Abstract
Background
The POZ/BTB and AT-hook-containing Zinc finger protein 1 (PATZ1) is a ubiquitously expressed transcription factor belonging to the POZ domain Krüppel-like zinc finger (POK) family. It is involved in the pathogenesis of a growing list of human diseases, including cancer. The effect of PATZ1 on serous ovarian carcinoma (SOC) remains unclear. This study initially explored the clinical significance of PATZ1 in patients with SOC, the relationship between its expression and the prognosis of SOC patients, and its role in tumor proliferation and invasion.
Material/Methods
Immunohistochemistry and quantitative real-time polymerase chain reaction (qPCR) were performed to characterize the expression of PATZ1 in SOC tissues. The relationship between PATZ1 expression and the clinicopathological features of patients with SOC was analyzed by chi-square test. Kaplan-Meier method and Cox regression analyses were utilized to evaluate the prognosis of SOC. PATZ1-constructed transfection-mediated overexpression was conducted. The CCK-8 assay was performed to examine the proliferation, while Transwell assay was used to detect the invasive capability.
Results
The results of IHC and qPCR analyses showed that the expression of PATZ1 in cancerous tissue was significantly lower than that in non-cancerous tissues. Meanwhile, PATZ1 expression was significantly associated with tumor differentiation and LN metastasis. Survival analysis showed that PATZ1 expression was one of the independent prognosis factors for overall survival of SOC patients. In addition, overexpression of PATZ1 inhibited the proliferation and invasion of OVCAR3 cells by in vitro experiments.
Conclusions
Our data suggest that PATZ1 is a novel prognostic marker in SOC.
MeSH Keywords: Cell Proliferation, Ovarian Neoplasms, Prognosis
Background
Epithelial ovarian carcinoma (EOC) is one of the most common gynecological malignancies in the world [1], and serous ovarian carcinoma (SOC) is the major histological type of EOC. As an extremely aggressive disease, EOC causes an estimate 125 000 annual deaths worldwide [2], and is one of the most common causes of female cancer mortality. Due to the difficulties of early diagnosis, most EOC patients have metastases or extensive local invasion at the time of diagnosis [3]. Combined with the high recurrence rate of EOC, the 5-year overall survival rate is less than 50% [4]. The clinical outcome of SOC still remains unsatisfactory. In order to improve the diagnosis and prognosis of SOC, more sensitive biomarkers and effective therapies are urgently needed.
The POZ/BTB and AT-hook-containing Zinc finger protein 1 (PATZ1) is a ubiquitously expressed transcription factor. In addition to the C-terminal zinc finger DNA binding domain, PATZ1 also contains a central DNA binding AT hook domain and an N-terminal BTB domain for protein interaction [5–7], which make it essential in the remodeling of chromatin during transcription. It was proved that PATZ1 acts as either an activator or a repressor, depending on the cellular context in the regulation of gene expressions [8] and thus is involved in a variety of physiological and pathophysiological processes. PATZ1 was first found to attenuate the RNF4-mediated enhancement of androgen receptor-dependent transcription [9]. While spermatogenesis is essentially dependent on the action of androgens, PATZ1 might be involved in spermatogenesis [10]. Previous animal studies revealed that knockout of PATZ1 resulted in most mice dying in utero or soon after birth, which was likely caused by the developmental defects in cardiac outflow tract and neurogenesis [11,12]. Although a few PATZ1 knockout mice grew to the adult stage, they showed a dwarf phenotype, as PATZ1 was shown to play a role in cell proliferation, premature senescence [11], and apoptosis [13,14]. In addition, PATZ1 serves as an important pluripotency regulator for embryonic stem cells by maintaining the expressions of NANOG and OCT4 [15]. Of note, more and more studies have revealed that PATZ1 participates in various cancers and it was reported to be both a oncogene and a tumor suppressor. The up-regulation and mislocalization of PATZ1 could be associated with the development of testicular germ cell tumors (TGCTs) [16]. Meanwhile, it was reported that knockdown of PATZ1 suppresses cell proliferation and PATZ1 could be a potential proto-oncogene in colorectal cancer [17]. Recently, PATZ1 was revealed to be a prognostic marker for glioblastoma. Although high expression level of PATZ1 was correlated with a poor prognosis of proneural glioblastoma, PATZ1 showed a low expression in another mesenchymal subtype, indicating PATZ1 plays complicated roles in regulating tumor progression [18]. The expression and roles of PATZ1 in SOC still remain unclear.
In this present study, we detected the expression of PATZ1 in SOC tissues and other cancerous tissues by immunohistochemistry (IHC) analysis and qPCR. Then, we evaluated the correlation of PATZ1 expression with the clinicopathologic features and survival of SOC patients. We found that PATZ1 expression was an independent prognostic factor for SOC. Of note, PATZ1 can inhibit tumor progression via suppressing the proliferation and invasion of tumor cells.
Material and Methods
Patients and samples
This study complied with the Helsinki Declaration and was approved by the Research Ethics Committee of Yidu Central Hospital of Weifang. Informed consent was collected from every patient enrolled in this study. A total of 208 tissue samples from SOC patients were obtained from the Department of Gynecology and Obstetrics, Yidu Central Hospital of Weifang (Weifang, China) from January 2015 to December 2016, including 104 SOC tissues and 104 adjacent non-tumor tissues. In addition, fresh SOC tissues and adjacent non-tumor tissues were resected from another 22 cases of SOC patients, which were used to compare the expressions of PATZ1 between cancerous and non-cancerous tissues.
All of the 104 SOC patients enrolled in our retrospective study were staged and graded according to the International Federation of Gynecology and Obstetrics (FIGO) criteria [19]. Among them, 14 patients were histologically graded as well differentiated (G1), while 44 and 46 patients were graded as moderately (G2) and poorly differentiated (G3), respectively. In terms of FIGO stage, 47 patients (45.2%) were in FIGO stage I/II while the other 57 cases (54.8%) were in FIGO stage III/IV. The detailed clinicopathological features of the enrolled patients are summarized in Table 1.
Table 1.
Clinical characteristics of SOC patients.
| Parameter | Patients | PATZ1 protein level by IHC | P value | |
|---|---|---|---|---|
| (n=104) | Low (n=41) | High (n=63) | ||
| Age (years) | 0.899 | |||
| ≤54.0 | 55 | 22 | 33 | |
| >54.0 | 49 | 19 | 30 | |
| CA-125 (U/ml) | 0.358 | |||
| ≤554.0 | 50 | 22 | 28 | |
| >554.0 | 54 | 19 | 35 | |
| Differentiation | 0.049* | |||
| Well/moderate | 58 | 18 | 40 | |
| Poor | 46 | 23 | 23 | |
| LN metastasis | <0.001* | |||
| Negative | 64 | 15 | 49 | |
| Positive | 40 | 26 | 14 | |
| FIGO stage | 0.155 | |||
| I–II | 47 | 15 | 32 | |
| III–IV | 57 | 26 | 31 | |
IHC – immunohistochemistry; LN – lymph node; FIGO – International Federation of Gynecology and Obstetrics.
Patient follow-up
Patient follow-up survival data were obtained retrospectively through medical record analyses. The follow-up period varied from 4 months to 120 months, and the median follow-up period was 60 months. The 5-year overall survival rate was 81.2%, and the median survival time was 91 months.
Immunohistochemistry (IHC) and IHC evaluation
The expression of PATZ1 in SOC tissues was detected by immunohistochemical staining, which was described previously [20], using PATZ1 antibody (1: 1000, #26225-1-AP, Proteintech, UK).
The expression of PATZ1 was assessed by the degree of staining and the percent of positively stained cells, which were examined and scored by 2 pathologists independently. Briefly, in terms of the degree of staining, no staining, weak staining (light yellow), moderate staining (dark yellow), and strong staining (brown) were scored as 0, 1, 2, and 3, respectively. Meanwhile, the percent of positively stained cells were scored as follows: 1 for no more than 25%, 2 for 26–50%, 3 for 51–75%, and 4 for 76–100%. Our final score equals the degree of staining scores multiplied by the score for the percent of positively stained cells. We considered it a low expression level if the final score was no more than 4; otherwise, we included it as the PATZ1 high expression cases.
Cell culture and transfection
SV-40, a normal ovarian epithelial cell line, was purchased from Applied Biological Materials Inc (#T1074, Canada) and human serous ovarian carcinoma (SOC) cell line (OVCAR3) was obtained from Thermo Fisher Scientific (PA, USA). They were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS and 100 IU/mL penicillin and 100 μg/mL streptomycin at 37°C and 5% CO2 in an incubator.
Human PATZ1 cDNA was isolated by RT-PCR and subcloned into a pcDNA vector to generate the pcDNA-PATZ1 construct. Then, the pcDNA-PATZ1 construct was transfected into the OVCAR3 cells by Lipo3000 (Invitrogen, PA) following the manufacturer’s instructions. Other experiments were performed with those cells after further incubation for 48 h.
Quantitative real-time PCR
Total RNA was extracted from cultured cells by utilizing TRIzol reagent (Life Technology, CA) following the manufacturer’s instructions. A High-capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, PA) was used for reverse transcription PCR. The resulting cDNA was used as the template for quantitative real-time PCR to examine the gene expressions. The gene expressions were analyzed by using the 2−ΔΔCT method [21]. GAPDH was used as an internal reference gene. The primer sequences are as followings:
PATZ1 forward, 5′-AAGTGTCAGACCTGCAATG-3′,
PATZ1 reverse, 5′-CAGATGCTACAGAAGTTGCT-3′;
GAPDH forward, 5′-AGGGCTGCTTTTAACTCTGGT-3′,
GAPDH reverse, 5′-CCCCACTTGATTTTGGAGGGA-3′.
Western blot analysis
Total proteins were extracted from cultured cells with RIPA buffer containing protease inhibitor and phosphatase inhibitors. Protein concentrations were measured with a BCA kit. We took 30 μg of proteins from each sample for SDS-PAGE, followed by the analysis of immunoblotting. After blocking the membrane with 5% BSA for 1 h at room temperature, anti β-actin and anti-PATZ1 primary antibodies (1: 1000, #26225-1-AP, Proteintech, UK) were incubated with the membrane at 4°C overnight. The secondary antibody was added and incubated at room temperature for 1 h. Finally, electrogenerated chemiluminescence (ECL) was added, followed by exposure to X-ray films.
Cell proliferation
The effect of PATZ1 on OVCAR3 cells proliferation was determined by use of a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Rockville, MA), as described previously [22]. Briefly, OVCAR3 cells transfected with pcDNA/PATZ1 or pCDNA vector plasmids were seeded in 96-well plates at a density of 20 000 per well and then incubated at 37°C in a humidified atmosphere with 5% CO2. At 48 h post-transfection, 10 μl CCK-8 solutions were added to each well and incubated for 2 h. Absorbance was measured at 450 nm to assess the number of viable cells. The results were derived from at least 3 independently repeated experiments.
Transwell assay
The effect of PATZ1 on OVCAR3 cells invasive capacity was determined by Transwell assay using a Boyden chamber (BD Bioscience, CA) as described by others [23]. In brief, OVCAR3 cells were transfected with pcDNA/PATZ1 construct and cultured for another 48 h; then cells were re-suspended, and about 1×105 cells were seeded in the upper chambers. After incubation for 8 h, when the cells in the upper chamber adhered, the medium in the upper chambers was changed to serum-free DMEM, while the bottom chamber was filled with DMEM containing 10% FBS. After incubation for further 24 h, the upper surfaces of the Transwell chambers were wiped with cotton swabs, and the cells that had migrated to the lower surface of the membrane were fixed with 4% paraformaldehyde and stained with Hoechst 33258 for 5 min. The stained cells were photographed and counted in 5 high-power fields per insert.
Statistics
All data were analyzed using SPSS 19.0 statistics software (SPSS, USA). Chi-square (χ2) tests were employed to evaluate whether PATZ1 expression was correlated with the clinicopathological parameters of SOC. Overall survival curves were constructed by the Kaplan-Meier method and subjected to the log-rank test, with factors including age, CA-125, pathological grade, LN metastasis, FIGO stage, and PATZ1 level. Among them, factors which were shown to be prognostic significant in univariate models were further analyzed in a multivariate Cox regression model to evaluate their independent prognostic values. The hazard ratio (HR) was calculated by setting the group with the lowest hazard as the reference group. The data from PCR and cell experiments were statistically analyzed utilizing the t test. For all analyses, a P-value <0.05 was considered statistically significant.
Results
PATZ1 expression in cancerous tissues
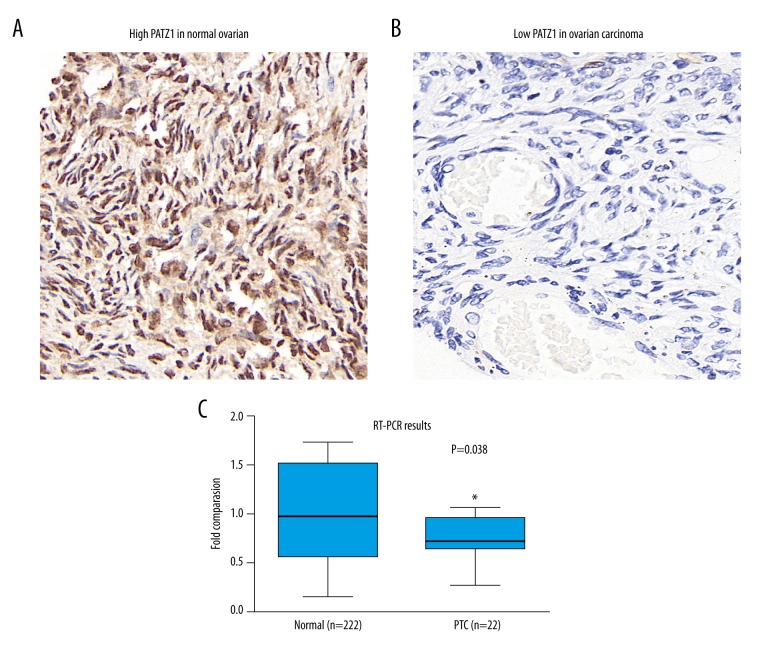
To elucidate the potential effect of PATZ1 in SOC, we first detected the expression of PATZ1 by performing immunohistochemical staining on normal ovarian tissues and SOC tissues. Generally, PATZ1 was highly expressed in normal tissue (Figure 1A) in which PATZ1 was mainly observed in the nucleus, with less extended expression in cytoplasm. Compared with normal ovarian tissue, there were far fewer positively stained cells in SOC tissues (Figure 1B), indicating the reduced expression of PATZ1 in SOC tissues. To further confirm the reduced expression of PATZ1 in cancerous tissues, we collected fresh resected SOC tissues and their adjacent non-tumor tissue from 22 SOC patients. By performing quantitative real-time PCR, we compared the expressions of PATZ1 between cancerous tissues and normal tissues at the mRNA level. As Figure 1C shows, PATZ1 was indeed significantly decreased in cancerous tissues.
Figure 1.
Expression of PATZ1 in normal and cancerous tissues. (A) Representative immunohistochemical expression of PATZ1 in normal ovarian tissue. 400× magnification. (B) Representative immunohistochemical expression of PATZ1 in ovarian cancer tissue. 400× magnification. (C) mRNA level of PATZ1 in serous ovarian carcinoma (SOC) tissues and adjacent non-tumor tissues were analyzed by qPCR. Data are mean ±SD from 3 independent experiments (* P<0.05).
Correlation between PATZ1 expression and clinicopathological features of SOC patients
Revealing the reduced expression of PATZ1 in cancerous tissues, we further explored the significance of PATZ1 expression in SOC patients. By evaluating the IHC staining for SOC tissues, among 104 SOC patients enrolled in our study, 63 patients had high PATZ1 expression and 41 patients had low PATZ1 expression. Then, the relationship of PATZ1 expression and the clinicopathological features of SOC patients were further analyzed. The results showed that low PATZ1 expression was significantly associated with poor tumor differentiation and positive LN metastasis. In contrast, there is no obvious relationship between PATZ1 expression and patient age, serum CA-125 level, or FIGO stage. The basic clinicopathological features of SOC patients are summarized in Table 1.
Prognostic potentials of PATZ1 in SOC
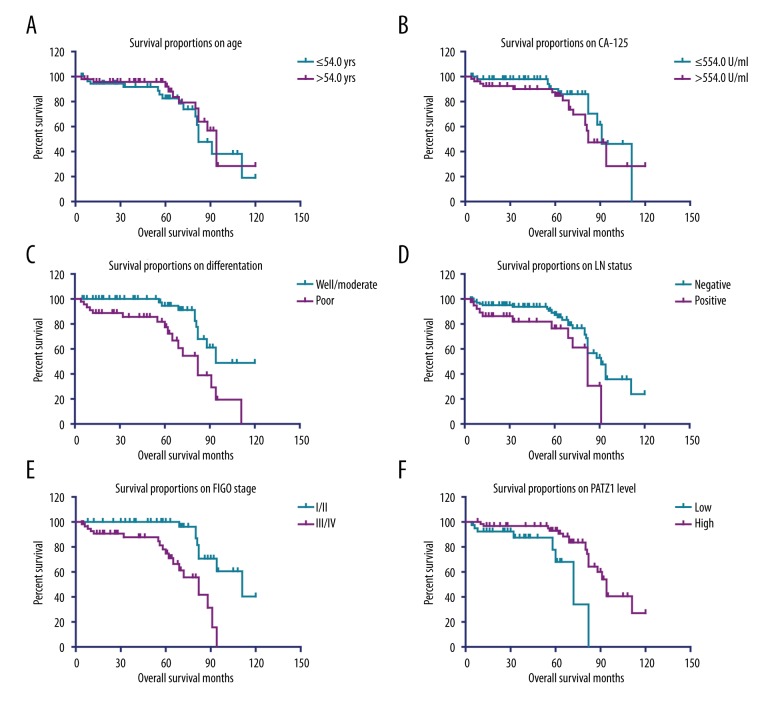
To further explore the importance of PATZ1 expression in the progression of SOC, we evaluated the survival the 104 SOC patients using Kaplan-Meier survival analysis. The 5-year overall survival rate was 81.2%, while the median survival time was 91 months. Of note, univariate analysis revealed that tumor differentiation, LN metastasis, FIGO stage, and PATZ1 expression were all significantly correlated with the overall survival time (Figure 2, Table 2). In contrast, no statistical correlations between patient age, serum CA-125 level, and survival time was found. Patients with high expression of PATZ1 showed a better clinical outcome compared with the patients with low PATZ1 expression (P=0.002, Table 2).
Figure 2.
Kaplan-Meier analysis of overall survival. Kaplan-Meier curve showed the correlations of overall survival of SOC patients with (A) patient age; (B) serum CA-125 level; (C) tumor differentiation; (D) LN status; (E) FIGO stage; and (F) PATZ1 expression.
Table 2.
Kaplan-Meier survival analysis of SOC patients.
| Parameter | Patients (n=104) | OS (months) Mean ±S.D. | 5-year OS | P value |
|---|---|---|---|---|
| Age (years) | ||||
| ≤54.0 | 55 | 86.6±5.7 | 82.6% | 0.459 |
| >54.0 | 49 | 90.5±5.7 | 92.1% | |
| CA-125 (U/ml) | ||||
| ≤554.0 | 50 | 92.9±5.3 | 90.1% | 0.240 |
| >554.0 | 54 | 84.8±5.5 | 84.6% | |
| Differentiation | ||||
| Well/moderate | 58 | 100.4±4.8 | 94.5% | 0.002* |
| Poor | 46 | 74.5±5.9 | 77.5% | |
| LN metastasis | ||||
| Negative | 64 | 97.4±4.3 | 93.1% | 0.002* |
| Positive | 40 | 69.3±5.4 | 76.4% | |
| FIGO stage | ||||
| I–II | 47 | 103.7±4.4 | 100.0% | <0.001* |
| III–IV | 57 | 71.6±4.1 | 74.8% | |
| PATZ1 expression | ||||
| Low | 41 | 65.9±5.0 | 68.1% | 0.002* |
| High | 63 | 93.7±4.2 | 92.9% | |
OS – overall survival; LN – lymph node; FIGO – International Federation of Gynecology and Obstetrics.
To further explore the role of PATZ1 expression in predicting the prognosis of SOC patients, we included those risk factors for the overall survival of SOC patients revealed by univariate analysis and performed the multivariate Cox proportional hazards regression analysis. Although univariate analysis showed that tumor differentiation, LN metastasis, FIGO stage, and PATZ1 expression were all significantly correlated with the overall survival time, only FIGO stage and PATZ1 expression were independent prognosis factors in multivariate survival analysis (Table 3). These results indicate the PATZ1 expression could serve as a potential biomarker for predicting the survival of patients with SOC.
Table 3.
Multivariate analysis for overall survival of SOC patients.
| Parameter | HR | 95% CI | P value |
|---|---|---|---|
| Differentiation | 1.80 | 0.76–4.26 | 0.181 |
| LN metastasis | 1.58 | 0.65–3.89 | 0.316 |
| FIGO stage | 3.53 | 1.33–9.38 | 0.011* |
| PATZ1 expression | 0.33 | 0.11–0.99 | 0.049* |
HR – hazard ratio; 95% CI – 95% confidence interval; LN – lymph node; FIGO – International Federation of Gynecology and Obstetrics.
PATZ1 inhibited the proliferation and invasion of ovarian carcinoma cells
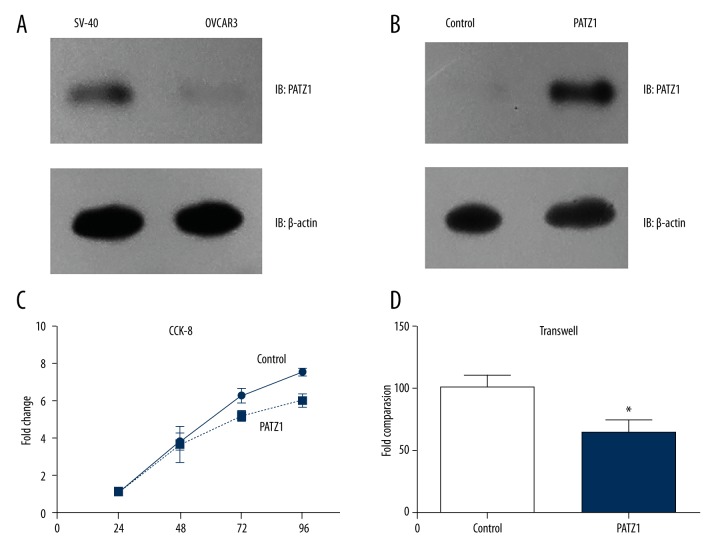
To elucidate how PATZ1 affects the progression of OSC, we further performed biological experiments in vitro, including cell proliferation and invasion assays. We utilized the normal ovarian cell line, SV-40 cells, and the human SOC cell line, OVCAR3 cells, that were established from the malignant ascites of a patient with ovarian epithelial serous adenocarcinoma. We compared the expressions of PATZ1 between SV-40 cells and OVCAR3 cells by Western blot analysis, and the PATZ1 expression in OVCAR3 cells was markedly decreased compared with that in SV-40 cells (Figure 3A). Next, we transfected OVCAR3 cells with pcDNA/PATZ1 construct to overexpress PATZ1. After confirming the effect of transfection (Figure 3B), we examined the proliferation of the transfected cells by use of a CCK-8 kit. As shown in Figure 3C, overexpression of PATZ1 significantly suppressed the proliferation of OVCAR3 cells. In addition, we also examined the effect of PATZ1 on invasion with Transwell assay. Results showed that overexpression of PATZ1 significantly inhibited the invasion capacity of OVCAR3 cells (Figure 3D). Since poorly differentiated tumors have more potent proliferation and invasion capacities [24], our cellular data are consistent with clinical findings showing that lower PATZ1 was associated with poor SOC differentiation. Additionally, lymph node metastasis is a clinical consequence of tumor cell invasion, which can also at least partially be explained by our cellular results.
Figure 3.
PATZ1 inhibited the proliferation and invasion of OVCAR3 cells. (A) Protein levels of PATZ1 in SV-40 cells and OVCAR3 cells analyzed by Western blot. (B) Transfection efficiency of pcDNA/PATZ1 construct in OVCAR3 cells was validated by Western blot. (C) The CCK-8 assay showed that overexpression of PATZ1 suppressed the proliferation of OVCAR3 cells. (D) Transwell assay showed that overexpression of PATZ1 inhibited the invasion of OVCAR3 cells. Data are mean ±SD from 3 independent experiments (* P<0.05).
Discussion
In the present study, we initially characterized the expression and functions of PATZ1 in SOC progression. As a transcriptional regulatory factor, PATZ1 has been shown to act as either an activator or a repressor in the regulation of gene expression, depending on the cellular context [14]. Accumulating evidence has shown that PATZ1 is involved in a variety of biological processes, such as embryogenesis, stemness, apoptosis, senescence, and proliferation [8]. Recently, increasing evidence has shown that PATZ1 plays essential roles in various malignancies [28–30]. For example, in thyroid cancer, the downregulation of PATZ1 results in the proceeding of differentiated to undifferentiated carcinomas, indicating its role in promoting cancer progression [29]. Notably, the roles of PATZ1 in cancer still remain controversial, for it acts as either a tumor suppressor or an oncogene. On one hand, as the inhibitor of p53 protein, it makes PATZ1 a proto-oncogene [13]; in addition, it functions in G1/S cycle transition and promotes cell growth of colon cancer cells [17]. On the other hand, it also serves as a tumor suppressor as mentioned above, exerting inhibiting effects. In addition to bench findings, the role of PATZ1 in clinical application is also being revealed. Its prognostic predictive significance has only been reported in glioblastoma [18]. Glioblastoma consists of 2 major subtypes: proneural and mesenchymal. Guadagno et al. showed that PATZ1 was upregulated in proneural glioblastoma but was downregulated in mesenchymal subtype. Their data suggested that a higher level of PATZ1 is an unfavorable prognostic marker for the overall survival of proneural glioblastoma patients, but functions as a tumor suppressor in the mesenchymal subtype [18]. Importantly, as a very important player in cancer, it has not been reported in SOC. Therefore, we aimed to explore PATZ1 expression in SOC tissues and to determine whether it was capable for serving as a biomarker to predict disease progression and prognosis.
By doing the IHC staining, we confirmed that PATZ1 is richly expressed in normal ovarian tissues. In contrast, PATZ1 expression was significantly reduced in SOC tissues, which is consistent with previous studies showing decreased levels of PATZ1 in cancerous tissue [28,29]. To confirm the PATZ1 expression pattern, we conducted qPCR with SOC tissues and their adjacent non-tumor tissue. Consistently, PATZ1 expression in cancerous tissues was much lower than it was in non-cancerous tissues. To further explore the function of PATZ1 in SOC, we utilized OVCAR3, a serous ovarian carcinoma cell line, and performed in vitro experiments. PATZ1 expression in OVCAR3 cells was also significantly lower than it was in normal ovarian cells and SV-40 cells. We further explored the function of PATZ1 in OVCAR3 cells by overexpressing PATZ1. OVCAR3 cells were transfected with pcDNA/PATZ1 construct with Lipo3000. The CCK-8 assay was performed and revealed that overexpression of PATZ1 obviously suppressed the proliferation of OVCAR3 cells. In addition, overexpression of PATZ1 inhibited the invasive capacity of OVCAR3 cells as revealed by Transwell assay. These results all indicate that PATZ1 serves as a tumor suppressor in OSC. Our results are consistent with its functions in thyroid carcinoma [29] and mesenchymal glioblastoma [18]. Details of the mechanisms of PATZ1 in SOC need to be further studied in the future, and the results will be of great importance in drug target discovery.
Besides the expression and function of PATZ1 in SOC, we also explored whether PATZ1 is a potential biomarker for predicting the progression and prognosis of SOC. By evaluating the IHC staining of the 104 SOC tissues, we divided the SOC patients into PATZ1 high-expression and PATZ1 low-expression groups. Kaplan-Meier survival analysis was then performed. Consistent with previous studies [31], tumor differentiation, LN metastasis, and FIGO stage were all significantly correlated with the overall survival time. We also found PATZ1 expression was significantly correlated with the overall survival time. By performing multivariate Cox proportional hazards regression analysis, we further determined that FIGO stage and PATZ1 expression are independent prognostic factors for SOC.
Although our results, together with previous studies, showed a tumor-suppressing role of PATZ1 in thyroid carcinoma [29] and ovarian carcinoma, it was also reported to be a tumor promoter in colon cancer and proneural glioblastoma. Its role in other malignancies remains to be elucidated. Since PATZ1 is a transcription factor, it is reasonable that it may contribute to various signaling pathways in different tissues by regulating distinct targeted genes. Therefore, we should always keep in mind that it is critical to balance its distinct roles in different tissues for its future application in tumor therapies. One possible strategy is the targeted route of drug administration combined with artery interventional therapy to minimize its distribution to other tissues.
Conclusions
We revealed that PATZ1 expression was markedly lower in SOC tissues, and PATZ1 expression was an independent prognosis factor for SOC. In addition, we showed that PATZ1 could suppress the progression of SOC via inhibiting the proliferation and invasion of ovarian carcinoma cells.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B, Cai FF, Zhong XY. An overview of biomarkers for the ovarian cancer diagnosis. Eur J Obstet Gynecol Reprod Biol. 2011;158(2):119–23. doi: 10.1016/j.ejogrb.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann C, Kramer B, Brucker SY, et al. Relevance of pelvic and para-aortic node metastases in early-stage ovarian cancer. Anticancer Res. 2014;34(11):6735–38. [PubMed] [Google Scholar]
- 4.Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204(6):466–78. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Siggs OM, Beutler B. The BTB-ZF transcription factors. Cell Cycle. 2012;11(18):3358–69. doi: 10.4161/cc.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedele M, Benvenuto G, Pero R, et al. A novel member of the BTB/POZ family, PATZ, associates with the RNF4 RING finger protein and acts as a transcriptional repressor. J Bio Chem. 2000;275(11):7894–901. doi: 10.1074/jbc.275.11.7894. [DOI] [PubMed] [Google Scholar]
- 7.Costoya JA. Functional analysis of the role of POK transcriptional repressors. Brief Funct Genomic Proteomic. 2007;6(1):8–18. doi: 10.1093/bfgp/elm002. [DOI] [PubMed] [Google Scholar]
- 8.Fedele M, Crescenzi E, Cerchia L. The POZ/BTB and AT-Hook Containing Zinc Finger 1 (PATZ1) transcription regulator: Physiological functions and disease involvement. Int J Mol Sci. 2017;18(12) doi: 10.3390/ijms18122524. pii: E2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pero R, Lembo F, Palmieri EA, et al. PATZ attenuates the RNF4-mediated enhancement of androgen receptor-dependent transcription. J Biol Chem. 2002;277(5):3280–85. doi: 10.1074/jbc.M109491200. [DOI] [PubMed] [Google Scholar]
- 10.O’Hara L, Smith LB. Androgen receptor roles in spermatogenesis and infertility. Best Pract Res Clin Endocrinol Metab. 2015;29(4):595–605. doi: 10.1016/j.beem.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Valentino T, Palmieri D, Vitiello M, et al. Embryonic defects and growth alteration in mice with homozygous disruption of the Patz1 gene. J Cell Physiol. 2013;228(3):646–53. doi: 10.1002/jcp.24174. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Hombauer M, Bilic I, et al. The zinc-finger protein MAZR is part of the transcription factor network that controls the CD4 versus CD8 lineage fate of double-positive thymocytes. Nat Immunol. 2010;11(5):442–48. doi: 10.1038/ni.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keskin N, Deniz E, Eryilmaz J, et al. PATZ1 is a DNA damage-responsive transcription factor that inhibits p53 function. Mol Cell Biol. 2015;35(10):1741–53. doi: 10.1128/MCB.01475-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valentino T, Palmieri D, Vitiello M, et al. PATZ1 interacts with p53 and regulates expression of p53-target genes enhancing apoptosis or cell survival based on the cellular context. Cell Death Dis. 2013;4:e963. doi: 10.1038/cddis.2013.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ow JR, Ma H, Jean A, et al. Patz1 regulates embryonic stem cell identity. Stem Cells Dev. 2014;23(10):1062–73. doi: 10.1089/scd.2013.0430. [DOI] [PubMed] [Google Scholar]
- 16.Fedele M, Franco R, Salvatore G, et al. PATZ1 gene has a critical role in the spermatogenesis and testicular tumours. J Pathol. 2008;215(1):39–47. doi: 10.1002/path.2323. [DOI] [PubMed] [Google Scholar]
- 17.Tian X, Sun D, Zhang Y, et al. Zinc finger protein 278, a potential oncogene in human colorectal cancer. Acta Biochim Biophys Sin (Shanghai) 2008;40(4):289–96. doi: 10.1111/j.1745-7270.2008.00405.x. [DOI] [PubMed] [Google Scholar]
- 18.Guadagno E, Vitiello M, Francesca P, et al. PATZ1 is a new prognostic marker of glioblastoma associated with the stem-like phenotype and enriched in the proneural subtype. Oncotarget. 2017;8(35):59282. doi: 10.18632/oncotarget.19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho KR, Shih Ie M. Ovarian cancer. Ann Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–90. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Guo X, Wang W, et al. Vset and transmembrane domaincontaining 1 is silenced in human hematopoietic malignancy cell lines with promoter methylation and has inhibitory effects on cell growth. Mol Med Rep. 2015;11(2):1344–51. doi: 10.3892/mmr.2014.2785. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: New insights into β-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–40. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40(5):499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coward JI, Middleton K, Murphy F. New perspectives on targeted therapy in ovarian cancer. Int J Womens Health. 2015;7:189–203. doi: 10.2147/IJWH.S52379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. Cancer J Clin. 2011;61(3):183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–88. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 28.Franco R, Scognamiglio G, Valentino E, et al. PATZ1 expression correlates positively with BAX and negatively with BCL6 and survival in human diffuse large B cell lymphomas. Oncotarget. 2016;7(37):59158–72. doi: 10.18632/oncotarget.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiappetta G, Valentino T, Vitiello M, et al. PATZ1 acts as a tumor suppressor in thyroid cancer via targeting p53-dependent genes involved in EMT and cell migration. Oncotarget. 2015;6(7):5310–23. doi: 10.18632/oncotarget.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burrow AA, Williams LE, Pierce LC, Wang YH. Over half of breakpoints in gene pairs involved in cancer-specific recurrent translocations are mapped to human chromosomal fragile sites. BMC Genomics. 2009;10:59. doi: 10.1186/1471-2164-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Xiao N, Li Z, Wang Q. Expression of inorganic pyrophosphatase (PPA1) correlates with poor prognosis of epithelial ovarian cancer. Tohoku J Exp Med. 2017;241(2):165–73. doi: 10.1620/tjem.241.165. [DOI] [PubMed] [Google Scholar]