Abstract
Background
In Zimbabwe, while the Xpert MTB/RIF assay is being used for diagnosing tuberculosis and rifampicin-resistance, re-treatment tuberculosis (TB) patients are still expected to have culture and drug sensitivity testing (CDST) performed at national reference laboratories for confirmation. The study aim was to document the Xpert MTB/RIF assay scale-up and assess how the CDST system functioned for re-treatment TB patients.
Methods
We performed an ecologic study using national aggregate data.
Results
Use of the Xpert MTB/RIF assay increased from 11 829 to 68 153 between 2012 and 2016. Xpert assays worked well, with successful tests in more than 90% of cases, TB detection rates at 15–17% and rifampicin resistance in <10%. During Xpert scale-up, the number of sputum specimens from re-treatment TB patients reaching national reference laboratories for CDST increased from 12% to 51%. In terms of laboratory performance, culture contamination increased from 3% to 17%, positive cultures from 13% to 17% and successful CDST from 6% to 14%: the proportion of CDST showing any resistance to rifampicin averaged 44%. From 2009 to 2016, the proportion of notified re-treatment TB patients with successful CDST increased from <1% to 7%.
Conclusions
While components of Zimbabwe’s CDST system for re-treatment TB patients showed some changes during the scale-up of the Xpert MTB/RIF assay, overall performance was poor. The country must either invest in improving CDST performance or in advanced molecular diagnostic technology.
Keywords: culture and drug sensitivity testing, Mycobacterium tuberculosis, re-treatment tuberculosis, rifampicin resistance, Xpert MTB/RIF, Zimbabwe
Introduction
Recurrent or re-treatment tuberculosis (TB) is a global public health challenge. It is an important risk factor for drug-resistant TB, especially multidrug-resistant TB (MDR-TB; defined as TB that does not respond to at least isoniazid and rifampicin, the two most powerful anti-TB drugs).1 Treating MDR-TB is more difficult and more expensive than drug-susceptible TB, with a regimen that is usually 24 months long and associated with numerous adverse drug events.2 While the overall TB burden is declining in Zimbabwe, the proportion of re-treatment TB patients remained at around 11% (n=5691) between 2015 and 2016, and treatment outcomes among these patients have tended to be worse than those observed in new TB cases.1,3
One of the reasons for worse treatment outcomes could be due to failure to detect drug resistance. It is thus essential that re-treatment TB patients are appropriately investigated through mycobacterial culture and drug susceptibility testing (CDST), the gold standard for TB diagnostic confirmation and drug susceptibility testing of anti-TB drugs. Global and national TB guidelines recommend that sputum specimens for every re-treatment TB patient should be sent to a reference laboratory to identify drug resistance and MDR-TB.4,5 In Zimbabwe, CDST is provided by the two national reference laboratories (NRLs): the National TB Reference Laboratory in Bulawayo and the National Microbiology Reference Laboratory in Harare, which serve the southern and northern regions, respectively. The process of getting sputum specimens from peripheral health facilities to the NRLs is not only difficult, but also inconsistent and unreliable.3 These challenges have been reported in both African and Asian countries.6–9
One of the major advances in the diagnosis of TB in the last 7 y has been the introduction, deployment and scale-up of the Xpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA). This is a highly sensitive, fully automated and commercially available nucleic acid amplification test for use with sputum and other body specimens.10 The Xpert MTB/RIF assay requires minimal expertise and has a short sample processing time of 2 h to confirm Mycobacterium tuberculosis (MTB) and to detect rifampicin resistance. However, this test is also more expensive and has infrastructure and technical issues related to deployment in peripheral settings.11,12 Initially, the World Health Organization (WHO) recommended use of Xpert MTB/RIF assay for those suspected of having MDR-TB and for those with presumptive TB who are also co-infected with human immunodeficiency virus (HIV).13 However, in 2013 the WHO made a conditional recommendation that the Xpert MTB/RIF assay be considered as the initial diagnostic test for all people requiring investigation for TB.14
In late 2011, the Xpert MTB/RIF assay was scaled up throughout Zimbabwe. High-risk groups such as HIV-infected persons with presumptive TB, those with a previous history of TB treatment, miners and prisoners were initially prioritised for Xpert MTB/RIF investigation. However, since 2014 the country has adopted the WHO recommendations and the Xpert MTB/RIF assay is now the initial diagnostic test for all presumptive TB patients. While those diagnosed with TB by the Xpert MTB/RIF assay are started on treatment, national guidelines still recommend that all notified re-treatment TB patients and those with rifampicin resistance also have sputum specimens sent to the NRLs for CDST to confirm the Xpert MTB/RIF results and to detect resistance to other drugs, especially isoniazid.5
Given the national recommendations and the scale-up of the Xpert MTB/RIF technology in Zimbabwe, it is highly likely that the number of sputum specimens being sent to and received by the NRLs has increased. During this time, the human resources in the NRLs did not change. We hypothesised that the increased workload may have led to a deterioration in CDST performance. There is no published information in Zimbabwe either before or during the scale-up of the Xpert MTB/RIF assay about the proportion of re-treatment TB cases that have sputum specimens sent to the NRLs or how well the NRLs have performed with respect to CDST.
The aim of this study was to assess how the Zimbabwe mycobacterial CDST system for re-treatment TB patients has functioned during the scale-up of the Xpert MTB/RIF assay. Specific objectives were to document and assess for the whole country the scale-up and performance of the Xpert MTB/RIF assay between 2011 and 2016 and, before and during the scale-up of the Xpert MTB/RIF assay, the number and proportion of notified re-treatment TB patients who had sputum specimens received by the NRLs along with the performance and results of CDST.
Methods
Study design
This was an ecologic study involving secondary analyses of aggregate data routinely collected by the national TB control programme.
Setting
General
Zimbabwe is a landlocked country situated in southern Africa that is bordered by high HIV- and TB-prevalent countries: South Africa, Botswana, Mozambique and Zambia. The country’s population is approximately 13 million according to the last census and it has a gross domestic product (GDP) of $924 per capita, compared with $1589 per capita for the sub-Saharan Africa region.15,16 Zimbabwe is divided into northern and southern regions, made up of five administrative provinces in each region, with a total of 62 administrative districts.
TB is a major public health threat, with the prevalence of disease (all forms) estimated at 275 per 100 000 population (95% confidence interval [CI] 217 to 334 per 100 000) according to the 2014 TB prevalence survey for Zimbabwe.5 TB treatment services are offered in all public health facilities, where they are integrated with other general health services. The laboratory network consists of at least one TB diagnosing centre per district (translating to 1.4 smear microscopy centres per 100 000 population) and two NRLs that conduct CDST, each NRL serving one region. Both NRLs provide culturing of MTB as well as phenotypic and molecular drug sensitivity testing (DST). Molecular tests such as the Hain’s line probe assay (LPA) were introduced at the two NRLs in 2015/2016 and are used to complement but not replace phenotypic DST.
Management of CDST in re-treatment patients
Re-treatment TB patients are those who previously received at least 1 month or more of anti-TB drugs and are diagnosed once again with TB. They include relapses, treatment after failure and treatment after loss to follow-up on a first-line treatment regimen.17 All patients diagnosed with re-treatment TB are supposed to be investigated by the Xpert MTB/RIF assay and are also supposed to submit an additional sputum specimen to one of the two NRLs for CDST, as part of government policy. Once rifampicin resistance is detected, patients are initiated on a standardised treatment regimen pending CDST results. The sputum specimens are collected at peripheral sites and transported through government and non-governmental organization partners to the NRLs. At the NRLs, all samples are decontaminated using sodium hydroxide to kill commensal bacteria, which may contaminate the cultures. The resultant sputum pellet is inoculated on both Lowenstein–Jensen (LJ) agar media and Mycobacteria Growth Indicator Tube (MGIT) 960 liquid media (Becton Dickinson, Franklin Lakes, NJ, USA) before being incubated for growth. All pure growths of MTB (with no contaminants) are subcultured and DSTs are carried out on both LJ and MGIT 960 media using the proportion method.18,19 The performances of both NRLs as measured by the results from an external quality assurance programme have hitherto been excellent.
Study population
The study included aggregate data on the number of Xpert MTB/RIF instruments deployed in Zimbabwe between 2011 and 2016 along with their performance and assay results and the number of patients notified with re-treatment TB in Zimbabwe between 2008 and 2016. The pre-Xpert era included the years 2008–2010 and the Xpert era included the years 2011–2016.
Data variables, sources of data and data collection
The following data variables were collected. For the scale-up of the Xpert MTB/RIF assay, data included year, instruments deployed each year, assays done each year, unsuccessful tests (errors reported by the instrument), successful tests in which valid Xpert assay results were produced, tests showing MTB and tests showing rifampicin-resistant TB (RR-TB). The source of data was the National Laboratory Annual Report for Tuberculosis. For notified patients with re-treatment TB, data included year, re-treatment TB patients registered nationally each year, sputum specimens received at the NRLs, culture contamination, MTB culture growth, CDST performed and results of CDST in terms of resistance to isoniazid and/or rifampicin. The sources of data were the WHO Global TB Reports (for Zimbabwe), the Laboratory Information Management System for each NRL and physical records (laboratory request forms for CDST) for each NRL. Data collection was carried out in August 2017 using a paper-based questionnaire.
Analysis and statistics
Data were double-entered from the paper-based questionnaire into EpiData (version 4.0.1.44 for data entry and version 2.2.2.186 for data analysis [EpiData Association, Odense, Denmark]). Numbers and proportions were reported for all numeric and categorical variables, and bar graphs were plotted to show annual trends for the relevant variables. The χ2 test was used to compare differences in categorical variables between the pre-Xpert and Xpert periods. Levels of significance were set at 5%.
Ethics
Ethics approval for this study was obtained from the Medical Research Council of Zimbabwe as well as the Ethics Advisory Group, International Union Against Tuberculosis and Lung Disease (The Union), Paris, France.
Results
Scale-up of the Xpert MTB/RIF assay in Zimbabwe
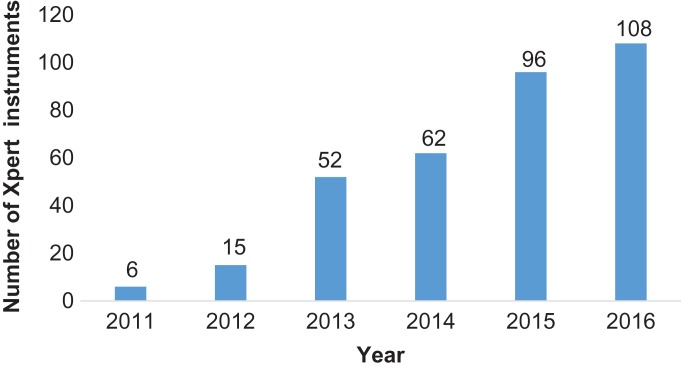
The number of Xpert MTB/RIF instruments deployed each year in Zimbabwe between 2011 and 2016 is shown in Figure 1. There was a large increase in the number of instruments in 2013 and from 2015 onwards. The number of Xpert MTB/RIF assays performed along with the results for each year between 2012 and 2016 are shown in Table 1. There were no data available for 2011. Between 2012 and 2016 there was a steady increase in the absolute number of tests performed, along with those tests detecting MTB and RR-TB. For the years 2012–2013 and 2015–2016, there were fairly similar results showing successful tests at greater than 90%, the proportion detecting MTB ranging from 15% to 17% and the proportion with RR-TB at less than 10%. In 2014, there was a large increase in errors, a reduction in successful tests and in the detection of MTB and an increase in RR-TB.
Figure 1.
Number of Xpert MTB/RIF instruments deployed each year in Zimbabwe, 2011–2016.
Table 1.
Number of Xpert MTB/RIF assays performed and test results in Zimbabwe from 2012–2016
| Xpert MTB/RIF assays and results | Year | ||||
|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Number of assays each year | 11 829 | 24 356 | 48 694 | 62 370 | 68 153 |
| Successful tests | 10 839 (92) | 22 552 (93) | 30 852 (63) | 57 598 (92) | 62 938 (92) |
| Tests showing MTB | 1797 (17) | 3754 (17) | 2963 (10) | 8739 (15) | 9549 (15) |
| Tests showing RR-MTB | 72 (4) | 300 (9) | 432 (15) | 488 (6) | 533 (6) |
| Tests showing errorsa | 990 (8) | 1804 (7) | 17 842 (37) | 4472 (8) | 5215 (8) |
aErrors include aborted assays due to probe check control failures, no results due to power failure or stoppage of the test by the operator or invalid results.
Re-treatment TB and receipt of sputum specimens at NRLs
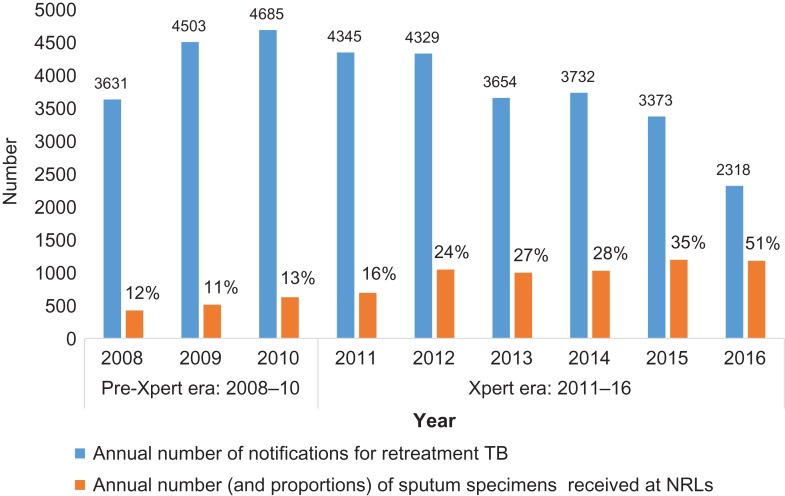
The annual number of notifications of re-treatment TB along with the number (and proportions) of sputum specimens from each patient received at the NRLs in the pre-Xpert era and the Xpert era are shown in Figure 2. The proportions of patients for whom sputum specimens were received at the NRLs remained fairly constant, at around 11–13% in the pre-Xpert era, but increased exponentially from 16% to 51% in the Xpert era. During the Xpert era, 28.2% of sputum specimens from re-treatment TB patients were received at the NRLs, which was significantly higher than the 12.1% that were received during the pre-Xpert era (p<0.001).
Figure 2.
Numbers of re-treatment TB patients who had sputum specimens received by NRLs in Zimbabwe before and during the scale-up of the Xpert MTB/RIF assay: 2008–2016.
Performance of CDST at the NRLs
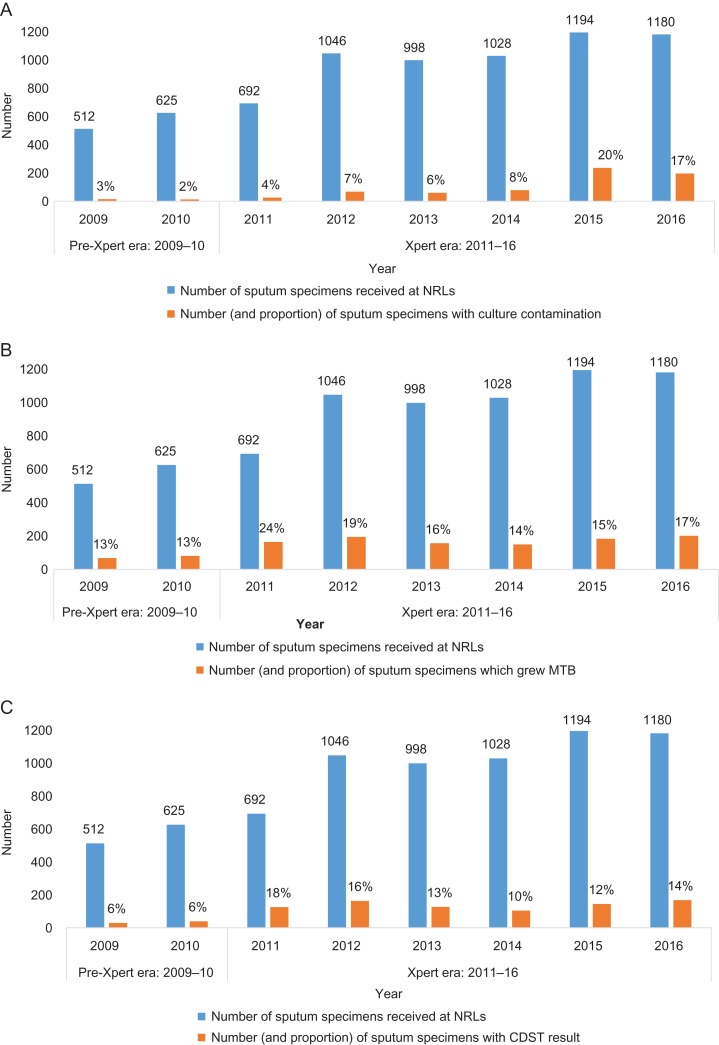
The annual numbers and proportions of sputum specimens from re-treatment TB patients received at the NRLs that had culture contamination, positive MTB growth and successful CDST are shown in Figure 3. There were no data for 2008.
Figure 3.
Performance of NRLs in Zimbabwe before and during the scale-up of the Xpert MTB/RIF assay: 2009–2016. (A) Number sputum specimens from re-treatment TB patients that showed culture contamination. (B) Number of sputum specimens from re-treatment TB patients that showed positive MTB cultures. (C) Number of sputum specimens from re-treatment TB patients with successful CDST.
Contamination rates each year were fairly similar in the pre-Xpert era, but these increased in the Xpert era, particularly in the last 2 y (Figure 3A). During the pre-Xpert era, 2.5% of specimens had culture contamination, and this increased significantly to 10.8% in the Xpert era (p<0.001).
Positive MTB cultures each year were similar in the pre-Xpert era and increased slightly in the Xpert era (Figure 3B). During the pre-Xpert era, 12.9% of sputum specimens had positive MTB cultures, which increased significantly to 17.1% in the Xpert era (p<0.001). The pattern of successful CDST mirrored that seen with positive MTB cultures (Figure 3C). During the pre-Xpert era, 5.9% of sputum specimens had successful CDST, which increased significantly to 13.5% in the Xpert era (p<0.001).
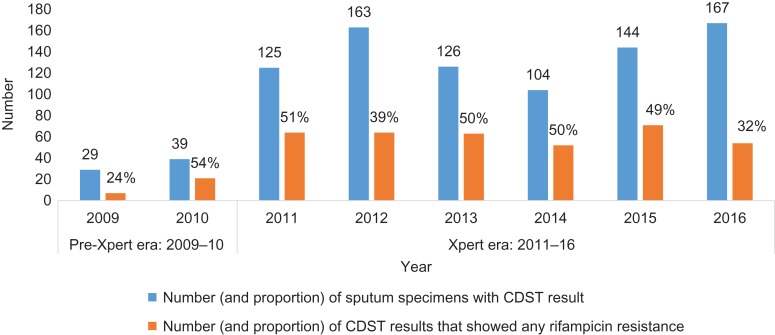
The number and proportion of CDST results that showed any resistance to rifampicin are shown in Figure 4. The proportions of CDST results showing any resistance to rifampicin were fairly similar in the pre-Xpert and Xpert eras. During the pre-Xpert era, 41% of CDST results showed any resistance to rifampicin, which was not significantly different to 44% in the Xpert era (p=0.61). During the pre-Xpert era, there were also 5 (7%) CDST results showing isoniazid monoresistance, which was not significantly different from 33 (4%) in the Xpert era (p=0.18).
Figure 4.
Mycobacterium tuberculosis cultures that showed any resistance to rifampicin on drug susceptibility testing in Zimbabwe: 2009–2016.
Re-treatment TB and CDST
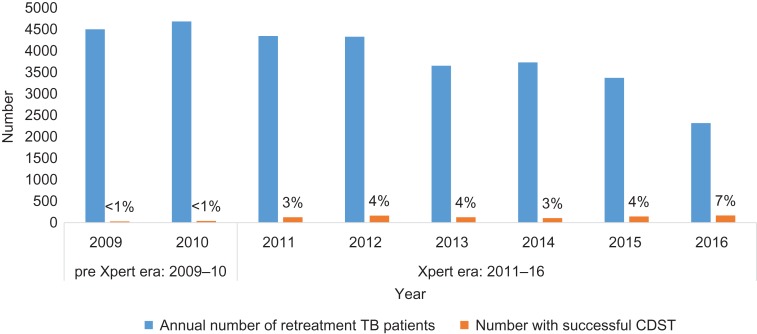
The number and proportion of notified re-treatment TB patients with a successful CDST result are shown in Figure 5. In the pre-Xpert era, less than 1% of notified re-treatment TB patients had a successful DST. This increased in the Xpert era from 3% in 2011 to 7% in 2016.
Figure 5.
Number of re-treatment TB patients who had successful CDST in NRLs in Zimbabwe before and during the scale-up of the Xpert MTB/RIF assay: 2009–2016.
Discussion
This is the first study from Zimbabwe documenting the countrywide scale-up of molecular diagnostic technology in the form of the Xpert MTB/RIF assay and assessing how the CDST system has functioned during this period. There were some interesting findings.
First, there has been a remarkable scale-up of the Xpert MTB/RIF assay in Zimbabwe and, with the exception of the year 2014, the assays performed well, with a high proportion of successful tests and good detection rates of MTB with rifampicin resistance generally staying below 10%. The error rates, which in effect are wasted assays each costing about US$10, also remained below 10%. These error rates are acceptable and similar to reports from other national programmes that have deployed the Xpert MTB/RIF assay in the field.20,21 In 2014 there was a high proportion of errors, possibly due to frequent power failures throughout the country, and these negatively impacted the numbers and proportions of successful tests and tests detecting MTB and RR-TB. The negative experience in 2014 was a wake-up call leading to the National Tuberculosis Control Programme installing backup solar-powered batteries to allow uninterrupted power supplies to Xpert instruments. This is an important lesson when it comes to decentralising this type of technology to more peripheral areas with limited infrastructure. Instrument errors may also result from poor training, inexperienced users as well as defective cartridges, and it is important to ensure that new batches of cartridges come from reliable suppliers and are subject to quality control checks.22
Second, during the scale-up of the Xpert MTB/RIF assay, there was indeed an increase in the number of sputum specimens from re-treatment TB patients reaching NRLs, supporting our initial hypothesis. However, implementation of the current policy was still far from satisfactory, with only 51% of specimens arriving at the NRLs in 2016. This is consistent with findings from a previous study in 2014 in two Zimbabwe provinces in which the proportion of specimens that reached the NRLs was 53%.23 We do not know the precise reasons for this suboptimal performance, but they could include health care workers not adhering to policy guidelines that stipulate all sputum specimens for re-treatment TB patients be sent for CDST, a preference to just send specimens that are rifampicin resistant, unreliable sputum specimen transportation systems and poor administrative systems at the NRLs. Research, especially of a qualitative nature, is needed to find the answers to these questions.
Third, the performance at the NRLs during the Xpert scale-up was patchy and marginal. Levels of culture contamination increased, especially in 2015 and 2016. At this time the laboratory workload was also increased by a national TB prevalence survey and the biosafety laboratory cabinets were overdue for service, and these factors probably negatively affected culture processes. MTB culture rates and CDST marginally improved during the Xpert era, which may have been due to additional technical support that was received during the prevalence survey and the implementation of improved laboratory quality management systems. In terms of CDST results, a high proportion showed any resistance to rifampicin, supporting our earlier assumption that there was a preference for sending sputum specimens from patients whose sputum specimens had RR-TB detected on the Xpert MTB/RIF assay.
Finally, while the proportion of re-treatment TB patients who had CDST increased in the Xpert era, the results were poor and consistently remained below 10%.
This study has several strengths. It was a countrywide study over 9 y using several data sources and was therefore representative of the situation in Zimbabwe with respect to specimen referral and NRL culture processing systems. Also, the reporting of the study was in line with Strengthening the Reporting of Observational Studies in Epidemiology guidelines.24
However, there were some limitations. First, re-treatment TB patients were regarded as a homogeneous group and we were unable to stratify by region or type of re-treatment, which would have been useful for more detailed analyses and possible policy formulation. Second, there were data challenges. For 2008 there were no data available for CDST in the NRLs. There were no data about system processes such as specimens arriving at the NRL but being rejected, time between specimen collection and arrival at the NRL, time to get CDST results or whether the results were sent back to referral facilities and reached the patients. Such information requires individual rather than aggregate data and should be the subject of future research. Third, we were only able to report on the total number of Xpert tests done each year and were unable to get disaggregated data on how many re-treatment TB patients received this assay, because the information was not available in the Xpert registers. Fourth, we did not manage to collect data on the different error codes for the Xpert MTP/RIF tests. Error codes were mainly due to power fluctuations during testing (error code 2127), poor sample preparation (error code 2008) and excessive temperatures (error code 1001). This information would have helped in instituting targeted interventions to reduce errors and hence wastages during Xpert MTB/RIF testing.
Despite these limitations, there are two important programmatic implications. First, it is essential that further scale-up and decentralisation of Xpert MTB/RIF is accompanied by reliable and stable backup power, and this must be accompanied by good quality control checks on cartridges, with the aim to reduce the number of errors and therefore costs. There also needs to be better documentation in Xpert registers about who is receiving the assay.
Second, Zimbabwe must decide whether it wants to invest in and try to improve the national CDST system or whether it should disband the system, which is clearly not functioning, at least for re-treatment TB patients. This decision must take into account several new developments.
The use of CDST as the gold standard for RR-TB was successfully challenged.25 We recommend having all RR-TB patients in Xpert tested for second-line drug resistance using LPA, in line with new TB management guidelines. This will help in identifying who needs an extensively drug-resistant TB regimen. There is already new molecular diagnostic technology available, such as the GeneXpert OMNI system (a portable, battery-operated, single-cartridge system) and Xpert MTB/RIF Ultra (an assay with higher sensitivity than Xpert MTB/RIF), that if used would allow programmes to be more confident about the feasibility of decentralization and the accuracy of diagnoses. Indeed, in 2017 the WHO recommended the use of Xpert MTB/RIF Ultra in all settings as a replacement for Xpert MTB/RIF.26 Use of Xpert MTB/RIF Ultra might obviate the need for confirmatory CDST for MDR-TB and RR-TB. However, there is the issue of isoniazid monoresistance and resistance to second-line anti-TB drugs. There is some evidence that isoniazid monoresistance (found in 5% of patients in our study and increasing in this part of Africa) is associated with poorer treatment outcomes,27 and the WHO recommends that high levels of isoniazid resistance warrant a change from rifampicin–isoniazid in the continuation phase to rifampicin–isoniazid and ethambutol.28 The WHO has also recently recommended a new short-course regimen of 9–12 months for MDR-TB, provided patients have not been treated with second-line drugs and/or have had resistance to fluoroquinolones and second-line injectable agents.29 Currently conventional CDST and molecular line probe assays performed in laboratories are the only ways of obtaining this information. However, a new automated, cartridge-based assay has been developed that accurately detects MTB mutations associated with resistance to isoniazid, fluoroquinolones and aminoglycosides, and this holds promise as a rapid future point-of-care test to guide therapeutic decisions for patients with TB.30 If these new molecular technology tests become available in the field, the need for CDST might disappear.
In conclusion, this study documented an impressive scale-up of the Xpert MTB/RIF assay in Zimbabwe over the last 6 y and, apart from 1 y, the assays have performed well. During the Xpert era there was a gradual increase in sputum specimens from re-treatment TB patients arriving at NRLs, although performance in terms of culture contamination, positive MTB cultures and successful CDST results was patchy and marginal. Zimbabwe needs to decide whether to continue supporting a suboptimal system or invest in new molecular diagnostic technology.
Acknowledgments
Authors’ contributions: CT, ADH and KCT conceived the study. CT, ADH, KCT and AMKV designed the study protocol and all authors read and approved the study protocol. CT, HM and BMM collected the data and all authors contributed to analysing and interpreting the data. CT, ADH and KCT drafted the manuscript and all authors critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. CT and CS are guarantors of the paper.
Acknowledgements: This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Medécins sans Frontières (MSF). The specific SORT IT program that resulted in this publication was implemented by MSF, Brussels Operational Centre, Luxembourg and the Centre for Operational Research, The Union, Paris, France. Mentorship and the coordination/facilitation of SORT IT workshops were provided through the Centre for Operational Research, The Union, Paris, France; the Operational Research Unit (LuxOR); AMPATH, Eldoret, Kenya; the Institute of Tropical Medicine, Antwerp, Belgium; the Centre for International Health, University of Bergen, Norway; and the National Institute for Medical Research, Muhimbili Medical Research Centre, Dar es Salaam, Tanzania.
Funding: The program was funded by the UK’s Department for International Development (DFID), The Union, MSF and La Fondation Veuve Emile Metz-Tesch (Luxembourg). The Global Fund to Fight AIDS, Tuberculosis and Malaria provided financial support for data collection. La Fondation Veuve Emile Metz-Tesch supported open access publication costs. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1. World Health Organisation What is multidrug-resistant tuberculosis (MDR-TB) and how do we control it?. http://www.who.int/features/qa/79/en/ (accessed 2 May 2018).
- 2. Caminero JA, editor. Guidelines for clinical and operational management of drug-resistant tuberculosis. Paris: International Union Against Tuberculosis and Lung Disease, 2013. [Google Scholar]
- 3. Ministry of Health and Child Care National tuberculosis control programme: external review report. Harare, Zimbabwe: Ministry of Health and Child Care, 2016.
- 4. World Health Organization Treatment of tuberculosis guidelines, 4th ed WHO/HTM/TB/2009.420. Geneva: World Health Organization, 2010. [Google Scholar]
- 5. Ministry of Health and Child Care National TB management guidelines for Zimbabwe. Harare, Zimbabwe: Ministry of Health and Child Care, 2016.
- 6. Harries AD, Michongwe J, Nyirenda TE et al. Using a bus service for transporting sputum specimens to the Central Reference Laboratory: effect on the routine TB culture service in Malawi. Int J Tuberc Lung Dis 2004;8(2):204–10. [PubMed] [Google Scholar]
- 7. Kilale AM, Ngowi BJ, Mfinanga GS, et al. Are sputum samples of ‘tuberculosis reaching the reference laboratories? A 9-year audit in Tanzania. Public Health Action 2013;3(2):156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chadha SS, Sharath BN, Reddy K, et al. Operational challenges in diagnosing multi-drug resistant TB and initiating treatment in Andhra Pradesh, India. PLoS One 2011;6(11):e26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qi W, Harries AD, Hinderaker SG. Performance of culture and drug susceptibility testing in pulmonary tuberculosis patients in northern China. Int J Tuberc Lung Dis 2011;15(1):137–9. [PubMed] [Google Scholar]
- 10. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363(11):1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trebucq A, Enarson DA, Chiang CY, et al. Xpert MTB/RIF for national tuberculosis programmes in low-income countries: when, where and how? Int J Tuberc Lung Dis 2011;15(12):1567–71. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’: practical considerations. WHO/HTM/TB/2014.1. Geneva: World Health Organization, 2014. [PubMed]
- 13. World Health Organization Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. WHO/HTM/TB/2011.4. Geneva: World Health Organization, 2011.
- 14. World Health Organization Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. WHO policy update. WHO/HTM/TB/2013.16. Geneva: World Health Organization, 2013.
- 15. Zimbabwe National Statistics Agency and Macro ICF Census 2012. National report. http://www.zimstat.co.zw/sites/default/files/img/National_Report.pdf (accessed 25 June 2018).
- 16. World Bank GDP per capita (current USD$). http://data.worldbank.org/indicator/NY.GDP.PCAP.CD (accessed 16 February 2017).
- 17. World Health Organization Definitions and reporting framework for tuberculosis—2013 revision. WHO/HTM/TB/2013.2. Geneva: World Health Organization, 2013. http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345eng.pdf.
- 18. Salman H, Rüsch-Gerdes S MGIT procedure manual for BACTECTM MGIT 960TM TB system. 2006. https://www.finddx.org/wp.../mgit_manual_nov2006.pdf (accessed 24 June 2018).
- 19. Stop TB Partnership Mycobacteriology laboratory manual, 1st ed. April 2014. Geneva: Global Laboratory Initiative, Stop TB Partnership.
- 20. Sikhondze W, Dlamini T, Khumalo D, et al. Countrywide roll-out of Xpert MTB/RIF in Swaziland: the first three years of implementation. Public Health Action 2015;5(2):140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gounder A, Gounder S, Reid SA. Evaluation of the implementation of the Xpert MTB/RIF assay in Fiji. Public Health Action 2014;4(3):179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ardizzoni E, Fajardo E, Saranchuk P, et al. Implementing the Xpert1MTB/RIF diagnostic test for tuberculosis and rifampicin resistance: outcomes and lessons learned in 18 countries. PLoS One 2015;10(12):e0144656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charambira K, Ade S, Harries AD, et al. Diagnosis and treatment of TB patients with rifampicin resistance detected using Xpert MTB/RIF in Zimbabwe. Public Health Action 2016;6(2):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Org 2007;85(11):867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Deun A, Kya A, Aung A, et al. Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol 2013;51(8):2633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. WHO/HTM/TB/2017.04. Geneva: World Health Organization, 2017. http://www.who.int/tb/publications/2017/XpertUltra/en/ (accessed 14 November 2017).
- 27. van der Hiejden YF, Karim F, Mufamadi G, et al. Isoniazid-monoresistant tuberculosis is associated with poorer treatment outcomes in Durban, South Africa. Int J Tuberc Lung Dis 2017;21(6):670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization Treatment of tuberculosis. Guidelines for treatment of drug-susceptible tuberculosis and patient care. WHO/HTM/TB/2017.05. Geneva: World Health Organization, 2017.
- 29. World Health Organization The shorter MDR-TB regimen. Geneva: World Health Organization, 2016. http://www.who.int/tb/Short_MDR_regimen_factsheet.pdf (accessed 14 November 2017).
- 30. Xie YL, Chakravorty DT, Amstrong SL, et al. Evaluation of a rapid molecular drug-susceptibility test for tuberculosis. N Engl J Med 2017;377(11):1043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]