Abstract
Objectives
Amino acid levels in serum or plasma are used for early detection and diagnosis of several diseases. The objective of this study was to analyze amino acid levels in serum exosomes, which have not been previously reported.
Design and methods
We investigated the amino acid composition of exosomes from human serum using HPLC with fluorescence detection.
Results
The composition ratios of His, Arg, Glu, Cys-Cys, Lys, and Tyr were significantly increased in the exosomes compared with those in the corresponding native serum. d-Ser, an endogenous co-agonist of the N-methyl-d-aspartate receptor, was also enriched in the exosome-eluted fraction.
Conclusions
Our results suggest that certain amino acids are enriched in the exosome-eluted fraction from human serum. These differences could have future diagnostic potential.
Abbreviations: EVs, extracellular vesicles; HPLC, high-performance liquid chromatography; NBD-F, 4-fluoro-7-nitro-2,1,3-benzoxadiazole; NMDA, N-methyl-D-aspartate; RIPA, radioimmunoprecipitation assay
Keywords: Exosomes, Human serum, Amino acid, Cystine, d-serine
Highlights
-
•
Serum amino acid levels are used for early detection and diagnosis of diseases.
-
•
The amino acid composition of exosomes from human serum was determined by HPLC.
-
•
The amino acid composition between exosomes and native serum was compared.
-
•
Certain amino acids were enriched in exosomes from human serum.
1. Introduction
Amino acid levels in the serum or plasma have been used for early detection and diagnosis of type 2 diabetes, cervical cancer, pancreatic cancer, and post-stroke cognitive impairment [1], [2], [3], [4]. Recently, exosomes (30–200 nm diameter), phospholipid bilayer-enclosed extracellular vesicles (EVs), have become the focus of disease diagnosis because they are released by exocytosis [5] into serum, urine, cerebrospinal fluid, saliva, and breast milk and may contain cellular information. According to the ExoCarta database [6], exosomes contain proteins, lipids, DNA, mRNA, and miRNA. In particular, miRNAs have been used as biomarkers for cancer diagnosis and treatment. Intracellular miRNAs present in EVs are essential for cell–cell communication [7], and an increase in miR-200c and miR-141 levels is related to the invasive ability of colorectal cancer cells [7]. However, to the best of our knowledge, there have been no reports published to date describing low-molecular-weight compounds, such as amino acids, in the exosomes from human serum.
In the present study, we determined the amino acid content of exosomes prepared from human serum using HPLC with fluorescence detection and compared those results with the amino acid content of human serum. Indeed, we found differences in amino acid composition between the exosomes and native serum.
2. Materials and methods
2.1. Chemicals
The DiagExo Serum Exosomal Protein Extraction Kit was obtained from 101 Bio (Palo Alto, CA, USA). The Exosome Antibody Kit was obtained from System Biosciences, LLC (Palo Alto, CA, USA). Methanol (MeOH), trifluoroacetic acid, and Immunostar LD Chemiluminescense Reagent were obtained from Wako Pure Chemical Industries (Osaka, Japan). Acetonitrile (CH3CN) was obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). Phosphate-buffered saline (PBS) was obtained from Nissui Pharmaceutical (Tokyo, Japan). e-PAGEL polyacrylamide gels (10%) were obtained from ATTO Corporation (Tokyo, Japan). Hybond-P polyvinylidene fluoride (PVDF) membranes and 1% skim milk were obtained from GE Healthcare Bio-Sciences AB (Uppsala, Sweden). Water (H2O) was purified using a Millipore purification system (Nihon Millipore K.K., Tokyo, Japan).
2.2. Serum samples
This study was approved by the ethics committee of the Faculty of Pharmaceutical Sciences, Toho University (approval number 23-1, 24-6). Written informed consent was obtained from all individuals prior to participation.
Blood (5 mL) was collected from healthy human volunteers (n = 9, 2 men and 7 women) before lunch (between 11:00 A.M. and 12:00 P.M.) in Venoject II AUTOSEP tubes (Terumo Corporation, Tokyo, Japan). Samples stood for 30 min at room temperature, and then were centrifuged at 1200 × g for 15 min and stored at − 80 °C until analysis.
2.3. Isolation of exosomes from serum
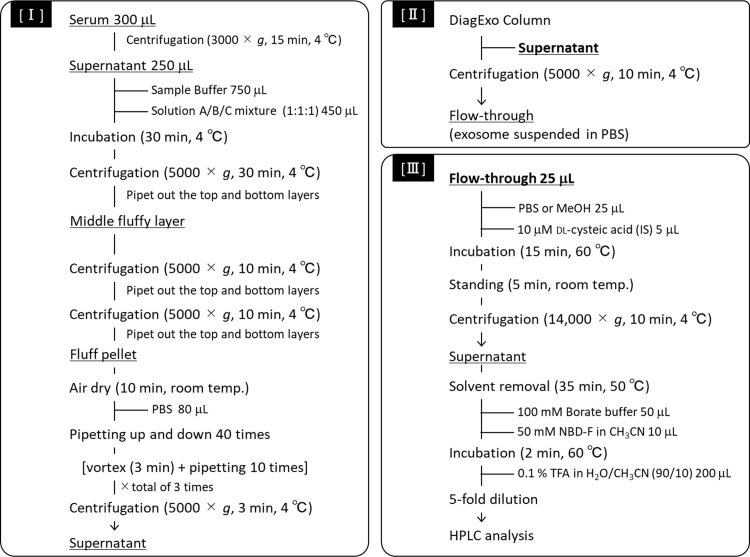
Exosome isolation was carried out according to the manufacturer's (101 Bio's) instructions; the procedure is outlined in Supplemental Scheme 1. Aliquots of the flow-through fractions were stored at − 80 °C until use.
2.4. Transmission electron microscopy (TEM)
Aliquots of the flow-through fractions were diluted 5-fold with PBS, placed on a Cu grid with supporting film (JEOL, Tokyo, Japan), subjected to hydrophilic treatment (HT-400; JEOL Datum), and negatively stained with 2 µL of aqueous uranyl acetate. Samples were examined with a JEM-1010 transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 100 kV.
2.5. Western blot analysis
Exosomes in PBS were lysed with radioimmunoprecipitation assay (RIPA) buffer, according to the manufacturer's instructions. Protein amounts were determined using the Bradford assay. Proteins (7.5 µg) were denatured, separated on a 10% e-PAGEL gel in Tris-Gly-SDS buffer (0.25 M Tris, 1.92 M Gly, and 1% SDS), and transferred to a Hybond-P PVDF membrane in Towbin buffer (25 mM Tris, pH 8.3, 192 mM Gly, and 15% MeOH). The membrane was blocked with 1% skim milk in Tris-buffered saline with Tween®20. Exosome-specific markers were detected using primary antibodies against CD63 and a secondary antibody conjugated with horseradish peroxidase. Blots were treated with Immunostar LD and visualized using the chemiluminescent image analyzer, EZ-Capture MG (Atto Corporation, Tokyo, Japan).
2.6. Determination of d- and l-Ser and other amino acids
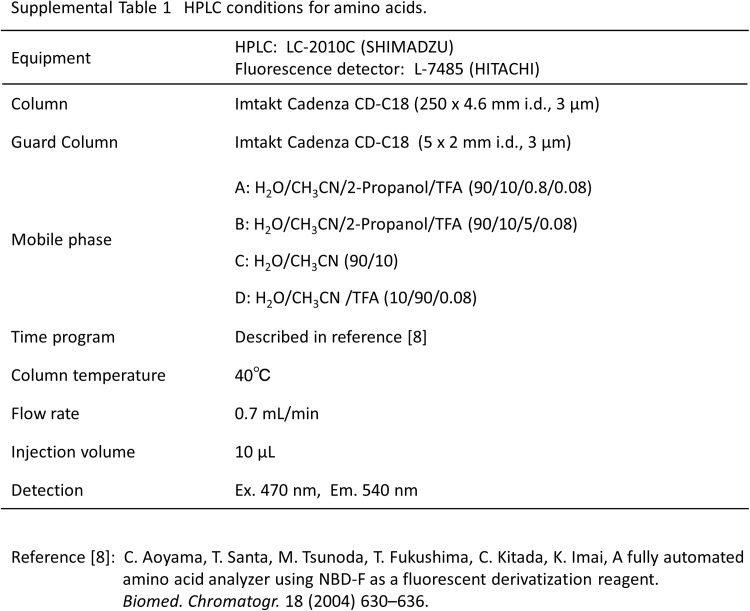
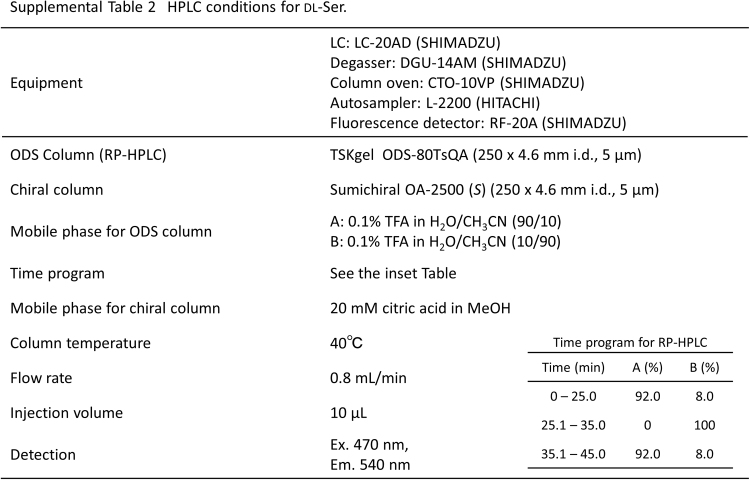
To disrupt exosomes, the flow-through fraction (25 µL), MeOH (25 µL), and 10 μM dl-cysteic acid (internal standard, 5 µL) were mixed and incubated for 15 min at 60 °C. The solution was centrifuged at 14,000 × g for 10 min at 4 °C, and the supernatant was subjected to fluorescence derivatization with 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F) as described previously [8] (Supplemental Scheme 1 [III]). Serum (10 µL) was subjected to the same fluorescence derivatization. Amino acid [[8], Supplemental Table 1] and d-Ser [[9], Supplemental Table 2] levels were determined using HPLC with fluorescence detection.
2.7. Statistical analysis
The ratios of d-Ser to total Ser and of each amino acid to total amino acids in the exosome-eluted fractions and serum samples were calculated as percentages and compared using the non-parametric Mann–Whitney U-test (2-tailed). A p value below 0.05 was considered statistically significant. The Spearman rank correlation test was performed to determine statistical correlations between variables.
3. Results and discussion
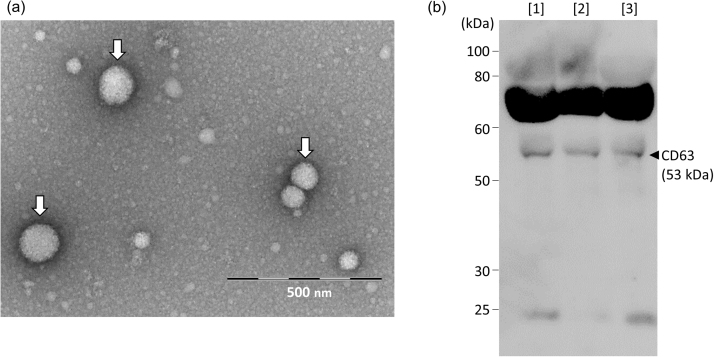
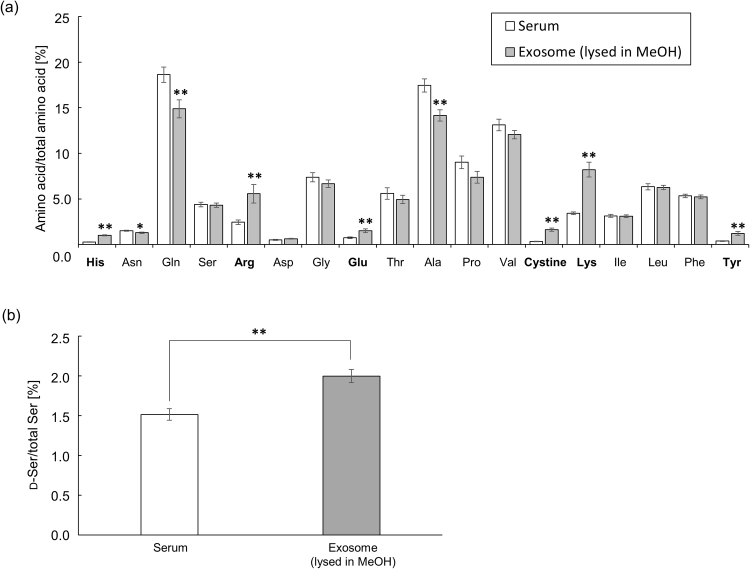
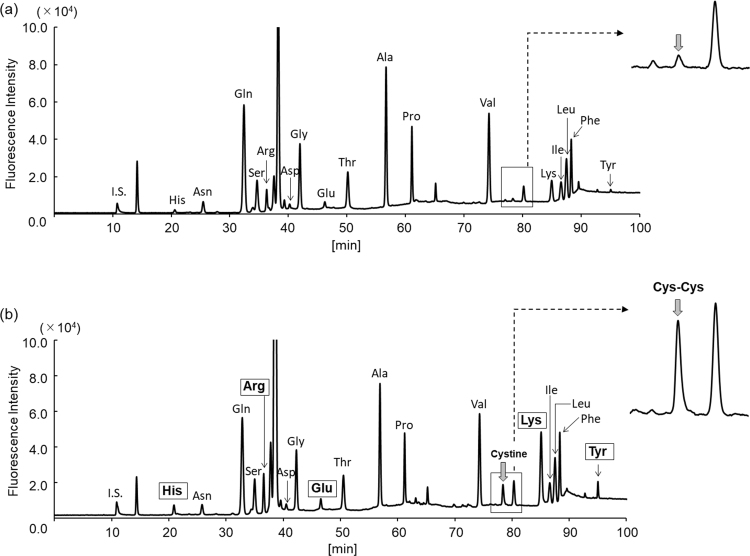
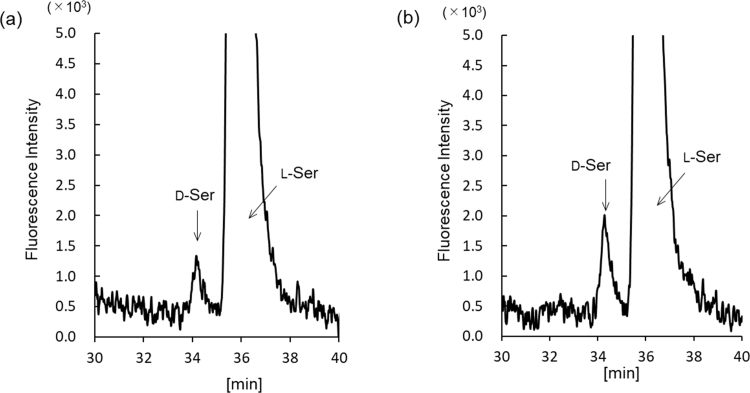
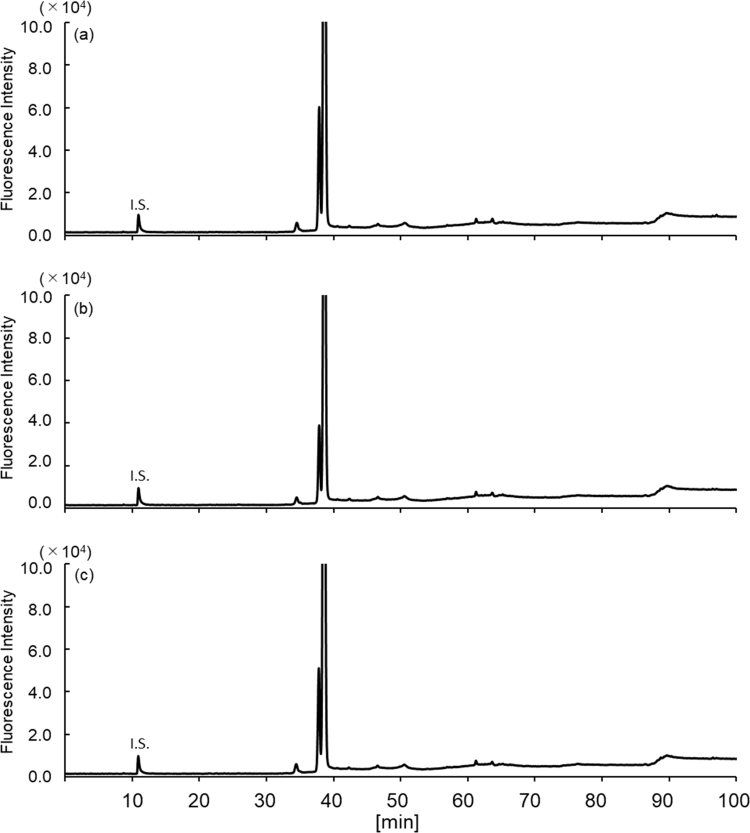
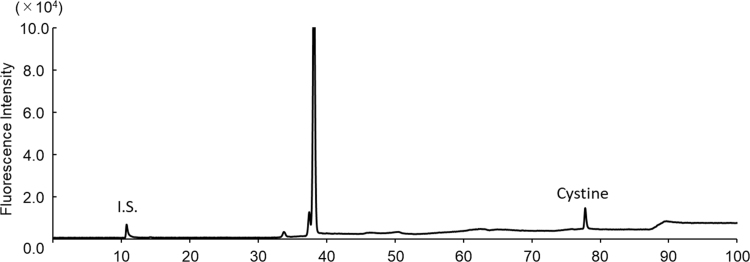
The exosomes obtained as shown in Supplemental Scheme 1 were round with a size range of 50–100 nm (Fig. 1a, arrows). Western blot analysis also verified the presence of these EVs (Fig. 1b) through the detection of CD63 (53 kDa), a marker protein of exosomes [10]. Owing to the presence of intact membranes, complete lysis of exosomes is required for amino acid analysis. Kumeda et al. previously reported that the membranes of salivary exosomes are stable at 4 °C for at least 28 days, and that this stability is maintained after freezing and thawing [11]. Complete lysis of exosomes is usually achieved using Triton-X100, RIPA buffer, SDS, or NP40 [11], [12]. In the present study, heating in a polar organic solvent (MeOH) was used to simultaneously lyse the exosomes and deproteinize the extract; precipitated proteins were removed by centrifugation. The exosome-eluted fractions contained 18 amino acids (Supplemental Fig. 1b) and d-Ser (Supplemental Fig. 2b), similar to those found in the native serum. Target peaks were not detected from blank samples containing PBS or the solutions from the commercial kit (Supplemental Figs. 3 and 4). The ratios of His, Arg, Glu, Cystine, Lys, and Tyr (Fig. 2a) were significantly increased in the exosome-eluted fraction compared with those in the corresponding native serum. In particular, cystine (Fig. 2a and Supplemental Fig. 5) was markedly enriched in the exosome-eluted fraction. These results suggest that certain amino acids are enriched in exosomes.
Fig. 1.
(a) Transmission electron microscopy of exosomes from human serum. Exosomes are indicated by arrows. (b) Western blot analysis of CD63 expression in exosomes (n = 3).
Fig. 2.
Percentage of each amino acid (mean ± SE, n = 9) relative to total amino acids in native human sera and exosome-eluted fractions (a). Percentage of d-Ser (mean ± SE, n = 9) relative to total Ser (d-Ser + l-Ser) in native human sera and exosome-eluted fractions (b). *p < 0.05, **p < 0.01 for exosome-eluted fraction vs. native serum.
The underlying reasons for this enrichment remain unclear, although future studies determining the functions of the enriched amino acids in the exosomes should clarify this issue. We also found that d-Ser, an endogenous co-agonist of the N-methyl-d-aspartate (NMDA) receptor that has been evaluated as a potential biomarker of schizophrenia, was significantly enriched in the exosome-eluted fraction (Fig. 2b). Significantly lower concentrations of serum d-Ser are observed in patients with schizophrenia [13]. Because the glutamate hypothesis proposes that the main symptoms of schizophrenia are caused by a hypofunction of the NMDA receptor in the central nervous system [14], [15], d-Ser deficiency may underlie the pathogenesis of this disease. Therefore, d-Ser levels in exosomes may discriminate between normal subjects and schizophrenia patients and become a useful tool for diagnosis of schizophrenia. Although serum amino acids could be transported into the exosomes through specific membrane transporters, our results suggest that some amino acids are enriched inside exosomes in human serum.
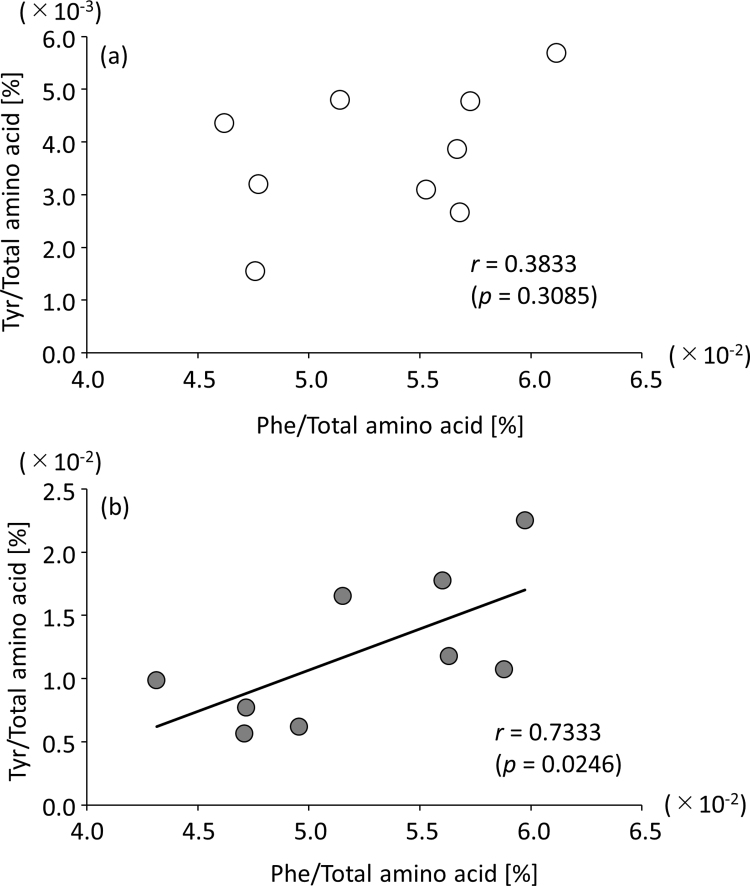
Tyr was also enriched in the exosome-eluted fraction (Fig. 2a); Tyr is biosynthesized from Phe by phenylalanine hydroxylase (EC 1. 14. 16. 1). In fact, Tyr had a statistically significant, positive correlation (p = 0.0246, r = 0.7333) with its precursor, Phe, in the exosome-eluted fraction (Supplemental Fig. 6b). However, this correlation was not observed in serum (Supplemental Fig. 6a). Our findings may provide a methodology to explore metabolic disorders by examining alterations in the amino acid composition of exosomes obtained from human serum.
A limitation of the present study is that we did not verify that the amino acids were located inside the exosomes, like miRNA and proteins. A size exclusion-chromatographic separation for the complete isolation of exosomes from human serum is therefore required to clarify this issue.
In conclusion, the present study revealed that certain amino acids might be enriched in the exosome-eluted fraction from human serum. Furthermore, the determination and comparison of low-molecular-weight compounds in exosomes released from the brain tissue of healthy controls and patients with schizophrenia may allow the assessment of abnormalities in metabolism based on fluctuations in exosomal metabolites. This new diagnotic tool could be useful for early diagnosis of severe psychiatric disorders.
Acknowledgements
The authors are grateful to Ms. C. Nakayama at JEOL (Tokyo, Japan) for TEM analysis and to Ms. M. Fukuda and Ms. A. Shoji, Toho University, for their technical assistance. This research was financially supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (Grant no. 25460224) awarded to T.F., the Faculty of Pharmaceutical Sciences, Toho University, and a Grant-in-Aid for Private University Research Branding Project from the MEXT awarded to M.K. We thank Editage (www.editage.jp) for English language editing.
Declarations of interest
None.
Supplementary materials
The online version of this article contains supplementary materials.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.plabm.2018.e00099.
Appendix A. Supplementary material
Supplemental Fig. 1.
Representative chromatograms of amino acids in the serum (a) and exosome-eluted fraction (b). Altered levels of amino acids and Cys-Cys (only detected in exosomes) are indicated by rectangle and arrows, respectively.
Supplemental Fig. 2.
Representative chromatograms of D- and L-Ser in serum (a) and exosome-eluted fraction (b).
Supplemental Fig. 3.
Representative chromatograms of blank samples for D- and L-Ser: PBS (a), Mixed Solution A/B/C (1:1:1) (b), and sample buffer (c).
Supplemental Fig. 4.
Representative chromatograms of blank samples for amino acids: PBS (a), Mixed Solution A/B/C (1:1:1) (b), and sample buffer (c).
Supplemental Fig. 5.
Representative chromatograms of cystine standard (200 μM). The cystine peak was detected at a retention time of 78 min.
Supplemental Fig. 6.
Correlations between Phe and Tyr in serum (a) and exosome-eluted fraction (b).
Supplemental Scheme 1.
Procedure for HPLC-composition analysis of amino acids in the exosome-eluted fraction from human serum by DiagExo Serum Exosomal Protein Extraction Kit. Isolation of exosomes from human serum [I], purification of isolated exosomes using a DiagExo Column [II], and lysis and fluorescence derivatization [III].
Supplemental Table 1.
HPLC conditions for amino acids.
Supplemental Table 2.
HPLC conditions for DL-Ser.
References
- 1.Sun L., Jiao H., Gao B., Yuanzi Q., Zhang H., Wang Y., Ou N., Yan Z., Zhou H. Hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry method for the simultaneous determination of L-valine, L-leucine, L-isoleucine, L-phenylalanine, and L-tyrosine in human serum. J. Sep. Sci. 2015;38:3876–3883. doi: 10.1002/jssc.201500512. [DOI] [PubMed] [Google Scholar]
- 2.Hou Y., Yin M., Sun F., Zhang T., Zhou X., Li H., Zheng J., Chen X., Li C., Ning X., Lou G., Li K. A metabolomics approach for predicting the response to neoadjuvant chemotherapy in cervical cancer patients. Mol. Biosyst. 2014;10:2126–2133. doi: 10.1039/c4mb00054d. [DOI] [PubMed] [Google Scholar]
- 3.Lin X., Zhan B., Wen S., Li Z., Huang H., Feng J. Metabonomic alterations from pancreatic intraepithelial neoplasia to pancreatic ductal adenocarcinoma facilitate the identification of biomarkers in serum for early diagnosis of pancreatic cancer. Mol. Biosyst. 2016;12:2883–2892. doi: 10.1039/c6mb00381h. [DOI] [PubMed] [Google Scholar]
- 4.Liu M., Zhou K., Li H., Dong X., Tan G., Chai Y., Wang W., Bi X. Potential of serum metabolites for diagnosing post-stroke cognitive impairment. Mol. Biosyst. 2015;11:3287–3296. doi: 10.1039/c5mb00470e. [DOI] [PubMed] [Google Scholar]
- 5.Denzer K., Kleijmeer M.J., Heijnen H.F.G., Stoorvogel W., Geuze H.J. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 6.Mathivanan S., Fahner C.J., Reid G.E., Simpson R.J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka S., Hosokawa M., Ueda K., Iwakawa S. Effects of decitabine on invasion and exosomal expression of miR-200c and miR-141 in oxaliplatin-resistant colorectal cancer cells. Biol. Pharm. Bull. 2015;38:1272–1279. doi: 10.1248/bpb.b15-00129. [DOI] [PubMed] [Google Scholar]
- 8.Aoyama C., Santa T., Tsunoda M., Fukushima T., Kitada C., Imai K. A fully automated amino acid analyzer using NBD-F as a fluorescent derivatization reagent. Biomed. Chromatogr. 2004;18:630–636. doi: 10.1002/bmc.365. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima T., Kawai J., Imai K., Toyo'oka T. Simultaneous determination of D- and L-serine in rat brain microdialysis sample using a column-switching HPLC with fluorimetric detection. Biomed. Chromatogr. 2004;18:813–819. doi: 10.1002/bmc.394. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A., Pulliam L. Exosomes as mediators of neuroinflammation. J. Neuroinflamm. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumeda N., Ogawa Y., Akimoto Y., Kawakami H., Tsujimoto M., Yanoshita R. Characterization of membrane integrity and morphological stability of human salivary exosomes. Biol. Pharm. Bull. 2017;40:1183–1191. doi: 10.1248/bpb.b16-00891. [DOI] [PubMed] [Google Scholar]
- 12.Pocsfalvi G., Stanly C., Fiume I., Vekey K. Chromatography and its hyphenation to mass spectrometry for extracellular vesicle analysis. J. Chromatogr. A. 2016;1439:26–41. doi: 10.1016/j.chroma.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima T., Iizuka H., Yokota A., Suzuki T., Ohno C., Kono Y., Nishikiori M., Seki A., Ichiba H., Watanabe Y., Hongo S., Utsunomiya M., Nakatani M., Sadamoto K., Yoshio T. Quantitative analyses of schizophrenia-associated metabolites in serum: serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS One. 2014;9:e101652. doi: 10.1371/journal.pone.0101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schell M.J., Brady R.O., Molliver M.E., Snyder S.H. D-Serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J. Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto K., Engberg G., Shimizu E., Nordin C., Lindstrom L.H., Iyo M. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005;29:767–769. doi: 10.1016/j.pnpbp.2005.04.023. [DOI] [PubMed] [Google Scholar]