Abstract
Background:
Toward improving the reporting quality of clinical case reports in the Saudi Journal of Anesthesia, we conducted this audit. The aim of this paper is to provide an overview of the different objectives for clinical case reports and to identify those subordinate items which seem most relevant from the CAse REport (CARE) checklist.
Methods:
We performed this pilot study on clinical case reports published in the Saudi Journal of Anesthesia (SJA) in the past 5 years from 2013 to 2017. The journal publishes 4 issues/year that means 20 issues were studied. We used one online source to gather the clinical case reports which is the SJA website. A total of 84 case reports were studied. We have applied the 13th items in the CARE checklist on the case reports to determine their representations. Two reviewers abstracted data from all included papers to determine the adaptation of the CARE checklist. Data are presented as percentages of different subordinate items of the CARE guidelines.
Results:
None of the 84 case reports met all subordinate items of CARE guidelines, and only 5 subordinate items were reported fully met (100%). Patient perspective subordinate item was not mentioned in our series due to lack of data in the studied case reports. Therefore, only 12 subordinate items were included. We reported those adaptation percentages of the 12th subordinate items of the CARE checklist as follows: (a) title, keywords, abstract patient's biodata, and conclusion 100%; (b) main symptoms of the patients 97.6%; (c) timeline 78.5%; (d) diagnosis 94.0%; (e) treatment 97.6%; (f) strengths 85.7%; (g) literature review 94.0%; and (h) patient consent 33.4%.
Conclusion:
We believe that the CAse REport guidelines can provide an international framework for the authors to follow in writing their case reports and for the editors to use to ensure the completeness and readiness of the peer-reviewed case reports for publication. For the SJA, we have to apply the CARE checklist and to ensure all subordinate items are adapted including the patient's perspective subordinate item and to make sure that the consent form obtained and accompanied each submitted case reports.
Keywords: CAse REport guidelines, CAse REports, Saudi Journal of Anesthesia
Introduction
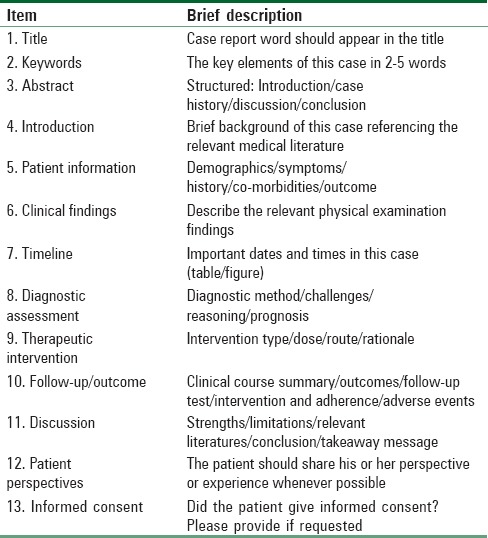
Case reports were previously recognized as a valuable tool for evidence-based medicine (EBM). Recently, the importance of case reports declined, and their impact in the field of research diminished to a serious level, to the extent that most of anesthesia journals nowadays do not accept case reports for peer reviewing and possible publication. Most of the editors now advise authors to write a letter to the editor rather than case reports. Case reports attract less citation from the researchers and that leads to lower impact factor of the target journal. That strange situation has opt editors in some journal to launch a separate edition for case reports only due to the believe in the importance of case reports to our readers. In our journal, we still accept case reports but with limitations. The report should address a rare and syndromic case. However, we also encourage authors to write letter to the editor more than case reports to improve the citations and hence the impact factor of the Saudi Journal of Anesthesia (SJA). To improve the consistency of information contained in case reports, the CAse Report (CARE) Group developed and published a set of guidelines for the medical community to facilitate systematic data collection (http://www.care-statement.org/).[1] It includes 13-subordinate items checklist which provides a framework for the competency and transparency of published case reports. The primary section of the CARE guidelines includes the title, abstract, and keywords, followed by the introduction section. The secondary section includes timeline, diagnostic assessment, follow-up and outcomes, and therapeutic intervention of the case report. In addition, two sections for the discussion, patient perspectives, and informed consent are included [Table 1].
Table 1.
CAre REport checklist
Toward improving the reporting quality of clinical case reports in the SJA, we conducted this audit. The aim of this paper is to provide an overview of the different objectives for clinical case reports and to identify those items which seem most relevant from the CARE checklist in an attempt to improve the quality of published reports in SJA and hence the impact factor.
Methods
We performed this pilot study on clinical case reports published in the SJA in the last 5 years from 2013 to 2017. The journal publishes 4 issues/year that means 20 issues were studied. We used one online source to gather the clinical case reports which is the SJA website. We have free access online to all articles of the journal. A total of 84 case reports were studied. We have applied the 13th subordinate items in the CARE checklist on the published case reports to determine their representations. Two reviewers abstracted data from all included papers to determine the adaptation of the CARE checklist. SJA has not yet obtained an impact factor, but its PubMed and Emerging Sources Citation Index indexed. Data are presented as percentages of different subordinate items of the CARE guidelines.
Results
The review of the structure of clinical case reports and their relative applicability to the CARE checklist is summarized in Figure 1. None of the 84 case reports met all subordinate items of CARE guidelines and only 5 subordinate items were reported fully met (100%). Patient perspective item was not mentioned in our series due to lack of data in the studied case reports. Therefore, only 12 subordinate items appeared in Figure 1. We reported those adaptation percentages of the 12th subordinate items of the CARE checklist as follows (a) title, keywords, abstract patient's biodata, and conclusion 100%; (b) main symptoms of the patients 97.6%; (c) timeline 78.5%; (d) diagnosis 94.0%; (e) treatment 97.6%; (f) strengths 85.7%; (g) literature review 94.0%; and (h) patient consent 33.4%.
Figure 1.

Percentages of the subordinate item of the CAre REport checklist
Discussion
We evaluated the reporting characteristics of case reports published in SJA in the past 5 years. The results showed that none of these case reports met all items of CARE guidelines. Only 5 subordinate items were reported fully adapted, namely, title, keywords, abstract, biodata, and conclusion. The other insufficient reported items included symptoms, timeline, diagnosis, treatment, strengths, literature, and consent. In one study, it was found that only 6 subordinate items were reported fully among 50% of the case reports while items that were reported insufficiently included title, keywords, abstract, introduction, patient information, timeline, follow-up, and outcomes.[2] During 2011–2012, a group of clinicians, researchers, and journal editors developed recommendations for the accurate reporting of information in case reports that resulted in the CARE statement and checklist. They were presented at the 2013 International Congress on Peer Review and Biomedical Publication and have been endorsed by multiple medical journals, and translated into nine languages. In an attempt to explain and elaborate on the CARE guidelines Riley et al. designed a study where each item from the CARE checklist was explained and accompanied by published examples. The explanation and examples were designed to support the writing of high-quality case reports by authors and their critical appraisal by editors, peer reviewers, and readers.[3] Gagnier et al. who developed the CARE guidelines, believed that the implementation of it by medical journals will improve the completeness and transparency of published case reports and that the systematic aggregation of information from case reports will inform clinical study design, provide early signals of effectiveness and harms, and improve healthcare delivery.[4] Case reports have been an essential component of medical education. In the era of EBM, their educational value has been questioned by many medical journal editors and publishers. In addition, case reports are a poor investment of their print page budgets because they use a disproportionately large number of pages for their rare citations (used in impact factor calculations) compared with the citations received for evidence-based original and review articles. Before the late 1990s, modern medicine was abandoning the case report as EBM was being more widely funded and supported by academicians. However, it became clear that EBM required more qualitative research and case reports to provide clinical context. The case report was reborn, but the same issues of educational value and page budget/citation existed. Individual journals each developed their own methods of dealing with this problem, with some shortening the reports and others limiting their publication to only the most novel cases.[5] There is renewed interest of some journals to republish case reports with reference to the Journal of Cutaneous Medicine and Surgery which provides an attractive platform for publishing case reports.[6] In another study, it was found that the scope and importance of clinical case reporting guidelines development were illustrated in different complementary and alternative medicine therapies.[7] Guidelines have also been developed for adverse-event case reports,[8] and all authors should be familiar with the Committee On Publication Ethics[9] and the Enhancing Quality and Transparency of Health Research Network.[10] Readers of SJA recognized the importance of unique and novel case reports as a valuable source of information and education. Our journal still accepting peer-reviewed case reports but with conditions. It should be novel case report not published before and has a clear message for the readers. To our surprise, the statement on obtaining the patient consent on publishing the case report in SJA scored the lowest percentages among the different subordinate items of the CARE checklist. That opt us to further reinforce on that item in the platform of CARE checklist and focus on its presence in the future publications. The limitation of our report is that it did not incorporate a comparison with other journals publishing peer-reviewed case reports.
Conclusion
We believe that the CARE guidelines can provide a framework for the authors to follow in writing their case reports and for the editors to ensure the completeness and readiness of the peer-reviewed case reports for publication. For the SJA, we have to apply the CARE checklist and to ensure all subordinate items are completed and adapted including the patient's perspective subordinate item and to make sure that the consent form obtained from patients and accompanied each submitted case report to the journal office.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rison RA, Kidd MR, Koch CA. The CARE (CAse REport) guidelines and the standardization of case reports. J Med Case Rep. 2013;7:261. doi: 10.1186/1752-1947-7-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An GH, Tang XT, Chen YL, Zhao Y. Reporting characteristics of case reports of acupuncture therapy with CARE guidelines. Chin J Integr Med. 2018;24:56–63. doi: 10.1007/s11655-017-2902-1. [DOI] [PubMed] [Google Scholar]
- 3.Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: Explanation and elaboration document. J Clin Epidemiol. 2017;89:218–35. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, et al. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2:38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akers KG. New journals for publishing medical case reports. J Med Libr Assoc. 2016;104:146–9. doi: 10.3163/1536-5050.104.2.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber K. 2018-changing our publication policies to emphasize value of case reports/2018-modifier nos politiques de publication pour souligner la valeur des rapports de cas. J Cutan Med Surg. 2018;22:12–3. doi: 10.1177/1203475417749595. [DOI] [PubMed] [Google Scholar]
- 7.van Haselen RA. Towards improving the reporting quality of clinical case reports in complementary medicine: Assessing and illustrating the need for guideline development. Complement Ther Med. 2015;23:141–8. doi: 10.1016/j.ctim.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Kelly WN, Arellano FM, Barnes J, Bergman U, Edwards IR, Fernandez AM, et al. Guidelines for submitting adverse event reports for publication. Pharmacoepidemiol Drug Saf. 2007;16:581–7. doi: 10.1002/pds.1399. [DOI] [PubMed] [Google Scholar]
- 9.COPE. Promoting Integrity in Research Publication. [Last Retrieved on 2018 Mar 07]. Available from: http://www.publicationethics.org/
- 10.EQUATOR Network. [Last Retrieved on 2018 Mar 07]. Available from: http://www.equator-network.org .


