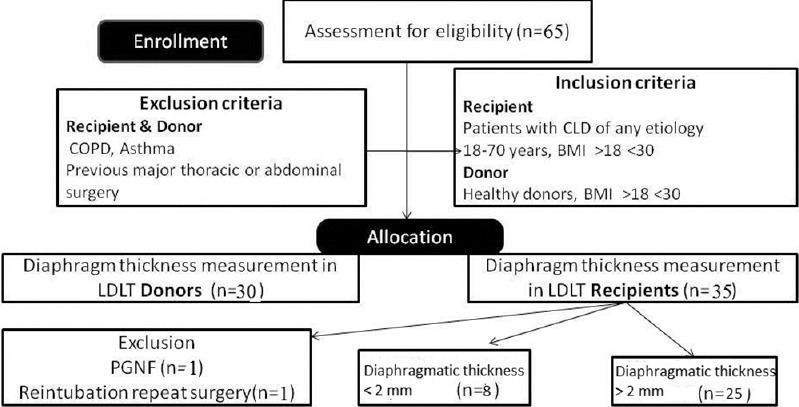
Consort diagram
Table 2.
Comparison between two subgroups of liver transplant recipients with diaphragmatic thickness less than or greater than 2 mm
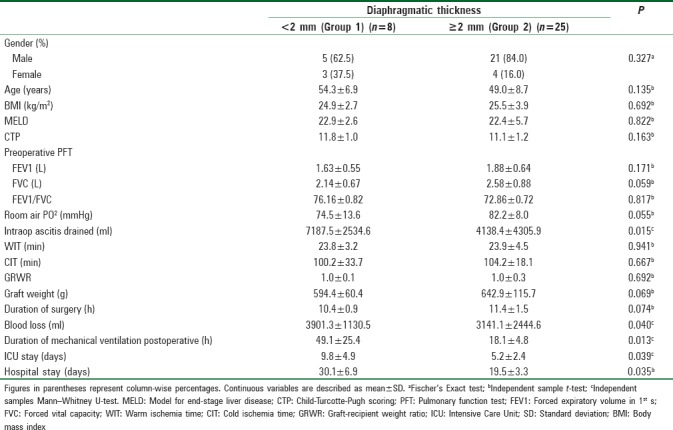
On multiple logistic regression, higher duration of mechanical ventilation was associated with diaphragmatic thickness <2 mm (adjusted odds ratio 0.86; 95% confidence interval: 0.75–0.99; P = 0.013) after adjusting for age, gender, and BMI.
The amount of ascitic fluid drained intraoperatively (P = 0.015), blood loss (P = 0.040), ICU stay (P = 0.039), and hospital stay (P = 0.035) was also significantly less in Group 2 (diaphragmatic thickness >2 mm) than Group 1 (diaphragmatic thickness <2 mm) [Table 2 and Figure 3]. There was no significant correlation between the subgroups of recipients for PFT (forced expiratory volume in 1st s [FEV1], forced vital capacity [FVC], and ratio of FEV1/FVC) and severity of decompensation (CTP, MELD).
Figure 3.
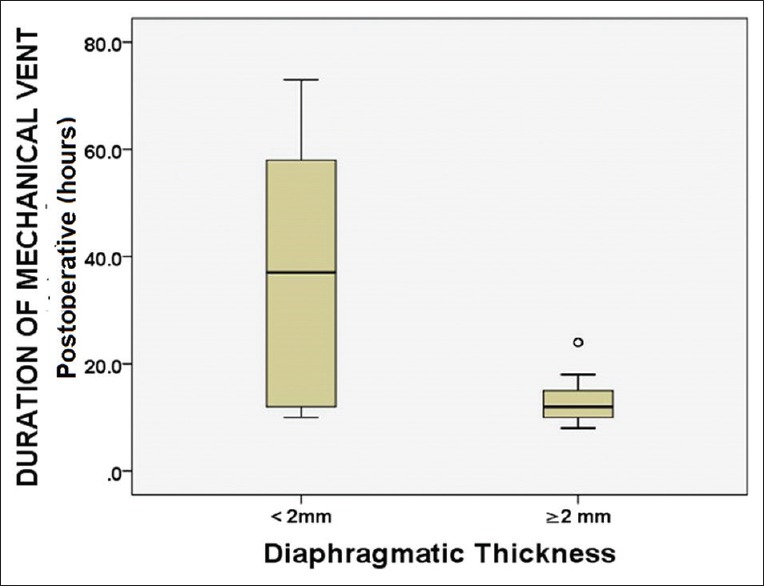
Diagram showing correlation between duration of postoperative ventilation and living donor liver transplantation recipients with diaphragmatic thickness less than and more than 2 mm
Discussion
Diaphragm is a unique skeletal muscle, essential for respiration, especially during vigorous activity and deep inspiration.[16] Real-time ultrasound (USG) of the diaphragm is a simple, radiation-free, inexpensive, and portable technique that can provide qualitative bedside anatomical information. Boon et al. in their study of 150 normal subjects found that a side-to-side difference in thickness at end-expiration of >0.33 cm is abnormal.[17] Goligher et al. measured the diaphragmatic thickness and thickening on deep inspiration in mechanically ventilated patients.[12] They found that right hemidiaphragm measurement was highly reproducible whereas thickening was only moderately reproducible. Furthermore, it was easier to obtain right hemidiaphragm thickness in 95% attempts whereas left hemidiaphragm measurements could not be obtained consistently. To eliminate bias due to variation in thickness between right and left hemidiaphragm, we studied only the right hemidiaphragm at 7th–9th intercostals space (zone of apposition). This further eliminated discrepancy due to artifacts secondary to gastric shadow.
Various authors have proposed different values for USG-guided thickness of diaphragm, but thickness <2.0 mm, measured at the end of expiration, has been proposed as the cutoff to define diaphragm atrophy. Carrillo-Esper et al.[16] showed that sonographic diaphragm evaluations can be applied to the general population. Goligher et al. in their study on five healthy subjects found that the mean diaphragmatic thickness in the zone of apposition at end-expiration was 2.1 + 0.3 mm whereas the average value observed by Boon et al. was 3.40 mm ± 1.80 mm.[12,17] In the present study, healthy donor ASA I/II population had diaphragm thickness of 3.70 ± 0.58 mm. Although sonographic thickness of diaphragm has been studied in healthy volunteer, patients with COPD, and critically ill patients on mechanical ventilation, Medline search did not reveal any study on diaphragmatic thickness in chronic liver disease patients. We observed a mean right hemidiaphragmatic thickness of 2.12 ± 0.54 mm in our cirrhotic patients scheduled for LT. The lower value of diaphragmatic thickness in the recipients as compared to healthy donors might be explained by sarcopenia which is highly prevalent in chronic liver disease patients.
In Group 1 of living donor liver transplantation (LDLT) recipients, (diaphragmatic thickness <2 mm) more ascitic fluid was drained intraoperatively than Group 2 (P = 0.015). It means that patients with decompensation, i.e., massive ascites, had more sarcopenia than patients who did not have ascites at the time of LDLT surgery.
Patients with thinner diaphragm (diaphragmatic thickness <2 mm) had prolonged duration of mechanical ventilation (P = 0.013). These patients also stayed longer in ICU (P = 0.039) and hospital (P = 0.035) in comparison to other group. This can be explained by reduction in diaphragm activity and diminished force generating capacity in patients with thinner diaphragm. It resulted in diaphragmatic atrophy which contributed to failure to wean from the ventilator and prolonged stay in ICU and hospital. More bleeding in patients with Group 1 (diaphragmatic thickness <2 mm) can be because of poor nutritional status which is a risk factor of sarcopenia in these patients.[9]
Cimsit et al.[14] evaluated thickness of the diaphragm by B-mode ultrasonography in 53 clinically stable patients with COPD to determine the relationship between diaphragm thickness measurement, PFT, and symptom scores. They found no correlation between diaphragmatic thickness in COPD patients and PFT, except for % FEV1 in mild COPD patients (r = 0.62, P = 0.017 < 0.05). In the present study, we also found no significant difference of PFT with USG-guided diaphragmatic thickness in both the LDLT recipients groups.
In the present study, there was significant difference in postoperative duration of mechanical ventilation in both the LDLT-recipient subgroups. Therefore, its preoperative assessment can predict postoperative weaning failure in these group of patients.
This was a pilot study aiming to explore the thickness of diaphragm in chronic liver disease recipients as compared to healthy donors and it's influence on weaning in the postoperative period after LT.
The major limitations of this study were very small group with diaphragmatic thickening of <2 mm, its small sample size, and that it was carried out in a single center. Extrapolation of results to the other study populations should be substantiated by further randomized controlled trials. The other limitation is that we studied the diaphragmatic thickness at end-expiration in a spontaneously breathing patient when the thickness is expected to be maximum. It did not study the thickening fraction/excursion of diaphragm as it depends on respiratory effort, which may be influenced by the presence of encephalopathy, massive ascites, or malnutrition and confound the findings. We suggest a multicenter randomized clinical trial for validation of the results of this pilot study to establish the utility of sonographic measurement of diaphragmatic thickness in patients with chronic liver disease and its correlation with sarcopenia and its effect on recovery of respiratory function.
Conclusions
Diaphragmatic thickness is decreased in patients with chronic liver disease as compared to healthy liver donors. Preoperative measurement of right hemidiaphragm thickness in the zone of apposition by ultrasound is an easy, radiation-free, bedside technique to prognosticate regarding weaning failure and to predict the duration of mechanical ventilation in cirrhotic recipients scheduled for LT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Levesque E, Hoti E, Azoulay D, Honore I, Guignard B, Vibert E, et al. Pulmonary complications after elective liver transplantation-incidence, risk factors, and outcome. Transplantation. 2012;94:532–8. doi: 10.1097/TP.0b013e31825c1d41. [DOI] [PubMed] [Google Scholar]
- 2.Ulubay G, Er Dedekarginoglu B, Kupeli E, Salman Sever O, Oner Eyuboglu F, Haberal M, et al. Postoperative pulmonary complications in living-liver donors: A retrospective analysis of 188 patients. Exp Clin Transplant. 2015;13(Suppl 1):340–5. doi: 10.6002/ect.mesot2014.p183. [DOI] [PubMed] [Google Scholar]
- 3.Bozbas SS, Eyuboglu FO, Ozturk Ergur F, Gullu Arslan N, Sevmis S, Karakayali H, et al. Pulmonary complications and mortality after liver transplant. Exp Clin Transplant. 2008;6:264–70. [PubMed] [Google Scholar]
- 4.O’Brien JD, Ettinger NA. Pulmonary complications of liver transplantation. Clin Chest Med. 1996;17:99–114. doi: 10.1016/s0272-5231(05)70301-4. [DOI] [PubMed] [Google Scholar]
- 5.Feltracco P, Carollo C, Barbieri S, Pettenuzzo T, Ori C. Early respiratory complications after liver transplantation. World J Gastroenterol. 2013;19:9271–81. doi: 10.3748/wjg.v19.i48.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doğrul MI, Akçay S, Savaş Bozbaş Ş, Er Dedekargınoğlu B, Öner Eyüboğlu F, Moray G, et al. Early pulmonary complications of liver transplant. Exp Clin Transplant. 2014;12(Suppl 1):153–5. [PubMed] [Google Scholar]
- 7.Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47:319–29. doi: 10.1002/mus.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen W. Proceeding of the First World Congress on Ultrasound Diagnostics in Medicine and SIDUO III. Vienna: 1969. Evaluation of the diaphragm by a subcostal B-scan technique; p. 63. [Google Scholar]
- 9.Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, et al. Inclusion of sarcopenia within MELD (MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. doi: 10.1038/ctg.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schepens T, Verbrugghe W, Dams K, Corthouts B, Parizel PM, Jorens PG, et al. The course of diaphragm atrophy in ventilated patients assessed with ultrasound: A longitudinal cohort study. Crit Care. 2015;19:422. doi: 10.1186/s13054-015-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zambon M, Beccaria P, Matsuno J, Gemma M, Frati E, Colombo S, et al. Mechanical ventilation and diaphragmatic atrophy in critically ill patients: An ultrasound study. Crit Care Med. 2016;44:1347–52. doi: 10.1097/CCM.0000000000001657. [DOI] [PubMed] [Google Scholar]
- 12.Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: Feasibility, reproducibility and validity. Intensive Care Med. 2015;41:642–9. doi: 10.1007/s00134-015-3687-3. [DOI] [PubMed] [Google Scholar]
- 13.Baria MR, Shahgholi L, Sorenson EJ, Harper CJ, Lim KG, Strommen JA, et al. B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest. 2014;146:680–5. doi: 10.1378/chest.13-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cimsit C, Bekir M, Karakurt S, Eryuksel E. Ultrasound assessment of diaphragm thickness in COPD. Marmara Med J. 2016;29:8–13. [Google Scholar]
- 15.Smargiassi A, Inchingolo R, Tagliaboschi L, Di Marco Berardino A, Valente S, Corbo GM, et al. Ultrasonographic assessment of the diaphragm in chronic obstructive pulmonary disease patients: Relationships with pulmonary function and the influence of body composition-a pilot study. Respiration. 2014;87:364–71. doi: 10.1159/000358564. [DOI] [PubMed] [Google Scholar]
- 16.Carrillo-Esper R, Pérez-Calatayud ÁA, Arch-Tirado E, Díaz-Carrillo MA, Garrido-Aguirre E, Tapia-Velazco R, et al. Standardization of sonographic diaphragm thickness evaluations in healthy volunteers. Respir Care. 2016;61:920–4. doi: 10.4187/respcare.03999. [DOI] [PubMed] [Google Scholar]
- 17.Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ, et al. Two-dimensional ultrasound imaging of the diaphragm: Quantitative values in normal subjects. Muscle Nerve. 2013;47:884–9. doi: 10.1002/mus.23702. [DOI] [PubMed] [Google Scholar]


