Abstract
Sinorhizobium meliloti CCNWSX0020, isolated from root nodules of Medicago lupulina growing in gold mine tailings in the northwest of China, displayed multiple heavy metal resistance and growth promotion of M. lupulina. In our previous work, the expression level of dmeR and dmeF genes were induced by Cu2+ through comparative transcriptome approach. Based on protein analysis, the dmeF encoded for a protein which showed a 37% similarity to the cation transporter DmeF of Cupriavidus metallidurans, whereas dmeR encoded transcriptional regulator which was highly homologous with DmeR belonging to RcnR/CsoR family metal-responsive transcriptional regulator. In addition to copper, quantitative real-time PCR analysis showed that dmeR and dmeF were also induced by nickel and cobalt. To investigate the functions of dmeR and dmeF in S. meliloti CCNWSX0020, the dmeR and dmeF deletion mutants were constructed. The dmeF mutant was more sensitive to Co2 + and Ni2 + than the wild type strain. Pot experiments were carried out to determine whether the growth of M. lupulina was affected when the dmeF gene was knocked out in the presence of nickel or cobalt. Results indicated that the nodule number of the host plant inoculated with the dmeF deletion mutant was significantly less than the S. meliloti CCNWSX0020 wild-type in the presence of Co2 + or Ni2 +. However, when standardized by nodule fresh weight, the nitrogenase activities of nodules infected by the dmeF deletion mutant was similar to nitrogenase activity of the wild type nodule.
Keywords: Sinorhizobium meliloti, Nickel, Cobalt, Resistance, Cation transporter
Introduction
Heavy metals are one of most common components in the environment. Some metals do not have a clear physiological function and are toxic to microorganisms even at low concentrations, such as lead and cadmium. Some heavy metals, such as cobalt, copper and nickel, are fundamental elements for living organisms and are involved in many physiological processes. In particular, cobalt is required for vitamin B12-dependent enzymes and proteins (Banerjee & Ragsdale, 2003), whereas nickel acts as a metal cofactor for some enzymes (Mulrooney & Hausinger, 2003; Guldan, Sterner & Babinger, 2008). However, in addition to being involved in some metabolic processes, an excess of cobalt and nickel can also damage cells by producing reactive oxygen species (Ahmad et al., 2015; Haferburg & Kothe, 2007; Liu et al., 2015). Therefore, releasing cobalt and nickel due to industrial and mining operations poses a significant threat to living organisms.
To prevent intracellular cobalt and nickel overload-mediated toxicity, many microorganisms have developed several mechanisms to protect themselves from an excess of metals (Rutherford, Cavet & Robinson, 1999; Pini et al., 2014; Matuszewska et al., 2008; Nies, 1999). Under normal conditions, these microelements are transported into cells. However, specific ion efflux systems have been used to eliminate excess metal ions from the cytoplasm. Therefore, efflux systems, uptake systems, the synthesis of ligand compounds and metallochaperones for regulating cobalt and nickel homeostasis play a crucial role in most cobalt/nickel-resistant organisms. Among these cobalt/nickel-resistant systems, resistance nodulation cell division efflux pumps (RND) (Stahler et al., 2006), cation diffusion facilitators (CDF) (Dokpikul et al., 2016) and P1b-type ATPases have been highlighted (Rutherford, Cavet & Robinson, 1999).
The first characterized CDF protein was CzcD, which was shown to participate in heavy metal tolerance in Cupriavidus metallidurans (Paulsen & Saier Jr, 1997). Later reports said that CDF family proteins were found to be ubiquitous in all living organisms (Nies, 2003). Proteins belonging to the cation diffusion facilitator (CDF) family have been implicated in metal tolerance. Most CDFs were located on internal membranes and catalysed the efflux of transition metal cations, including Zn2+, Co2+, Fe2+, Cd2+, Ni2+, or Mn2+, from the cytoplasm to the outside of the cell (Kolajrobin et al., 2015). Based on phylogenetic analysis, the CDF family was divided into Mn2+-transporting CDF, Fe2+/Zn2+-transporting CDF, Zn2+ and other metal transporting CDF according to the metal ion specificity (Montanini et al., 2007). The majority of CDF proteins from diverse sources have the following features in common: (1) they possess six putative transmembrane domains (TMDs) and share a signature sequence between TMD1 and TMD2; and (2) they share a C-terminal cation efflux domain. Many CDF transporters also contain a histidine-rich domain. Such domains are predicted to allow more efficient metal binding. In addition, it has been suggested that bacterial CDFs may participate in other biological functions. For instance, CepA confers chlorhexidine resistance to Keumonia (Fang et al., 2002). MamB and MamM of Magnetospirillum gryphiswaldense have been linked to magnetosome formation (Uebe et al., 2011). Sinorhizobium meliloti is a widely investigated model rhizobium species for symbiosis with leguminous plants. However, high concentrations of heavy metals have adverse effects on the rhizobia population (Sharaff & Archana, 2015; Wani, Khan & Zaidi, 2008). Our laboratory team recently isolated and sequenced the genome of heavy metal-resistant S. meliloti CCNWSX0020 that significantly improved the growth of Medicago lupulina in copper-contaminated soil. The genomic sequence data gave us information about the genes encoding putative proteins involved in heavy metal resistance in Sinorhizobium. So far, there is scarce functional evidence about genetically determined mechanisms of cobalt and nickel resistance in S. meliloti CCNWSX0020. In this study, molecular determinants responsible for cobalt and nickel resistance in S. meliloti were investigated. We also investigated whether cobalt- and nickel-sensitive mutants generated by homologous recombination affected the symbiotic nodulation capability with the host plant under cobalt or nickel stress conditions.
Materials & Methods
Bacterial strains, media and growth conditions
All bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli DH5 α was grown in Luria-Bertani (LB) medium at 37 °C. S. meliloti CCNWSX0020 and the mutants were grown at 28 °C in tryptone-yeast extract medium (TY medium: 5 g tryptone, 3 g yeast extract, 0.7 g CaCl2 ⋅2 H2O and 15 g agar per litre). Liquid culture of the cells was carried out in shaken tubes or Erlenmeyer flasks at 180 rpm. When necessary, media were supplemented with 100 µg/mL ampicillin (Amp), 50 µg/mL kanamycin (Km) or 50 µg/mL gentamicin (Gm).
Table 1. Bacteria, plasmids and primers used in the work.
| Bacteria, plasmids or primer | Features | Source |
|---|---|---|
| Strains | ||
| S. meliloti CNWSX0020 | Wild type, Ampr | Fan et al. (2011) |
| E. coli DH5a | lacZ4M15, recA1, gyrA96, hsdR17 | Hanahan (1983) |
| SM0020 ΔdmeF | dmeF deleted in S. meliloti CNWSX0020 | This work |
| SM0020 ΔdmeR | dmeR deleted in S. meliloti CNWSX0020 | This work |
| Plasmids | ||
| pk18 mob sacB | Suicide vector, Mob+, Kmr | Schafer et al. (1994) |
| pBBR1MCS-5 | Broad-host-range cloning vector, Gmr | Kovach et al. (1995) |
| pRK2013 | Helper pasmid, Kmr | University of York, Prof. Tanya Soule |
| pK18-ΔdmeR | Containing dmeR deletion fragment | This study |
| pK18-ΔdmeF | Containing dmeF deletion fragment | This study |
| pBBR-dmeF | pBBR1MCS-5 contain entire dmeF | This study |
| pBBR-dmeR | pBBR1MCS-5 contain entire dmeR | This study |
| Primers | ||
| dmeFF1 | CGGGATCCTTGGCACCAGAAAGAAGACGA | |
| dmeFR1 | GCTATGGTGGTGCTCGTGATGCCATCATTCC | |
| CGCAGTCAGT | ||
| dmeFF2 | ACTGACTGCGGGAATGATGGCATCACGAG | |
| CACCACCATAGC | ||
| dmeFR2 | GCTCTAGATCCTCTTCCGCATTCACGAC | |
| dmeRF1 | CGGGATCCAAGCCGCGACTGGGAAGA | |
| dmeRR1 | TCTCCCTGGGTTTCGTGGGGAGGCGACG | |
| AGGTTGAGA | ||
| dmeRF2 | TCTCAACCTCGTCGCCTCCCCACGAAAC | |
| CCAGGGAGA | ||
| dmeRR2 | GCTCTAGAGCAGAGCGATCAAGGCAAGTA | |
| dmeH1 | ATCCCGGGGTTTGGCACCAGAAAGAAGACGA | |
| dmeH2 | GCTCTAGAGCAGAATGCAGCCGCTAAGAT |
Notes.
Underlined indicates the restriction site.
qRT-PCR assays
S. meliloti CCNWSX0020 was cultured to logarithmic phase in TY liquid medium. Different heavy metals were added to the logarithmic phase medium, and the final concentrations of CoCl2, NiCl2, CuSO4, ZnCl2, Pb(NO3) 2 andCdCl2 were adjusted to 0.3, 0.5, 0.5, 0.5, 0.5 and 0.2 mM. Then, culture medium was incubated for 15 min before the total RNA was extracted. Residual DNA in the total RNA was removed by DNase I. A TakaRa reverse transcription kit and SYBR Premix ExTaqTM II (Tli RNaseH Plus) kit were used for reverse transcription and qRT-PCR. All experimental operations were carried out according to the manufacturer’s instructions. To standardize the results, 16S rRNA was used as an internal standard and the relative levels of transcription were calculated using the 2−ΔΔCtmethod (Livak & Schmittgen, 2001).
Bioinformation analyses
The draft genome of S. meliloti CCNWSX0020 (AGVV00000000) was previously sequenced and deposited in GenBank. The known DmeF protein sequences of most bacterial genomes used in this study were obtained from NCBI (https://www.ncbi.nlm.nih.gov). The whole set of bacterial DmeF sequences was aligned using ClustalW2 and the phylogenetic tree visualized with MEGA 6.0 (http://www.megasoftware.net). The DmeF membrane topology of strain CCNWSX0020 was generated and visualized by HMMTOP (version 2.0; http://www.enzim.hu/hmmtop/).
Generation of deletion mutants in dmeR and dmeF
The total genomic DNA of S. meliloti CCNWSX0020 was extracted according to the protocol of Wilson & Carson (2001). A 940-bp upstream and a 590-bp downstream fragment of dmeF were amplified using the primer pairs dmeFF1/dmeFR1 and dmeFF2/dmeFR2, respectively. The upstream and downstream PCR products were ligated by crossover PCR with primer pairs dmeFF1/dmeFR2 (Fig. 1B). The resulting 1.53-kb fragment was digested with Bam HI/XbaI and cloned into the Bam HI/XbaI site of the suicide vector pK18mobsacB to produce pK18-ΔdmeF. For the construction of pK18-ΔdmeR, 455-bp upstream and 405-bp downstream fragments of dmeR were amplified using the primer pairs dmeRF1/dmeRR1 and dmeRF2/dmeRR2, respectively. The upstream and downstream PCR products were ligated by crossover PCR with primers dmeRF1/dmeRR2. The resulting 860-bp fragment was digested with BamHI/XbaI and cloned into the BamHI/XbaI site of the suicide vector pK18mobsacB. All primers used in this study are listed in Table 1. The constructed suicide plasmid pK18-ΔdmeF or pK18-ΔdmeR was transferred into S. meliloti CCNWSX0020 by triparental mating, which included S. meliloti CCNWSX0020 (Amp r) as the recipient, E. coli JM109 cells containing pK18- ΔdmeF (Kmr) as the donor, and E. coli DH5α cells containing pRK2013 as helper cells. A single clone of transferred S. meliloti CCNWSX0020, which was resistant to both kanamycin and ampicillin, was grown in TY solid medium containing ampicillin and 10% (w/v) sucrose. Double crossover recombinants were confirmed by PCR using dmeFF1 and dmeFR2 as primers, and then the correct PCR products were sequenced. The resulting mutants were designated as SM0020ΔdmeF and SM0020ΔdmeR (Table 1).
Figure 1. Gene organization of S. meliloti CCNWSX0020 dmeRF and transmembrane structure of.
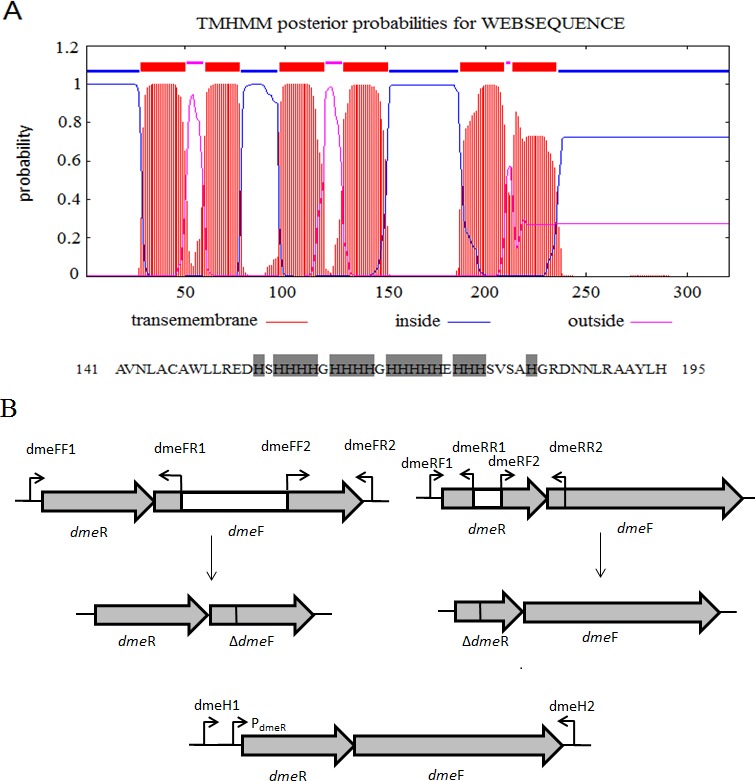
(A) Predicted transmembrane structure of DmeF, a histidine-rich stretch locates between TMD4 and TMD5 of DmeF. (B) Gene organization of S. meliloti CCNWSX0020 dmeRF. The dmeF (SM0020_17742) is located downstream of the dmeR (SM0020_17737). White region represents deletion sequence; PdmeR represents dmeR promoter.
dmeF/dmeR deletion mutant complementation experiment
To complement the dmeF and dmeR mutants, the entire dmeRF including the dmeR promoter was amplified from S. meliloti CCNWSX0020 with primers dmeH1/dmeH2 (Fig. 1B). The PCR products were digested with SmaI/XbaI and inserted into a broad-range plasmid pBBR1MCS-5 to generate pBBR-dmeF. The complement plasmids were transformed into SM0020 ΔdmeF, and single clones harbouring pBBR-dmeF were selected on TY solid medium supplemented with 50 µg/mL Gm. The presence of the entire dmeF gene in the mutant strain was confirmed by PCR.
Determination of the metal sensitivity of the defective mutant
Heavy metal sensitive assays of CCNWSX0020 strain, SM0020 ΔdmeF and the complementary strain were carried out on TY solid medium. The wild-type strain and dmeF mutant were grown to mid-exponential phase in TY liquid medium at 28 °C with shaking at 150 rpm. Cells were grown to the exponential phase in TY liquid medium and diluted to an OD600 of 0.1. Five 10-fold dilutions were spotted on the TY solid medium and incubated at 28 °C for 24 h. Each experiment was repeated three times.
Determination of Maximum Tolerable Concentrations (MTCs)
S. meliloti CCNWSX0020, dmeF mutant and dmeF mutant carrying pBBR-dmeF plasmid were grown to mid-exponential phase in TY liquid medium at 28 °C with shaking at 200 rpm, and cell suspensions were prepared at the same OD600 of 1.0 (optical density at 600 nm). Then, 1% of the cell suspensions was added to fresh TY medium supplemented with a different concentration of CuCl2, ZnCl2, CoCl2 and NiCl2. The cells were incubated with shaking at 200 rpm for 48 h, and the growth was monitored at OD600. The data are shown as the means of biological triplicates±SD.
Plant tests
M. lupulina seeds were surface sterilized and germinated in petri dishes with water agar (5 g agar per litre) at 28 °C for 48 h, and then seedlings were sown in pots filled with sterilized perlite-vermiculite (3:2) supplemented with different concentrations of CoCl2 or NiCl2 and grown in a greenhouse at 25 °C. When the first main leaf grew out, suspensions of either S. meliloti CCNWSX0020 or dmeF mutant were added to each plant root with a final concentration of 108 CFU per root. Plants were harvested 21 days after inoculation, the number of nodules on the plant roots was counted, and the lengths of the shoots and roots were measured. Nitrogenase activity in nodules was measured by the acetylene reduction assay as described by Hardy et al. (1968). Fresh nodules from M. lupulina inoculated with S. meliloti CCNWSX0020 and the dmeF mutant in the presence of 100 mg/kg cobalt or nickel were fixed in FAA solution (90 mL 70% ethanol, 5 mL acetic acid, and 5 mL 40% methanol) for 16 h. Dehydrating and clearing processing were carried out through a graded ethanol series and chloroform series, respectively, followed by embedding and sectioning of the paraffin blocks. Paraffin-embedded nodule sections of 5–10-µm thickness were stained by 0.05% (w/v) toluidine blue solution for observation with a BX53 biological microscope (Olympus, Tokyo, Japan).
Statistical analysis
SPSS 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the data. Data were compared by analysis of variance and multiple comparison tests.
Results
Identification of nickel- and cobalt-resistant genes
S. meliloti CCNWSX0020, isolated from the root nodules of M. lupulina growing in gold mine tailings in northwest China, could be resistant to many types of heavy metals, such as Cu2+, Zn2+, Pb2+ and Cd2+ (Fan et al., 2011). In our previous work, we found that the expression of two putative genes SM0020-17742 and SM0020-17737 was induced by Cu2+ through transcriptome sequencing. The 966-bp-long open reading frame of SM0020-17742 encodes a 321-amino-acid protein, and the deduced protein shows high identity with several previously characterized cobalt- and nickel-resistant proteins: DmeF of C. metallidurans (ABF07084, 37%) and DmeF of A. fabrum (AAK86697, 52%), so we designated the SM0020-17742 gene dmeF. In C. metallidurans and A. tumefaciens, the DmeF protein has an important role in cobalt and nickel resistance (Dokpikul et al., 2016; Munkelt, Grass & Nies, 2004). However, the phylogenetic tree based on the DmeF protein sequence showed that the DmeF proteins of S. meliloti CCNWSX0020, S. arboris and S. medicae are more closely related to each other than that of C. metallidurans and A. tumefaciens (Fig. S1). DmeF of S. meliloti SM0020 contained six predicted transmembrane segments (http://www.cbs.dtu.dk/services/TMHMM), with the histidine-rich stretch located between TMD4 and TMD5 (Fig. 1A). Another gene, SM0020_17737, is located directly upstream of dmeF (SM0020_17742) and predicted to be in the same operon with dmeF (Fig. 1B). SM0020_17737 encodes a 90-amino-acid protein that is highly homologous with DmeR belonging to the RcnR/CsoR family of metal-responsive transcriptional regulators. E. coli RncR binds to the rncA promoter DNA fragment in the absence of Ni2+ or Co2+, and the affinity of RncR for this promoter is reduced in the presence of excess nickel or cobalt. Alignment of sequences revealed that the upstream region of SM0020_17737 contains an inverted repeat (ATAGGGTACCCCCCTATGCTATG) between -35 and -10 similar to the dmeRF promoter of A. tumefaciens (Dokpikul et al., 2016). These observations suggest that the expression of both SM0020_17737 and SM0020_17742 (dmeF) might be regulated by the SM0020_17737 gene product, so the SM0020_17737 gene was designated as dmeR.
Nickel and cobalt induced dmeRF gene transcription in S. meliloti CCNWSX0020
Since heavy metal efflux systems of other bacteria are activated in the presence of the corresponding metal cation, we decided to investigate which metal could affect the expression of the dmeR and dmeF genes in S. meliloti CCNWSX0020 besides Cu2+. Expression of the dme R and dmeF genes was analysed first in free-living cells from S. meliloti CCNWSX0020 under different metal stresses. The expression of dmeF was strongly up-regulated by Ni2+, Co2+ and Cu2+ exposure (∼30-fold for 0.5 mM Ni2+, ∼40-fold for 0.3 mM Co2+ and ∼25-fold for 0.5 mM Cu2+), while the expression of dmeR was induced by Ni2+ (30-fold), Co2+ (30-fold) and Cu2+ (20-fold) (Fig. 2A). No significant induction of dmeR and dmeF was observed when Zn2+, Pb2+, or Cd2+ was added at concentrations up to 0.5 mM (Cd2+, 0.2 mM). The dmeR gene was located directly upstream of dmeF, and deletion of dmeR led to enhancement of the dmeF gene expression with or without nickel/cobalt stresses; meanwhile, the expression of dme RF was increased by cobalt and nickel treatment (Figs. 2B and 2C). These results suggested that DmeR is a cobalt/nickel sensor and regulates the expression of dmeF and its own.
Figure 2. dmeFR PCR analysis.

Induction of dmeF and dmeR of wild type or dmeR mutant by various metals examined through quantitative real-time PCR analysis. Wild type and dmeR mutants of S. meliloti CCNWSX0020 strains at OD600 of 1.0 were incubated with 0.5 mM CuSO4, ZnCl2, Pb(NO3)2, NiCl2, 0.2 mM CdCl2 and 0.3 mM CoCl2 for 30 min. The fold changes in dmeF and demR expression are expressed relative to the untreated control. Samples were then processed for qPCR analysis and normalized against the ribosomal 16 S rRNA. Error bars represent standard deviations of three biological repeats.
Functional analysis of dmeF in the CCNWSX0020 strain
To determine the function of DmeF in S. meliloti CCNWSX0020, the dmeF mutants were constructed by homologous recombination, resulting in strain SM0020ΔdmeF. Since the DmeF protein, belonging to a cation diffusion facilitator, was responsible for resistance to nickel and cobalt in A. tumefaciens C58 (Dokpikul et al., 2016), the sensitivities of S. meliloti CCNWSX0020 and dmeF mutant were characterized using metal-tolerance growth assays in TY solid medium. The dmeF mutant was more sensitive to 0.3 mM CoCl2 (10-fold) and 0.5 mM NiCl2 (103-fold) than the wild type, and the growth of the dmeF mutant was completely inhibited by 0.4 mM CoCl2 (Fig. 3). However, the resistance of the dmeF mutant to other metals, including CuSO4, CdCl2, Pb(NO3)2, and ZnCl2, was similar to the wild type. Surprisingly, the gene expression of dmeF was strongly induced by 0.5 mM CuSO4 (Fig. 2A), but there was no difference in the growth of the dmeF mutant and wild type under copper stress. It is probable that Cu2+ could bind to DmeR and induce the expression of the dmeRF operon, but DmeF was only the specific transporter of Co2+ and Ni2+. To verify the presence of the dmeF genes that were responsible for cobalt and nickel resistance, the dmeF PCR fragments containing the 550-bp upstream sequence of dmeR were inserted into the pBBR1MCS-5 vector, transformed into the corresponding mutant and then tested for these metal tolerances. Figure 3 shows that complemented strains could restore the cobalt and nickel resistance of the mutants. These results demonstrated that DmeF plays an important role in resistance to cobalt and nickel in S. meliloti CCNWSX0020.
Figure 3. Sensitivity of wild type and dmeF mutant to cobalt/nickel.
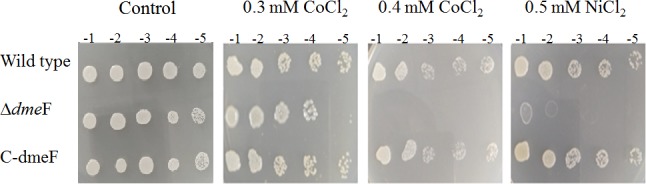
Sensitivity of wild type, dmeF mutant (Δ dmeF) and complemented strains (C- dmeF) of S. meliloti CCNWSX0020 to cobalt and nickel. Log-phase cells grown in TY were adjusted, serially 10-fold diluted and spotted onto TY plates in the presence of the indicated concentrations of CoCl2 (0.3 mM or 0.4 mM) and NiCl2 (0.5 mM).
Maximum tolerable concentration of the dmeF mutant
S. meliloti CCNWSX0020 and the dmeF mutant were cultured in TY medium supplemented with increasing concentrations of CoCl2, NiCl2, ZnCl2 and CuSO4, and the growth of the wild type and dmeF mutant was analysed after 48 h. As shown in Fig. 4, the dmeF mutant exhibited sensitivity to different concentrations of Co2+ and Ni2+ but not to other metals. The growth of the dmeF mutant was significantly inhibited if the concentration of nickel or cobalt was higher than 0.8 mM or 0.6 mM, respectively. The maximum tolerances of the S. meliloti CCNWSX0020 wild type to Co2+ and Ni2+ were 1.0 mM and 1.2 mM in TY liquid medium. In contrast, ZnCl2 and CuCl2 had no obvious effect on the growth of the wild type or mutant.
Figure 4. Growth of S. meliloti CCNWSX0020 and dmeF mutant under different metal stress.

Growth of S. meliloti CCNWSX0020 and the dmeF mutant which had been incubated with the different heavy metals in TY medium for 48 h. (A) NiCl2; (B) CoCl2; (C) CuSO4; (D) ZnCl2. S. meliloti CCNWSX0020 wild type (⧫), dmeF mutant(■), dmeF mutant carrying pBBR-dmeF plasmid (▴).
Deletions of dmeF decreased nodule number
To determine the effects of dmeF on the symbiotic capacity of S. meliloti CCNWSX0020, M. lupulina seedlings were inoculated with the wild-type strain CCNWSX0020 or the dmeF mutant. The plant length, nodule numbers and nitrogenase activities were determined to evaluate the symbiotic efficiency. Both S. meliloti CCNWSX0020 and the dmeF mutant can form well-defined rod-shaped pink nodules with M. lupulina. No significant difference was observed in the nodule number between the wild-type strain and the dmeF mutant without Ni2+ or Co2+ stress. However, the number of nodules produced by the dmeF mutant decreased significantly (P < 0.01) compared to the nodule numbers generated by the wild-type strain under Ni2+ or Co2+ stress (Fig. 5).
Figure 5. Influence of deletions in dmeF on symbiosis.
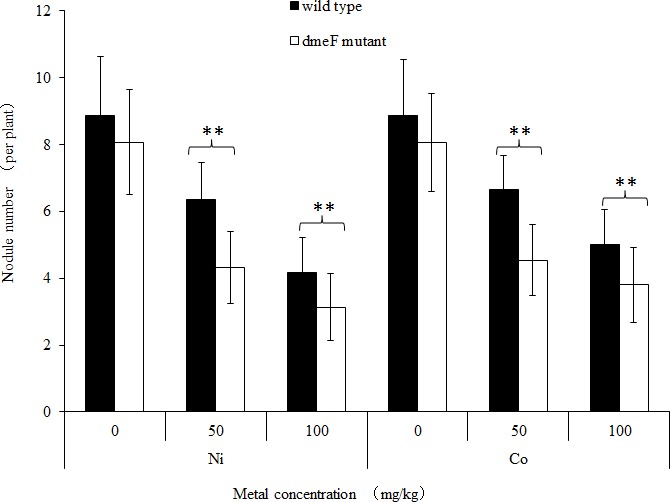
Influence of deletions in dmeF on symbiotic nodulation. M. lupulina seedlings were sown in pots supplied with NiCl2 (A) or CoCl2 (B). The nodule number, length of roots and shoots were determined at 21 DAI.
For single metal treatment, the nodule number of the plant inoculated with the dmeF mutant decreased by about 32.2% or 24.9% compared to the wild-type strain under 50 mg/kg or 100 mg/kg nickel stress, respectively. The same trend was observed when perlite-vermiculite were supplemented with CoCl2. The nodule number of M. lupulina inoculated with the dmeF mutant was about 31.9% or 24% less than those inoculated with S. meliloti CCNWSX0020 in the presence of 50 mg/kg or 100 mg/kg CoCl2, respectively. No significant decreases (P < 0.05) in the root and shoot length of M. lupulina inoculated with the dmeF mutant were observed compared with those inoculated with S. meliloti CCNWSX0020 in the presence of Ni2+ or Co2+ (Fig. S2). Since the nodulation of plants inoculated with the dmeF mutant showed a significant reduction by treatment with CoCl2 and NiCl2 compared to the controls, the nitrogenase activity of the nodules formed by the wild-type strain and dmeF mutant were determined. Table 2 indicates that the nitrogenase activities per plant for M. lupulina inoculated with dmeF mutant were reduced by ∼44% or 40% compared to that of S. meliloti CCNWSX0020 under 100 mg/kg Ni2+ or Co2+ stress. However, when standardized by nodule wet weight, the rate of acetylene reduction by nodules infected with the dmeF mutant was not statistically different from nodules infected with the wild-type strain. When rhizobium successfully infected the nodule cells, it would be dyed blue by toluidine blue. The histological organization of nodules showed that the proportion of infected cells (blue-stained N-fixing cells) within the nodule tissue induced by the dmeF mutant was not significantly lower than that of the wild-type strain (Fig. 6).
Table 2. Nitrogenase activities of nodule infected by S. meliloti CCNWSX0020 or dmeF mutant under Co2+ or Ni2+ stress.
| Bacterial strain | Nodule fresh weight (mg plant−1) | Nitrogenase activity (nmol h−1 plant−1) | Nitrogenase activity (nmol h−1 [mg nodule mass]−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 mg/kg NiCl2 | 50 mg/kg NiCl2 | 100 mg/kg NiCl2 | 0mg/kg NiCl2 | 50 mg/kg NiCl2 | 100 mg/kg NiCl2 | 0 mg/kg NiCl2 | 50 mg/kg NiCl2 | 100 mg/kg NiCl2 | |
| Wild-type strain dmeF mutant |
5.32 ± 0.5a 4.84 ± 0.8a |
4.30 ± 0.4a 2.72 ± 0.5a |
3.99 ± 0.5a 2.28 ± 0.3b |
54.31 ± 4.1a 48.78 ± 4.7a |
42.24 ± 3.9a 30.50 ± 3.2b |
41.88 ± 4.5a 23.64 ± 3b |
9.96 ± 1.7a 9.91 ± 1.1a |
9.53 ± 1.5a 11.03 ± 1.4a |
10.28 ± 1.6a 10.09 ± 1.4a |
| 0 mg/kg CoCl2 | 50 mg/kg CoCl2 | 100 mg/kg CoCl2 | 0 mg/kg CoCl2 | 50 mg/kg CoCl2 | 100 mg/kg CoCl2 | 0 mg/kg CoCl2 | 50 mg/kg CoCl2 | 100 mg/kg CoCl2 | |
| Wild-type strain dmeF mutant |
5.14 ± 0.5a 4.91 ± 0.8a |
3.82 ± 0.4a 2.50 ± 0.5a |
2.60 ± 0.5a 1.88 ± 0.3b |
52.22 ± 5a 50.08 ± 4.9a |
38.06 ± 3.6a 28.99 ± 3.2b |
30.41 ± 3.5a 18.32 ± 3.1b |
10.11 ± 1.6a 10.18 ± 1.4a |
9.95 ± 1.3a 11.2 ± 1.4a |
11.53 ± 1.2a 9.57 ± 1.3a |
Notes.
The values indicate the means ± standard error of triplicate samples.
The letters a/b are significant difference (p < 0.05) from plants inoculated with S. meliloti CCNWSX0020 or dmeF mutant under nickel or cobalt stress conditions.
Figure 6. Light micrographs of nodule sections produced by S. meliloti wild-type strain and dmeF mutant.
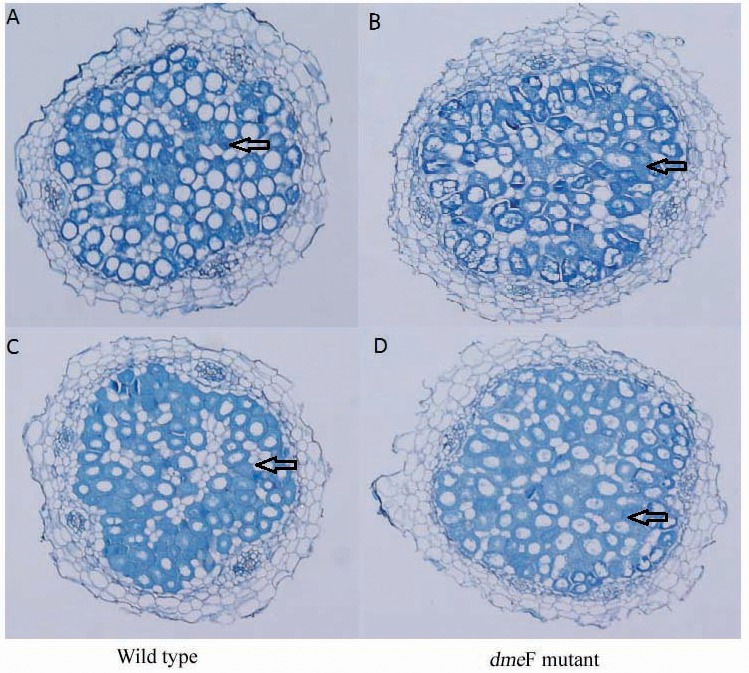
Light micrographs of nodule sections produced by S. meliloti CCNWSX0020 wild-type strain and the dmeF mutant with 100 mg/kg nickel (A and B) or cobalt (C and D).
Discussion
S. meliloti CCNWSX0020 is a bacterium that is resistant to multiple heavy metals isolated from root nodules of M. lupulina growing in mine tailings in the northwest of China (Fan et al., 2011). The genome of S. meliloti CCNWSX0020 was sequenced, and some copper and zinc resistance genes have been analysed in previous studies (Li et al., 2012; Lu et al., 2016; Lu et al., 2017). Here, we characterized the mechanisms of cobalt and nickel resistance and the potential of harnessing these mechanisms for phytostabilization.
In a previous study, Ni2+ transporter homologues of NreB in C. metallidurans 31A were found in the genomes of S. meliloti Rm1021, S. meliloti AK83 and S. meliloti BL225C through comparative genome analysis (Galardini et al., 2011). Recently, P1b-5 ATPase, which prevents excessive accumulation of iron and nickel in the cytoplasm, has been discovered in S. meliloti (Zielazinski et al., 2013). However, we have not found nreB (encoding a Ni2+ transporter) in the genome of S. meliloti CCNWSX0020 in previous work, and there is no difference in NiCl2 resistance between the mutant and the wild type when the nia gene (encoding a P1b-5 ATPase) was knocked out. Analysis of the S. meliloti CCNWSX0020 genome led to the identification of a dmeF-like gene (SM0020-17742). This open reading frame encodes a protein that has 37% similarity to cobalt- and nickel-resistant proteins (DmeF) of C. metallidurans. Upstream of SM0020-17742, an ORF SM0020-17737 encodes an RcnR/CsoR family of metal-responsive transcriptional regulators (DmeR). DmeR could negatively regulate the expression of dmeRF in the presence of nickel and cobalt in A. tumefaciens.
RT-PCR showed that the expression profiles of the dmeR and dmeF genes in S. meliloti were not only induced by cobalt and nickel but were also induced by copper. However, the expression of dmeR and dmeF was significantly up-regulated by nickel or cobalt, whereas no induction of other metal ions was observed (Dokpikul et al., 2016). Previous studies showed that the metal-responsive transcriptional regulator, encoded by dmeR, could combine with the promoter region of dmeRF and repress the expression of dem RF in the absence of metal. In contrast, nickel and cobalt bind to DmeR and inhibit the interaction of this protein with the dmeR promoter, and thus, transcriptional repression was relieved in R. leguminosarum (Rubiosanz et al., 2013). Similar to A. tumefaciens and R. leguminosarum, the gene dmeR (SM0020-17737) was located directly upstream of dmeF (SM0020-17742) and the non-coding sequences between SM0020-17737 and SM0020-17732 contain a conserved inverted repeat (ATA-GG-GTA-CCCCCC-TAT-GC-TAT) overlapping the −10 sequences, similar to the A. tumefaciens dmeRF promoter. Meanwhile, DmeR (SM0020-17737) exhibits the residues His3, Cys35, His60 and His64 for Ni2+ or Co2+ coordination and DmeF (SM0020-17742) contains six predicted transmembrane domains, with two conservative motifs HX3H and HX3D at the beginning of TM2 and TM5, and a histidine-rich stretch having Co2+ and Ni2+ as substrates (Fig. 1). According to the analysis of the mutants generated by homologous recombination, the dmeF deletion mutant was most sensitive to cobalt and nickel compared to the wild type, the dmeF deletion mutant was most sensitive to cobalt and nickel compared to the wild type, but there was no difference in the growth between the dmeF mutant and wild type under Zn2+ or Cu2+ stresses, showing the critical role of the DmeF transporter in cobalt and nickel resistance in S. meliloti CCNWSX0020. The experimental results of heavy metal resistance are not consistent with the RT-PCR (Figs. 2 and 3). A possible reason is that DmeR belongs to the RcnR/CsoR metal responsive transcriptional regulatory family. RcnR is thought to act as a tetramer and bind to one Ni2+ or Co2+ per monomer (Iwig & Chivers, 2009). RcnR is structurally similar to CsoR, which is the Cu+-responsive repressor of the copper efflux gene copA (encoding a Cu+/Ag+ efflux P1b-type ATPase) (Ma et al., 2009). We speculated that DmeR from S. meliloti could bind Cu+ in addition to Ni2+ and Co2+ with high affinity and up-regulated the expression of dmeF in vitro. But DmeF can only transfer nickel and cobalt from the cytoplasm to outside the cell.
There was a different cobalt/nickel tolerance ability in the agar plate assay, where the growth of the dmeF mutant was completely inhibited by 0.4 mM CoCl2/0.5 mM NiCl2, but in the liquid medium growth test, the growth of the dmeF mutant was significantly inhibited by 0.6 mM CoCl2/0.8 mM NiCl2 (Figs. 3 and 4). The first reason for this phenomenon was that the liquid medium was cultured for 48 h, while solid medium was only cultured for 24 h. Second, the nutrient assimilation rate is favoured in liquid media and agar could reduce nutrient diffusion throughout the medium (Romberger & Tabor, 1971). Thus, the growth rate of bacteria on solid medium was less than that of liquid medium. So we hypothesized that if the incubation time of solid medium was prolonged, the tolerance to cobalt/nickel in these two assays would be similar.
The effective nodules are directly related to nitrogen fixation efficiency and affect the growth of the legume plant. So the application of rhizobium-legume symbiosis systems for host plant growth promotion and heavy metal absorption in metal-contaminated soils have attracted much attention (Zribi et al., 2013). However, high-concentration heavy metals could inhibit rhizobium growth and associate with the host plant. Metal resistance determinants might protect rhizobia and thereby ensure the ability to build an effective symbiosis relationship under heavy metal stress conditions. The shoot and root biomass of M. sativa inoculated with the Zn-tolerant strain S. meiloti S532 was higher than plants with the Zn-intolerant strain S. meiloti S112. Stan et al. (2011) found that the biomass and nitrogen content of clover inoculated with R. leguminosarum biovar trifolii were increased compared with untreated plants. Although S. meliloti CCNWSX0020 displayed resistance to various heavy metals, the cobalt and nickel resistance mechanisms of this strain were not characterized. Moreover, whether metal-resistant genes affect the symbiotic relationship between rhizobia and plants is not clear under heavy metal stress. Our results showed that excess nickel and cobalt indeed reduced the number of functional nodules, which agreed with other reports that rhizobia-legume symbioses were inhibited by excess metal (Sanchez-Pardo, Fernandez-Pascual & Zornoza, 2012). Although some reports indicated that Ni is used as a structural component of urease and hydrogenase (Brito et al., 1994), Co is mainly used as a component of vitamin B12. Processes in the development of some root nodules specifically require nickel and cobalt. The low supply of Ni2+ and Co2+ may result in increasing hydrogenase and urease activities in leaves and nitrogenase activities in root nodules (Lavres, Franco & Câmara, 2016). The dmeF gene deletion aggravated the inhibition of nodulation in the presence of nickel or cobalt (Fig. 5). The dmeF mutant decreased nitrogenase activity of the Medicago plants under nickel or cobalt stress conditions. However, the rate of acetylene reduction by nodules infected with the dmeF mutant was similar to that of the wild-type strain when the rate of acetylene reduction was standardized by nodule fresh weight. These results suggest that DmeF could relieve the toxicity of nickel/cobalt to free-living rhizobial cells and help to infect host plants but did not participate in the nitrogen fixation process. This was different from copper-resistant determinants. For example, the lipA mutant was not only sensitive to Cu2+ but also reduced functional nodule numbers, infected cells, leghaemoglobin expression and N fixation in nodules (Hao et al., 2015). These data suggest that S. meliloti selected dmeRF as a general strategy to maintain nickel and cobalt homeostasis in the cytoplasm.
Conclusion
In this work, two heavy metal resistance genes (sm0020-17737 and sm0020-17742) of S. meiloti CCNWSX0020 were identified with high homology to the dmeRF operon in Cupriavidus metallidurans. The dmeR and dmeF genes encoded a transcriptional regulator and cation transporter, respectively. Although the dmeRF of the CCNWSX0020 strain was induced by Cu2+, Ni2+ and Co2+, the dmeF mutant exhibited more sensitivity to Co2+ or Ni 2+than the wild type. Also, there was no difference in the growth between the dmeF mutant and the wild type under other metal stress conditions. Plant experiment results showed that the nodule number of the host plant inoculated with the dmeF deletion mutant was significantly decreased in the presence of Co2+ or Ni2+. However, the nitrogenase activities of nodules infected by the dmeF deletion mutant were not reduced when standardized by the nodule fresh weight. These results indicated that the dmeF gene confers Co2+ or Ni2+ resistance to bacteria and does not participate in the symbiosis with the host plant.
Supplemental Information
Protein sequences of DmeF or their orthologs in each strain were concatenated and used for drawing the tree.
A. Stem length of Medicago lupulina plants under control, nickel/cobalt (50 mg kg−1) and 100 mg kg−1) stress conditions. of three replicates. B. Root length of Medicago lupulina plants under control, nickel/cobalt (50 mg kg−1) and 100 mg kg−1) stress conditions. The values indicate the mean±S.E. of three replicates.
Funding Statement
This study was funded by the National Key Research and Development Program of China (Award No: 2018YFD0200103 and 2016YFD0800706) and the Natural Science Basic Research Plan in the Shaanxi province of China (Program NO. 2018JM3004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Zhefei Li conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper.
Xiuyong Song performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables.
Juanjuan Wang, Xiaoli Bai and Engting Gao performed the experiments.
Gehong Wei approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The dmeRF sequence is deposited at NCBI with the accession number: AGVV01000035.1.
References
- Ahmad et al. (2015).Ahmad J, Alhadlaq HA, Siddiqui MA, Saquib Q, Alkhedhairy AA, Musarrat J, Ahamed M. Concentration-dependent induction of reactive oxygen species, cell cycle arrest and apoptosis in human liver cells after nickel nanoparticles exposure. Environmental Toxicology. 2015;30(2):137–148. doi: 10.1002/tox.21879. [DOI] [PubMed] [Google Scholar]
- Banerjee & Ragsdale (2003).Banerjee R, Ragsdale SW. The many faces of vitamin B12: catalysis by Cobalamin-dependent nzymes. Annual Review of Biochemistry. 2003;72(1):209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- Brito et al. (1994).Brito B, Palacios JM, Hidalgo E, Imperial J, Ruiz-Argüeso T. Nickel availability to pea (Pisum sativum L.) plants limits hydrogenase activity of Rhizobium leguminosarum bv. viciae bacteroids by affecting the processing of the hydrogenase structural subunits. Journal of Bacteriology. 1994;176(17):5297–5303. doi: 10.1128/jb.176.17.5297-5303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokpikul et al. (2016).Dokpikul T, Chaoprasid P, Saninjuk K, Sirirakphaisarn S, Johnrod J, Nookabkaew S, Mongkolsuk S. Regulation of the Cobalt/Nickel Efflux Operon dmeRF in Agrobacterium tumefaciens and a ink between the iron-sensing regulator RirA and Cobalt/Nickel resistance. Applied and Environmental Microbiology. 2016;82(15):4732–4742. doi: 10.1128/AEM.01262-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan et al. (2011).Fan L, Ma Z, Liang J, Li H, Wang ET, Wei G. Characterization of a copper-resistant symbiotic bacterium isolated from Medicago lupulina growing in mine tailings. Bioresource Technology. 2011;102(2):703–709. doi: 10.1016/j.biortech.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Fang et al. (2002).Fang C, Chen H, Chuang Y, Chang S, Wang J. Cloning of a cation efflux pump gene associated with Chlorhexidine esistance in Klebsiella pneumoniae. Antimicrobial Agents and Chemotherapy. 2002;46(6):2024–2028. doi: 10.1128/AAC.46.6.2024-2028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardini et al. (2011).Galardini M, Mengoni A, Brilli M, Pini F, Fioravanti A, Lucas S, Biondi EG. Exploring the symbiotic pangenome of the nitrogen-fixing bacterium Sinorhizobium meliloti. BMC Genomics. 2011;12(1):235–235. doi: 10.1186/1471-2164-12-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldan, Sterner & Babinger (2008).Guldan H, Sterner R, Babinger P. Identification and characterization of a bacterial glycerol-1-phosphate dehydrogenase: Ni2+-dependent AraM from Bacillus subtilis. Biochemistry. 2008;47:7376–7384. doi: 10.1021/bi8005779. [DOI] [PubMed] [Google Scholar]
- Haferburg & Kothe (2007).Haferburg G, Kothe E. Microbes and metals: interactions in the environment. Journal of Basic Microbiology. 2007;47:453–467. doi: 10.1002/jobm.200700275. [DOI] [PubMed] [Google Scholar]
- Hanahan (1983).Hanahan D. Studies on transformation of Escherichia coli with plasmids. Journal of Molecular Biology. 1983;166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hao et al. (2015).Hao X, Xie P, Zhu Y, Taghavi S, Wei G, Rensing C. Copper tolerance mechanisms of Mesorhizobium amorphae and its role in aiding phytostabilization by Robinia pseudoacacia in Copper contaminated soil. Environmental Science & Technology. 2015;49(4):2328–2340. doi: 10.1021/es504956a. [DOI] [PubMed] [Google Scholar]
- Hardy et al. (1968).Hardy RW, Holsten RD, Jackson EK, Burns RC. The Acetylene-Ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiology. 1968;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwig & Chivers (2009).Iwig JS, Chivers PT. DNA recognition and wrapping by Escherichia coli RcnR. Journal of Molecular Biology. 2009;393(2):514–526. doi: 10.1016/j.jmb.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Kolajrobin et al. (2015).Kolajrobin O, Russell D, Hayes K, Pembroke JT, Soulimane T. Cation diffusion facilitator family: structure and function. FEBS Letters. 2015;589(12):1283–1295. doi: 10.1016/j.febslet.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Kovach et al. (1995).Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- Lavres, Franco & Câmara (2016).Lavres J, Franco GC, Câmara GMS. Soybean seed treatment with nickel improves biological nitrogen fixation and urease activity. Frontiers in Environmental Science. 2016;4 doi: 10.3389/fenvs.2016.00037. Article 37. [DOI] [Google Scholar]
- Li et al. (2012).Li Z, Ma Z, Hao X, Wei G. Draft genome sequence of Sinorhizobium meliloti CCNWSX0020, a nitrogen-fixing symbiont with copper tolerance capability isolated from lead-zinc mine tailings. Journal of Bacteriology. 2012;194(5):1267–1268. doi: 10.1128/JB.06682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu LZ, Ding M, Zheng JZ, Zhu Y, Fenderson BA, Li B, Yu JJ, Jiang BH. Tungsten carbide-cobalt nanoparticles induce reactive oxygen species, AKT, ERK, AP-1, NF-κB, VEGF, and angiogenesis. Biological Trace Element Research. 2015;166(1):57–65. doi: 10.1007/s12011-015-0331-6. [DOI] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2017).Lu M, Jiao S, Gao E, Song X, Li Z, Hao X, Rensing C, Wei G. Transcriptome response to heavy metals in Sinorhizobium meliloti CCNWSX0020 reveals new metal resistance determinants that also promote bioremediation by Medicago lupulina in metalcontaminated soil. Applied and Environmental Microbiology. 2017;83(20):e01244-17. doi: 10.1128/AEM.01244-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2016).Lu M, Li Z, Liang J, Wei Y, Rensing C, Wei G. Zinc resistance mechanisms of P1B-type ATPases in Sinorhizobium meliloti CCNWSX0020. Scientific Reports. 2016;6:29355. doi: 10.1038/srep29355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2009).Ma Z, Cowart DM, Scott RA, Giedroc DP. Molecular insights into the metal selectivity of the copper (I)-sensing repressor CsoR from Bacillus subtilis. Biochemistry. 2009;48:3325–3334. doi: 10.1021/bi900115w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewska et al. (2008).Matuszewska E, Kwiatkowska J, Kuczynska-Wisnik D, Laskowska E. Escherichia coli heat-shock proteins IbpA/B are involved in resistance to oxidative stress induced by copper. Microbiology. 2008;154:1739–1747. doi: 10.1099/mic.0.2007/014696-0. [DOI] [PubMed] [Google Scholar]
- Montanini et al. (2007).Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics. 2007;8:107–123. doi: 10.1186/1471-2164-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney & Hausinger (2003).Mulrooney SB, Hausinger RP. Nickel uptake and utilization by microorganisms. FEMS Microbiology Reviews. 2003;27(2):239–261. doi: 10.1016/S0168-6445(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Munkelt, Grass & Nies (2004).Munkelt D, Grass G, Nies DH. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. Journal of Bacteriology. 2004;186:8036–8043. doi: 10.1128/JB.186.23.8036-8043.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies (1999).Nies DH. Microbial heavy-metal resistance. Applied Microbiology and Biotechnology. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- Nies (2003).Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. Fems Microbiology Reviews. 2003;27(2):313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Paulsen & Saier Jr (1997).Paulsen IT, Saier Jr MH. A novel family of ubiquitous heavy metal ion transport proteins. Journal of Membrane Biology. 1997;156(2):99–103. doi: 10.1007/s002329900192. [DOI] [PubMed] [Google Scholar]
- Pini et al. (2014).Pini F, Spini G, Galardini M, Bazzicalupo M, Benedetti A, Chiancianesi M, Mengoni A. Molecular phylogeny of the nickel-resistance gene nreB and functional role in the nickel sensitive symbiotic nitrogen fixing bacterium Sinorhizobium meliloti. Plant and Soil. 2014:189–201. doi: 10.1007/s11104-013-1979-3. [DOI] [Google Scholar]
- Romberger & Tabor (1971).Romberger JA, Tabor CA. The picea abies shoot apicalmeristem in culture. IL agar and autoclavingeffects. American Journal of Botany. 1971;58(2):131–140. doi: 10.2307/2441456. [DOI] [Google Scholar]
- Rubiosanz et al. (2013).Rubiosanz L, Prieto RI, Imperial J, Palacios JM, Brito B. Functional and expression analysis of the metal-inducible dmeRF system from Rhizobium leguminosarum bv. viciae. Applied and Environmental Microbiology. 2013;79(20):6414–6422. doi: 10.1128/AEM.01954-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, Cavet & Robinson (1999).Rutherford JC, Cavet JS, Robinson NJ. Cobalt-dependent transcriptional switching by a dual-effector MerR-like protein regulates a cobalt-exporting variant CPx-type ATPase. Journal of Biological Chemistry. 1999;274:25827–25832. doi: 10.1074/jbc.274.36.25827. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pardo, Fernandez-Pascual & Zornoza (2012).Sanchez-Pardo B, Fernandez-Pascual M, Zornoza P. Copper microlocalisation, ultrastructural alterations and antioxidant responses in the nodules of white lupin and soybean plants grown under conditions of copper excess. Environmental and Experimental Botany. 2012;84:52–60. doi: 10.1016/j.envexpbot.2012.04.017. [DOI] [Google Scholar]
- Schafer et al. (1994).Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145(1):69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Sharaff & Archana (2015).Sharaff M, Archana G. Copper-induced modifications in early symbiotic signaling factors of Ensifer (Sinorhizobium)—Medicago interactions. Archives of Microbiology. 2015;198(7):701–709. doi: 10.1007/s00203-016-1242-4. [DOI] [PubMed] [Google Scholar]
- Stahler et al. (2006).Stahler F, Odenbreit S, Haas R, Wilrich J, Van Vliet AH, Kusters JG, Bereswill S. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infection and Immunity. 2006;74(7):3845–3852. doi: 10.1128/IAI.02025-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan et al. (2011).Stan V, Cornea CP, Gament E, Voaides C, Pop A. Heavy metal resistant Rhizobium leguminosarum biovar trifolii isolates: characterization and use in rhizoremediation of polluted soils. Current Opinion in Biotechnology. 2011;22:s74. doi: 10.1016/j.copbio.2011.05.217. [DOI] [Google Scholar]
- Uebe et al. (2011).Uebe R, Junge K, Henn V, Poxleitner G, Katzmann E, Plitzko JM, Schuler D. The cation diffusion facilitator proteins MamB and MamM of Magnetospirillum gryphiswaldense have distinct and complex functions, and are involved in magnetite biomineralization and magnetosome membrane assembly. Molecular Microbiology. 2011;82(4):818–835. doi: 10.1111/j.1365-2958.2011.07863.x. [DOI] [PubMed] [Google Scholar]
- Wani, Khan & Zaidi (2008).Wani PA, Khan MS, Zaidi A. Effects of heavy metal toxicity on growth, symbiosis, seed yield and metal uptake in pea grown in metal amended soil. Bulletin of Environmental Contamination and Toxicology. 2008;81(2):152–158. doi: 10.1007/s00128-008-9383-z. [DOI] [PubMed] [Google Scholar]
- Wilson & Carson (2001).Wilson T, Carson J. Rapid, high-throughput extraction of bacterial genomic DNA from selective-enrichment culture media. Letters in Applied Microbiology. 2001;32:326–330. doi: 10.1046/j.1472-765X.2001.00906.x. [DOI] [PubMed] [Google Scholar]
- Zielazinski et al. (2013).Zielazinski EL, González-Guerrero M, Subramanian P, Stemmler TL, Arguello JM, Rosenzweig AC. Sinorhizobium meliloti Nia is a P1B-5-ATPase expressed in the nodule during plant symbiosis and is involved in Ni and Fe transport. Metallomics. 2013;5(12):1614–1623. doi: 10.1039/c3mt00195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zribi et al. (2013).Zribi K, Nouairi I, Slama I, Talbi-Zribi O, Mhadhbi H. Medicago sativa-Sinorhizobium meliloti symbiosis promotes the bioaccumulation of zinc in nodulated roots. International Journal of Phytoremediation. 2013;17(1):49–55. doi: 10.1080/15226514.2013.828017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein sequences of DmeF or their orthologs in each strain were concatenated and used for drawing the tree.
A. Stem length of Medicago lupulina plants under control, nickel/cobalt (50 mg kg−1) and 100 mg kg−1) stress conditions. of three replicates. B. Root length of Medicago lupulina plants under control, nickel/cobalt (50 mg kg−1) and 100 mg kg−1) stress conditions. The values indicate the mean±S.E. of three replicates.
Data Availability Statement
The following information was supplied regarding data availability:
The dmeRF sequence is deposited at NCBI with the accession number: AGVV01000035.1.


