Abstract
Background
Factor VII-activating protease (FSAP) has a role in vascular inflammation and may have a role coronary artery disease (CAD). The aim of this study was to investigate the association between two naturally occurring single nucleotide polymorphisms (SNPs) in the FSAP gene and the risk of coronary artery disease (CAD).
Material/Methods
Of 733 patients, 173 patients had symptoms of angina, and 560 patients had CAD confirmed by coronary angiography. All patients were genotyped for SNPs of the FSAP gene, Marburg I (MI-SNP) and Marburg II (MII-SNP), using 5′ exonuclease TaqMan assays. Logistic regression analysis was used to evaluate the association between two gene polymorphisms, metabolic and other cardiovascular risk factors in patients with CAD.
Results
The presence of MI-SNP and MII-SNP FSAP gene polymorphisms were not associated with the presence of CAD. However, the MII-SNP polymorphism was significantly associated with a reduced risk of developing CAD (OR=0.422; 95% CI, 0.194–0.920; P=0.035); the MI-SNP polymorphism was associated with absence of hyperlipoproteinemia (OR=0.601; 95% CI, 0.344–1.051; P=0.074). There was no significant association between expression of the MI-SNP and MII-SNP FSAP gene polymorphisms and the incidence of myocardial infarction, or of a history of diabetes mellitus, arterial hypertension, obesity, or smoking.
Conclusions
The MI-SNP and MII-SNP FSAP gene polymorphisms were not predictive or prognostic biomarkers for CAD or its main risk factors. However, the presence of the MII-SNP polymorphism was associated with a reduced risk of developing CAD.
MeSH Keywords: Cardiovascular Diseases, Genetics, Risk Factors
Background
Sudden or unexpected coronary artery atherothrombotic events can occur in individuals with few or no risk factors for coronary artery disease (CAD), which suggests that unrecognized genetic factors may be involved [1–3]. It is now recognized that a genetic predisposition is an important risk factor for atherosclerosis leading to CAD, and to myocardial ischemia, and myocardial infarction. Several previously published studies have now investigated the associations between single nucleotide polymorphisms (SNPs) and the clinical development of coronary artery atherosclerosis [4,5]. Candidate genes in the pathways involved in disease initiation and progression in CAD have been explored, including those related to lipid metabolism, coagulation, and inflammation [5,6]. Despite these advances, the understanding of the coronary artery atherothrombotic processes is incomplete, and the ability to predict cardiovascular events, especially in individual patients, continues to be limited [4–7].
Recently, there has bee increasing published evidence from experimental and epidemiological data that factor VII-activating protease (FSAP), a serine protease involved in both coagulation and fibrinolysis [8–10], plays a key role in the inflammatory diseases, such as sepsis and atherosclerosis [11–13]. Increased expression of the FSAP gene has been localized in human atherosclerotic plaque and is associated with plaque instability [13]. Recently, we have shown that baseline circulating FSAP levels were strongly predictive of future cardiovascular mortality in patients with CAD [14]. Elevation of FSAP protein levels in young pre-menopausal female smokers and non-smokers, without any additional risk factors for atherosclerosis, but with oral contraceptive use, has also been reported [15,16].
The FSAP gene is mapped on chromosome 10q25-q26 as a single copy gene [17]. A single nucleotide polymorphism (SNP) at position 1601 (G→A) in exon 13 was discovered in the gene coding for the FSAP protein, and results in the substitution of glycine 511 by glutamic acid; this polymorphism, G534E, is also known as the Marburg I (MI-SNP), and is present in 9% of the Caucasian population [17]. Half of the SNP 1601 positive donors displayed the additional SNP at position 1177 resulting in an E370Q amino acid exchange, the Marburg II (MII-SNP), which does not affect fibrinolysis. MII is commonly co-expressed with the MI polymorphism. The protease expressed by the FSAP gene carrying the MI-SNP has a reduced ability to activate pro-urokinase-type plasminogen activator (uPA) and FVII, and inactivate tissue factor pathway inhibitor (TFPI) [17,18]. The MI-SNP was found to be an independent risk factor for the development of late complications of carotid stenosis [19], and may also be related to the occurrence of venous thromboembolism, in some but not all studies [20–23], and is associated with increased risk for stroke, and may represent a risk factor for CAD [24,25].
The aim of this study was to investigate the association between the two naturally occurring SNPs in the FSAP gene, MI-SNP, and MII-SNP, and the risk factors associated with CAD in a population in Germany.
Material and Methods
Study design and patient selection
This study was a single center retrospective clinical study and included 733 patients with symptoms of angina pectoris and coronary artery disease (CAD), who were tested for the presence of factor VII-activating protease (FSAP) gene polymorphisms (Figure 1). This study population included 173 patients who were admitted to the Department of Internal Medicine I Cardiology/Angiology, University Hospital of Giessen and underwent diagnostic or therapeutic coronary angiography; 560 patients had CAD which was confirmed by therapeutic coronary angiography. All study participants were genetically tested for FSAP gene polymorphisms consisting of one gene database from the Institute of Clinical Chemistry of the University of Giessen. The study was approved by the Ethics Committee of the University of Giessen and informed written consent was given by all patients.
Figure 1.
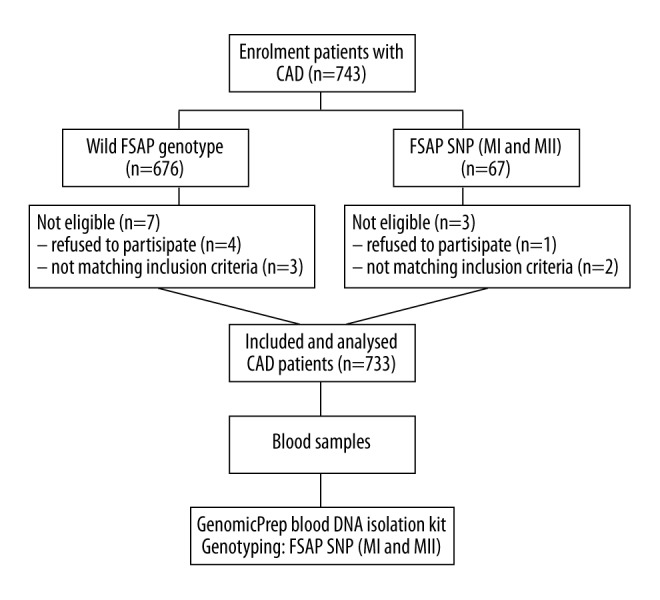
Flowchart of the study design and study population. CAD – coronary artery disease; FSAP – factor VII-activating protease; SNP – single nucleotide polymorphisms; MI – Marburg I; MII – Marburg II.
Patients with significant coronary artery stenosis (≥50%) in at least one main branch of the coronary artery were defined as patients with CAD. Angiographic multivessel CAD was defined as stenosis ≥50% and was qualified as one-vessel, two-vessel, or three-vessel disease, if one, two, or three coronary arteries were affected, respectively. Age, sex, obesity, defined as a body mass index (BMI) between 25.0 and 29.9 kg/m2, cardiovascular risk factors including systemic hypertension (systolic blood pressure >140 mmHg’ diastolic blood pressure >90 mmHg), diabetes mellitus (fasting blood glucose ≥126 mg/dl or postprandial blood glucose ≥200 mg/dl), current smoking habit (10 or more cigarettes daily), and hyperlipidemia (total cholesterol ≥240 mg/dl or fasting triglyceride concentration ≥150 mg/dl) were assessed and recorded.
There were 733 consecutive patients with CAD who were included in the study, with the following inclusion criteria: age >18 years; diagnosis of CAD by coronary angiography during percutaneous coronary intervention (PCI); the presence of at least one FSAP SNP polymorphism by genotyping assays. Exclusion criteria included: the presence of recent (>6 months) myocardial infarction; a recent history (>6 months) of percutaneous coronary intervention (PCI), or previous coronary artery bypass graft (CABG); contraindications for revascularization (PCI and/or CABG); the presence of infectious disease, or autoimmune diseases; the presence of malignancy; women who were pregnant or breastfeeding; patients with recent major surgery; patients who were unwilling to provide written informed consent to participate in the study.
Genotyping
A peripheral venous blood sample from each individual was collected in sterile tubes containing a disodium-EDTA-dihydrate anticoagulant, and stored at −80°C. Genomic DNA was prepared from frozen whole blood with the GenomicPrep blood DNA isolation kit (Amersham Pharmacia Biotech, Vienna, Austria) and DNA analysis was performed, as described previously [14,26].
Laboratory methods
Routine blood chemistry and lipids (total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides) were analyzed using fresh blood samples according to established enzymatic methods, standardized at the local laboratories, as previously described [14].
Statistical analysis
Categorical variables were reported as counts (percentages), and continuous variables were reported as the mean ± standard deviation (SD), or median. The normal distribution of parameters was assessed by the Kolmogorov-Smirnov test. Student’s unpaired t-test was used for normally distributed continuous variables, and Pearson’s chi-squared test was used for comparison of categorical variables. Group comparisons were performed using analysis of variance (ANOVA). The association of polymorphisms with CAD and cardiovascular risk factors under different inheritance models was tested using logistic regression analysis. Data were analyzed with Statistical Package for the Social Sciences (SPSS) software, version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Distribution of the Factor VII-activating protease (FSAP) gene single nucleotide polymorphisms (SNPs)
The clinical characteristics of the study population are shown in Table 1. In the cluster of 733 patients with coronary artery disease (CAD), 64 subjects were heterozygous for the both FSAP SNP polymorphisms, corresponding to a carriage rate of 8.7%: MI-SNP 7.8% (n=57) and MII-SNP 3.8% (n=28), respectively. The frequency of the MI-SNP and MII-SNP were similar to that reported previously [19].
Table 1.
Associations of FSAP gene polymorphisms with metabolic and cardiovascular risk factors in individual with coronary artery disease (CAD).
| Clinical parameters | MI-SNP (n=64) | OR (95% CI) | p-Value | MII-SNP (n=28) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Obesity (%) | 6.4 | 0.644 (0.375–1.108) | 0.125 | 3.1 | 0.631 (0.296–1.346) | 0.247 |
| Type 2 diabetes mellitus (%) | 7.6 | 0.969 (0.509–1.844) | 1.0 | 3.5 | 0.893 (0.356–2.238) | 1.0 |
| Smoking (%) | 7.5 | 0.935 (0.475–1.642) | 0.951 | 3.2 | 0.763 (0.271–2.041) | 0.891 |
| Hyperlipoproteinemia (%) | 5.9 | 0.601 (0.344–1.051) | 0.074 | 2.8 | 0.583 (0.265–1.281) | 0.184 |
| Arterial hypertension (%) | 8.3 | 1.382 (0.715–2.671) | 0.335 | 4.5 | 2.214 (0.758–6.463) | 0.189 |
FASAP – Factor VII-activating protease; SNP – single nucleotide polymorphism; MI-SNP – Marburg I; MII-SNP – Marburg II; CI – confidence interval; OR – overall response.
Association of the FSAP gene MI-SNP and MII-SNP with coronary artery disease (CAD)
The distribution of the wild-type FSAP gene and FSAP SNPs was similar in patients with early CAD, and no associations of FSAP SNPs with CAD were found (Figure 2). Also, MII-SNP polymorphism had a protective effect in CAD and was significantly associated with the absence of premature CAD (OR=0.422; 95% CI, 0.194–0.920; P=0.035). Within the CAD study group, subgroups were compared that included varying severity of CAD, defined as none, or one-vessel, or two-vessel, or three-vessel disease. There was no association between the two FSAP gene polymorphisms and different degrees of CAD and of the incidence of myocardial infarction (MI) (Figure 2).
Figure 2.
Effects of wild-type Factor VII-activating protease (FSAP) gene, and single nucleotide polymorphisms (SNPs), Marburg I (MI-SNP) and Marburg II (MII-SNP). Effects on the risk of myocardial infarction (MI) (A), coronary artery disease (CAD) (B), and one-vessel, two-vessel, or three-vessel disease (VD) (C). All associations were tested using logistic regression when compared with the wild-type FSAP group, adjusted for age, gender, body mass index (BMI); (n) represents the number of cases with each single nucleotide polymorphisms (SNP).
Association of FSAP MI-SNP and FSAP MII-SNP with cardiovascular risk factors
The effect of the FSAP gene polymorphisms on different cardiovascular risk factors was investigated separately in the CAD patients. No significant associations were found with hypertension, diabetes mellitus, smoking, and obesity; however negative association with some metabolic parameters was detected in the CAD patients (Table 1). The MI-SNP showed a small but significant association with the lack of hyperlipoproteinemia (OR=0.601; 95% CI, 0.344–1.051; P=0.074) (Figure 3).
Figure 3.
Effects of wild-type Factor VII-activating protease (FSAP) gene, and single nucleotide polymorphisms (SNPs), Marburg I (MI-SNP) and Marburg II (MII-SNP). Effects on the risk of obesity (A), smoking (B), diabetes mellitus (DM) (C), hypolipoproteinaemia (HLP) (D), and arterial hypertension (AH) (E). All associations were tested using logistic regression when compared with the wild-type FSAP group, adjusted for age, gender, body mass index (BMI); (n) represents the number of cases with each single nucleotide polymorphisms (SNP).
Discussion
Early and accurate identification of individuals with a genetic predisposition for developing coronary artery disease (CAD) is important for effective implementation of preventative lifestyle modifications and medical interventions [4–6]. Recent studies have shown that the use of a genomic risk score (GRS), based on the identification of a large number of single nucleotide polymorphisms (SNPs) can more efficiently stratify risk for the development of CAD, and could provide clinically relevant predictive data [4–6]. The aim of the present preliminary study was to attempt to determine whether SNPs of the Factor VII-activating protease (FSAP) gene, Marburg I (MI-SNP) and Marburg II (MII-SNP), were associated with CAD, and with its associated cardiovascular and metabolic risk factors. Because the findings of this study could have implications in the future risk assessment for patient assessment and management, the role of the FSAP gene SNPs was investigated in a population-based study but did not demonstrate a causal role of MI-SNP and MII-SNP in cases of established CAD.
The frequency of MI-SNP has been reported to range from between 4.3–9% in European populations [17]. The present study confirmed these previous findings, as a population frequency of MI-SNP of 7.8%, and a population frequency of MII-SNP of 3.8% was found in the German population studied. FSAP, a plasma-hyaluronan-binding protein with serine protease activity, cleaves urinary plasminogen activator (UPA), coagulation factor VII, and tissue factor pathway inhibitor (TFPI), and helps regulate coagulation, tissue remodeling, and vascular inflammation [8–11]. Also, a previously published study has shown that an increased FSAP plasma level is a marker of plaque instability [13]. Importantly, increased plasma levels of FSAP protein in patients with acute coronary syndrome (ACS) has been shown to be a predictive biomarker for the late occurrence of an acute ischemic event caused by plaque instability (<1 year) [14]. The FSAP gene carrying the MI-SNP has previously been reported to reduce the ability to activate pro-uPA and FVII, and inactivate TFPI, which may result in a prothrombotic state [17,18].
Despite the fact that the FSAP gene and the FSAP protein are involved in vascular inflammation and disease [13–16], only one clinical study has examined whether FSAP SNPs play a role in the development and progression of human atherosclerosis [17]. Willeit et al. studied the FSAP gene polymorphisms, MI-SNP and MII-SNP, in the Bruneck Study population, and reported no significant association between the MI-SNP and early atherogenesis. However, the polymorphism MI-SNP has previously been shown to be a strong predictor of risk for atherogenesis, and a significant association between MI-SNP expression and with carotid artery stenosis (lumen narrowing of >40%) has been shown [19]. In the same previously published study, MII-SNP of the FSAP gene, which is commonly co-segregated with the MI-SNP, has been shown to be unrelated to atherogenesis and the progression of atherosclerosis [19]. However, in their study, Willeit et al. did not examine the association between the FSAP gene SNPs and coronary atherosclerosis [19].
It remains unknown, whether or not there is a relationship between expression of the MI-SNP and MII-SNP of FSAP and metabolic parameters or other cardiovascular risk factors. In the present study, MI-SNP and MII-SNP of FSAP were not associated with the early development of CAD and their association remained non-statistically significant when adjusting the logistic regression model for other cardiovascular risk predictors (Figure 3). Also, the MII-SNP polymorphism showed a protective effect on the development of CAD and was significantly associated with a reduced risk of developing CAD in the German population of participants in this study (Figure 2). Therefore, the findings of this preliminary study support the view that MII-SNP expression might be a genetic marker for reduced risk of developing CAD, and that people who carry the MII-SNP may a reduced risk of developing CAD. Also, the findings of the present study showed that there was no association between expression of the two FSAP gene polymorphisms, and different vessels affected, or the incidence of myocardial infarction (Figure 2). These conclusions were further supported by the association analysis, which did not affect the association between FSAP SNPs and metabolic parameters or other cardiovascular risk factors, using both case-control and cases-only analysis.
A previously reported study from Ireland et al. investigated the effect of MI-SNP on CAD and found no significant effect in a population, but observed an interactive effect on risk between the MI-SNP and elevated levels of cholesterol and triglyceride [23]. In agreement with this finding, in the present study, there was no association between CAD and the incidence of myocardial infarction in the population studied. In contrast, in this study, the MI-SNP polymorphism was associated with absence of hyperlipoproteinemia (OR=0.601; 95% CI, 0.344–1.051; P=0.074) (Figure 3; Table 1). In this study, there was an association between MII-SNP with a reduced risk of developing CAD in the German population studied. Also, no significant associations were found between expression of the two FSAP gene polymorphisms and other cardiovascular risk factors, including diabetes mellitus, arterial hypertension, obesity, and smoking in patients with CAD in the German population studied (Figure 3; Table 1).
This study has several limitations. Two naturally occurring SNP polymorphisms of the FSAP gene were studied in a small population of patients in a single center in Germany. Because this study investigated these gene polymorphisms and their association with CAD and risk factors for CAD, which can vary between populations, the findings of the study should be compared with the findings of future studies that include larger, multicentre, and varied populations worldwide. The findings of this preliminary study should be interpreted with caution, as expression analysis studies were not performed and no evidence was found to support whether the expression of FSAP gene polymorphisms was different in patients with angiographically-confirmed CAD or symptomatic patients with angina. This study was not designed to study the association between the expression of the FSAP polymorphism and survival in patients with ACS, which would require a long-term longitudinal epidemiological study, to determine whether carriers of the polymorphism face a higher risk of thromboembolic events, non-fatal or fatal myocardial infarction. Further studies are required on the associations between the expression of SNPs of the FSAP gene, vascular pathophysiology, and risk stratification for clinical outcome in patients with CAD. Finally, the predictive functional consequences of expression of the FSAP gene polymorphisms, using bioinformatics tools, may be required to support clinical study findings in future.
We have previously shown that the activity of the FSAP protein was increased in the plasma of patients with recent acute coronary syndrome (ACS) [14]. This previous study demonstrated that a single FSAP measurement had some predictive value in a population of patients with ACS at high risk for myocardial ischemia and infarction [14]. Increased inflammation could affect the most important cardiovascular parameters, explaining the associations observed in patients with early-onset CAD [14,27]. In view of the findings of our previous study and the present study, it might be possible to hypothesize that expression of FSAP SNP plays a role in the chronic and repeated inflammatory stimuli typical of CAD in patients. In the present study, although the two functional SNPs of FSAP were not related to cardiovascular risk and the occurrence of CAD, the protective effect of FSAP MII-SNP in patients treated for CAD is clinically difficult to explain. These combined genetic and functional studies of the MI-SNP and MII-SNP polymorphism might add new information to the statistical and biological risk profile driving the progression of CAD. Further studies are needed to identify the causal variants of these associated SNPs, as well as their functional properties, leading to the increased risk of CAD and ACS.
Conclusions
The findings of this preliminary study in a single population in Germany showed that for naturally occurring single nucleotide polymorphisms (SNPs) of the Factor VII-activating protease (FSAP) gene, Marburg I (MI-SNP) and Marburg II (MII-SNP), neither gene polymorphism was predictive or prognostic for coronary artery disease (CAD) or its risk factors. However, the presence of the MII-SNP polymorphism was associated with a reduced risk of developing CAD. This preliminary study was limited by the small study size and the single population studied. The study findings should be further investigated in future large-scale, multicentre, global populations, before assigning any association between SNP polymorphisms of the FSAP gene to CAD risk.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Williams RR, Hunt SC, Heiss G, et al. Usefulness of cardiovascular family history data for population-based preventive medicine and medical research (the Health Family Tree Study and the NHLBI Family Heart Study) Am J Cardiol. 2001;87:129–35. doi: 10.1016/s0002-9149(00)01303-5. [DOI] [PubMed] [Google Scholar]
- 2.Dong P, Yang X, Bian S. Genetic polymorphism of CYP2C19 and inhibitory effects of ticagrelor and clopidogrel towards post-percutaneous coronary intervention (PCI) platelet aggregation in patients with acute coronary syndromes. Med Sci Monit. 2016;22:4929–36. doi: 10.12659/MSM.902120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohmueller KE, Pearce CL, Pike M, et al. Metaanalysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–82. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 4.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham G, Havulinna AS, Bhalala OG, et al. Genomic prediction of coronary heart disease. Eur Heart J. 2016;37:3267–78. doi: 10.1093/eurheartj/ehw450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Pan Y, Dai L, et al. Association of genetic polymorphisms on vascular endothelial growth factor and its receptor genes with susceptibility to coronary heart disease. Med Sci Monit. 2016;22:31–40. doi: 10.12659/MSM.895163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li JF, Peng DY, Ling M, Yin Y. Evaluation of adenosine triphosphate-binding cassette transporter A1 (ABCA1) R219K and C-reactive protein gene (CRP) +1059G/C gene polymorphisms in susceptibility to coronary heart disease. Med Sci Monit. 2016;22:2999–3008. doi: 10.12659/MSM.897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi-Miura NH, Tobe T, Sumiya J, et al. Purification and characterization of a novel hyaluronan-binding protein (PHBP) from human plasma: it has three EGF, a kringle and a serine protease domain, similar to hepatocyte growth factor activator. J Biochem. 1996;119:1157–65. doi: 10.1093/oxfordjournals.jbchem.a021362. [DOI] [PubMed] [Google Scholar]
- 9.Kannemeier C, Feussner A, Stöhr H-A, et al. Factor VII and single-chain plasminogen activator-activating protease: Activation and autoactivation of the proenzyme. Eur J Biochem. 2001;268:3789–96. doi: 10.1046/j.1432-1327.2001.02285.x. [DOI] [PubMed] [Google Scholar]
- 10.Römisch J, Feussner A, Vermöhlen S, Stöhr HA. A protease isolated from human plasma activating Factor VII independent of tissue factor. Blood Coagul Fibrinolysis. 1999;10:471–79. [PubMed] [Google Scholar]
- 11.Etscheid M, Kress J, Seitz R, Dodt J. The hyaluronic acid-binding protease: A novel vascular and inflammatory mediator? Int Immunopharmacol. 2008;8:166–70. doi: 10.1016/j.intimp.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Stephan F, Hazelzet JA, Bulder I, et al. Activation of factor VII-activating protease in human inflammation: A sensor for cell death. Crit Care. 2011;15:R110. doi: 10.1186/cc10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parahuleva MS, Kanse SM, Parviz B, et al. Factor seven activating protease (FSAP) expression in human monocytes and accumulation in unstable coronary atherosclerotic plaques. Atherosclerosis. 2008;196:164–71. doi: 10.1016/j.atherosclerosis.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Parahuleva MS, Hölschermann H, Zandt D, et al. Circulating Factor VII activating protease (FSAP) is associated with clinical outcome in acute coronary syndrome. Circ J. 2012;76:2653–61. doi: 10.1253/circj.cj-11-1502. [DOI] [PubMed] [Google Scholar]
- 15.Parahuleva MS, Hölschermann H, Erdogan A, et al. Factor seven ativating potease (FSAP) levels during normal pregnancy and in women using oral contraceptives. Thromb Res. 2010;126:e36–40. doi: 10.1016/j.thromres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Parahuleva MS, Langanke E, Holschermann H, et al. Nicotine modulation of Factor VII activating protease (FSAP) expression in human monocytes. J Atheroscler Thromb. 2012;19:962–69. doi: 10.5551/jat.9589. [DOI] [PubMed] [Google Scholar]
- 17.Etscheid M, Muhl L, Pons D, et al. The Marburg I polymorphism of factor VII activating protease is associated with low proteolytic and low pro-coagulant activity. Thromb Res. 2012;130:935–41. doi: 10.1016/j.thromres.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Kanse SM, Declerck PJ, Ruf W, et al. Factor VII-activating protease promotes the proteolysis and inhibition of tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2012;32:427–33. doi: 10.1161/ATVBAHA.111.238394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willeit J, Kiechl S, Weimer T, et al. Marburg I polymorphism of factor VII activating protease: A prominent risk predictor of carotid stenosis. Circulation. 2003;107:667–70. doi: 10.1161/01.cir.0000055189.18831.b1. [DOI] [PubMed] [Google Scholar]
- 20.Hanson E, Kanse SM, Joshi A, et al. Plasma factor VII-activating protease antigen levels and activity are increased in ischemic stroke. J Thromb Haemost. 2012;10:848–56. doi: 10.1111/j.1538-7836.2012.04692.x. [DOI] [PubMed] [Google Scholar]
- 21.Sidelmann JJ, Vitzthum F, Funding E, et al. Factor VII-activating protease in patients with acute deep venous thrombosis. Thromb Res. 2008;122:848–53. doi: 10.1016/j.thromres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Hoppe B, Tolou F, Radtke H, et al. Marburg I polymorphism of factor VII-activating protease is associated with idiopathic venous thromboembolism. Blood. 2005;105:1549–51. doi: 10.1182/blood-2004-08-3328. [DOI] [PubMed] [Google Scholar]
- 23.Gulesserian T, Hron G, Endler G, et al. Marburg I polymorphism of factor VII-activating protease and risk of recurrent venous thromboembolism. Thromb Haemost. 2006;95:65–67. [PubMed] [Google Scholar]
- 24.Trompet S, Pons D, Kanse SM, et al. Factor VII activating protease polymorphism (G534E) is associated with increased risk for stroke and mortality. Stroke Res Treat. 2011;2011:424759. doi: 10.4061/2011/424759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ireland H, Miller GJ, Webb KE, et al. The factor VII activating protease G511E (Marburg) variant and cardiovascular risk. Thromb Haemost. 2004;92:986–92. doi: 10.1160/TH04-05-0275. [DOI] [PubMed] [Google Scholar]
- 26.Tag CG, Mengsteab S, Weiskirchen R, Kanse SM. Rapid genotyping of the G534E polymorphism (Marburg I) of the gene encoding the factor VII-activating protease (FSAP) by LightCycler PCR. Clin Biochem. 2007;40:1063–64. doi: 10.1016/j.clinbiochem.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Parahuleva MS, Maj R, Hölschermann H, et al. Regulation of monocyte/macrophage function by factor VII activating protease (FSAP) Atherosclerosis. 2013;230:365–72. doi: 10.1016/j.atherosclerosis.2013.08.007. [DOI] [PubMed] [Google Scholar]




