Abstract
Branched-chain amino acids increase the brain perfusion of patients with hepatic encephalopathy (HE), but the amino acid and the mechanisms involved are still unknown. This study compared brain perfusion and clinical improvement during leucine or isoleucine supplementation. After randomization, 27 subjects with cirrhosis and HE received leucine or isoleucine supplements for one year. Brain single Photon Emission Computed Tomography (SPECT) and dynamic brain scintigraphy (DBS) were performed pretreatment and at 1, 8 and 12 months of supplementation. Brain perfusion was increased only in the isoleucine group at 8 months of treatment by both SPECT and DBS (p < 0.001 and p = 0.05, respectively) and by SPECT at the 12th month (p < 0.05). This was associated with hepatic encephalopathy improvement at 8 and 12 months (p = 0.008 and 0.004, respectively), which was not observed in the leucine group (p = 0.313 and 0.055, respectively). Isoleucine supplementation achieved a better impact on brain perfusion restoration in HE.
Abbreviations: AC, arm circumference; APMT, adductor pollicis muscle thickness; BCAA, branched-chain amino acids; BCKA, branched-chain ketoacids; BMI, body mass index; CAMA, corrected mid-arm muscle area; CBF, cerebral blood flow; EEG, electroencephalogram; FDR, false discovery rate; GDH, glutamate dehydrogenase; GLN, glutamine; GLU, glutamate; HE, hepatic encephalopathy; HGS, handgrip strength; HPLC, high-performance liquid chromatography; HRQoL, health-related quality of life; MAMC, mid-arm muscle circumference; MELD, Model of End-Stage Liver Disease; NH3, ammonia; PDH, pyruvate dehydrogenase complex; ROIs, regions of interest; ROS, reactive oxygen species; SF-36, 36-item Short-Form General Health Survey; SPECT, Single Photon Emission Computed Tomography; SPM12, Statistical Parametrical Mapping 12; TCA, tricarboxylic acid; TSF, triceps skinfold; α-KG, α-ketoglutarate; αKGDH, α-ketoglutarate dehydrogenase complex
Keywords: Hepatic encephalopathy, Liver cirrhosis, Branched-chain amino acids, Cerebral blood flow
Highlights
-
•
The cerebral effect of BCAA on hepatic encephalopathy is unknown.
-
•
Isoleucine increased brain perfusion and improved the hepatic encephalopathy grade.
-
•
The hypothesized mechanism involves the energy metabolism of astrocytic mitochondria.
-
•
The mechanism of manganese accumulation in the brain of such patients is hypothesized.
1. Introduction
Hepatic encephalopathy (HE) has a harmful impact on chronic and acute liver illnesses and affects more than one third of patients with liver cirrhosis. After overt episodes, the one-year mortality of these patients varies from 42 to 64%, which is even worse than the values attributed to other complications (Bustamante et al., 1999; Jepsen et al., 2010; Stewart et al., 2007). Hyperammonemia is the chief disturbance in HE physiopathology, when the conversion of ammonia to glutamine (GLN) is fully activated in extrahepatic tissues, thus increasing the influx of intracellular water into astrocytes due to GLN osmoregulation (Brusilow et al., 2010). Furthermore, the high permeability of the blood-brain barrier facilitates the influx of ammonia to the brain under inflammatory conditions (Alonso et al., 2014). For practical purposes, in this article the word ammonia is used to represent ammonia free base (NH3) plus ammonium (NH4+).
HE treatment is based on controlling the trigger factors and managing ammonia production and absorption from the gut, by using disaccharides or antibiotics. Since many patients do not achieve sufficient improvement, branched-chain amino acids (BCAA) and drugs aiming to increase ammonia conversion to urea are often added as adjuvant therapies.
Among all these options, BCAA (leucine, isoleucine and valine) have the least understood mechanism of action. The efficacy of these substances in HE treatment is well established from systematic reviews and meta-analyses (Gluud et al., 2015). Even so, the amount of BCAA and the proportion of each amino acid that patients should receive is still a matter of controversy, because all clinical trials until now used them together, making it impossible to analyze the influence of each one on HE manifestations. Of note, BCAA are not only linked to HE improvement but also to increased cerebral perfusion (Iwasa et al., 2003).
The key disturbance in aminoacidemia related to HE is ammonia accumulation, which is present in >80% of patients according to the diagnostic methods adopted (Al Sibae and McGuire, 2009). The main ammonia source in cirrhotic patients is the intestinal catabolism of GLN from the blood, in addition to a lesser content produced by intestinal bacteria (Olde Damink et al., 2002; Weber Jr and Veach, 1979). In normal adults, the daily amount produced is approximately 1000 mmol (Walker, 2014). When the conversion of ammonia to urea is insufficient, hyperammonemia increases GLN production in skeletal muscles and the brain (Lockwood et al., 1979). Since most patients with cirrhosis present moderate to severe malnutrition and loss of muscle mass, the high levels of circulating ammonia will increase glutamine production in the brain, thereby worsening HE manifestations (Merli et al., 2013; Romeiro and Augusti, 2015).
The chief extrahepatic detoxifying process in mammals is the activity of glutamine synthetase (Cooper, 2012), which begins with the conversion of glutamate (GLU) and ammonia into GLN (Eq. (1)). This reaction requires GLU provided from BCAA catabolism in skeletal muscles, where BCAA and α-ketoglutarate (α-KG) can be converted to GLU and branched-chain ketoacids (Eq. (2)) (Holecek, 2015).
| (1) |
| (2) |
Hyperammonemia decreases extracellular BCAA and increases the release of branched-chain ketoacids from the muscle (Holecek et al., 2011). The reversible nature of these reactions makes the concentrations of substrates and products the main regulators of these processes (Holecek et al., 2011). Consequently, the patients would have high levels of ammonia, GLN and branched-chain ketoacids, but lower levels of BCAA in the extracellular fluid.
BCAA deficiency has a large impact on the tricarboxylic acid (TCA) cycle, because leucine and valine are involved in the generation of Acetyl-CoA and Succinyl-CoA, respectively, whereas isoleucine is the only amino acid that generates both of these products (Fig. 1). Therefore, the BCAA deficiency can lead to an impaired TCA cycle.
Fig. 1.
Schematic representation of relevant metabolic reactions related to hyperammonemia and branched-chain amino acids. A = Pyruvate dehydrogenase, B = α-ketoglutarate dehydrogenase, C = glutamate dehydrogenase (the predominant reaction is the formation of α-ketoglutarate), D = glutamine synthetase, E = phosphate-activated glutaminase, F = pyruvate carboxylase, (→→→) = multiple steps, (-------) = mitochondrial membrane. Some of the most important steps related to this study are shown in the gray rectangle.
The effects of BCAA in the context of HE have been extensively studied (Bak et al., 2013; Holecek, 2015; Les et al., 2011; Yamamoto et al., 2005). Nevertheless, there is no standardization as to BCAA doses. In general, leucine, isoleucine and valine are administered together and in different proportions (Les et al., 2011; Marchesini et al., 2003). As a result, the appropriate amount of each amino acid to be prescribed is still unknown, but leucine and isoleucine are important to the oxidative metabolism of astrocytes (Bak et al., 2013; Johansen et al., 2007; Murin et al., 2009; Nissen et al., 2015).
The present study hypothesizes that the long-term effects of isoleucine leads to a higher increase in cerebral perfusion than that obtained by leucine, thus promoting different degrees of recovery from HE according to the amino acid received by the subjects. Hence, the study was focused on brain areas that are known for being more affected by hypoperfusion, such as the basal ganglia (Bizzi et al., 1996; Kumar et al., 1991; Sims and Pulsinelli, 1987), as well as regions where altered perfusion was already reported in HE and/or acquired hepatocerebral degeneration, such as the hippocampus, prefrontal cortex, medial temporal cortex, anterior cingulate cortex and parietooccipital regions (Lockwood et al., 1993; O'Carroll et al., 1991; Sunil et al., 2012; Ueki et al., 2002; Zafiris et al., 2004). Perfusion alterations in some of these regions were already associated with HE symptoms in prior studies, while some areas were reported as having dissimilar blood flow responses after BCAA administration (Catafau et al., 2000; Iwasa et al., 2003; Lockwood et al., 1993; O'Carroll et al., 1991; Ueki et al., 2002). Therefore, the primary aim of this study was to evaluate the effects of leucine and isoleucine supplementation on cerebral perfusion in patients with persistent HE. Secondary aims were to assess HE grade, body composition measurements and quality of life. The assessments were performed at 1, 8 and 12 months of treatment, and the serum amino acid levels were measured before and after the trial.
2. Materials and methods
Patients aged >18 years with cirrhosis and persistent HE who attended the Hepatology units at UNESP Hospital (Botucatu, São Paulo state, Brazil) from 2014 to 2015 were invited to participate. Patients with other neurological diseases, hepatocellular carcinoma, prior liver transplantation or acute-on-chronic liver failure were excluded, as well as those who were already taking BCAA or had started/changed medications for HE treatment in the last week before entering the trial. Thus, only patients taking the same medications and doses for at least one week were included. The study was approved by the Comitê de Ética em Pesquisa (protocol number 4334/2012 at 09/03/2012) and received the Brazilian trial registration number RBR-8t4bf3. The study protocol was conducted according to the Declaration of Helsinki and its revisions. All the subjects or their caregivers signed the informed consent. When the patient was not able to sign, the signature of a family member was obtained.
Liver cirrhosis was established from a liver biopsy or from clinical and complementary exams. Clinical and nutritional evaluations were carried out before the supplementation and bimonthly afterwards, including anthropometric evaluation and handgrip strength measures.
HE was graded according to the West Haven criteria proposed by Amodio et al. and endorsed by the American and European Guidelines (Amodio et al., 2004; Vilstrup et al., 2014a, Vilstrup et al., 2014b). Grade 0 comprised subjects without clinical signals of HE, in whom minimal HE was diagnosed according to specific exams (electroencephalogram). Grades I and II were categorized according to the current Guidelines (Vilstrup et al., 2014a, Vilstrup et al., 2014b), thus classifying each neurological finding into a specific form. Since only outpatients were recruited, those with HE Grades III and IV were not included.
2.1. Sample size calculation and randomization plan
In a similar study, the brain perfusion standard deviation in the parietal lobe was 14% and increased by 20% after BCAA administration (Iwasa et al., 2003). Considering these same values, with respective alpha and beta errors of 0.05 and 0.2, the sample size required to find significant differences between the groups would be 16 subjects.
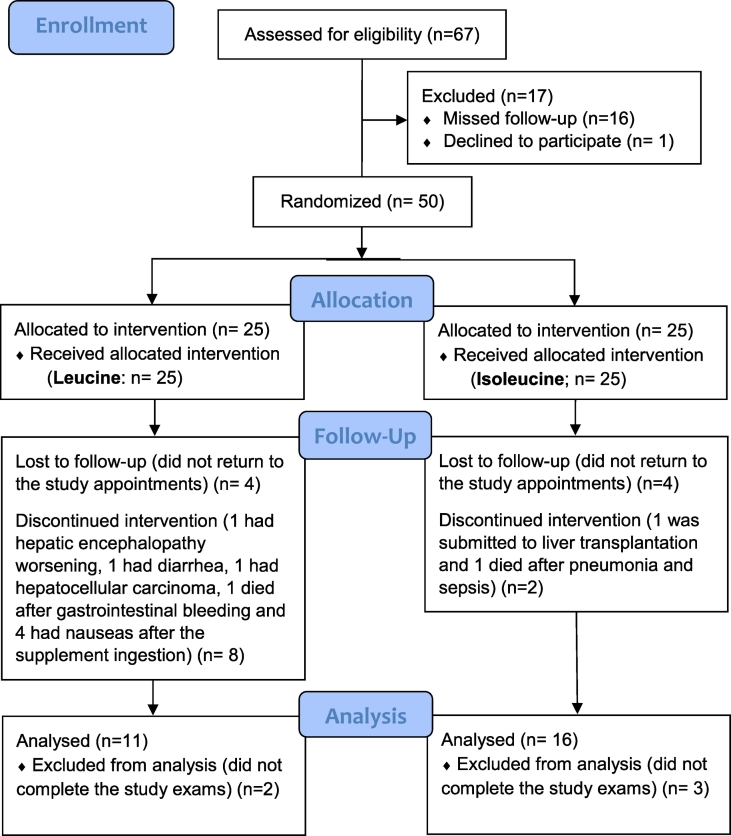
The subjects were randomized to participate in one of two groups. Additional information is included as Supplementary material. A control group under placebo treatment was not included due to ethical concerns, in agreement with prior recommendations (Als-Nielsen et al., 2004; Romeiro et al., 2013). The flow diagram of the patients included is shown in Fig. 2.
Fig. 2.
Flow diagram of the subjects.
Each packet contained 5.0 g of maltodextrin, 1.8 g of maltitol and 1.2 g of flavoring plus 10.0 g of the amino acid, which was l-leucine or l-isoleucine according to the subjects' randomization (Ajinomoto® and Basecol Mix Indústria e Comércio de Alimentos LTDA, Brazil).
After starting the supplementation, the suggested schedule was to take 3 packets daily mixed in 200 mL of juice or dairy drinks ingested during breakfast, lunch and an evening snack for 12 months. BCAA doses of 30 g were previously given daily to HE patients by Les et al. (2011). Each subject was reevaluated every other month, when the adherence to treatment and the remaining packets were checked. Monthly phone calls were made to assure that the subjects were taking the supplement according to the trial schedule.
Before the amino-acid supplementation, the subjects were submitted to clinical exams, anthropometric assessment, handgrip strength measures, brain SPECT, dynamic brain scintigraphy, electroencephalogram and lab tests. The clinical exam was focused on grading HE and ascites. Laboratory exams included venous ammonia and the tests needed to calculate both the Model of End Stage Liver Disease score and the Child-Pugh classification.
2.2. Electroencephalogram
Electroencephalogram exams were used to diagnose minimal hepatic encephalopathy. Acquisition information is included as Supplementary Material.
2.3. Single Photon Emission Computed Tomography (SPECT)
The subject was kept in a supine position and free from audiovisual stimuli for at least 20 min before performing the brain SPECT acquisitions. Caffeine, alcohol and stimulants were not allowed starting on the day before the exam. Each patient received 1110 MBq (30 mCi) of 99mTc-bicisate ethyl cysteinate dimer through a peripheral vein one hour before the acquisition. A dual-headed gamma camera (Millennium MG system, General Electric, UK) was employed to acquire the images.
The photopeak was centered at 140 keV with 20% energy using low-energy and high-resolution collimators. The acquisition was made in a 128 × 128 matrix with voxel sizes of 2.26 × 2.26 × 2.26 mm3, thus resulting in a resolution of 0.44 pixels/mm over 360° rotation performing 65 frames of 25 s each. A fifth-order Butterworth high-pass filter with cutoff frequency of 0.5 cycles/min was applied to perform the filtered backprojection reconstruction (Morano and Seibyl, 2003; Sunil et al., 2012). A specialist who was unaware of the supplement given performed the SPECT analysis (details included as Supplementary material).
2.4. Dynamic brain scintigraphy
Dynamic brain scintigraphy was also acquired with the patients in a supine position. Immediately before the acquisition, the subjects received 1110 MBq (30 mCi) of 99mTc through a peripheral vein. Images including head, neck and proximal thorax were acquired by a dual-headed gamma camera (Millennium MG system, General Electric, United Kingdom) equipped with low-energy high-resolution photopeak centered at 140 keV and 20% energy. Acquisition information is included as Supplementary material.
2.5. Nutritional assessment and quality-of-life survey
Nutritional status was evaluated through anthropometric measures and handgrip strength. Quality of life was assessed using the Medical Outcomes Study 36-item Short-Form General Health Survey (SF-36) validated for Portuguese. Additional information is included as Supplementary material.
2.6. Amino-acid dosages
Serum levels of leucine and isoleucine from the subjects before and after the trial were obtained by high-performance liquid chromatography (SCL-10Avp control system, RF-10AXL fluorescence detector, Shimadzu, Japan). Chromatographic separation was achieved in a Shim-pack ISC-07/S1504 Na type sulfone group analysis column (4 mm × 150 mm i.d., 7 μm, Shimadzu, JPN), which was operated at 25 °C. A Shim-pack ISC-30/S0504 ammonia trap column was also used (4 mm × 50 mm i.d.) for suppressing baseline fluctuations that are often experienced in amino acid analysis. Additional information is included as Supplementary material.
2.7. Statistical analysis
Descriptive analysis was presented as median and interquartile ranges. Pretreatment comparisons between the groups were assessed by Mann-Whitney U test, whereas the individual results throughout the trial were evaluated by the Wilcoxon U test. The software Sigma Stat 3.5 (Systat Software Inc., San Jose, CA, USA) for Windows was used for the analysis.
Brain perfusion was assessed via the software SPM 12. The paired t-test was used to compare acquisitions at 1, 8 and 12 months of treatment with those before the supplementation. The anatomic visualization of the brain perfusion results from all patients of each group was obtained by a t statistic image, which was constructed and projected onto a standard high-resolution T1-weighted Magnetic Resonance Image (Song et al., 2014). The statistical significance level was defined as p < 0.05 for all the assays. For the SPECT comparisons throughout time, the p value was corrected using the false discovery rate (FDR), and some additional analyses were performed with p < 0.001.
3. Results
The subjects' initial characteristics did not differ between the groups. The number of male/female subjects was 8/3 in the leucine group and 12/4 in the isoleucine group (p = 0.152). The mean age was 61 years (52.5–65.7) in the leucine group and 57 years (52.0–62.0) in the isoleucine group (p = 0.767). Child-Pugh and MELD scores in the leucine and isoleucine groups were 8.00 (6.00–10.0) and 12.0 (8.50–15.5) in the former and 7.0 (6.5–8.5) and 11.0 (9.5–12.5) in the latter group, respectively (p = 0.218 and 0.535). The HE grade was 1 (0–1) in both groups (p = 0.909). Seven subjects in the leucine group and nine in the isoleucine group had minimal HE. Four subjects in the leucine group and six in the isoleucine group presented Grade I HE. One subject in the isoleucine group had Grade II HE.
In the SPECT analysis, there were no differences in cerebral perfusion after 1 month of treatment; the results obtained at 8 and 12 months of treatment were not significantly different from the baseline for patients receiving leucine supplements (data not shown). In contrast, a significant increase in cerebral perfusion was registered in the isoleucine group at 8 months of supplementation (p < 0.001) (Fig. 3A).
Fig. 3.
Voxel-wise paired t-test comparison of cerebral perfusion between baseline and 8 months (A) and between baseline and 12 months (B) of isoleucine supplementation. Differences observed by brain SPECT are shown as color-coded regions in a total of 346 mm3 for 8 months (hot color scale, threshold p < 0.001 uncorrected) and 48 mm3 for 12 months (cold color scale, threshold p < 0.05 uncorrected). In the first row, results are overlaid in an anatomical MRI rendered in the axial orientation from inferior to superior and neurological orientation (right on right). In the second row, the results are overlaid in a tridimensional rendered model of an inflated brain (orientations are described below the renderings in panel B).
The main clusters of increased perfusion at 8 months were localized at: the right temporal lobe/inferior temporal gyrus (516 mm3, p FDR corrected = 0.028, T value = 6.78, maximal value at x = 63 y = − 40 z = −34 Montreal Neurological Institute coordinates); right cerebral white matter mainly involving the pre-central gyrus region (1356 mm3, p FDR corrected <0.0001, T value = 5.46, x = 21 y = −19 z = 50); left cerebral white matter primarily involving the supplementary motor cortex, superior frontal gyrus, pre-central gyrus, middle cingulate gyrus and pre-central gyrus medial segment (8265 mm3, p FDR corrected <0.0001, T value = 3.80, x = −18 y = −13 z = 47); deep right cerebral white matter mainly involving the caudate, putamen, thalamus and pallidum (2181 mm3, p FDR corrected <0.0001, T value = 3.52, x = 21 y = −4 z = 14).
This significant effect of isoleucine was also documented at 12 months of treatment. The difference at this time was less prominent when compared to the previous results, as displayed in Fig. 3B (p = 0.05). The main clusters of increased perfusion were localized at: the left cerebral white matter primarily involving the caudate and anterior cingulate gyrus (1119 mm3, p uncorrected = 0.006, T value = 2.49, maximal value at x = −18 y = 20 z = 20), right cerebral white matter (1983 mm3, p uncorrected = 0.007, T value = 2.78, x = 21 y = 35 z = 2) and left cerebral posterior white matter mainly involving the thalamus, middle and posterior cingulate gyrus (744 mm3, p uncorrected = 0.009, T value = 2.35, x = −21 y = −40 z = 17).
To analyze the brain scintigraphy, the slope of the ascending curves of carotids and brain hemispheres were measured in order to quantify the cerebral blood flow. Again, the results obtained at the first month did not differ from those before the trial, and the results obtained at 8 and 12 months of treatment were not significantly different from the baseline for patients receiving leucine supplements (data not shown). In contrast, a clear enhancement of cerebral blood flow at 8 months of supplementation was observed in the isoleucine group, which was shown by the increase of slope curves of both hemispheres. However, it did not reach the significance level at the 12th month (Table 1).
Table 1.
Dynamic brain scintigraphy analyses comparing the slope of the ascending curves obtained from carotids and brain hemispheres. The comparisons were performed between pretreatment values and the results at 8 and 12 months of supplementation.
| Amino acid |
|||||||
|---|---|---|---|---|---|---|---|
| Leucine group (n = 11) |
Isoleucine group (n = 16) |
||||||
| Pretreatment | 8 months | 12 months | Pretreatment | 8 months | 12 months | ||
| CA | R | 72.8 (67.5–73.4) | 76.6 (61.1–79.2) | 69.4 (57.4–73.5) | 72.0 (62.9–77.0) | 75.5 (71.2–81.5) | 71.9 (60.2–79.6) |
| L | 71.2 (58.7–78.8) | 72.8 (58.5–75.9) | 63.7 (59.9–73.5) | 66.5 (54.6–75.2) | 70.8 (57.7–77.9) | 71.5 (55.9–80.1) | |
| BH | R | 59.9 (41.1–65.3) | 60.9 (46.5–63.8) | 52.8 (39.8–57.7) | 50.2 (38.8–61.6) | 62.8 (48.7–75.4)⁎ | 56.4 (30.8–65.3) |
| L | 60.6 (39.8–64.1) | 56.1 (47.2–63.4) | 55.9 (35.2–61.6) | 49.2 (30.8–56.7) | 59.8 (44.9–73.8)# | 52.0 (40.7–66.2) | |
CA = carotids; BH = brain hemispheres; R = right; L = left. Data are expressed as median (1st quartile–3rd quartile).
p = 0.05.
p < 0.029.
The clinical evaluation findings at 8 and 12 months are described in Table 2. There were no significant changes in the Model of End Stage Liver Disease score during the trial. Reductions in Child-Pugh classification and HE grade did not achieve the significance level in the leucine group. In contrast, both Child-Pugh classification points and HE grade decreased at 8 and 12 months in the isoleucine group. Of note, ammonia levels did not change significantly during the trial.
Table 2.
Clinical evaluation findings and laboratory tests comparing pretreatment values with the results at 8 and 12 months of supplementation.
| Variables | Amino acid |
|||||
|---|---|---|---|---|---|---|
| Leucine group (n = 11) |
Isoleucine group (n = 16) |
|||||
| Pretreatment | 8 months | 12 months | Pretreatment | 8 months | 12 months | |
| MELD | 12.0 (8.50–15.5) | 11.0 (8.00–12.8) | 12.0 (8.00–12.8) | 11.0 (9.50–12.5) | 11.5 (9.50–13.0) | 12.0 (10.5–13.5) |
| Child-Pugh | 8.00 (6.00–10.0) | 7.00 (5.25–7.75) | 7.00 (6.25–7.75) | 7.00 (6.50–8.50) | 6.00 (5.00–6.50)⁎ | 6.00 (5.00–7.50) |
| HE grade | 1.00 (0.00–1.00) | 0.00 (0.00–1.00) | 0.00 (0.00–0.00)§ | 1.00 (0.00–1.00) | 0.00 (0.00–0.00)§§ | 0.00 (0.00–0.00)§§§ |
| Ammonia (μmol/L) | 52.0 (27.8–78.2) | 66.5 (44.0–94.0) | 77.0 (46.5–114) | 58.0 (26.5–95.5) | 55.0 (13.8–94.5) | 34.0 (20.8–66.0) |
MELD = model for end-stage liver disease; Child-Pugh = Child-Pugh classification; HE = hepatic encephalopathy. Data are expressed as median (1st quartile–3rd quartile). The upper normal limit for plasma ammonia levels is 35 μmol/L.
p < 0.001.
p = 0.055.
p = 0.008.
p = 0.004.
Body assessment measures are shown in Table 3. For most nutritional variables, there were no significant differences between pretreatment values and those measured at the 8th and 12th months, whereas triceps skinfold and handgrip strength increased significantly in both groups. Quality of life survey results are displayed in the Supplementary Material.
Table 3.
Body assessment measures comparing pretreatment values with the results at 8 and 12 months of supplementation.
| Variables | Amino acid |
|||||
|---|---|---|---|---|---|---|
| Leucine group (n = 11) |
Isoleucine group (n = 16) |
|||||
| Pretreatment | 8 months | 12 months | Pretreatment | 8 months | 12 months | |
| Weight (kg) | 82.2 (69.6–90.0) | 92.4 (78.8–95.4) | 91.6 (80.4–99.8) | 69.7 (59.8–77.7) | 78.0 (69.4–87.3) | 75.6 (69.8–86.7) |
| BMI (kg/m2) | 30.1 (25.1–34.5) | 31.5 (29.1–36.9) | 31.6 (30.3–36.8) | 26.0 (24.9–28.6) | 29.7 (26.9–32.3) | 29.4 (27.4–31.3) |
| AC (cm) | 30.0 (26.8–34.5) | 34.0 (30.2–36.5) | 32.5 (29.5–35.8) | 30.2 (26.6–32.2) | 31.0 (28.4–33.9) | 31.8 (27.0–32.9) |
| TSF (mm) | 17.0 (10.0–19.8) | 25.0 (22.0–32.5)⁎ | 27.0 (20.8–33.6)⁎ | 16.5 (8.50–21.0) | 22.0 (17.0–30.0)⁎ | 23.5 (15.0–28.5)⁎ |
| MAMC (cm) | 25.7 (22.0–27.9) | 24.2 (22.2–27.4) | 23.9 (22.7–25.4) | 25.5 (23.9–27.0) | 23.7 (21.5–25.8) | 23.9 (22.4–25.0) |
| CAMA (cm2) | 42.5 (30.4–53.5) | 39.9 (29.2–49.6) | 36.4 (31.2–41.9) | 41.9 (35.3–48.3) | 34.7 (26.8–43.2) | 35.4 (29.8–40.6) |
| APMT (mm) | 6.00 (4.50–9.00) | 8.00 (6.50–9.00) | 9.00 (7.00–10.0) | 5.50 (4.75–7.25) | 7.00 (5.00–9.00) | 7.00 (6.00–8.00) |
| HGS (kg) | 18.0 (16.5–22.0) | 28.0 (22.0–38.8)§ | 28.0 (20.5–41.5)§§ | 25.5 (18.0–28.5) | 33.0 (26.5–40.0)§§§ | 32.0 (26.5–41.0)§§ |
BMI = body mass index; AC = arm circumference; TSF = triceps skinfold; MAMC = mid-arm muscle circumference; CAMA = correct mid-arm muscle area; APMT = adductor pollicis muscle thickness; HGS = handgrip strength; kg = kilogram, kg/m2 = kilogram per square meter, cm = centimeter, mm = millimeter. Data are expressed as median (1st quartile–3rd quartile).
p ≤ 0.001.
p = 0.010.
p = 0.002.
p = 0.001.
Serum level analysis from subjects under leucine supplementation demonstrated that leucine levels increased from 34.2 nmol/L (23.1–45.5) to 63.0 nmol/L (30.2–120) at 12 months of treatment (p = 0.049). In contrast, isoleucine levels changed from 21.2 nmol/L (13.8–22.4) to 21.7 nmol/L (10.9–89.3) in this group (non-significant difference). At this same time, patients under isoleucine supplementation were evidencing a marked increase of both serum amino acids: levels of leucine increased from 27.9 nmol/L (19.9–37.0) to 40.1 nmol/L (27.2–58.9) (p = 0.030) while those of isoleucine increased from 14.4 nmol/L (11.3–20.7) to 88.5 nmol/L (20.8–207) (p = 0.003).
4. Discussion
The results of this trial suggest that hyperammonemia has a large impact on cerebral energy metabolism, in agreement with previous studies presented by Bessman and Bessman (1955); Cooper and Plum (1987); Lai and Cooper (1986); Ott and Vilstrup (2014). Furthermore, in the sample evaluated, isoleucine was able to reduce HE manifestations. The data demonstrate an increase of serum leucine levels at 12 months of supplementation in both groups, accompanied by gains in body assessment measures such as triceps skinfold after 8 and 12 months of supplementation.
Ammonia detoxification inside astrocytes causes an important influx of water to these glial cells due to GLN accumulation and the osmotic effect of this amino acid (Cichoz-Lach and Michalak, 2013). Concurrently, ammonia metabolism in muscle cells seems to be linked to strength loss, which is proportional to HE severity (Augusti et al., 2016; Merli et al., 2013). Thus, the results of strength restoration, triceps skinfold augmentation and HE grading improvement during isoleucine supplementation are inexplicable without considering a significant effect of BCAA on energy metabolism, thus decreasing cytotoxic brain edema and restoring muscle strength.
Taken together, these findings on muscular and astrocytic metabolism suggest that ammonia toxicity is related to a disruption in the cellular mechanisms involved in energy metabolism, especially in the brain. Glutamate dehydrogenase (GDH) activity depends on the amount of α-KG and energy from NAD/NADP provided by the TCA cycle, whereas glutamine synthetase activity requires ATP. However, excessive ammonia inhibits the α-ketoglutarate dehydrogenase complex (αKGDH) in this cycle (Ott and Vilstrup, 2014), shifting α-KG to GLN production and decreasing the α-KG oxidation to SuccinylCoA and CO2, thus contributing to curbing aerobic energy production (Lai and Cooper, 1986) (Eq. (3)).
| (3) |
Another piece of evidence that energy metabolism is the key to understanding how leucine and isoleucine can decrease HE manifestations is the formation of α-KG, which is produced when isocitrate is oxidized to α-KG and CO2 (Eqs. (4), (5)), a step that requires the bonding of a manganese ion to the carbonyl group before the decarboxylation. Thus, αKGDH inhibition by hyperammonemia leads not only to GLN accumulation but also to an interruption in the TCA cycle at α-KG formation (Fig. 1). Therefore, in advanced liver disease, both the high ammonia levels and the lack of BCAA may act together to impair α-KG production from oxalosuccinate (Eq. (5)). In tissues that depend on aerobic processes to obtain energy, such as brain cells, the reduced α-KG formation supposedly decreases the manganese uptake in this process. Consequently, the remaining manganese may accumulate in regions such as basal nuclei and cortical areas, a common finding in patients with HE and acquired hepatocerebral degeneration.
| (4) |
| (5) |
As shown in Fig. 1, αKGDH inhibition is an additional cause of oxidative metabolism impairment in organs that depend on aerobic processes to obtain energy, such as the brain (Bak et al., 2013; Cooper and Plum, 1987; Lai and Cooper, 1986), thus impairing ATP generation from the TCA cycle. Despite the fact that GLN accumulation in astrocytes causes cytotoxic edema due to the osmotic effect of GLN (Brusilow et al., 2010; Parekh and Balart, 2015), it may be insufficient to account for the astrocytic swelling or the manganese deposits in HE, which only could be explained by additional disturbances, such as metabolic impairment and oxidative stress.
Even though brain perfusion control is not fully understood, there are important reports showing that cerebral blood flow (CBF) is regulated by astrocytes according to brain metabolism by controlling vasoconstriction and vasodilation (Belanger et al., 2011; Carmignoto and Gomez-Gonzalo, 2010). The GLU release by neurons raises intracellular Ca+2 in astrocytes, which activates the production of arachidonic acid and vasodilators such as prostaglandins and epoxyeicosatrienoic acids. On the other hand, arachidonic acid can be converted to 20-hydroxyeicosatetraenoic acid in arteriolar smooth muscle, resulting in vasoconstriction. Therefore, the direction toward vasodilation or vasoconstriction in the brain is mediated by metabolic conditions (Belanger et al., 2011; Carmignoto and Gomez-Gonzalo, 2010).
The findings obtained in this trial suggest that astrocytic dysfunction is probably the cause of the cerebral perfusion changes observed in HE. In the SPECT analysis, an augmentation of cerebral perfusion was documented after isoleucine supplementation at 8 and 12 months of treatment as shown in Fig. 3. The slope of the ascending curves obtained from brain hemispheres, depicting the CBF rise, constituted additional evidence of blood flow improvement at 8 months of isoleucine supplementation (Table 1). Cytotoxic edema recovery seems to constitute a reasonable explanation for the increased perfusion found at 8 and 12 months of treatment in the isoleucine group. However, the CBF improvement observed was greater at 8 months than at 12 months of treatment (Fig. 3, Table 1).
The imaging technique used in this work was the same employed by Iwasa et al. (2003), who also reported a CBF improvement in patients with cirrhosis under BCAA supplementation. Nevertheless, given that all BCAA (valine, leucine and isoleucine) were administered together in this previous study, the role of each one in this effect on cerebral perfusion was difficult to distinguish. In this present trial, long-term isoleucine supplementation has led to significant changes in cerebral perfusion, mainly in the white matter. Involvement of the cingulate gyrus and basal ganglia was observed as well. These brain areas were previously reported as being associated with HE symptoms, such as cognitive impairment, lack of attention, memory loss and deficits in visual-spatial perception (Catafau et al., 2000; Lockwood et al., 1993; O'Carroll et al., 1991).
The reason that the results from isoleucine supplementation were better than those obtained from leucine can only be understood by analyzing the effects of ammonia on the TCA cycle. Isoleucine and leucine can both be converted to Acetyl-CoA, but isoleucine is also converted to Succinyl-CoA, providing additional carbon to this cycle at the step when αKGDH is inhibited by ammonia (Bak et al., 2013; Holecek, 2010; Nissen et al., 2015; Ott et al., 2005). Due to this unique property of isoleucine, it can serve as an ideal substrate for maintaining TCA as a source of energy, even in the presence of hyperammonemia, by reestablishing oxaloacetate to form citrate from Acetyl-CoA (Johansen et al., 2007). Therefore, BCAA supplementation is capable of restoring the oxidative metabolism in neural cells, especially when a larger amount of isoleucine is administered, because it can reduce the disruption in ion transportation through the cell membranes, thus maintaining the osmotic regulation and probably reducing the cytotoxic edema observed in HE.
In addition to its role in TCA cycle maintenance, isoleucine is also involved in ammonia detoxification when α-KGDH is inhibited (Ott et al., 2005). This additional role of isoleucine might reduce the GLN concentration in astrocytes, thereby avoiding the cytotoxic edema. The evidence supporting this property of isoleucine is that the subjects who received isoleucine presented elevated serum levels of both isoleucine and leucine. On the other hand, leucine supplementation led to an increase only in this same amino-acid serum level, probably due to the fact that leucine supplies Acetyl-CoA only for the TCA cycle, which requires isoleucine to provide Succinyl-CoA and keep the cycle running (Fig. 1) (Johansen et al., 2007; Ott et al., 2005).
Both leucine and isoleucine supplementations improved the quality of life related to physical functioning, most likely by restoring energy generation and strength, as shown by the handgrip strength elevation in both groups, indicating that some symptoms were ameliorated. Although the increase in cerebral perfusion was more pronounced at 8 months, the clinical improvement was maintained until the end of the trial and the subjects did not suffer HE deterioration. Therefore, the increase obtained in cerebral perfusion may be a compensatory mechanism induced by isoleucine in the first months, leading to a partial restoration in the metabolic coupling between cerebral regions. The results suggest that this mechanism was activated to a lesser degree when the subjects achieved improvement in their HE manifestations, a hypothesis in line with relevant studies on this issue (Catafau et al., 2000; Zafiris et al., 2004). Last but not least, four subjects in the leucine group exited the trial because they had experienced nausea and vomiting. The lack of such terminations in the isoleucine group leads to the suspicion that isoleucine preparations may be more palatable than leucine preparations.
In conclusion, the results of this double-blind randomized clinical trial showed that isoleucine supplementation facilitates the achievement of a better impact on brain perfusion and HE grade than that obtained by leucine, suggesting that patients with cirrhosis and HE should receive supplements enriched with a higher level of isoleucine than leucine.
Conflict of interest
None.
Acknowledgements
The study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Capes, Pró-Reitoria de Pesquisa – PROPe/UNESP and the São Paulo Research Foundation – FAPESP grant numbers 2013/11761-2, 2013/15121-8, 2014/22572-9 and 2016/07117-9. The authors are grateful to Professor Eduardo Maffud Cilli and Leandro Alves dos Santos for dosing the amino acids and to James Richard Welsh for the English language editing of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.03.028.
Contributor Information
Fernando Gomes Romeiro, Email: fgromeiro@fmb.unesp.br.
Matheus Alvarez, Email: matheus@consult.med.br.
Kátia Hiromoto Koga, Email: khkoga@fmb.unesp.br.
Giovanni Faria Silva, Email: giovanni@fmb.unesp.br.
Luiz Eduardo Gomes Garcia Betting, Email: betting@fmb.unesp.br.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4
Supplementary material 5
Brain regions where the increase in cerebral perfusion was statistically significant were compared via SPECT images taken before and after 8 months of isoleucine supplementation.
Brain regions where the increase in cerebral perfusion was statistically significant were compared via SPECT images taken before and after 12 months of isoleucine supplementation.
References
- Al Sibae M.R., McGuire B.M. Current trends in the treatment of hepatic encephalopathy. Ther. Clin. Risk Manag. 2009;5:617–626. doi: 10.2147/tcrm.s4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J., Cordoba J., Rovira A. Brain magnetic resonance in hepatic encephalopathy. Semin. Ultrasound CT MR. 2014;35:136–152. doi: 10.1053/j.sult.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Als-Nielsen B., Gluud L.L., Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328:1046. doi: 10.1136/bmj.38048.506134.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio P., Montagnese S., Gatta A., Morgan M.Y. Characteristics of minimal hepatic encephalopathy. Metab. Brain Dis. 2004;19:253–267. doi: 10.1023/b:mebr.0000043975.01841.de. [DOI] [PubMed] [Google Scholar]
- Augusti L., Franzoni L.C., Santos L.A., Lima T.B., Ietsugu M.V., Koga K.H., Moriguchi S.M., Betting L.E., Caramori C.A., Silva G.F., Romeiro F.G. Lower values of handgrip strength and adductor pollicis muscle thickness are associated with hepatic encephalopathy manifestations in cirrhotic patients. Metab. Brain Dis. 2016;31:909–915. doi: 10.1007/s11011-016-9828-8. [DOI] [PubMed] [Google Scholar]
- Bak L.K., Waagepetersen H.S., Sorensen M., Ott P., Vilstrup H., Keiding S., Schousboe A. Role of branched chain amino acids in cerebral ammonia homeostasis related to hepatic encephalopathy. Metab. Brain Dis. 2013;28:209–215. doi: 10.1007/s11011-013-9381-7. [DOI] [PubMed] [Google Scholar]
- Belanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bessman S.P., Bessman A.N. The cerebral and peripheral uptake of ammonia in liver disease with an hypothesis for the mechanism of hepatic coma. J. Clin. Invest. 1955;34:622–628. doi: 10.1172/JCI103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi A., Righini A., Turner R., Le Bihan D., Bockhorst K.H., Alger J.R. Imaging focal reperfusion injury following global ischemia with diffusion-weighted magnetic resonance imaging and 1H-magnetic resonance spectroscopy. Magn. Reson. Imaging. 1996;14:581–592. doi: 10.1016/0730-725x(96)00094-x. [DOI] [PubMed] [Google Scholar]
- Brusilow S.W., Koehler R.C., Traystman R.J., Cooper A.J. Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. 2010;7:452–470. doi: 10.1016/j.nurt.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J., Rimola A., Ventura P.J., Navasa M., Cirera I., Reggiardo V., Rodes J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J. Hepatol. 1999;30:890–895. doi: 10.1016/s0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- Carmignoto G., Gomez-Gonzalo M. The contribution of astrocyte signalling to neurovascular coupling. Brain Res. Rev. 2010;63:138–148. doi: 10.1016/j.brainresrev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Catafau A.M., Kulisevsky J., Berna L., Pujol J., Martin J.C., Otermin P., Balanzo J., Carrio I. Relationship between cerebral perfusion in frontal-limbic-basal ganglia circuits and neuropsychologic impairment in patients with subclinical hepatic encephalopathy. J. Nucl. Med. 2000;41:405–410. [PubMed] [Google Scholar]
- Cichoz-Lach H., Michalak A. Current pathogenetic aspects of hepatic encephalopathy and noncirrhotic hyperammonemic encephalopathy. World J. Gastroenterol. 2013;19:26–34. doi: 10.3748/wjg.v19.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A.J. The role of glutamine synthetase and glutamate dehydrogenase in cerebral ammonia homeostasis. Neurochem. Res. 2012;37:2439–2455. doi: 10.1007/s11064-012-0803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A.J., Plum F. Biochemistry and physiology of brain ammonia. Physiol. Rev. 1987;67:440–519. doi: 10.1152/physrev.1987.67.2.440. [DOI] [PubMed] [Google Scholar]
- Gluud L.L., Dam G., Les I., Marchesini G., Borre M., Aagaard N.K., Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2017;(5):1–54. doi: 10.1002/14651858.CD001939.pub4. (Art. No.: CD001939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holecek M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition. 2010;26:482–490. doi: 10.1016/j.nut.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition. 2015;31:14–20. doi: 10.1016/j.nut.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Holecek M., Kandar R., Sispera L., Kovarik M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids. 2011;40:575–584. doi: 10.1007/s00726-010-0679-z. [DOI] [PubMed] [Google Scholar]
- Iwasa M., Matsumura K., Watanabe Y., Yamamoto M., Kaito M., Ikoma J., Gabazza E.C., Takeda K., Adachi Y. Improvement of regional cerebral blood flow after treatment with branched-chain amino acid solutions in patients with cirrhosis. Eur. J. Gastroenterol. Hepatol. 2003;15:733–737. doi: 10.1097/01.meg.0000059162.46867.f0. [DOI] [PubMed] [Google Scholar]
- Jepsen P., Ott P., Andersen P.K., Sorensen H.T., Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- Johansen M.L., Bak L.K., Schousboe A., Iversen P., Sorensen M., Keiding S., Vilstrup H., Gjedde A., Ott P., Waagepetersen H.S. The metabolic role of isoleucine in detoxification of ammonia in cultured mouse neurons and astrocytes. Neurochem. Int. 2007;50:1042–1051. doi: 10.1016/j.neuint.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kumar K., Goodrich J., Marcoux F. Comparison of vascular perfusion in ischemia-sensitive and ischemia-resistant regions of gerbil brain by an automated laser cytometric device: a preliminary study. J. Neurosci. Methods. 1991;39:1–8. doi: 10.1016/0165-0270(91)90087-g. [DOI] [PubMed] [Google Scholar]
- Lai J.C., Cooper A.J. Brain alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution, and effects of inhibitors. J. Neurochem. 1986;47:1376–1386. doi: 10.1111/j.1471-4159.1986.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Les I., Doval E., Garcia-Martinez R., Planas M., Cardenas G., Gomez P., Flavia M., Jacas C., Minguez B., Vergara M., Soriano G., Vila C., Esteban R., Cordoba J. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am. J. Gastroenterol. 2011;106:1081–1088. doi: 10.1038/ajg.2011.9. [DOI] [PubMed] [Google Scholar]
- Lockwood A.H., McDonald J.M., Reiman R.E., Gelbard A.S., Laughlin J.S., Duffy T.E., Plum F. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia. J. Clin. Invest. 1979;63:449–460. doi: 10.1172/JCI109322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood A.H., Murphy B.W., Donnelly K.Z., Mahl T.C., Perini S. Positron-emission tomographic localization of abnormalities of brain metabolism in patients with minimal hepatic encephalopathy. Hepatology. 1993;18:1061–1068. [PubMed] [Google Scholar]
- Marchesini G., Bianchi G., Merli M., Amodio P., Panella C., Loguercio C., Rossi Fanelli F., Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Merli M., Giusto M., Lucidi C., Giannelli V., Pentassuglio I., Di Gregorio V., Lattanzi B., Riggio O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab. Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- Morano G.N., Seibyl J.P. Technical overview of brain SPECT imaging: improving acquisition and processing of data. J. Nucl. Med. Technol. 2003;31:191–195. (quiz 202-193) [PubMed] [Google Scholar]
- Murin R., Mohammadi G., Leibfritz D., Hamprecht B. Glial metabolism of isoleucine. Neurochem. Res. 2009;34:194–204. doi: 10.1007/s11064-008-9840-4. [DOI] [PubMed] [Google Scholar]
- Nissen J.D., Pajecka K., Stridh M.H., Skytt D.M., Waagepetersen H.S. Dysfunctional TCA-cycle metabolism in glutamate dehydrogenase deficient astrocytes. Glia. 2015;63:2313–2326. doi: 10.1002/glia.22895. [DOI] [PubMed] [Google Scholar]
- O'Carroll R.E., Hayes P.C., Ebmeier K.P., Dougall N., Murray C., Best J.J., Bouchier I.A., Goodwin G.M. Regional cerebral blood flow and cognitive function in patients with chronic liver disease. Lancet. 1991;337:1250–1253. doi: 10.1016/0140-6736(91)92920-w. [DOI] [PubMed] [Google Scholar]
- Olde Damink S.W., Jalan R., Redhead D.N., Hayes P.C., Deutz N.E., Soeters P.B. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology. 2002;36:1163–1171. doi: 10.1053/jhep.2002.36497. [DOI] [PubMed] [Google Scholar]
- Ott P., Vilstrup H. Cerebral effects of ammonia in liver disease: current hypotheses. Metab. Brain Dis. 2014;29:901–911. doi: 10.1007/s11011-014-9494-7. [DOI] [PubMed] [Google Scholar]
- Ott P., Clemmesen O., Larsen F.S. Cerebral metabolic disturbances in the brain during acute liver failure: from hyperammonemia to energy failure and proteolysis. Neurochem. Int. 2005;47:13–18. doi: 10.1016/j.neuint.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Parekh P.J., Balart L.A. Ammonia and its role in the pathogenesis of hepatic encephalopathy. Clin. Liver Dis. 2015;19:529–537. doi: 10.1016/j.cld.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Romeiro F.G., Augusti L. Nutritional assessment in cirrhotic patients with hepatic encephalopathy. World J. Hepatol. 2015;7:2940–2954. doi: 10.4254/wjh.v7.i30.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeiro F.G., da Silva Yamashiro F., Americo M.F., Cora L.A., Silva G.F., Miranda J.R., Caramori C.A. Erythromycin versus neomycin in the treatment of hepatic encephalopathy in cirrhosis: a randomized double-blind study. BMC Gastroenterol. 2013;13:13. doi: 10.1186/1471-230X-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N.R., Pulsinelli W.A. Altered mitochondrial respiration in selectively vulnerable brain subregions following transient forebrain ischemia in the rat. J. Neurochem. 1987;49:1367–1374. doi: 10.1111/j.1471-4159.1987.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Song I.U., Park J.W., Chung S.W., Chung Y.A. Differences in cerebral perfusion according to phenotypes of essential tremor: brain perfusion SPECT study using SPM analysis. Neurol. Sci. 2014;35:767–772. doi: 10.1007/s10072-013-1600-9. [DOI] [PubMed] [Google Scholar]
- Stewart C.A., Malinchoc M., Kim W.R., Kamath P.S. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl. 2007;13:1366–1371. doi: 10.1002/lt.21129. [DOI] [PubMed] [Google Scholar]
- Sunil H.V., Mittal B.R., Kurmi R., Chawla Y.K., Dhiman R.K. Brain perfusion single photon emission computed tomography abnormalities in patients with minimal hepatic encephalopathy. J. Clin. Exp. Hepatol. 2012;2:116–121. doi: 10.1016/S0973-6883(12)60099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y., Isozaki E., Miyazaki Y., Koide R., Shimizu T., Yagi K., Hirai S. Clinical and neuroradiological improvement in chronic acquired hepatocerebral degeneration after branched-chain amino acid therapy. Acta Neurol. Scand. 2002;106:113–116. doi: 10.1034/j.1600-0404.2002.01230.x. [DOI] [PubMed] [Google Scholar]
- Vilstrup H., Amodio P., Bajaj J., Cordoba J., Ferenci P., Mullen K.D., Weissenborn K., Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- Vilstrup H., Amodio P., Bajaj J., Cordoba J., Ferenci P., Mullen K.D., Weissenborn K., Wong P. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J. Hepatol. 2014;61:642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Walker V. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 2014;67:73–150. doi: 10.1016/bs.acc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Weber F.L., Jr., Veach G.L. The importance of the small intestine in gut ammonium production in the fasting dog. Gastroenterology. 1979;77:235–240. [PubMed] [Google Scholar]
- Yamamoto M., Iwasa M., Matsumura K., Nakagawa Y., Fujita N., Kobayashi Y., Kaito M., Takeda K., Adachi Y. Improvement of regional cerebral blood flow after oral intake of branched-chain amino acids in patients with cirrhosis. World J. Gastroenterol. 2005;11:6792–6799. doi: 10.3748/wjg.v11.i43.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafiris O., Kircheis G., Rood H.A., Boers F., Haussinger D., Zilles K. Neural mechanism underlying impaired visual judgement in the dysmetabolic brain: an fMRI study. NeuroImage. 2004;22:541–552. doi: 10.1016/j.neuroimage.2004.01.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4
Supplementary material 5
Brain regions where the increase in cerebral perfusion was statistically significant were compared via SPECT images taken before and after 8 months of isoleucine supplementation.
Brain regions where the increase in cerebral perfusion was statistically significant were compared via SPECT images taken before and after 12 months of isoleucine supplementation.