Abstract
Purpose
Myopia progression is thought to involve biomechanical weakening of the sclera, which leads to irreversible deformations and axial elongation of the eye. Scleral crosslinking has been proposed as a potential treatment option for myopia control by strengthening the mechanically weakened sclera. The biomechanical mechanism by which the sclera weakens during myopia and strengthens after crosslinking is not fully understood. Here, we assess the effect of lens-induced myopia and exogenous crosslinking using genipin on the inelastic mechanical properties of the tree shrew sclera measured by cyclic tensile tests.
Methods
Cyclic tensile tests were performed on 2-mm wide scleral strips at physiological loading conditions (50 cycles, 0–3.3 g, 30 sec/cycle). Two scleral strips were obtained from each eye of juvenile tree shrews exposed to two different visual conditions: normal and 4 days of monocular −5 D lens wear to accelerate scleral remodeling and induce myopia. Scleral strips were mechanically tested at three alternative conditions: immediately after enucleation; after incubation in phosphate buffered saline (PBS) for 24 hours at 37°C; and after incubation for 24 hours in PBS supplemented with genipin at a low cytotoxicity concentration (0.25 mM). Cyclic softening was defined as the incremental strain increase from one cycle to the next.
Results
−5D lens treatment significantly increased the cyclic softening response of the sclera when compared to contralateral control eyes (0.10 ± 0.029, mean ± standard error, p = 0.037). Exogenous crosslinking of the lens treated sclera significantly decreased the cyclic softening response (−0.12 ± 0.014, p = 2.2×10−5). Contrary to all other groups, the genipin-crosslinked tissue did not exhibit cyclic softening significantly different from zero within the 50-cycle test.
Conclusions
Results indicated that cyclic tensile loading leads to an inelastic, cyclic softening of the juvenile tree shrew sclera. The softening rate increased during lens-induced myopia and was diminished after genipin crosslinking. This finding suggests that axial elongation in myopia may involve a biomechanical weakening mechanism that increased the cyclic softening response of the sclera, which was inhibited by scleral crosslinking using genipin.
Keywords: Myopia, Sclera, Biomechanics, Scleral Crosslinking
1. Introduction
Myopia is the most commonly occurring eye disability, affecting more than 30% of American adults and ~50% in some other adult populations around the globe.1 High myopia is a significant risk factor in ocular pathologies, such as glaucoma and retinal detachment.2–5 Often referred to as near-sightedness, myopia is an optical pathology characterized by an eye that is too long for its optical system (cornea and lens). Myopia is typically caused by an over-elongation of the posterior segment of the eye, placing the retina behind the focal plane.6 The elongation of the myopia developing eye involves scleral remodeling and several biomechanical changes of the sclera.7–9 Recently, we have shown that the cyclic application of tensile loads to scleral strips leads to progressive deformations (cyclic softening) of the normal juvenile tree shrew sclera.8 Here, we investigate whether or not the cyclic softening response of the tree shrew sclera increases during lens induced myopia and if artificial scleral crosslinking (SXL) using genipin inhibits this biomechanical response.
The biomechanical properties of the sclera are complex, nonlinear, anisotropic, time, and disease dependent. While multiple studies have investigated the biomechanics of the sclera, few have explored its inelastic properties.10 Common mechanical tests provide measures of the tissue elasticity and mechanical properties that relate to reversible deformations. A change in tissue elasticity cannot explain the slow but progressive elongation of the eye during myopia, which are characterized by inelastic and irreversible deformations. In contrast, softening due to cyclic loading is an inelastic response that leads to inelastic deformations, which can explain the lasting elongation of the eye in myopia.
Mechanical testing protocols often use cyclic loading to precondition the tissue before the actual experiment is recorded. Preconditioning is typically only performed to obtain a repeatable experimental result. Typically, the nonlinear mechanical response of soft tissues changes (“softens”) during preconditioning but stabilizes after a certain number of cycles.11–14 The extent of the preconditioning effect depends on the tissue properties and the mechanical testing protocol. In the case of scleral tissue, strip testing seems to amplify this effect8 compared to inflation testing.15 In some cases, preconditioning is not feasible as the mechanical response of some soft tissues leads to continuous cyclic softening and irreversible deformations, which never stabilize. The juvenile tree shrew sclera is one of these exceptions.8 However, whether the cyclic softening response changes during myopia development or after scleral crosslinking is unknown and investigated here.
The sclera of tree shrews is similar to that of humans and mainly composed of interwoven lamellae of type I collagen.16–19 The nonlinear mechanical response (hyperelasticity) of the sclera is thought to be mainly driven by the crimping response of collagen fibrils.20, 21 The collagen fibrils crimp and buckle when the sclera is unloaded. With increasing tensile load, the sclera stiffens as the crimp is removed. The sclera also contains glycosaminoglycans (GAG) and proteoglycans (PG), such as aggrecan, decorin, and biglycan.19, 22 Animal experiments suggest that scleral remodeling is accelerated during experimental myopia, which involves several structural, biochemical, and biomechanical changes: (i) scleral thinning18, 23; (ii) reduction in scleral dry weight (3–5%)23–25; (iii) lower hyaluronan and sulfated GAG levels25; (iv) upregulated enzymatic degradation26–30; (v) downregulated collagen type I synthesis31; (vi) downregulation of aggrecan32; (vii) increased creep rate7; and (viii) increased collagen fibril crimping.8 In vivo, the sclera is subjected to tensile forces that result from the intraocular pressure (IOP). It is thought that the increased enzymatic activity and biochemical alterations in the GAG and PG composition lead to a biomechanical weakening of the sclera during experimental myopia.33 We and others hypothesize that scleral remodeling and biomechanical weakening of the sclera in myopia involve collagen sliding between scleral lamellae and/or collagen fibrils.8, 9, 22, 34 It is thought that the biomechanical weakening of the sclera in myopia induces a creep-like elongation of the sclera at baseline IOP. However, the eye is constantly exposed to high and low frequency IOP fluctuations.35 Cyclic loading is known to weaken man-made materials such as steel in a different way than constant loading. It has never been explored if biomechanical weakening during myopia alters the inelastic deformation response of the sclera to cyclic loading. We hypothesize that the myopic sclera is more susceptible to inelastic deformations due to cyclic loading (cyclic softening) and that these deformations contribute to the axial elongation of the eye during myopia progression.
Collagen crosslinking has been used to mechanically stabilize collagenous tissues and hydrogels.36 Biomechanical weakening of the cornea has been hypothesized to underlie keratoconus,37 leading to the clinical use of riboflavin and UV light to crosslink and mechanically stabilize the cornea. This has proven to be an effective therapy for keratoconus patients.38, 39 SXL has been suggested as a potential treatment to control progressive myopia by mechanically stabilizing the sclera.40 Progressive myopia is one of the leading causes of blindness worldwide.41–45 While the underlying mechanism is unclear, progressive or pathologic myopia is thought to be due to uncontrolled, progressive scleral remodeling leading to posterior staphylomas. Scleral reinforcement is currently the only available treatment option to slow the progressive remodeling, but this treatment option remains controversial due to the risk of complications.46–50 Given the lack of treatment options available to halt progressive scleral remodeling and the morbidity of progressive myopia, an effective clinical solution is becoming increasingly necessary. Though crosslinking itself is simple, cytotoxicity is often a concern with the agents utilized.51, 52 Of the known low-cytotoxic collagen crosslinking agents, genipin is one of the best characterized and most potent crosslinking agents.51, 53 It is a naturally occurring organic compound derived from the fruit of the gardenia plant (Gardenia jasminoides).51 Genipin has been shown to alter the scleral material properties of porcine sclera54 and to slow myopia progression in guinea pigs.55 Campbell et al. have shown that a much lower concentration of genipin is required to achieve a comparable scleral stiffening effect using glyceraldehyde (7 fold) or methylglyoxal (30 fold), which are alternative low-cytotoxic collagen crosslinking agents. Recently, SXL was shown to reduce collagen fibril crimping,56 supporting the notion that SXL can counteract biomechanical and micro-structural changes in the sclera during myopia.8 Only one study has investigated the biomechanics of the sclera in animal eyes with induced myopia and after SXL.55 While this study showed that SXL stiffens the myopic sclera, the effect of SXL on inelastic scleral material properties was not investigated. To the best of our knowledge, it has never been investigated if SXL can counteract a biomechanical weakening response that involves cyclic softening. To gain insight into the biomechanical strengthening mechanism of SXL, we investigate here for the first time if SXL using genipin can reduce or even halt cyclic softening in juvenile tree shrew scleras with experimental myopia.
2. Materials and Methods
2.1 Subjects
Two groups of juvenile tree shrews (Tupaia glis belangeri) were used in this study (10 animals in total). A total of 11 animals were tested, but one animal had to be removed from the analysis as one of its scleral strips failed during mechanical testing. The animals were bred and raised in the colony of the University of Alabama at Birmingham in accordance with The American Association for the Advancement of Laboratory Animal Care guidelines. The normal group (n = 5) had a normal visual experience. The animals of the treated group (n = 5) were exposed to 4 days of −5 D monocular lens wear starting at 24 days of visual experience (DVE) to accelerate scleral remodeling and induce myopia. Daily refractive measurements of each animals’ eyes were taken from 24 to 28 DVE using an autorefractor (Nidek ARK-700A, http://usa.nidek.com/). At 28 DVE, animals were sacrificed and both eyes enucleated. The untreated eye of the lens-wearing animal was used as a contralateral control – which will be referred to as control hereafter. A detailed description of the subject care and the experimental techniques including the goggle system used to induce myopia can be found in previous publications.7, 18, 57–59
2.2 Tissue Preparation
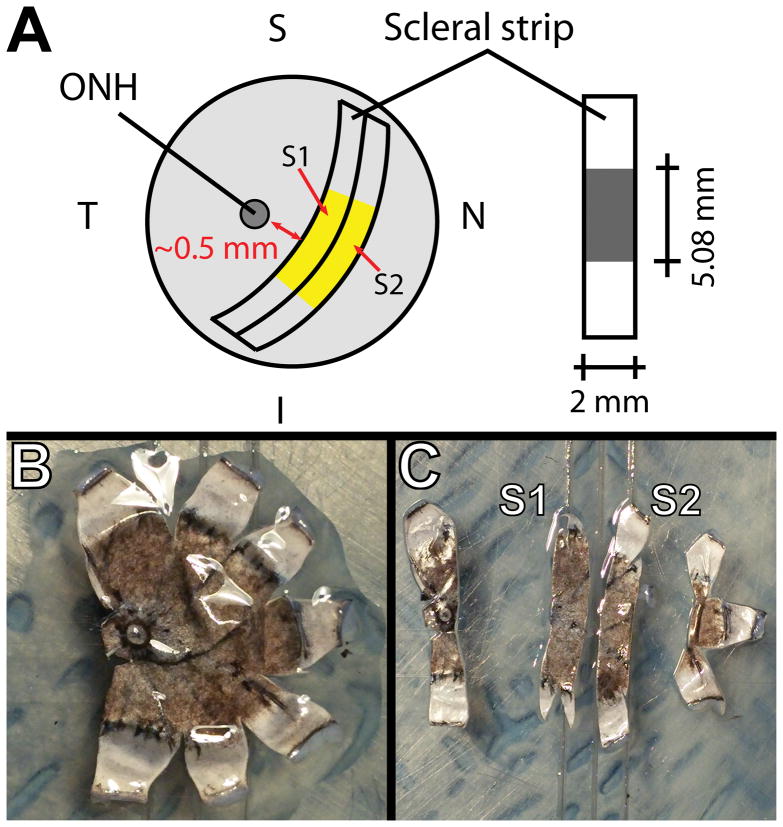
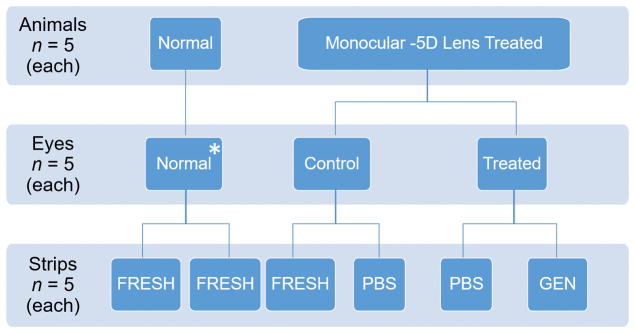
Each eye was first cleaned of all extraneous fat and connective tissue before making an incision at the limbus to remove the cornea, lens, retina, and retinal pigmented epithelium (RPE). Small, radial incisions were then made from the limbus toward the posterior pole of the sclera, allowing it to lay flat. Using a custom razor blade jig, two 2 mm-wide and approximately 12 mm long strips were cut from the posterior pole at a similar orientation (approximately superior-inferior) from each eye as shown in Figure 1. To obtain the thickness of the strips, each strip was placed in a pool of 1x phosphate buffered saline (PBS; ThermoFisher Scientific) between two cover slips. PBS was pH balanced to 7.2 and isotonic (KH2PO4: 1.54 mM; NaCl: 155.17 mM; Na2HPO4-7H2O: 2.71 mM) to minimize potential swelling or shrinkage of the scleral strips. The thickness of each strip was estimated by measuring the distance between the top surface of the bottom cover slip and bottom surface of the top coverslip using a microscope.7 To account for potential differences in tissue anisotropy and material properties between the two strip locations (one strip is closer to the optic nerve head than the other), tissue treatment conditions were randomly assigned to strips taken at the two different locations. Three different strip conditions were investigated: no tissue treatment (FRESH); 24 hours incubation in 2 ml of 1x PBS; or 24 hours incubation in 2 ml of 1x PBS supplemented with 0.25 mM (0.006 wt%) genipin (GEN; Wako Pure Chemical Industries, Ltd.). All strips of normal animals were tested immediately after enucleation (normal FRESH). For the control eye, strips were either tested immediately after enucleation (control FRESH) or after 24 hours incubation in PBS (control PBS). The treated eye strips were tested either after 24 hours incubation in PBS (treated PBS) or after 24 hours incubation in PBS supplemented with genipin (treated GEN). The animal and tissue treatment groups are illustrated in Figure 2. All incubation was done at 37 °C in closed microcentrifuge tubes to prevent evaporation. Strips were mechanically tested within 4 hours after enucleation (FRESH groups) or within 4 hours after completing the incubation (all other groups). Strips were stored in fresh PBS at 4 °C after enucleation (FRESH groups) or incubation (all other groups) until testing.
Figure 1.
Scheme for acquiring two 2-mm strips from a tree shrew eye (A). Each strip was 2 mm wide and approximately 15 mm long, where 5.08 mm was the initial clamp-to-clamp distance. Images of a flattened posterior scleral shell before (B) and after (C) the strips were cut.
Figure 2.
The experimental groups in this study are represented by a combination of different in vivo eye treatment conditions (normal, control, treated) and ex vivo strip treatments (FRESH, PBS, GEN). Five of the possible nine combinations of eye and strip treatments were tested (normal FRESH, control FRESH, control PBS, treated PBS, treated GEN). Both strips from normal animal eyes were mechanically tested after enucleation (normal FRESH). Strips of the control eyes of −5D lens treated animals were either tested after enucleation (control FRESH) or after incubation for 24 hours in PBS (control PBS). The strips of the lens treated eyes were tested after 24 incubation hours in PBS (treated PBS) or PBS supplemented with genipin (treated GEN). * Only one eye of each normal animal (randomly chosen between left and right eye) was used in this study.
2.3 Mechanical Testing
The strips were then mounted in a Vitrodyne V-200 material tester (Liveco Biomechanical Instruments, Figure 3A) for uniaxial tensile testing as described in previous work.7, 8 This material tester was designed for load-controlled mechanical testing of small samples with a load and displacement resolution of 0.01 g and 2.5μm, respectively. Pre-existing stresses were minimized by floating the strip in saline then wicking away the fluid, leaving the strip in place on a platform of known length (5.08 mm) between the clamps (Figure 3B). After the strip was clamped, the displacement readout was zeroed, the mounting platform was removed (Figure 3C), and the strip was immersed in room temperature PBS. To remove any slack in the strip, a minimal pre-load of 0.01 grams was applied. The load reading of the machine was tared after the pre-load was applied. Cyclic uniaxial tensile tests at physiological loads were performed while the tissue remained immersed in 1x PBS throughout testing. All strips were loaded from 0 to 3.33 grams, then unloaded back to 0 load in a 30-second cycle for 50 cycles. 3.33 grams corresponds to approximately 60 mmHg IOP as estimated by Laplace’s Law.60 Load and displacement were recorded throughout testing at 3 Hz. Cyclic softening was described as the increment in strain (Δεmax) at maximum load (3.33 grams) between two consecutive cycles.8 Increments between strains (Δεmin) at minimum load (0 grams) were also calculated and investigated.
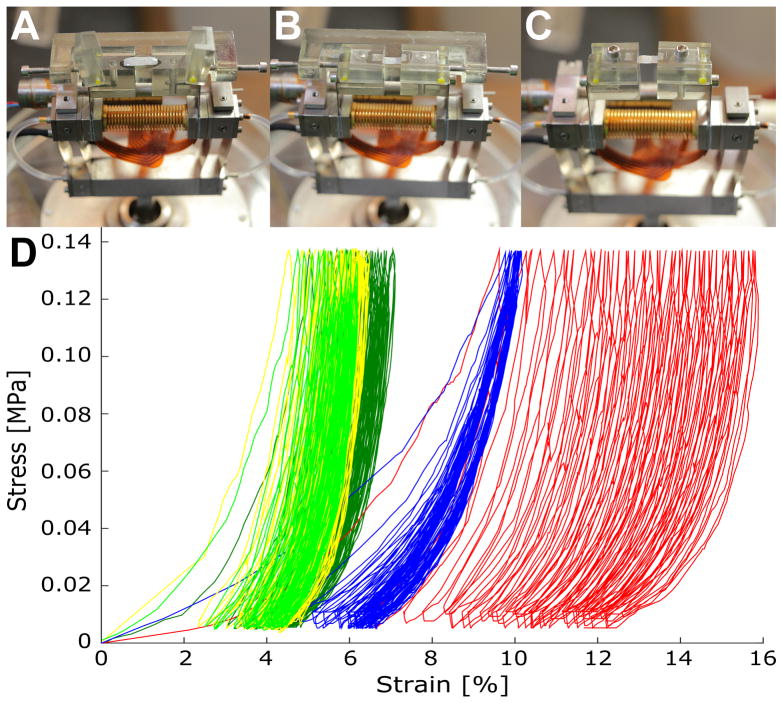
Figure 3.
Uniaxial material tester and representative stress-strain curves. Sample strip is floating between clamps and across the jig (A). Strip is clamped before (B) and after jig was removed (C). Representative stress-strain curves for 50 cycles of loading and unloading are shown for each experimental group (D): normal FRESH (yellow), control FRESH (light green), control PBS (dark green), treated PBS (red), and treated GEN (blue).
2.4 Statistical Analysis
Linear mixed-effects models were constructed to test for significant differences in Δεmin and Δεmax, due to strip location S1 versus S2 (Figure 1), left versus right eye, tissue incubation (FRESH versus incubation for 24 hours in PBS), normal versus control eyes, lens treatment (control versus lens treated eyes), and SXL using genipin (incubation in PBS versus incubation in PBS supplemented with GEN). To minimize the number of estimated parameters in the regression analysis, only the intercept was treated as random. The model was run for each of these effects considering the investigated effect as fixed while all other effects were treated as random. In addition to the above listed effects, variations between animals and between cycle numbers were considered in the hierarchical correlation structure of the model effects. The correlation of the strain values across the consecutive cyclic loadings was taken into account by an autoregressive correlation structure. The null hypothesis was rejected and a significant difference in Δεmin or Δεmax was reported if the p-values were less than 0.05.
To investigate if the scleral strips reached a preconditioned state during the 50 cycles, we tested against the null hypothesis that the Δεmin or Δεmax was different from zero. Each experimental group (normal FRESH, control FRESH, control PBS, treated PBS, treated GEN) was treated as a cluster in a hierarchical linear mixed effect model; significance of each cluster mean was tested against a common intercept representing the zero-strain value.
For illustration purposes, box plots and curve plots showing the cycle-dependent change in Δεmin and Δεmax were generated for each experimental group. A variety of non-linear models were tested, where the following asymptotic function was selected as it provided the best Akaike information criterion (AIC) score:
| (1) |
This function was fitted to the strain increments Δεmin and Δεmax of each experimental group. Curve plots were generated by using the mean values and confidence intervals of the fitting parameters a, b, and c.
3. Results
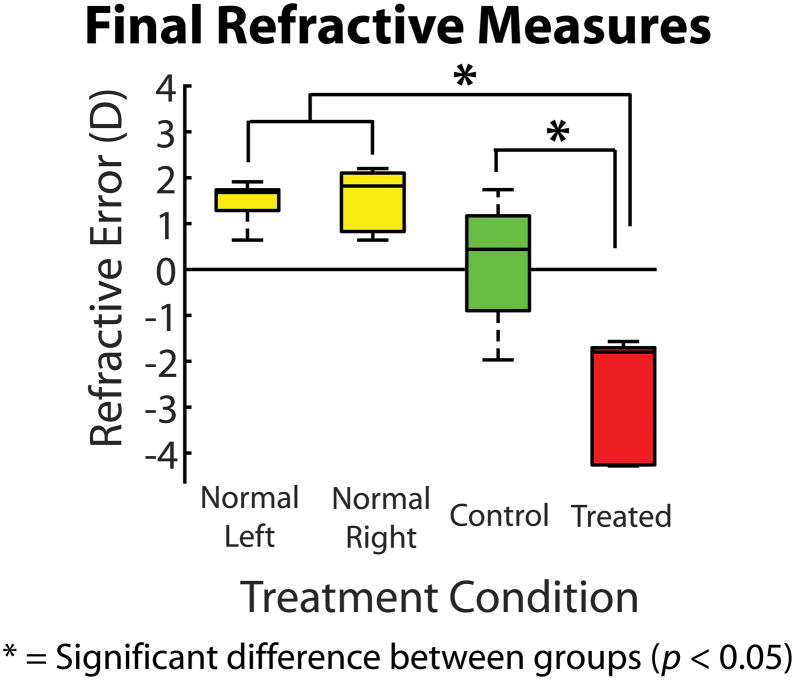
A boxplot of final refractive errors for each in vivo treatment condition is shown in Figure 4. No significant differences in refraction were found between left and right normal eyes, and between normal and control eyes. The refractive errors of both normal and control eyes were significantly different from lens treated eyes (−2.73 D ± 0.60 D; p = 0.013).
Figure 4.
Boxplot showing the refractive errors for the treatment groups at the day of enucleation. Mean refractive errors of treated eyes were significantly different from normal and fellow control eyes (p = 0.013).
We utilized a two-sample t-test to test whether or not the overnight PBS incubation had an effect on scleral thickness. The means of scleral thicknesses of fresh samples were not found to be significantly different from the thicknesses of overnight incubated samples (p = 0.76). In addition, the effects of myopia treatment on scleral strip thickness was also examined using a two-sample t-test. The mean thickness of myopic scleral samples from lens-wearing eyes was not found to be significantly different from normal and control samples (p = 0.94). This finding is consistent with previous observations by Siegwart and Norton,7 who also found no significant difference in scleral thickness after 2, 4, 11, and 21 days of −5D lens treatment. Incubation in PBS supplemented with genipin also had no significant effect on scleral thickness compared to incubation in PBS alone (p = 0.90). Table 1 summarizes the thickness values of our different groups.
Table 1.
Mean scleral thickness values of each experimental group, reported with the standard error of the mean.
| Group | Thickness [μm] | S.E.M. [μm] |
|---|---|---|
| normal FRESH | 117.12 | 5.57 |
| control FRESH | 122.47 | 5.25 |
| control PBS | 115.00 | 4.46 |
| treated PBS | 115.27 | 6.59 |
| treated GEN | 115.27 | 5.49 |
Representative stress-strain curves for each experimental group are shown in Figure 3D. A similar softening response was seen in the normal FRESH, control FRESH, and control PBS groups. Lens treatment (treated PBS) increased the cyclic softening response, while SXL using genipin reduced the softening induced by −5D lens wear.
Results of the statistical analysis are summarized in Table 2. Differences in Δεmin and Δεmax between Strips 1 and 2 and between left and right eyes were insignificant. Amongst FRESH tissues, Δεmin and Δεmax were not significantly different between control and normal eyes. Amongst control eyes, overnight incubation in PBS did not have a significant effect on Δεmin and Δεmax. Among eyes incubated for 24 hours in PBS, myopia treatment had a significant effect on both Δεmin and Δεmax. Amongst lens treated eyes, incubation in genipin had a significant effect on the cyclic softening response Δεmax but not on Δεmin. When comparing genipin-treated scleral strips with all other scleral tissues, genipin had a significant effect on both Δεmin and Δεmax.
Table 2.
Results of the linear mixed effect models (p-value) testing for significant differences in Δεmin and Δεmax with strip number, left or right eye, normal versus control eyes, incubation time, lens treatment versus control, and incubation in genipin versus PBS as fixed effects. For each test, unfixed effects were treated as random effects in the models. The experimental groups that were included for each test are indicated. Results are shown as mean ± standard error (p value). Statistically significant differences (p < 0.05) are indicated by bold typeface.
| Fixed Effect | Strip 1 vs. Strip 2 | Left vs. Right Eye | Normal vs. Control | 0 vs. 24 hrs. Incubation |
|---|---|---|---|---|
| Included Groups | all | all | normal FRESH, control FRESH | control FRESH, control PBS |
| Δεmin | 0.0025 ± 0.010 (0.81) | −0.019 ± 0.016 (0.33) | 0.0062 ± 0.0093 (0.52) | 0.036 ± 0.022 (0.16) |
| Δεmax | 0.0020 ± 0.0072 (0.79) | −0.021 ± 0.018 (0.34) | 0.0054 ± 0.0084 (0.54) | 0.057 ± 0.0030 (0.11) |
| Fixed Effects | Control vs. Lens Treated | Genipin vs. PBS in lens treated eyes | Genipin vs. PBS+FRESH | |
| Included Groups | control PBS, treated PBS | treated PBS, treated GEN | all | |
| Δεmin | 0.095 ± 0.029 (0.048) | −0.044 ± 0.016 (0.074) | −0.047 ± 0.012 (0.0039) | |
| Δεmax | 0.10 ± 0.029 (0.037) | −0.10 ± 0.022 (0.020) | −0.12 ± 0.014 (2.2×10−5) |
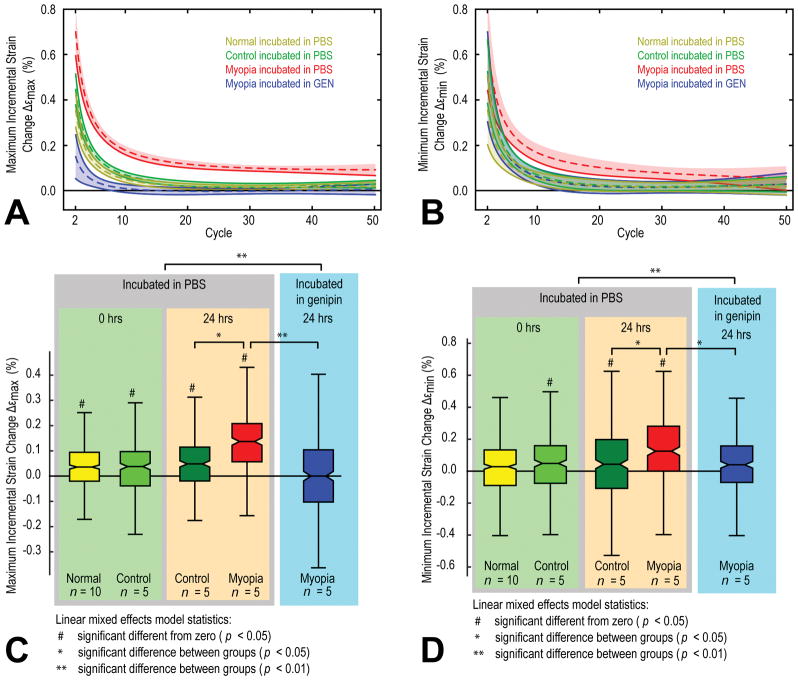
Best-fit models of the cycle-dependent change in the mean value of Δεmax and Δεmin using equation (1) are shown for each group in Figure 5A and Figure 5B, respectively. Boxplots of Δεmax and Δεmin are shown in Figure 5C and Figure 5D, respectively. The cyclic softening response (Δεmax) of all experimental groups except the genipin treated group (treated GEN) was significantly different from zero (Figure 5C). The cyclic softening was significantly increased in the lens treated group (treated PBS) when compared to control (control PBS). The cyclic softening decreased in all groups during the first ten cycles and the 95% confidence intervals of all groups apart from the treated PBS group touched the zero line with higher cycles (Figure 5A). Instead, the cyclic softening response in the lens treatment group (treated PBS) converged to a value far above the zero line, suggesting that cyclic softening would have continued beyond the tested 50 cycles and most likely resulted in tissue failure (Figure 5A). Not only did genipin treatment significantly lower the cyclic softening response of the lens treated scleral strips (treated PBS versus treated GEN, Figure 5C) but inhibited any increase in Δεmax beyond cycle ten (Figure 5A). The genipin treated group (treated GEN) was the only group that showed no significant difference in Δεmax with respect to zero within the 50 cycles. The analysis of Δεmin (Figure 5B,D) showed very similar results compared to Δεmax. The only difference in the analysis of Δεmin was that, in addition to the treated GEN group, the normal FRESH group was also not significantly different from zero (Figure 5D).
Figure 5.
The fitted incremental maximum (A) and minimum (B) strain changes versus load cycles for the different combinations of in vivo eye treatments (normal, control, and treated) and ex vivo strip treatments (FRESH, PBS, GEN). The 95 % confidence intervals of the fitted means of Δεmax and Δεmin are indicated by the color-shaded areas around each curve. The control FRESH group is not shown as it was very similar to the control PBS group. Box plots of the incremental maximum (C) and minimum (D) strain changes for the different experimental groups. Significant differences between groups are indicated by asterisks (values are found in Table 2) while significant differences from zero for individual groups are indicated by hashes.
4. Discussion
We have tested the compliance of juvenile tree shrew scleral strips using cyclic, uniaxial tensile loading. We have defined cyclic softening as the incremental strain increase from one to the next cycle at maximum load. The results suggest that the cyclic softening response of the tree shrew sclera is strongly altered during in vivo induced experimental myopia and ex vivo SXL using genipin. In vivo −5D lens treatment significantly increased the cyclic softening of the sclera while ex vivo SXL using genipin inhibited this response. These findings support the notion that the sclera is biomechanically weakened and more susceptible to inelastic deformations due to cyclic loading during myopia development and that SXL can counteract this weakening. The observed biomechanical weakening during myopia and strengthening after SXL was due to an alteration of the inelastic material properties of the sclera and not due to a macro-structural thinning and thickening of the tissue, respectively.
Siegwart and Norton have shown that the creep rate of the sclera increases during experimental myopia and decreases during recovery from myopia.7 McBrien, Jobling et al. suggested that scleral remodeling is accelerated during myopia development by means of a creep-like elongation of the sclera at normal IOP.33 The creep-like elongation was hypothesized to involve collagen sliding between scleral lamellae and/or collagen fibrils.8, 9, 22, 34 The data presented here suggests that in addition to a creep-like elongation of the sclera at normal IOP, IOP fluctuations may promote scleral softening and, as such, promote inelastic deformations and scleral elongation during myopia development. Continuous IOP telemetry measurements in non-human primates have shown that IOP is extremely dynamic with high and low frequency fluctuations. IOP fluctuates as much as 10 mmHg day-to-day and hour-to-hour, where saccades and blinks lead to high frequency IOP fluctuations that occur frequently throughout the day.35 Other sources of IOP change such as squinting and eye rubbing can lead to even larger IOP fluctuations – up to 100 and 300 mmHg, respectively.61, 62 The cyclic application of tensile loads to the sclera due to IOP fluctuations may play a critical role in promoting collagen sliding and accelerating scleral remodeling during myopia development.
In our recent publication, we have shown that induced myopia leads to a right-ward shift of the stress-strain response of the sclera, increasing the strain needed to uncrimp the collagen fibrils.8 The here observed cyclic softening leads to a similar right-ward shift of the stress-strain curve with every load cycle, which is most pronounced in the myopia group (Figure 3). The cyclic nature of IOP fluctuations may contribute to a similar shift in the scleral stress-strain response and increase the collagen fibril crimp during myopia development in vivo.
We have previously reported that the sclera of normal, juvenile tree shrews cannot be preconditioned at supra-physiological loads and may require a high number of load cycles (~135) to reach a repeatable response at physiological loads using uniaxial tensile testing.8 Here, we have solely investigated physiological loads, finding that the cyclic softening response increases in animals that develop myopia, and the scleras of these animals cannot be preconditioned even at physiological loads. In adult human sclera, the preconditioning effect was found to be very small, where repeatable results can be obtained after only 3 cycles.63 We speculate that the natural accumulation of collagen crosslinks may lead to an age-dependent decrease in the cyclic softening response. Our results here support this notion as ex vivo artificial crosslinking of myopia developing scleras showed a repeatable stress-strain response after approximately 10 cycles. As natural collagen crosslinking is unlikely to be modulated during myopia development, other tissue constituents must have altered the preconditioning effect and increased the cyclic softening in our myopic group. Experimental myopia is known to decrease GAGs, both sulfated and unsulfated, and aggrecan levels in tree shrew scleras.18, 24, 25, 32 The reduction of these constituents may have promoted cyclic softening in the scleras of our myopic group. Murienne et al. saw a right-ward shift in the stress-strain response of juvenile porcine sclera after artificial digestion of about 80% of GAGs.64 This shift in the stress-strain curve is similar to the cycle dependent shift seen here in the myopic group. Unfortunately, Murienne et al. have not yet investigated the effect of GAG removal on the preconditioning effect as their previous experiments showed a negligible preconditioning effect in normal porcine scleras during inflation testing.15 Our results suggest that the preconditioning effect can change dramatically from one treatment condition to another (e.g. during myopia development) and should be reevaluated whenever in vivo or ex vivo conditions are altered. We suspect that in addition to collagen crosslinks, the amount of GAGs and aggrecan may alter the cyclic softening response of the sclera and determine whether or not the sclera can be preconditioned. However, the exact mechanism and what constituents control cyclic softening in the sclera remains unclear.
SXL has been suggested as a potential treatment option for progressive myopia by biomechanical strengthening the sclera and inhibiting inelastic deformations. Our results support this idea as SXL using genipin strengthened the myopic scleras by inhibiting inelastic deformations due to cyclic loading. Wang and Corpuz55 have successfully used subconjunctival injection of 0.5% (22.1 mM) genipin to slow form-deprivation myopia in guinea pigs. We have used a much lower concentration of 0.25 mM in this study suggesting that a low but sustained delivery (24 hours in this study) of genipin to the sclera may lead to significant biomechanical strengthening. However, SXL in our study was performed ex vivo. In vivo SXL may require a much higher dosage due to natural periocular circulations (blood and lymphatic), which may compromise the delivery of genipin to the sclera. Amrite et al. performed subconjunctival injections of nanoparticles (20 nm) in dead and alive rats.65 In dead animals, the particle concentration was 19 fold higher in the sclera compared to living animals, suggesting that periocular circulations play a critical role in the ability to deliver particles or a drug to the sclera via subconjunctival injections. Consequently, in vivo SXL for myopia control using subconjunctival injections of genipin may indeed require a higher dosage than used here. No in vivo study has been performed so far that investigates the dosage-dependent effect of SXL on myopia control.
The present study has several limitations, which should be considered when interpreting the results. We performed uniaxial tensile tests on scleral strips. Cutting the strips out of the sclera may have altered the integrity of the collagen architecture and released residual stresses. This potential loss of integrity due to cutting out the strips may have increased the cyclic softening effect in our experiments. Even though cutting scleral strips may have impacted the softening response in an unmeasured way, the observed significant differences due to experimental myopia and collagen crosslinking reflect alterations of the inelastic scleral biomechanical properties. However, it remains unclear if similar alterations in scleral biomechanics can be detected using inflation testing.
Our loading protocol used a cyclic load that corresponds to approximately 60 mmHg IOP. Although high and low frequency IOP fluctuations occur throughout the day due to blinks, saccades, squinting, and eye rubbing, these IOP fluctuations are typically of smaller magnitude than the cyclic load used here. It remains unclear if the normal and voluntary IOP fluctuations could lead to a similar cyclic softening response in vivo as observed here.
We have carefully designed the experimental groups to obtain reliable results while minimizing the number of animals. Based on this design, every combination of in vivo and ex vivo treatments was not explored in this study (Figure 2). While unlikely, an unexplored combination may lead to an unexpected interaction between in vivo and ex vivo treatments. E.g. we tested whether or not control and normal eyes had a statistically different cyclic softening response. This test was mainly driven by the “normal FRESH” and “control FRESH” groups with matching ex vivo treatment conditions. While possible, it seems unlikely that incubation in PBS would have different effects on the cyclic softening response in normal versus control scleras. Therefore, the normal strips were not investigated after 24 hours of incubation in PBS. Furthermore, the effect of genipin on the cyclic softening response of scleras from normal animals remains unstudied. Future experiments including in vivo crosslinking studies will evaluate these effects.
In conclusion, we have presented further evidence that the sclera is biomechanically weakened during myopia development and that this weakening leads to inelastic scleral deformations when exposed to cyclic tensile loading (cyclic softening). This finding suggests that scleral remodeling in myopia may involve a mechanism that increases the cyclic softening response of the sclera. We have shown that SXL using genipin at a low concentration can effectively inhibit the cyclic softening response of myopia-developing scleras. This finding supports the potential use of genipin for biomechanically strengthening the sclera and slowing scleral remodeling in progressive myopes.
Highlights.
Experimental myopia leads to inelastic cyclic softening of the sclera
Scleral crosslinking using genipin can diminish the cyclic softening response
Acknowledgments
Support: This work was supported by the National Institutes of Health Grants R01 EY026588, R01 EY027759, P30 EY003039; Eye Sight Foundation of Alabama, Birmingham, Alabama; and Research to Prevent Blindness, New York, New York.
References
- 1.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012 Jan;32(1):3–16. doi: 10.1111/j.1475-1313.2011.00884.x. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 2.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005 Sep;25(5):381–91. doi: 10.1111/j.1475-1313.2005.00298.x. Epub 2005/08/17. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999 Oct;106(10):2010–5. doi: 10.1016/s0161-6420(99)90416-5. Epub 1999/10/16. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology. 2007 Feb;114(2):216–20. doi: 10.1016/j.ophtha.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Qiu M, Wang SY, Singh K, Lin SC. Association between myopia and glaucoma in the United States population. Invest Ophthalmol Vis Sci. 2013 Jan 28;54(1):830–5. doi: 10.1167/iovs.12-11158. Epub 2013/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine L. Beitrage zur Anatomie des myopischen Auges. Archiv für Augenheilkunde. 1899;38:277–90. [Google Scholar]
- 7.Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999 Jan;39(2):387–407. doi: 10.1016/s0042-6989(98)00150-3. Epub 1999/05/18. [DOI] [PubMed] [Google Scholar]
- 8.Grytz R, Siegwart JT., Jr Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2015 Mar 03;56(3):2065–78. doi: 10.1167/iovs.14-15352. Epub 2015/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldivia S, Levy A, Hegde S, Aper SJ, Merkx M, Grytz R. A Novel Organ Culture Model to Quantify Collagen Remodeling in Tree Shrew Sclera. PLoS One. 2016;11(11):e0166644. doi: 10.1371/journal.pone.0166644. Epub 2016/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downs JC, Suh JK, Thomas KA, Bellezza AJ, Burgoyne CF, Hart RT. Viscoelastic characterization of peripapillary sclera: material properties by quadrant in rabbit and monkey eyes. J Biomech Eng. 2003 Feb;125(1):124–31. doi: 10.1115/1.1536930. Epub 2003/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung Y. Biomechanics - Mechanical Properties of Living Tissues. New York: Springer-Verlag; 1993. [Google Scholar]
- 12.Ehret AEIM. Modeling of anisotropic softening phenomena: Application to soft biological tissues. International Journal of Plasticity. 2009;25(5):901–19. [Google Scholar]
- 13.Sverdlik A, Lanir Y. Time-dependent mechanical behavior of sheep digital tendons, including the effects of preconditioning. J Biomech Eng. 2002 Feb;124(1):78–84. doi: 10.1115/1.1427699. Epub 2002/03/02. [DOI] [PubMed] [Google Scholar]
- 14.Lokshin O, Lanir Y. Viscoelasticity and preconditioning of rat skin under uniaxial stretch: microstructural constitutive characterization. J Biomech Eng. 2009 Mar;131(3):031009. doi: 10.1115/1.3049479. Epub 2009/01/22. [DOI] [PubMed] [Google Scholar]
- 15.Tonge TK, Murienne BJ, Coudrillier B, Alexander S, Rothkopf W, Nguyen TD. Minimal preconditioning effects observed for inflation tests of planar tissues. J Biomech Eng. 2013 Nov;135(11):114502. doi: 10.1115/1.4025105. Epub 2013/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walls GL. The vertebrate eye and its adaptive radiation. Bloomfield Hills, Mich: Cranbrook Institute of Science; 1942. pp. xivpp. 1l–785. [Google Scholar]
- 17.Torczynski E, editor. Progress in Clinical and Biological Research. New York, New York: 1982. Normal and abnormal ocular development in man. [PubMed] [Google Scholar]
- 18.Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995 May;35(9):1271–81. doi: 10.1016/0042-6989(94)00243-f. Epub 1995/05/01. [DOI] [PubMed] [Google Scholar]
- 19.Rada JA, Achen VR, Perry CA, Fox PW. Proteoglycans in the human sclera. Evidence for the presence of aggrecan. Invest Ophthalmol Vis Sci. 1997 Aug;38(9):1740–51. Epub 1997/08/01. [PubMed] [Google Scholar]
- 20.Grytz R, Fazio MA, Girard MJ, Libertiaux V, Bruno L, Gardiner S, et al. Material properties of the posterior human sclera. J Mech Behav Biomed Mater. 2014 Jan;29:602–17. doi: 10.1016/j.jmbbm.2013.03.027. Epub 2013/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho LC, Sigal IA, Jan NJ, Squires A, Tse Z, Wu EX, et al. Magic angle-enhanced MRI of fibrous microstructures in sclera and cornea with and without intraocular pressure loading. Invest Ophthalmol Vis Sci. 2014 Aug 07;55(9):5662–72. doi: 10.1167/iovs.14-14561. Epub 2014/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006 Feb;82(2):185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 23.McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001 Sep;42(10):2179–87. Epub 2001/08/31. [PubMed] [Google Scholar]
- 24.McBrien NA, Lawlor P, Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000 Nov;41(12):3713–9. Epub 2000/10/29. [PubMed] [Google Scholar]
- 25.Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Invest Ophthalmol Vis Sci. 2007 Jul;48(7):2947–56. doi: 10.1167/iovs.06-0906. Epub 2007/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996 Jun;37(7):1380–95. Epub 1996/06/01. [PubMed] [Google Scholar]
- 27.Gao H, Frost MR, Siegwart JT, Jr, Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis. 2011;17:903–19. [PMC free article] [PubMed] [Google Scholar]
- 28.Guo L, Frost MR, He L, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013;54(10):6806–19. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegwart JT, Jr, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005 Oct;46(10):3484–92. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegwart JT, Jr, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002 Jul;43(7):2067–75. [PMC free article] [PubMed] [Google Scholar]
- 31.Gentle A, Liu Y, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003 May 09;278(19):16587–94. doi: 10.1074/jbc.M300970200. Epub 2003/02/28. [DOI] [PubMed] [Google Scholar]
- 32.Siegwart JT, Jr, Strang CE. Selective modulation of scleral proteoglycan mRNA levels during minus lens compensation and recovery. Mol Vis. 2007;13:1878–86. [PubMed] [Google Scholar]
- 33.McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009 Jan;86(1):E23–30. doi: 10.1097/OPX.0b013e3181940669. Epub 2008/12/24. [DOI] [PubMed] [Google Scholar]
- 34.Grytz R, El Hamdaoui M. Multi-Scale Modeling of Vision-Guided Remodeling and Age-Dependent Growth of the Tree Shrew Sclera During Eye Development and Lens-Induced Myopia. J Elast. 2017 Dec;129(1–2):171–95. doi: 10.1007/s10659-016-9603-4. Epub 2017/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011 Sep 21;52(10):7365–75. doi: 10.1167/iovs.11-7955. Epub 2011/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lima EG, Tan AR, Tai T, Marra KG, DeFail A, Ateshian GA, et al. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res A. 2009 Dec;91(3):692–700. doi: 10.1002/jbm.a.32305. Epub 2008/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes S, Boote C, Tuft SJ, Quantock AJ, Meek KM. A study of corneal thickness, shape and collagen organisation in keratoconus using videokeratography and X-ray scattering techniques. Exp Eye Res. 2007 Mar;84(3):423–34. doi: 10.1016/j.exer.2006.10.014. Epub 2006/12/21. [DOI] [PubMed] [Google Scholar]
- 38.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003 May;135(5):620–7. doi: 10.1016/s0002-9394(02)02220-1. Epub 2003/04/30. [DOI] [PubMed] [Google Scholar]
- 39.Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015 Jan;41(1):41–6. doi: 10.1016/j.jcrs.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 40.Wollensak G, Iomdina E. Crosslinking of scleral collagen in the rabbit using glyceraldehyde. J Cataract Refract Surg. 2008 Apr;34(4):651–6. doi: 10.1016/j.jcrs.2007.12.030. Epub 2008/03/26. [DOI] [PubMed] [Google Scholar]
- 41.Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Nielsen NV. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004 Jan;111(1):53–61. doi: 10.1016/j.ophtha.2003.05.010. Epub 2004/01/09. [DOI] [PubMed] [Google Scholar]
- 42.Cotter SA, Varma R, Ying-Lai M, Azen SP, Klein R Los Angeles Latino Eye Study G. Causes of low vision and blindness in adult Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2006 Sep;113(9):1574–82. doi: 10.1016/j.ophtha.2006.05.002. Epub 2006/09/05. [DOI] [PubMed] [Google Scholar]
- 43.Green JS, Bear JC, Johnson GJ. The burden of genetically determined eye disease. Br J Ophthalmol. 1986 Sep;70(9):696–9. doi: 10.1136/bjo.70.9.696. Epub 1986/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krumpaszky HG, Ludtke R, Mickler A, Klauss V, Selbmann HK. Blindness incidence in Germany. A population-based study from Wurttemberg-Hohenzollern. Ophthalmologica. 1999;213(3):176–82. doi: 10.1159/000027415. Epub 1999/04/15. [DOI] [PubMed] [Google Scholar]
- 45.Munier A, Gunning T, Kenny D, O’Keefe M. Causes of blindness in the adult population of the Republic of Ireland. Br J Ophthalmol. 1998 Jun;82(6):630–3. doi: 10.1136/bjo.82.6.630. Epub 1998/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avetisov ES, Tarutta EP, Iomdina EN, Vinetskaya MI, Andreyeva LD. Nonsurgical and surgical methods of sclera reinforcement in progressive myopia. Acta Ophthalmol Scand. 1997 Dec;75(6):618–23. doi: 10.1111/j.1600-0420.1997.tb00617.x. Epub 1998/04/04. [DOI] [PubMed] [Google Scholar]
- 47.Chen M, Dai J, Chu R, Qian Y. The efficacy and safety of modified Snyder-Thompson posterior scleral reinforcement in extensive high myopia of Chinese children. Graefes Arch Clin Exp Ophthalmol. 2013 Nov;251(11):2633–8. doi: 10.1007/s00417-013-2429-x. Epub 2013/08/03. [DOI] [PubMed] [Google Scholar]
- 48.Curtin BJ, Whitmore WG. Long-term results of scleral reinforcement surgery. Am J Ophthalmol. 1987 Apr 15;103(4):544–8. doi: 10.1016/s0002-9394(14)74278-3. Epub 1987/04/15. [DOI] [PubMed] [Google Scholar]
- 49.Thompson FB, Ward B. Long-term results of scleral reinforcement surgery. Am J Ophthalmol. 1987 Oct 15;104(4):442–3. doi: 10.1016/0002-9394(87)90253-4. Epub 1987/10/15. [DOI] [PubMed] [Google Scholar]
- 50.Ward B, Tarutta EP, Mayer MJ. The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye (Lond) 2009 Dec;23(12):2169–74. doi: 10.1038/eye.2008.433. Epub 2009/02/21. [DOI] [PubMed] [Google Scholar]
- 51.Fessel G, Cadby J, Wunderli S, van Weeren R, Snedeker JG. Dose- and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics - toward clinical application for tendon repair. Acta Biomater. 2014 May;10(5):1897–906. doi: 10.1016/j.actbio.2013.12.048. Epub 2014/01/05. [DOI] [PubMed] [Google Scholar]
- 52.Kim M, Takaoka A, Hoang QV, Trokel SL, Paik DC. Pharmacologic alternatives to riboflavin photochemical corneal cross-linking: a comparison study of cell toxicity thresholds. Invest Ophthalmol Vis Sci. 2014 Apr 10;55(5):3247–57. doi: 10.1167/iovs.13-13703. Epub 2014/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell IC, Hannon BG, Read AT, Sherwood JM, Schwaner SA, Ethier CR. Quantification of the efficacy of collagen cross-linking agents to induce stiffening of rat sclera. J R Soc Interface. 2017 Apr;14(129):20170014. doi: 10.1098/rsif.2017.0014. Epub 2017/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu TX, Wang Z. Collagen crosslinking of porcine sclera using genipin. Acta Ophthalmol. 2013 Jun;91(4):e253–7. doi: 10.1111/aos.12172. Epub 2013/05/29. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Corpuz CC. Effects of scleral cross-linking using genipin on the process of form-deprivation myopia in the guinea pig: a randomized controlled experimental study. BMC Ophthalmol. 2015 Jul 29;15:89. doi: 10.1186/s12886-015-0086-z. Epub 2015/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zyablitskaya M, Takaoka A, Munteanu EL, Nagasaki T, Trokel SL, Paik DC. Evaluation of Therapeutic Tissue Crosslinking (TXL) for Myopia Using Second Harmonic Generation Signal Microscopy in Rabbit Sclera. Invest Ophthalmol Vis Sci. 2017 Jan 01;58(1):21–9. doi: 10.1167/iovs.16-20241. Epub 2017/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siegwart JT, Jr, Norton TT. Goggles for controlling the visual environment of small animals. Lab Anim Sci. 1994 Jun;44(3):292–4. Epub 1994/06/01. [PubMed] [Google Scholar]
- 58.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992 May;32(5):833–42. doi: 10.1016/0042-6989(92)90026-f. Epub 1992/05/01. [DOI] [PubMed] [Google Scholar]
- 59.Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998 Nov;38(22):3505–15. doi: 10.1016/s0042-6989(98)00053-4. Epub 1999/01/20. [DOI] [PubMed] [Google Scholar]
- 60.Friedman B. Stress upon the ocular coats: effects of scleral curvature scleral thickness, and intra-ocular pressure. Eye Ear Nose Throat Mon. 1966 Sep;45(9):59–66. Epub 1966/09/01. [PubMed] [Google Scholar]
- 61.Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969 Nov;82(5):637–40. doi: 10.1001/archopht.1969.00990020633011. Epub 1969/11/01. [DOI] [PubMed] [Google Scholar]
- 62.Turner D, Girkin CA, Downs JCC. IOP Elevations Associated with Eye Rubbing. Investigative Ophthalmology & Visual Science. 2016;57(12):6433. [Google Scholar]
- 63.Geraghty B, Jones SW, Rama P, Akhtar R, Elsheikh A. Age-related variations in the biomechanical properties of human sclera. J Mech Behav Biomed Mater. 2012 Dec;16:181–91. doi: 10.1016/j.jmbbm.2012.10.011. Epub 2012/11/28. [DOI] [PubMed] [Google Scholar]
- 64.Murienne BJ, Chen ML, Quigley HA, Nguyen TD. The contribution of glycosaminoglycans to the mechanical behaviour of the posterior human sclera. J R Soc Interface. 2016 Jun;13(119):20160367. doi: 10.1098/rsif.2016.0367. Epub 2016/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis. 2008 Jan 29;14:150–60. Epub 2008/03/13. [PMC free article] [PubMed] [Google Scholar]