Abstract
Background.
The purpose of this study was to report the response to and toxicity of ultra–low-dose radiotherapy (RT) for B-cell ocular adnexal lymphoma (OAL).
Methods.
We conducted a retrospective review of patients with indolent B-cell and mantle cell OAL treated with 4 Gy to the orbit(s) in two 2-Gy fractions. Disease response was assessed clinically and/or radiographically at 2 to 4-month intervals after RT. Data collected included rates of overall response, complete response (CR), partial response (PR), and treatment-related toxic effects.
Results.
Twenty-two patients (median age, 65 years) had the following histologic subtypes: mucosa-associated lymphoid tissue (MALT; 14 patients; 64%); follicular lymphoma (5 patients; 23%); mantle cell lymphoma (MCL; 2 patients; 9%); and unclassifiable (1 patient, 4%). The overall response rate was 100%; 19 patients (86%) had a CR and 3 patients (14%) had a PR. The only acute toxic effect was grade 1 dry eye syndrome in 1 patient.
Conclusion.
Ultra–low-dose RT in patients with OAL is associated with high response rates and minimal toxic effects, and is much shorter in duration and cost.
Keywords: mucosa-associated lymphoid tumor, ocular adnexal lymphoma, orbital lymphoma, radiotherapy, mantle cell lymphoma
INTRODUCTION
Non-Hodgkin lymphoma can arise in the orbit or can spread to the orbit in patients with advanced or relapsed disease.1 Although orbital involvement by non-Hodgkin lymphoma is rare, it represents the most common primary orbital and ocular adnexal malignant neoplasm.2 Ocular adnexal lymphoma (OAL) includes lesions of the conjunctiva, lacrimal gland, orbital soft tissue, or eyelid2 and are predominantly low-grade non-Hodgkin lymphoma. These types of lesions must be distinguished from intraocular lymphoma, a subtype of primary central nervous system lymphoma; that represents a high-grade process with a distinct therapeutic strategy.3,4 Standard-of-care management of localized low-grade B-cell lymphoma of the ocular adnexa is external-beam radiotherapy (RT). External-beam RT is also used to treat ocular adnexal mantle cell lymphoma (MCL), a subtype of B-cell lymphoma that is not low grade but that has been demonstrated in numerous studies to be exquisitely radiosensitive. The optimal radiation dose for OAL is one that achieves optimal local control without increasing the risk of long-term morbidity in the treated eye.
Historically, OALs were treated with moderate to high doses of radiation, ranging from 35 to 54 Gy5–7; however, evidence is increasing that low-grade OAL can be treated successfully and with minimal morbidity to lower doses of radiation. With historical doses of 35 to 54 Gy, local control rates were excellent, ranging from 90% to 100%. In most series, however, doses greater than 35 Gy resulted in considerable late toxic effects, including keratitis, severe dry eye syndrome, glaucoma, retinopathy, and cataract formation.7,8 Several single-institution series have reported similar high local control rates but decreased toxicity with lower doses of RT, suggesting that the effective dose for the definitive management of ocular adnexal low-grade lymphoma has not been defined.7–10 In a series from Princess Margaret Hospital of 89 patients with localized mucosa-associated lymphoid tissue (MALT) lymphoma of the ocular adnexa, 86 patients (97%) were treated with a dose of 25 Gy, and the 7-year local control rate was 97%.9 In a similar series of 54 patients with OAL treated to doses of 24 to 25.5 Gy, the 5-year local control rate was 95%.10 In patients with indolent OAL treated for palliation or in cases of reirradiation, doses of 4 Gy have been reported to yield excellent control rates. Fasola et al11 reported a complete response (CR) rate of roughly 85% for 27 sites of ocular adnexal involvement in 20 patients with non-Hodgkin lymphoma treated with 4 Gy total dose of radiation. For the orbital sites in which a CR was observed, the 2-year local control rate was 100%.11
Given the radiosensitivity of indolent non-Hodgkin lymphoma and MCL, and the fact that local relapses of these diseases are salvageable and non-life-threatening, in 2013, we began offering ultra–low-dose RT for indolent B-cell lymphoma and MCL affecting the ocular adnexa. Here, we report the results of a retrospective evaluation of the toxicity and efficacy of delivering 4 Gy in 2 fractions of external beam RT in patients with OAL treated at our institution.
MATERIALS AND METHODS
Patients
All patients treated with ultra–low-dose external beam RT for ocular adnexal MCL and low-grade B-cell lymphoma, including MALT lymphoma and follicular lymphoma, at The University of Texas MD Anderson Cancer Center between March 2013 and November 2015 were included in this analysis after Institutional Review Board approval was obtained. The work was done under strict Health Insurance Portability and Accountability Act compliance.
Disease evaluation and treatment
Disease was confirmed by review of a diagnostic biopsy specimen by a specialized hematopathologist. Full workup consisted of a comprehensive ocular examination by a licensed ophthalmologist (B.E.), basic laboratory studies, baseline volumetric imaging (fluorodeoxyglucose 18F [FDG] positron emission tomography-CT or CT imaging of the neck, chest, abdomen, and pelvis), and bone marrow biopsy. The orbits were imaged with either CT or MRI. Many patients with MALT lymphoma and MCL underwent baseline esophagogastroduodenoscopy to exclude gastric involvement. Patients with synchronous bilateral ocular adnexal involvement in the absence of distant lymphoma were considered to have stage IE disease.
All patients underwent CT-based radiation planning and were immobilized with a custom thermoplastic mask. For patients with disease limited to the conjunctiva, the clinical target volume was defined as the entire palpebral and bulbar conjunctiva. For all other patients, the clinical target volume included the entire orbit. Patients were treated with electrons or photons to 4 Gy in two 2-Gy fractions delivered on consecutive days. Lens shielding was not utilized. Bolus material was utilized as necessary to achieve adequate coverage of disease in cases of superficial involvement.
Response assessment
Response was assessed with clinical examination with or without radiographic imaging at 2-month to 4-month intervals with the same imaging modality used at diagnosis to define the lymphoma. Given the low yield of MRI for the identification of conjunctival lymphoma,12 baseline and response assessments were clinical for patients with conjunctival disease who did not have a detectable radiographic abnormality on baseline evaluation.
CR was defined as resolution of tumor by clinical examination in the case of conjunctival lymphomas and by radiographic studies in the case of orbital and bulky eyelid lymphomas. In cases of ocular adnexal tumors that were FDG avid at baseline, CR was defined as resolution of FDG avidity even in the setting of a radiographic residual mass, in keeping with the Lugano classification for response assessment of non-Hodgkin lymphoma.13 Partial response (PR) was defined as a decrease in size (or FDG avidity if positron emission tomography-CT was available) of the disease burden with or without radiographic studies. Stable disease was defined as no change in measurable disease clinically or radiographically. Progressive disease was defined as any increase in OAL on clinical examination or imaging studies. The overall response rate was defined as the rate of CR and PR.
Toxicity
Acute and chronic ocular toxic effects were assessed at 2-month to 4-month intervals and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.14
Statistical analysis
Freedom from local recurrence was measured from the date when RT ended to the date of disease recurrence within the irradiated orbit. Actuarial relapse rates were estimated using the Kaplan–Meier method. Analyses were performed using SPSS Statistics software, version 22 (IBM, Armonk, NY).
RESULTS
Between March 2013 and November 2015, 22 patients with B-cell OAL were treated with 4 Gy to the orbit in two 2-Gy fractions delivered on consecutive days. Patient, disease, and treatment characteristics are summarized in Table 1. The median age of the patients at diagnosis of OAL was 64.5 years (range, 25–88 years). Twelve patients were men. Fifteen patients had stage IE disease, and 5 of these patients had synchronous bilateral disease at diagnosis. Fourteen patients had MALT lymphoma, and 2 patients had MCL. Sixteen patients had radiographic evidence of lymphoma, and the median lesion size was 2.1 cm (range, 1.2–3.2cm). Four patients had preexisting ocular conditions: 2 had Sjögren disease, 1 had glaucoma, and 1 had hereditary ptosis. Staging esophagogastroduodenoscopy was performed in both patients with MCL and showed evidence of gastric MCL in 1 patient. Staging esophagogastroduodenoscopy was performed in 6 of the 14 patients with MALT lymphoma and did not show evidence of gastric MALT lymphoma in any case. Ten patients had a history of systemic therapy for lymphoma, and 1 patient had a history of RT to the contralateral orbit.
Table 1.
Patient, disease, and treatment characteristics.*
| Characteristics | Values |
|---|---|
| Age, y, median (range) | 64.5 (25–88) |
| Sex | |
| Male | 12 (55) |
| Female | 10 (45) |
| Race/ethnicity | |
| White | 16 (73) |
| Hispanic | 4 (18) |
| African American | 2 (9) |
| Performance status | |
| 0 | 16 (73) |
| 1 | 4 (18) |
| 2 | 2 (9) |
| Stage | |
| IE | 15 (68) |
| IV | 7 (32) |
| Histologic subtype | |
| MALT | 14 (64) |
| Follicular, grade 1–2 | 5 (23) |
| MCL | 2 (9) |
| Low-grade unclassifiable | 1 (4) |
| Orbital symptoms | |
| Yes | 11 (50) |
| No | 11 (50) |
| Disease detected on clinical examination | |
| Yes | 18 (82) |
| No | 4 (18) |
| Disease detected on radiographic examination | |
| Yes | 16 (73) |
| No | 6 (27) |
| Orbital site | |
| Conjunctiva | 6 (27) |
| Lacrimal gland | 6 (27) |
| Soft tissue | 9 (41) |
| Mixed | 1 (5) |
| Laterality | |
| Left | 7 (32) |
| Right | 9 (41) |
| Bilateral | 6 (27) |
| Lesion size, cm, median (range) | 2.1 (1.2–3.2) |
| Preexisting orbital condition | |
| Sjögren syndrome | 2 (9) |
| Glaucoma | 1 (5) |
| Hereditary ptosis | 1 (5) |
| None | 18 (81) |
| Prior systemic therapy for lymphoma | |
| Yes | 10 (45) |
| No | 12 (55) |
| Prior orbital RT | |
| Yes | 1 (5) |
| No | 21 (95) |
| Radiation modality | |
| Electrons | 8 (36) |
| Photons | 14 (64) |
Abbreviations: MALT, mucosa-associated lymphoid tissue; MCL, mantle cell lymphoma; RT, radiotherapy.
Values are the number of patients (percentage), unless otherwise indicated.
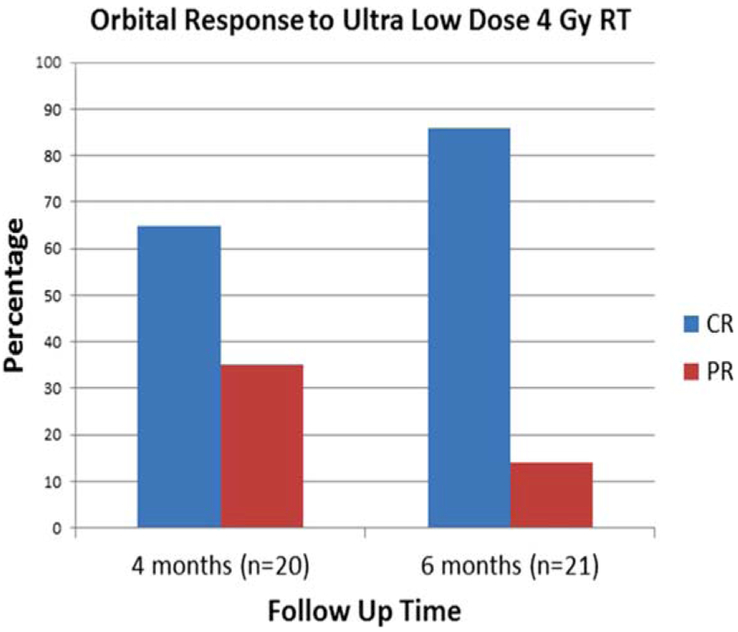
The median follow-up for all 22 patients was 14.1 months (range, 3.7–29.9 months). The median time to first follow-up response evaluation was 3.0 months (range, 1.4–5.6 months). Twenty patients (91%) had an initial evaluation within 4 months after completion of RT. Of these 20 patients, 13 (65%) had a CR, and 7 (35%) had a PR (see Figure 1). Of the 7 patients with only a PR at 4 months, 4 patients eventually achieved a CR at a median of 6.6 months (range, 5.07–10.5 months) after RT (see Figure 2). Twenty-one patients were evaluated within 6 months after RT: 18 patients (86%) had a CR, and 3 (14%) had a PR. Ultimately, for all 22 patients, the overall response rate was 100%: 19 patients (86%) had a CR, and 3 patients (14%) had a PR. Of the 19 patients with a CR, only 1 patient received systemic therapy after ultra–low-dose RT before achievement of the CR in the orbit. Of the 3 patients who did not achieve a CR after ultra–low-dose RT, 1 patient with unclassifiable low grade B-cell lymphoma received weekly rituximab and an additional 20 Gy of orbital RT and achieved a CR; 1 patient with stage IV follicular lymphoma and a right superior orbital lesion had persistent soft tissue fullness on CT imaging and, as of this writing, has been followed up for 1 year after ultra–low-dose RT without receiving additional therapy; and 1 patient had evidence of persistent low-burden asymptomatic bilateral conjunctival follicular lymphoma at the 3-month follow-up visit and, as of this writing, is scheduled for a 6-month follow-up visit.
FIGURE 1.
Orbital response to 4 Gy orbital radiotherapy (RT) rates of complete response (CR) and partial response (PR) after completion of ultra–low-dose radiotherapy to the orbit. At 4 months, 20 patients were evaluated for disease response and CR was achieved among 65% of patients (n = 13). At 6 months, 21 patients were evaluated and CR was apparent in 86% of patients (n = 19). The 1 patient who was not evaluated at 6 months was seen at 3-month and 6-month follow-ups, and was pending at the time of manuscript preparation of this study. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
Complete response to ultra–low-dose orbital radiotherapy (RT) for mantle cell lymphoma (MCL) achieved 10 months after completion of RT. (A) A 70-year-old woman with stage IV MCL had a dominant enhancing left lacrimal gland mass measuring 2.6 × 1.5 cm on axial postcontrast CT imaging. (B) She was treated with 4 Gy in 2 fractions with 16-MeV electrons. (C) She had a partial response to RT at 4 months after completion of RT. (D) She had a complete response to RT at 10.5 months after completion of RT without any interval treatment. [Color figure can be viewed at wileyonlinelibrary.com]
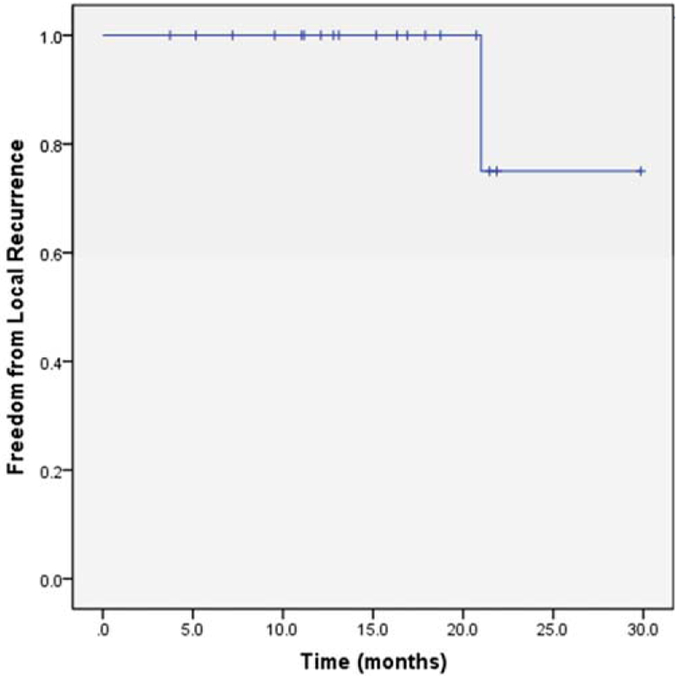
For the 19 patients who achieved a CR, the median follow-up time was 15.2 months (range, 3.7–29.9 months). The median time to achievement of CR was 3.73 months (range, 2.07–10.5 months). The 1-year and 2-year rates of freedom from local recurrence were 100% and 75%, respectively (see Figure 3). There was one local recurrence in this series. A patient with MCL of the bilateral lacrimal glands experienced a recurrence in the left lacrimal gland approximately 21 months after completion of ultra–low-dose RT. Retreatment with 4 Gy in 2 fractions to the left orbit was given with follow-up pending. An additional patient developed a relapse of MALT lymphoma in the contralateral orbit roughly 19 months after completion of ultra–low-dose RT for MALT lymphoma. This patient was treated with 4 Gy in 2 fractions to the contralateral orbit and had a CR to therapy.
FIGURE 3.
Rate of freedom from local recurrence among patients with ocular adnexal low-grade B-cell lymphoma and mantle cell lymphoma who achieved a complete response after treatment with 4 Gy in 2 fractions (n = 5 19). [Color figure can be viewed at wileyonlinelibrary.com]
Two patients died of causes unrelated to lymphoma. One patient died of complications related to therapy for metastatic melanoma. The second patient died of renal failure without evidence of lymphoma at the time of death.
Ultra–low-dose orbital RT was well tolerated. One patient developed grade 1 dry eye syndrome. No other toxic effects were noted.
DISCUSSION
For primary RT for indolent B-cell OAL, the current standard dose is 24 Gy. Most of the support for this practice comes from single retrospective studies and a randomized trial among patients with nodal low-grade lymphoma.9,15–17 In the current study, we used ultra–low-dose RT consisting of 4 Gy to the orbit in two 2-Gy fractions delivered on consecutive days for definitive management of B-cell OAL. We observed CRs in 86% of cases. Although the follow-up time was relatively short, only 1 local recurrence was observed, and ocular toxicity was minimal. Results with this low dose were comparable to results in the literature with doses as high as 45 Gy.
The standard dose for the management of low grade B-cell lymphoma has decreased over the past several decades. A randomized phase III trial comparing 24 Gy versus 40 Gy to 45 Gy for indolent systemic lymphoma found no difference in rates of overall response or progression within the radiation field between the standard and lower-dose arms.16 Overall survival and progression-free survival were also not significantly different between the arms, but there was a trend toward reduced toxicity with 24 Gy. It is unclear how many patients in this multi-institutional trial were treated for extranodal disease involving the orbit. Single-institution series have reported excellent local control rates, in excess of 90%, with doses of 24 Gy to 25.5 Gy to the orbit for B-cell OAL.9,15 Although 24 Gy to the orbit is generally well tolerated, the majority of patients do experience acute toxic effects. The risk of long-term toxic effects of RT is lower, but such effects can be permanent for some patients. In a single-institution series from Princess Margaret Hospital, in which 97% of patients received 25 Gy, late toxic effects occurred in 45% of patients and included grade 1 to 3 cataracts, dry eye syndrome, keratitis, macular degeneration, and vitreous detachment.9 Management of the late effects was effective, however: only 10% of patients experienced unresolved chronic toxic effects of RT.
Given the lower but persistent risk of long-term orbital toxic effects with RT doses of 24 Gy, together with the increasing recognition of the radiosensitivity of low-grade B-cell lymphoma and MCL, there is increasing interest in lower doses of radiation for treatment of these forms of lymphoma. Several single-institutional series have demonstrated considerable overall response rates to doses of 4 Gy for treatment of systemic (extraocular) nodal masses from indolent B-cell lymphoma.18–21 Furthermore, a retrospective study from Stanford by Fasola et al11 showed the effectiveness of 4 Gy to the orbit for palliation or reirradiation of low-grade B-cell OAL. In that study, 20 patients with low-grade B-cell OAL with 27 sites of orbital involvement were treated with 4 Gy in 2 fractions.11 With a median follow-up time of just over 2 years, the overall response rate was 96%, and the CR rate was 85%. Among patients who achieved a CR, the local control rate was 100% with no in-field relapses. As expected, there were no long-term toxic effects.
In the current study, 86% of patients achieved a CR after treatment with 4 Gy; only 3 patients did not have a CR after this ultra–low-dose treatment. One of the 3 patients who did not have a CR after 4 Gy to the orbit had an unclassifiable low-grade B-cell lymphoma with 3 monoclonal B-cell populations detected in the biopsy specimen, including cells suggestive of small lymphocytic lymphoma/chronic lymphocytic leukemia, a disease that has been shown not to respond well to palliative doses of 4 Gy.18 Ultimately, this patient had complete regression of his orbital lymphoma after an additional 20 Gy was administered. This staged approach to therapy, in which patients with suboptimal responses to 4 Gy go on to receive an additional 20 Gy, is currently the focus of an ongoing prospective trial at our institution (NCT02494700). The purpose of this study was to identify patients where ultra–low-dose RT is curative, while maintaining excellent local control among patients who do require full doses of 24 Gy to eradicate ocular adnexal disease. It should be noted that, in 4 patients in our series, CR to ultra–low-dose RT was not apparent at the initial follow-up visit at 3 to 4 months, and became apparent only later (in 1 patient, not until 10 months without intervening systemic therapy). This highlights the need to allow adequate follow-up time for maximal response to occur before decisions are made about additional therapy.
Although MCL is not a low-grade lymphoma, numerous studies have demonstrated that MCL is exquisitely radiosensitive to RT. In a study by Russo et al18 of the predictors of response to 4 Gy delivered in 2 fractions for treatment of mainly systemic nodal non-Hodgkin lymphoma, the response rate in patients with MCL was over 90%, and patients with MCL had the lowest rate of local recurrence. Systemic failures were significant, however, highlighting the fact that patients with MCL almost always have systemic involvement of their disease. In the Stanford series of ultra–low-dose treatment of orbital low-grade non-Hodgkin lymphoma, 1 patient was treated for MCL, and this patient did not have an in-field relapse.11 In our current study, the only in-field relapse occurred in a patient treated for MCL. Despite this relapse, we still consider patients with MCL for ultra–low-dose therapy given the known radiosensitivity of MCL and the almost uniform existence of systemic disease in patients with MCL. In patients with ocular adnexal MCL, a 2-day low-toxicity therapy is attractive because it offers the possibility of local control of orbital disease and does not interfere with essential aggressive systemic therapy.
There was 1 patient in our study who developed relapsed disease of MALT lymphoma in the contralateral orbit almost 2 years after completion of ultra–low-dose RT. The contralateral orbit is a well-recognized pattern of failure among patients with ocular adnexal MALT lymphoma, even when orbital doses of 25 to 40 Gy are administered and local control is achieved in the treated orbit.9,22–24 Because contralateral ocular adnexal relapses are known to occur when local control rates are excellent, the goal of therapy should be achievement of CR within the affected orbit with the lowest dose of radiation possible to reduce the potential for toxicity. One could even consider investigating the efficacy of 1 fraction of 2 Gy if long-term follow-up of our study is promising.
Ultra–low-dose RT for B-cell OAL offers distinct benefits for different patient subgroups. For all patients, this treatment offers the opportunity for durable local control within the treated field without significant long-term ocular morbidity. Obvious advantages of ultra–low-dose RT compared to standard-dose RT are shorter duration of treatment (2 days as opposed to 12 days), lower cost of therapy, and decreased ocular toxic effects. In patients newly diagnosed with non–life-threatening, limited-stage OAL, the fact that ultra–low-dose RT minimizes long-term ocular morbidity is important. In patients with advanced disease and relapsed indolent OAL, a 2-day course of therapy permits patients to pursue systemic therapy without a prolonged break for RT. Furthermore, patients with autoimmune disease, such as Sjögren syndrome, have an increased risk for the development of MALT lymphoma, which often affects the eyes.25–27 In these patients, radiation is generally considered, but with reservations because RT may worsen dry eye syndrome. With ultra–low-dose RT, patients with autoimmune disease, such as Sjögren syndrome, can receive RT with less concern about ocular damage. In fact, 2 patients in the current study had Sjögren syndrome, and neither experienced exacerbation of baseline ocular symptoms.
In the present study, we observed a CR rate of 86% among 22 patients with ocular adnexal low-grade B-cell lymphoma and MCL treated with 4 Gy in 2 fractions to the orbit for definitive management of disease. Although additional follow-up is required to document durable local control, the high CR rates to this low dose of RT are encouraging. This strategy has the potential to be curative while reducing the risk of toxic effects and is currently being explored in a prospective trial at MD Anderson Cancer Center. Findings in our study reported here and in the previous report from Fasola et al19 suggest a potential paradigm shift in the treatment of ocular adnexal low-grade B-cell lymphoma and MCL.
REFERENCES
- 1.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer 1972;29:252–260. [DOI] [PubMed] [Google Scholar]
- 2.Margo CE, Mulla ZD. Malignant tumors of the orbit. Analysis of the Florida Cancer Registry. Ophthalmology 1998;105:185–190. [DOI] [PubMed] [Google Scholar]
- 3.Ferreri AJ, Blay JY, Reni M, et al. Relevance of intraocular involvement in the management of primary central nervous system lymphomas. Ann Oncol 2002;13:531–538. [DOI] [PubMed] [Google Scholar]
- 4.Cheah CY, Milgrom S, Chihara D, et al. Intensive chemoimmunotherapy and bilateral globe irradiation as initial therapy for primary intraocular lymphoma. Neuro Oncol 2016;18:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolek TW, Moyses HM, Marcus RB Jr, et al. Radiotherapy in the management of orbital lymphoma. Int J Radiat Oncol Biol Phys 1999;44:31–36. [DOI] [PubMed] [Google Scholar]
- 6.Pelloski CE, Wilder RB, Ha CS, Hess MA, Cabanillas FF, Cox JD. Clinical stage IEA-IIEA orbital lymphomas: outcomes in the era of modern staging and treatment. Radiother Oncol 2001;59:145–151. [DOI] [PubMed] [Google Scholar]
- 7.Stafford SL, Kozelsky TF, Garrity JA, et al. Orbital lymphoma: radiotherapy outcome and complications. Radiother Oncol 2001;59:139–144. [DOI] [PubMed] [Google Scholar]
- 8.Minehan KJ, Martenson JA Jr, Garrity JA, et al. Local control and complications after radiation therapy for primary orbital lymphoma: a case for low-dose treatment. Int J Radial Oncol Biol Phys 1991;20:791–796. [DOI] [PubMed] [Google Scholar]
- 9.Goda JS, Le LW, Lapperriere NJ, et al. Localized orbital mucosa-associated lymphoma tissue lymphoma managed with primary radiation therapy: efficacy and toxicity. Int J Radiat Oncol Biol Phys 2011;81:e659–e666. [DOI] [PubMed] [Google Scholar]
- 10.Kennerdell JS, Flores NE, Hartsock RJ. Low-dose radiotherapy for lymphoid lesions of the orbit and ocular adnexa. Ophthal Plast Reconstr Surg 1999;15:129–133. [DOI] [PubMed] [Google Scholar]
- 11.Fasola CE, Jones JC, Huang DD, Le QT, Hoppe RT, Donaldson SS. Low-dose radiation therapy (2 Gy × 2) in the treatment of orbital lymphoma. Int J Radiat Oncol Biol Phys 2013;86:930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasser QJ, Pfeiffer ML, Romaguera J, et al. Clinical value of magnetic resonance imaging and other baseline testing for conjunctival mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma 2014;55:1013–1017. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181. [DOI] [PubMed] [Google Scholar]
- 15.Tran KH, Campbell BA, Fua T, et al. Efficacy of low dose radiotherapy for primary orbital marginal zone lymphoma. Leuk Lymphoma 2013;54:491–496. [DOI] [PubMed] [Google Scholar]
- 16.Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol 2011;100:86–92. [DOI] [PubMed] [Google Scholar]
- 17.Haque W, Voong KR, Shihadeh F, et al. Radiation therapy is an effective modality in the treatment of mantle cell lymphoma, even in heavily pretreated patients. Clin Lymphoma Myeloma Leuk 2014;14:474–479. [DOI] [PubMed] [Google Scholar]
- 18.Russo AL, Chen YH, Martin NE, et al. Low-dose involved-field radiation in the treatment of non-Hodgkin lymphoma: predictors of response and treatment failure. Int J Radiat Oncol Biol Phys 2013;86:121–127. [DOI] [PubMed] [Google Scholar]
- 19.Rossier C, Schick U, Miralbell R, Mirimanoff RO, Weber DC, Ozsahin M. Low-dose radiotherapy in indolent lymphoma. Int J Radiat Oncol Biol Phys 2011;81:e1–e6. [DOI] [PubMed] [Google Scholar]
- 20.Chan EK, Fung S, Gospodarowicz M, et al. Palliation by low-dose local radiation therapy for indolent non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2011;81:e781–e786. [DOI] [PubMed] [Google Scholar]
- 21.Girinsky T, Guillot-Vals D, Koscielny S, et al. A high and sustained response rate in refractory or relapsing low-grade lymphoma masses after low-dose radiation: analysis of predictive parameters of response to treatment. Int J Radiat Oncol Biol Phys 2001;51:148–155. [DOI] [PubMed] [Google Scholar]
- 22.Parikh RR, Moskowitz BK, Maher E, et al. Long-term outcomes and patterns of failure in orbital lymphoma treated with primary radiotherapy. Leuk Lymphoma 2015;56:1266–1270. [DOI] [PubMed] [Google Scholar]
- 23.Le QT, Eulau SM, George TI, et al. Primary radiotherapy for localized orbital MALT lymphoma. Int J Radiat Oncol Biol Phys 2002;52:657–663. [DOI] [PubMed] [Google Scholar]
- 24.Tsang RW, Gospodarowicz MK, Pintilie M, et al. Stage I and II MALT lymphoma: results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys 2001;50:1258–1264. [DOI] [PubMed] [Google Scholar]
- 25.Pinnix CC, Reed V, Dabaja B. Gastric MALT lymphoma treated with primary radiotherapy in the setting of autoimmune disease. J Natl Compr Canc Netw 2012;10:815–819. [DOI] [PubMed] [Google Scholar]
- 26.Voulgarelis M, Ziakas PD, Papageorgiou A, Baimpa E, Tzioufas AG, Moutsopoulos HM. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjogren Syndrome. Medicine (Baltimore) 2012;91:1–9. [DOI] [PubMed] [Google Scholar]
- 27.Tonami H, Matoba M, Kuginuki Y, et al. Clinical and imaging findings of lymphoma in patients with Sjögren syndrome. J Comput Assist Tomogr 2003;27:517–524. [DOI] [PubMed] [Google Scholar]