Abstract
Background
Circulating tumor cells (CTCs) are associated with worse prognosis in metastatic breast cancer (BC). We evaluated the association of metabolic, inflammatory, and tumor markers with CTCs in women with metastatic BC before commencing a new systemic therapy.
Methods
Ninety-six patients with newly diagnosed or progressing metastatic BC without current diabetes or use of anti-inflammatory agents were recruited from four Ontario hospitals. Women provided fasting blood for measurement of metabolic, inflammatory, and tumor markers and CTCs. CTCs were assayed within 72 hours of collection using CellSearch. Other blood was frozen at –80°C, and assays were performed in a single batch. Associations between CTC counts with study factors were evaluated using Spearman correlation, and the chi-square or Fisher exact test. All statistical tests were two-sided and P value ≤ .05 was considered statistically significant.
Results
The median age was 60.5 years; 90.6% were postmenopausal. The cohort included hormone receptor–positive (87.5%), HER2–positive (15.6%), and triple-negative (10.4%) BCs. Patients were starting firstline (35.5%), second-line (26.0%), or third-or-later-line therapy (38.5%). CTC counts (per 7.5 mL of blood) ranged from 0 to 1238 (median 2); an elevated CTC count, defined as five or more CTCs, was detected in 42 (43.8%) patients. Those with liver metastases (vs not) more frequently had an elevated CTC count (59.0% vs 33.3%, P = .02). CTCs were significantly associated with C-reactive protein (R = .22, P = .02), interleukin (IL)-6 (R = .25, P = .01), IL-8 (R = .38, P = .0001), plasminogen activator inhibitor 1 (R = .31, P = .001), carcinoembryonic antigen (R = .31, P = .002), and cancer antigen 15-3 (R = .40, P = .0001) and inversely associated with body mass index (R = –.23, P = .02) and leptin (R = –.26, P = .01).
Conclusions
CTC counts were positively associated with tumor and inflammatory markers and inversely associated with some metabolic markers, potentially reflecting tumor burden and cachexia.
Elevated circulating tumor cell (CTC) counts, defined as five or more CTCs in 7.5 mL of peripheral blood, have been associated with worse outcome (1) and poor treatment benefit in metastatic breast cancer (BC; eg, median progression-free survival = 7 vs 2.7 months, median overall survival > 18 vs 10.1 months in nonelevated vs elevated cases, before the initiation of a new course of therapy) (2). Also, obesity and obesity-related markers, such as some inflammatory and metabolic variables, are associated with BC risk (3) and worse prognosis after diagnosis of early BC (4–6). Elevated C-reactive protein, for example, is a risk factor for postmenopausal BC (7), whereas high fasting insulin, leptin, and glucose are associated with poor BC outcome in nondiabetic women (8). It is possible that obesity and CTCs are linked because of changes in tumor microenvironment (9), and this interaction is of interest because these factors may enhance CTC release and contribute to poor outcome. CTC counts have also been associated with tumor markers in metastatic BC, including carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA15-3) (10), potentially reflecting higher tumor burden.
We examined the association of an elevated CTC count with inflammatory, metabolic, and tumor markers in a cross-sectional study of women with newly diagnosed or progressing metastatic BC about to commence a new systemic treatment. We hypothesized a priori that an elevated CTC count in metastatic BC would be positively associated with tumor markers, body mass index (BMI), and other host metabolic factors, as well as with inflammatory markers. As one of the first studies to explore these associations, we did not restrict patients by site of metastasis, breast cancer subtype, line of therapy, performance, or metabolic status; in this sense, the study is exploratory.
Methods
Patients
Eligible patients had histologically confirmed BC (at primary diagnosis or metastasis) with newly diagnosed or progressing metastatic BC and were about to commence a new systemic treatment (chemotherapy, hormone therapy, or biologic therapy). Patients were enrolled at four participating hospitals in Ontario, Canada (Mount Sinai Hospital, Princess Margaret Cancer Centre, London Health Sciences Centre, St. Michael’s Hospital). Women were excluded if they met any of the following criteria: received drug treatment for diabetes or anti-inflammatory agents (including corticosteroids) within the past two weeks (these medications may alter levels of the blood factors of interest). In accordance with a protocol approved by the Ethics Board of each institution, all subjects provided written informed consent.
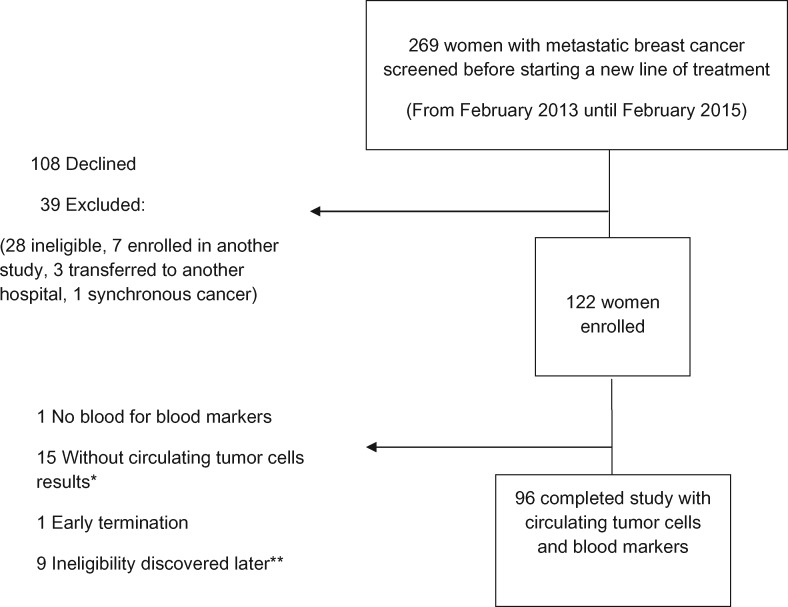
Two hundred sixty-nine women were screened, 108 declined participation, and 39 were excluded (28 did not meet patient selection criteria, seven were enrolled in another study, three were transferred to another hospital, and one had synchronous second cancer) (Figure 1). One hundred twenty-two patients were enrolled, but 26 were subsequently excluded from the analysis (nine due to ineligibility discovered late [three did not have evidence of BC progression, three did not have metastatic BC, one had previously undiagnosed diabetes, and two patients received prednisone before study visit], one early termination, one with no blood available for blood markers, and 15 without available CTC counts due to insufficient blood or technical problems such as CTC-ferrofluid aggregation and blood clot).
Figure 1.
A patient flow chart for association of metabolic, inflammatory, and tumor markers with circulating tumor cells in metastatic breast cancer—a cross-sectional study. *Insufficient blood or technical problems due to ferrofluid aggregation or blood clot. **Nine patients excluded after charts were reviewed (three did not have evidence of breast cancer progression, three did not have metastatic breast cancer, one had diabetes, and two received prednisone before the study visit).
Data and Blood Collection
Blood was collected after an overnight fast of at least 12 hours, and subjects provided 15 mL of blood for CTC analysis, 4 mL of serum for glucose, and 10 mL of serum. Glucose was assayed immediately after blood collection, whereas analysis of CTC counts was performed within 72 hours of blood collection. All other serum was frozen at –80°C until assays were performed in a single batch. Clinical data were collected using an interviewer-administered questionnaire and extraction from medical records.
Measurements
CTC Analysis
Blood was drawn into each of two CellSave tubes (CTCs were analyzed using the CellSearch platform. Based on this Food and Drug Administration–approved technology, CTCs were identified as nucleated cells expressing the epithelial cell adhesion molecule (EpCAM) and cytokeratins (CKs) 8, 18, and/or 19 but lacking CD45 expression. This determination is achieved using EpCAM antibody-conjugated magnetic beads to enrich the sample for epithelial cells and subsequent assessment of these cells for CKs, a leukocyte marker (CD45), and nuclei (DAPI) via fluorescent staining. All results were reviewed by a laboratory technologist and a pathologist (MCC), both with training in CTC analysis.
Blood Assays
Glucose was assayed in the clinical laboratory of each local hospital. Homeostasis model assessment (HOMA), a measure of insulin resistance, was calculated as insulin (mU/L) × glucose (mmol/L)/22.5.
All other blood assays were performed at Mount Sinai Hospital, Toronto, using commercial assay kits. Serum vascular endothelial growth factor (VEGF), tumor necrosis factor–alpha (TNF-α), and interleukin 6 (IL-6) were quantified by R&D Systems–Luminex Performance Assay–Custom Premix Multiplex (interassay coefficient of variation [CV] < 15%, sensitivity < 1.2 pg/mL, < 2.1 pg/mL, and < 1.7 pg/mL, respectively). Interleukin-8 (IL-8) was analyzed using emd Millipore High-Performance Multiplex assay–enzyme-linked immunosorbent assay (ELISA; CV < 3.5%, sensitivity < 0.4 pg/mL).
High-sensitivity C-reactive protein (hs-CRP) was measured using particle-enhanced immunoturbidimetric assay by Roche Modular P (CV = 4%, sensitivity < 0.1 mg/L). An Invitrogen-ELISA kit was used to quantify plasminogen activator inhibitor 1 (PAI-1; CV = 6%–9%, sensitivity < 30 pg/mL). An R&D Systems Magnetic Luminex Screening Assay, Premixed Multiplex, was used to measure CA15-3 (CV < 9%, sensitivity < 0.051 pg/mL). CEA and cancer antigen 125 (CA125) were evaluated using a Roche Modular E170 electrochemiluminescense immunoassay (CV < 10% for CEA and = 6% for CA125, sensitivity = <0.200 ug/L to >1000 ug/L and <0.600 U/mL to >5000 U/mL, respectively).
Insulin, leptin, and adiponectin were measured by Magnetic Luminex Screening Assays (CV < 15% for all the variables, sensitivity < 9.91 pg/mL, < 10.2 pg/mL, and = 0.25 ng/mL, respectively). Albumin, free fatty acids (FFAs), and creatine kinase (CK) were measured to assess the nutritional status of the patients and to further explore the relationship of obesity-related factors and CTCs. CK and FFA were assessed in serum using a Roche Modular P colorimetric assay. Reference ranges were CK 0 to 190 U/L and FFA 0.1 to 0.9 mmol/L. Albumin was measured with bromocresol green, with a reference range of 40 to 53 g/L.
Statistical Analyses
Patient characteristics were reported descriptively. A total of 100 patients were required to provide 80% power to detect a nonzero, true correlation of 0.28 between CTC counts and insulin when tested at the 5% level. An elevated CTC count was defined as five or more CTCs per blood sample of 7.5 mL, and associations of an elevated CTC count with categorical patient and tumor characteristics were assessed using the chi-square test or Fisher exact test. Five or more CTCs (as measured using Cell Search) is a US Food and Drug Administration–approved test with well-documented limits of detection and prognostic association established for the threshold of 5 cells2. For greater power, associations of CTCs with continuous tumor, metabolic, and inflammatory factors were evaluated using Spearman rank correlations based on the CTC counts themselves (rank correlations were used because of the skew distribution of the CTC counts and many of the factors).
Based on our hypotheses, we expected positive associations between CTC counts and tumor markers (CA15-3, CEA, CA 125), as well as body mass index, insulin, glucose, and leptin, and a negative association with adiponectin. We also hypothesized that elevated CTC counts would be positively associated with inflammatory markers such as VEGF, IL-6, IL-8, TNF-α, hs-CRP, and PAI-1. Prespecified hypotheses, effect sizes (ie, size of percentages, direction, and size of correlation coefficients), and group effects were taken into account when interpreting results. PAI-1 and CA15-3 exceeded the upper limit of the assay in 11 and five cases, respectively; the upper limit of the assay was used in these cases.
To evaluate the strength of the effect size, Cohen’s standard was used (11). For correlation analysis, coefficients between .10 and .29 represent a weak association, coefficients between .30 and .49 represent a moderate association, and coefficients of .50 and greater represent a strong association.
Results
Patient, treatment, and metastatic tumor characteristics are shown in Table 1. The median age of patients (interquartile range [IQR]) was 60.5 (55–71.2) years; most subjects were postmenopausal (87/96, 90.6%). In 84 patients (87.5%), tumors were hormone receptor (HR) positive, 15 (15.6%), human epidermal growth factor receptor 2 (HER2) positive (regardless of HR status), and 10 (10.4%) triple negative. Most primary tumors were grade 2 or 3 (81.2%). Bone, lung, liver, and brain metastases were present in 79.2%, 43.8%, 40.6%, and 6.3% of patients, respectively, with 54%, 37%, 35%, and 3% exhibiting progression at these sites, respectively. Thirty-five (35.5%) patients were starting first line therapy, 25 (26%) were starting second-line therapy, and 37 (38.5%) had third-or-higher-line therapy. Chemotherapy was the most common regimen about to be initiated 53 (55.21%).
Table 1.
Patient and tumor characteristics*
| Characteristics | Value (n = 96) |
|---|---|
| Age, median (IQR), y | 60.5 (55–71.2) |
| Height, median (IQR), cm | 161 (156.8–164.7) |
| Weight, median (IQR), kg | 67 (57.7–75.4) |
| BMI, median (IQR), kg/m2 | 25.6 (22.6–28.9) |
| Menopausal status, No. (%) | |
| Pre/Peri | 9 (9.4) |
| Post | 87 (90.6) |
| ER/PR status, No. (%) | |
| Positive | 84 (87.5) |
| Negative | 12 (12.5) |
| HER2 status, No. (%) | |
| Positive | 15 (15.6) |
| Negative | 79 (82.3) |
| Unknown | 2 (2.1) |
| Subtype, No. (%) | |
| HR+ and HER2- | 69 (71.9) |
| HER2+ and any HR | 15 (15.6) |
| Triple-negative | 10 (10.4) |
| Unknown | 2 (2.1) |
| Grade, No. (%) | |
| Grade I | 9 (9.4) |
| Grade II | 44 (45.8) |
| Grade III | 34 (35.4) |
| Unknown | 9 (9.4) |
| Line of treatment starting, No. (%) | |
| 1 | 34 (35.5) |
| 2 | 25 (26.0) |
| 3+ | 37 (38.5) |
| Type of treatment to be initiated, No. (%) | |
| Chemotherapy | 53 (55.2) |
| Anti-HER2 agent + chemotherapy | 2 (2.1) |
| Only anti-HER2 agent | 6 (6.2) |
| Hormone therapy | 33 (34.4) |
| Unknown | 2 (2.1) |
| Lung metastases, No. (%) | |
| Yes | 42 (43.8) |
| No | 54 (56.3) |
| Liver metastases, No. (%) | |
| Yes | 39 (40.6) |
| No | 57 (59.4) |
| Brain metastases, No. (%) | |
| Yes | 6 (6.3) |
| No | 90 (93.7) |
| Bone metastases, No. (%) | |
| Yes | 76 (79.2) |
| No | 20 (20.8) |
ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; HR = hormone receptor; IQR = interquartile range; PR = progesterone receptor.
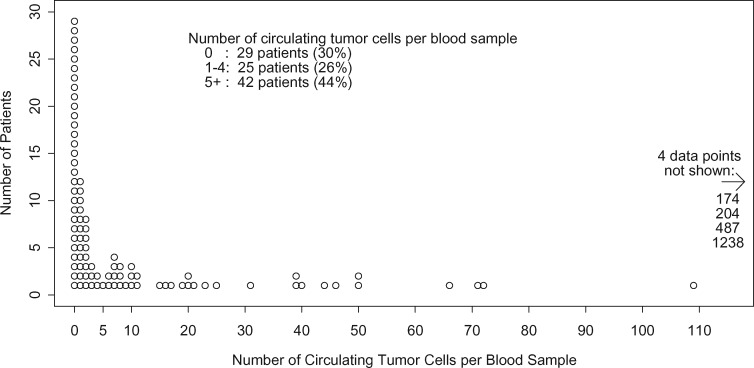
CTC counts (per 7.5 mL) ranged from 0 to 1238 (median = 2, geometric mean = 3.63) (Figure 2). Elevated CTC counts, defined as five or more CTCs, were present in 42 (44%) patients. The associations of tumor and treatment characteristics with elevated CTC counts are shown in Table 2. An elevated CTC count was present in 46.4% of HR-positive patients vs in 25% of HR-negative patients (P = .21), in 40% of HER2-positive vs 45.6% of HER2-negative patients (P = .56), and in 30% of triple-negative patients vs 45.3% of non-triple-negative patients (P = .56). In patients starting their first, second, and third or higher lines of treatment, rates of elevated CTC counts were 41.2%, 44%, and 45.9%, respectively (P = .16). An elevated CTC count was present in 40.5% of patients with lung metastases (vs in 46.3% without, P = .67), in 59% of patients with liver metastases (vs 33.3% without, P = .02), and in 47.4% patients with bone metastases (vs 30% without, P = .2). Only six patients had brain metastases, but four (66.7%) had an elevated CTC count, vs 38 of 90 (42.2%) of those without brain metastases (P = .39).
Figure 2.
Distribution of circulating tumor cells.
Table 2.
Association of elevated CTC count with metastatic tumor characteristics*
| Characteristic | CTC < 5(n = 54)(56.2%)No. (%) | CTC ≥ 5(n = 42)(43.8%)No. (%) | Total(n = 96) | P |
|---|---|---|---|---|
| ER/PR status† | ||||
| ER/PR+ | 45 (53.6) | 39 (46.4) | 84 | .21‡ |
| ER/PR- | 9 (75.0) | 3 (25.0) | 12 | |
| HER2 status† | ||||
| HER2+ | 9 (60.0) | 6 (40.0) | 15 | .56§ |
| HER2- | 43 (54.4) | 36 (45.6) | 79 | |
| Unknown | 2 (100.0) | 0 (0.0) | 2 | |
| Triple-negative† | ||||
| Yes | 7 (70.0) | 3 (30.0) | 10 | .56§ |
| No | 47 (54.7) | 39 (45.3) | 86 | |
| Line of starting treatment | ||||
| 1 | 20 (58.8) | 14 (41.2) | 34 | .16§ |
| 2 | 14 (56.0) | 11 (44.0) | 25 | |
| ≥3 | 20 (54.1) | 17 (45.9) | 37 | |
| Site of metastases | ||||
| Lung yes | 25 (59.5) | 17 (40.5) | 42 | .67§ |
| Lung no | 29 (53.7) | 25 (46.3) | 54 | |
| Liver yes | 16 (41) | 23 (59) | 39 | .02§ |
| Liver no | 38 (66.7) | 19 (33.3) | 57 | |
| Bone yes | 40 (52.6) | 36 (47.4) | 76 | .20§ |
| Bone no | 14 (70) | 6 (30) | 20 | |
| Brain yes | 2 (33.3) | 4 (66.7) | 6 | .39‡ |
| Brain no | 52 (57.8) | 38 (42.2) | 90 |
Percentages in each row add up to 100%. CTC = circulating tumor cell; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor.
Current metastatic disease status.
Fisher exact test for analysis of contingency tables, less than five expected counts.
Chi-square test for contingency tables.
The correlations of CTC counts with tumor, inflammatory, and metabolic factors are shown in Table 3. CTC counts were moderately positively correlated with tumor factors CA15-3 (R = .40, P = .0001) and CEA (R = .31, P = .002). The correlation with CA125 was weak and not statistically significant (R = .15, P = .14). CTC counts were positively correlated with most of the inflammatory factors, namely weak correlations with hs-CRP (R = .22, P = .02) and IL-6 (R = .25, P = .01) and moderate correlations with IL-8 (R = .38, P = .0001) and PAI-1 (R = .31, P = .001), but they were not correlated with TNF-α (R = .18, P = .06) or VEGF (R = .13, P = .18).
Table 3.
Association of CTC counts with metabolic, inflammatory, and tumor markers
| Spearman correlation | P* | |
|---|---|---|
| CA15-3, pg/mL | .40 | .0001 |
| CEA, µg/mL | .31 | .002 |
| CA125, U/mL | .15 | .14 |
| hs-CRP, mg/L | .22 | .02 |
| TNF-α, pg/mL | .18 | .06 |
| VEGF, pg/mL | .13 | .18 |
| PAI-1, ng/mL | .31 | .001 |
| IL-6, pg/mL | .25 | .01 |
| IL-8, pg/mL | .38 | .0001 |
| BMI, kg/m2 | –.23 | .02 |
| Glucose, mmol/L | –.08 | .41 |
| Insulin, pmol/L | –.17 | .08 |
| HOMA | –.17 | .08 |
| Leptin, ng/mL | –.26 | .01 |
| Leptin:adiponectin ratio | –.26 | .01 |
| Adiponectin, µg/mL | .10 | .32 |
| Albumin, g/L | –.14 | .178 |
| Creatinine kinase, U/L | .13 | .194 |
| Free fatty acids, mmol/L | .29 | .004 |
P value from testing the hypothesis that the Spearman rank correlation is zero. BMI = body mass index; CA15-3 = cancer antigen 15-3; CEA = carcinoembryonic antigen; CRP = C-reactive protein; CTC = circulating tumor cell; HOMA = homeostasis model assessment; IL = interleukin; PAI-1 = plasminogen activator inhibitor 1; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
For the metabolic factors, negative weak correlations with CTC counts were observed for BMI (R = –.23, P = .02), leptin, and the leptin-to-adiponectin ratio (both R = –.26, P = .01). Insulin, HOMA (both R = –.17, P = .08), adiponectin (R = .1, P = .32), and glucose (R = –.08, P = .41) were not significantly correlated with CTC counts.
In terms of body size, 3.1% of patients were underweight (BMI ≤ 18.5 kg/m2), 43.8% normal weight (BMI = 20–25 kg/m2), 32.3% overweight (BMI = 25–30 kg/m2), and 20.8% obese (BMI ≥ 30 kg/m2). Median albumin level (IQR) was 43 (41–45) g/L with 20.2% below 40 g/L, and the lower limit of the reference range and median CK was 69 (51–95) U/L, with 4.3% above upper limit of 190 U/L. Median FFA (IQR) was 0.43 (0.31–0.56) mmol/L, with 3.3% above 0.9 mmol/L, the upper limit of the reference range. The correlation of CTC counts with FFA was weakly positive (R = .29, P = .004). However, neither albumin nor CK was correlated with CTC counts (R = –.14, P = .178, and R = .13, P = .194, respectively).
Discussion
In our cohort of heavily pretreated postmenopausal patients with advanced BC, we observed an elevated CTC count rate of 44%, which is consistent with previous reports ranging from 38% to 49% (10,12). In a European pooled analysis, Bidard reported elevated CTC count rates in patients with liver, bone, lung, and central nervous system metastases of 57%, 56.2%, 44.3%, and 45.4%, respectively (10). We observed similar percentages of 59%, 47.4%, and 40.5% for liver, bone, and lung metastases. For patients with brain metastases, we observed a higher percentage of 66.7%, but this was based on only six patients.
In advanced BC, tumor markers, such as CA15-3, CEA, and CA125, have been used as noninvasive tools for measuring treatment response (13). Although these markers are used and, for some patients, can be a tool to measure treatment response, due to low sensitivity, they are not recommended as sole assessments of metastatic BC for the majority of international guidelines (14–16). Similar to a European pooled analysis by Bidard et al., we found positive correlations between CTC counts and the tumor markers CA15-3 and CEA (10). However, CTC count is a better prognostic marker, and its use is better established compared with the above protein-based tumor markers (1,10,17).
To our knowledge, this is the first study to evaluate the association of obesity-related factors and CTCs in metastatic BC. Obesity is associated with changes in a variety of metabolic markers, including dysregulated glycemic control, insulin resistance, altered adipokines, and inflammation (6,7). Preclinical data suggest that chronic inflammation can facilitate tumor growth and metastasis (9,18). Recent studies have suggested that inflammation may be associated with BC risk and adverse prognosis (7,20,21). The extent to which these inflammatory markers identified locally in the tumor microenvironment or systemically in the bloodstream mediate a potential association of BC with CTCs is not clear, but this is of interest because it could enhance CTCs’ release. We hypothesized that CTC counts would be positively associated with inflammatory factors. In our study, positive but weak to moderate correlations were found with the inflammatory markers IL-8, IL-6, PAI1, and hs-CRP.
Some metabolic markers may also mediate the adverse effects of obesity in BC. Receptors for insulin and leptin, for example, are expressed by nearly all breast tumors and lie upstream of some of the most frequently dysregulated pathways in human cancer, namely the PI3K/AKT/mTOR and JAK/STAT signaling networks, supporting a strong role for these hormones in breast tumorigenesis and disease progression (21–23). Alterations in the microenvironment related to metabolic factors can also lead to tumor growth and progression (24) and could be related to CTC release. We hypothesized a priori that an elevated CTC count would be positively associated with the metabolic factors BMI, insulin, and leptin and negatively associated with adiponectin. However, we observed the opposite for BMI and leptin, namely weak negative correlations. This finding was unexpected, and we postulated that these findings may be due to weight loss, cachexia, and/or sarcopenia in women with elevated CTC counts who have higher disease burden, but we cannot confirm this in our cross-sectional study. Another possibility that could be explored in future research is that metabolic factors may affect sensitivity and response to hormonal treatments, which may then affect CTC counts, especially in receptor-positive metastatic BC. It is also possible that inflammation is more important in CTC release than obesity/metabolic factors in advanced BC. Based on our findings, future studies should investigate the association of CTCs and weight loss/cachexia or explore additional markers of inflammation in metastatic BC.
Our study has limitations, including reliance on a single blood measurement. This was an exploratory cross-sectional study, with predominantly subjects with hormone receptor–positive BC. We did not evaluate the association of progressive weight loss with elevated CTC counts, and as in any cross-sectional study, associations between study variables and CTCs do not provide information on causality but can raise questions and hypotheses. We had too few subjects to do subset analyses for particular sites of metastasis or BC subtypes; future studies are needed to address these. A recognized limitation of CellSearch is that epithelial to mesenchymal transition can lead to loss of epCAM, resulting in the inability to detect epCAM-negative CTCs that may be present. However, understanding the associations we have studied could elucidate the processes involved in CTC release and may identify intervention strategies that could lower CTC counts and potentially improve prognosis.
We have shown that CTC counts were moderately correlated with the tumor markers CA15-3 and CEA and positively associated with liver metastases. Additionally, CTCs were positively and weakly correlated with inflammatory markers and inversely correlated with obesity-associated markers, the latter potentially reflecting weight loss or a cachexic state, which needs to be explored in further studies. Additional research focused on CTC phenotypes and circulating DNA is also needed to elucidate the significance of CTCs in BC development and progression.
Funding
This work was supported by the Breast Cancer Research Foundation and Hold ‘EM for Life Translating Research Discoveries into Breast Cancer Cures. The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Dr. Lohmann’s work is supported Hold’Em for Life Translating Discoveries into Breast Cancer Cures (Canada). Dr. Stambolic’s work is supported by the Canadian Institutes of Health Research (CIHR), Canadian Cancer Society Research Institute (CCSRI), and Hold’Em for Life Translating Discoveries into Breast Cancer Cures (Canada). Dr. Goodwin’s work is supported by The Breast Cancer Research Foundation (United States) and Hold’Em for Life Translating Discoveries into Breast Cancer Cures (Canada).
Notes
Affiliations of authors: Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Sinai Health System (AEL, CE, EL, KF, PJG, MCC); Department of Medicine (AEL, EA, CE, CBM, PJG), St. Michael’s Hospital (CBM), Department of Medical Biophysics (VS), and Department of Laboratory Medicine and Pathobiology (MCC), University of Toronto, Toronto, ON, Canada; University Health Network, Princess Margaret Cancer Centre, Toronto, ON, Canada (RJOD, EA, CE, VS); Applied Statistician, Markham, ON, Canada (ME); London Regional Cancer Program, London, ON, Canada (TV).
The authors also wish to acknowledge the efforts of Michelle Cornect and Peter Bokaei, our CTC technologists.
The authors have no relevant or potential conflicts of interest to declare.
All procedures performed in the study were in accordance with the ethical standards of the institutional review boards of the participating institutions and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients provided written informed consent to participate.
The results of this manuscript were presented as part of two posters at the San Antonio Breast Cancer Symposium 2015.
References
- 1. Bidard FC, Hajage D, Bachelot T, et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: A prospective observational study. Breast Cancer Res. 2012;14:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. [DOI] [PubMed] [Google Scholar]
- 3. Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niraula S, Ocana A, Ennis M, et al. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: A meta-analysis. Breast Cancer Res Treat. 2012;134:769–781. [DOI] [PubMed] [Google Scholar]
- 5. Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. [DOI] [PubMed] [Google Scholar]
- 6. Goodwin PJ, Stambolic V.. Impact of the obesity epidemic on cancer. Annu Rev Med. 2015;66:281–296. [DOI] [PubMed] [Google Scholar]
- 7. Gunter MJ, Wang T, Cushman M, et al. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J Natl Cancer Inst. 2015;107(9):djy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: Correlations and time course of prognostic associations. J Clin Oncol. 2012;30:164–171. [DOI] [PubMed] [Google Scholar]
- 9. Joyce JA, Pollard JW.. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bidard F, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. [DOI] [PubMed] [Google Scholar]
- 11. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Florence: Routledge; 2013. [Google Scholar]
- 12. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. [DOI] [PubMed] [Google Scholar]
- 13. Lee J, Park S, Park J, et al. Elevated levels of serum tumor markers CA 15-3 and CEA are prognostic factors for diagnosis of metastatic breast cancers. Breast Cancer Res Treat. 2013;141:477–484. [DOI] [PubMed] [Google Scholar]
- 14. Lauro S, Trasatti L, Bordin F, et al. Comparison of CEA, MCA, CA 15-3 and CA 27-29 in follow-up and monitoring therapeutic response in breast cancer patients. Anticancer Res. 1999;19:3511. [PubMed] [Google Scholar]
- 15. Duffy MJ, Evoy D, McDermott EW.. CA 15-3: Uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411:1869–1874. [DOI] [PubMed] [Google Scholar]
- 16. Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. [DOI] [PubMed] [Google Scholar]
- 17. Pierga J, Hajage D, Bachelot T, et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol. 2012;23:618–624. [DOI] [PubMed] [Google Scholar]
- 18. O'Byrne KJ, Dalgleish AG.. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho YA, Sung MK, Yeon JY, et al. Prognostic role of interleukin-6, interleukin-8, and leptin levels according to breast cancer subtype. Cancer Res Treat. 2013;45:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Villasenor A, Flatt SW, Marinac C, et al. Postdiagnosis C-reactive protein and breast cancer survivorship: Findings from the WHEL study. Cancer Epidemiol Biomarkers Prev. 2014;23:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lohmann AE, Goodwin PJ, Chlebowski RT, et al. Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol. 2016;34:4249–4255. [DOI] [PubMed] [Google Scholar]
- 22. Law JH, Habibi G, Hu K, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–10246. [DOI] [PubMed] [Google Scholar]
- 23. Chang M, Ennis M, Dowling R, et al. Abstract P6-02-03: Leptin receptor (OB-R) in breast carcinoma tissue: Ubiquitous expression and correlation with leptin-mediated signaling, but not with systemic markers of obesity. Cancer Res. 2017;77:03. [Google Scholar]
- 24. Iyengar NM, Gucalp A, Dannenberg AJ, et al. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]