Abstract
Research on the functions of interferon tau (IFNT) led to the theory of pregnancy recognition signaling in ruminant species. But IFNT does much more as it induces expression of interferon regulatory factor 2 (IRF2) in uterine luminal (LE), superficial glandular (sGE), but not glandular (GE) epithelia. First, IRF2 silences transcription of the estrogen receptor alpha gene and, indirectly, transcription of the oxytocin receptor gene to abrogate development of the luteolytic mechanism to prevent regression of the corpus luteum and its production of progesterone for establishing and maintaining pregnancy. Second, IRF2 silences expression of classical interferon-stimulated genes in uterine LE and sGE; however, uterine LE and sGE respond to progesterone (P4) and IFNT to increase expression of genes for transport of nutrients into the uterine lumen such as amino acids and glucose. Other genes expressed by uterine LE and sGE encode for adhesion molecules such as galectin 15, cathepsins, and cystatins for tissue remodeling, and hypoxia-inducible factor relevant to angiogenesis and survival of blastocysts in a hypoxic environment. IFNT is also key to a servomechanism that allows uterine epithelia, particularly GE, to proliferate and to express genes in response to placental lactogen and placental growth hormone in sheep. The roles of secreted phosphoprotein 1 are also discussed regarding its role in implantation in sheep and pigs, as well as its stimulation of expression of mechanistic target of rapamycin mRNA and protein which is central to proliferation, migration, and gene expression in the trophectoderm cells.
Keywords: interferon tau, pregnancy, nutrient transport, arginine, secreted phosphoprotein 1, implantation, placentation
Interferon tau and pregnancy in ruminant species.
Introduction
Transition of the laboratory of Fuller W. Bazer from University of Florida to Texas A&M University
Interdisciplinary research in reproductive biology at the University of Florida resulted from the coming together of scientists to establish a strong and productive program through sharing of physical and intellectual resources. This approach to research on interferon tau (IFNT) and other areas continued in 1992 after Dr Bazer moved to Texas A&M University. A key factor in the move was the mission of the Institute of Biosciences and Technology in the Texas Medical Center in Houston, Texas, to integrate research in agricultural and life sciences, engineering, veterinary medicine, and medicine through interactions among faculties in a land grant university and institutions in the Texas Medical Center. Establishing a new laboratory at Texas A&M University required considerable time and effort of a number of key individuals with whom Dr Bazer has worked at Texas A&M University. In alphabetical order, they were or are Drs Robert Burghardt, Greg Johnson, Troy Ott, Thomas Spencer, Wenbin Tuo, Guoyao Wu, and Daming Zhou. With their leadership and dedication to the goals of the laboratory, a stable and mature research environment was established to foster graduate education and establishment of the Interdisciplinary Faculty for Reproductive Biology that serves the entire Texas A&M University System.
Overview of mechanisms for establishment of pregnancy in ruminant species
IFNT and pregnancy recognition in ruminants
In collaboration with Dr William W. Thatcher at the University of Florida, experiments were designed to unravel the mechanism whereby IFNT prevents luteal regression to allow establishment of pregnancy in ewes and other ruminants. Dr John McCracken's model of the “progesterone block” for regulation of the estrous cycle in ewes in 1983 [1] was key to our working hypothesis regarding the mechanism whereby IFNT acts to signal pregnancy recognition in sheep and other ruminant species. Our working hypothesis was that IFNT inhibited expression of estrogen receptor alpha (ESR1) and oxytocin receptor (OXTR) and stabilized expression of progesterone receptors (PGR) in uterine epithelia to extend the period of the progesterone (P4) block proposed by McCracken [1]. When that working hypothesis was tested, the results indicated that steady-state levels of PGR mRNA were not different between pregnant and cyclic ewes [2]. Those results were from the last experiments conducted at the University of Florida. However, our research at Texas A&M University using in situ hybridization and immunohistochemical analyses revealed loss of expression of PGR mRNA and protein in all uterine luminal (LE) and then glandular (GE) epithelia between Days 10 and 12–13 of both the estrous cycle and pregnancy, while expression of PGR mRNA and protein were maintained in both uterine stromal cells and myometrial cells during pregnancy [3]. Those results provided a clear message for future studies of pregnancy in ewes that one must examine temporal and cell-specific (spatial) aspects of gene expression in the uterus to understand mechanisms critical to pregnancy recognition signaling, conceptus development and implantation.
Our hypothesis at Texas A&M University was that P4 blocks expression of ESR1 and OXTR for a finite period of time, 10–12 days, after which time P4 downregulates expression of its own receptor (PGR) that allows upregulation of expression of ESR1 and OXTR genes in uterine epithelia of cyclic ewes. Then, pulsatile release of oxytocin from the posterior pituitary gland and corpus luteum (CL) elicit secretion of luteolytic pulses of prostaglandin F2-alpha (PGF) from uterine epithelia on Days 15 and 16 to cause regression of the CL and allow the ewe to again exhibit estrus. Those results led to the present theory of how IFNT signals pregnancy recognition in ewes and other ruminants that: (1) IFNT silences transcription of the ESR1 gene and, therefore, prevents E2-induced expression of OXTR in uterine LE, superficial glandular epithelium (sGE), and GE to abrogate endometrial production of oxytocin-induced luteolytic pulses of prostaglandin F2α (PGF); (2) basal production of PGF and PGE2 is greater in pregnant than cyclic ewes due to continued higher levels of prostaglandin endoperoxide synthase 2 (PTGS2) by the endometria LE and production of prostaglandins by conceptus trophectoderm; (3) IFNT silencing of ESR1 expression prevents E2 from inducing PGR in endometrial epithelia; and (4) loss of PGR by uterine epithelia is required for expression of P4-induced and nonclassical IFNT-stimulated genes that support development of the conceptus and implantation [4–24].
In ewes, IFNT is secreted between Days 10 and 21 of gestation (see 4), whereas IFNT is secreted between Days 16 and 21 of gestation as the pregnancy recognition signal that prevents pulsatile release of luteolytic PGF for maintenance of the CL in goats [18]. In cattle, IFNT, secreted between Days 16 and 38 of pregnancy, prevents secretion of luteolytic pulses of PGF by uterine epithelia, and blocks effects of exogenous estradiol (E2) and oxytocin to stimulate uterine release of PGF. Thus, ESR1 and OXTR mRNAs are either less abundant or not responsive to E2 and oxytocin in endometria of pregnant as compared to cyclic cows or cows that receive intrauterine injections of either ovine or bovine IFNT [19–24].
Given that P4 is the hormone of pregnancy and that expression of PGR is limited to stromal and myometrial cells, research was conducted to try and identify progestamedins from stromal cells that regulate function of their overlying uterine epithelia. Fibroblast growth factor 7 (FGF7), FGF10, and hepatocyte growth factor (HGF) are progestamedins secreted by uterine stromal cells based on work in the primate [25,26]. In the ovine uterus, FGF7 is expressed primarily by tunica intima of blood vessels, whereas FGF10 and HGF are expressed by stromal cells while their receptors, fibroblast growth factor receptor 2IIIb (FGFR2IIIb), and oncogene met (MET), respectively, are expressed by both uterine epithelia and conceptus trophectoderm [25,26]. A series of studies found that FGF10 is a bona fide progestamedin in the ovine uterus [27,28]. In the ewe, the uterine LE is lost during the latter part of the peri-implantation period (beginning on day 17) which allows trophectoderm to achieve intimate contact with and be directly affected by uterine stromal cells that express FGF10 and HGF that may be important for epithelial cell functions and conceptus development.
In comparative research with pigs, it was discovered that FGF7 is an estramedin in early pregnancy, but expressed by uterine LE [29,30]. That finding was contrary to the dogma that FGF7, FGF10, and HGF are expressed only by stromal cells; however, the uterine LE of pigs express FGF7 during the peri-implantation period and that FGFR2IIIb is expressed by both uterine epithelia and conceptus trophectoderm. The pig has a true epitheliochorial placenta and many genes are expressed by uterine LE in direct apposition to the trophectoderm/chorion until around Day 25 of pregnancy when the uterine GE/placental areolae complex is established for transport of uterine secretions across the placenta and into the fetal-placental vasculature [28,29]. The uterine LE that secretes FGF7 in pigs is not eroded and is in direct contact with trophectoderm cells that expresses FGFR2IIIb.
IFNT and the servomechanism for function of uterine GE
Studies on the role of IFNT further identified a servomechanism for maintenance of pregnancy that requires reciprocal communication between the conceptus and endometrium during implantation and synepitheliochorial placentation in ewes [31–36]. This concept was initially derived from studies to determine if the antiluteolytic effects of IFNT to extend lifespan of CL were reinforced by actions of placental lactogen (CSH1) and/or placental growth hormone (GH1) secreted from Day 15 to term and Days 35–65 of pregnancy, respectively. Neither independent nor interactive effects of CSH1 and GH1 on luteal maintenance and function in ewes were detected, but both CSH1 and GH1 affected development and function of uterine glands [31]. Of note, the responses of uterine GE to CSH1 and GH1 only occurs if the lumen of the uterus is first exposed to IFNT between Days 11 and 21 and then to intrauterine CSH1, GH1 or CSH1, and GH1 between Days 16 and 29 after onset of estrus. Accordingly, uterine gland hyperplasia occurs between Days 15 and 50 of pregnancy and that hypertrophy of the uterine glands increases the surface area of the uterine glands for maximal production of histotroph after Day 60 of gestation [36]. Thus, it was proposed that sequential exposure of the ovine endometrium to E2, P4, IFNT, CSH1, and GH1 constituted a “servomechanism” for those respective hormones to activate and maintain endometrial remodeling, secretory function, and uterine growth during gestation. The effects of IFNT may be attributed, in part, to increasing expression of prolactin receptors, which mediate effects of CSH1, in the glands [37]. The pathways mediating the interactive effects of IFNT and CSH1 on uterine gland function in ruminants remain unknown. This servomechanism operating primarily in the uterine glands is proposed to increase production of histotroph that is absorbed by the areolae of the placenta and used for fetal–placental growth during gestation.
Another key to research on the roles of IFNT at Texas A&M University followed the cloning and sequencing of the IFNT gene [38,39]. Initially, Day 16 sheep conceptuses were recovered from ewes throughout the sheep breeding season and cultured in vitro to yield enough highly purified native IFNT to investigate its potent antiviral, antiproliferative, and immunosuppressive activities, and determine its structural motif [40–44]. In order to enable studies on the biological actions of IFNT in vivo, a synthetic gene for IFNT was used to express and produced large amounts of ovine IFNT in the Pichia pastoris yeast system [45,46]. Subsequent experiments confirmed that recombinant IFNT had immunosuppressive, antiviral, antiproliferative, and antiluteolytic properties identical to those for native IFNT [40–44].
Cooperative effects of IFNT and P4 on gene expression by uterine epithelia
IFNT is the only known IFN to act as a pregnancy recognition signal in ruminants, but IFNs or IFNT-stimulated genes (ISGs) may have a biological role in establishment of pregnancy in other species including mice and humans [47,48]. The paracrine actions of IFNT on the endometrium stimulate expression of classical and nonclassical ISGs in the endometrium that are hypothesized to regulate uterine functions important for conceptus elongation, implantation, and establishment of pregnancy [47–50]. The interferon (alpha and beta) receptor (IFNAR) mediates IFNT actions [51]. The elongating ovine conceptus expresses IFNAR1/2 [52], and subsequent in vitro studies with ovine trophectoderm cells found that IFNT stimulates their proliferation and certain ISGs [53], supporting the idea that IFNT has an autocrine role in conceptus elongation and implantation. A recent in vivo loss of function study challenged that idea. Translation of IFNT or IFNAR1/2 mRNAs was inhibited in the trophectoderm of the ovine conceptus using morpholino antisense oligonucleotides delivered into the uterine lumen via osmotic pumps from days 8 to 14 postmating [54]. Elongating and filamentous type conceptuses were recovered from ewes infused with a control morpholino or IFNAR1/2 morpholinos. In contrast, severely growth-retarded and malformed conceptuses were recovered from IFNT morpholino-infused ewes with increased numbers of apoptotic trophectoderm cells. Thus, available studies support the idea that IFNT is a critical regulator of conceptus elongation and implantation, but those effects are indirect via paracrine effects on the endometrium, which is similar to the dual roles of the human pregnancy recognition signal, chorionic gonadotropin [55].
Classical type I IFN-stimulated genes in the endometrium
A combination of transcriptional profiling and proteomic experiments elucidated genes stimulated or uniquely induced by IFNT in human cells, ovine endometrium, bovine endometrium, and bovine peripheral blood mononuclear cells during early pregnancy [48,56–65]. The majority of genes induced or upregulated by IFNT are classical ISGs also associated with the effects of other Type I IFNs, such as IFNA and IFNB, that are activated during innate immune responses to invading pathogens (RNA viruses, DNA viruses, intracellular bacteria, parasites) [66].
An archetypal ISG that responds to IFNT in early pregnant ruminants is ISG15 (ISG15 ubiquitin-like modifier; also known as ubiquitin cross-reactive protein or ISG17). On Days 10 or 11 of the estrous cycle and pregnancy in sheep, ISG15 is expressed in the uterine LE, but disappears from the LE by Days 12–13 of pregnancy [67]. As the conceptus begins to elongate on day 12 and secrete more IFNT, ISG15 is upregulated in the upper stroma and GE by days 13–14, while expression extends to the lower stroma, deep glands, and myometrium as well as resident immune cells of the ovine uterus by days 15–16 of pregnancy [67,68]. As IFNT production by the conceptus declines, expression of ISG15 in the stroma and GE also declines between days 20 to 25 of pregnancy. Similar spatiotemporal alterations in ISG15 expression occur in the bovine uterus during early pregnancy [69,70].
A combination of studies with bovine endometrium, ovine endometrium, and human fibroblast cells found that IFNT activates the canonical janus kinase-signal transducer and activator of transcription-IFN regulatory factor (JAK-STAT-IRF) signaling pathway used by other Type I IFNs to induce expression of classical ISGs [71]. All endometrial cell types express IFNAR1 and IFNAR2 subunits in sheep [52]; however, in vivo studies revealed that most classical ISGs are not induced or upregulated by IFNT in the LE of the ovine uterus during early pregnancy [67,72–75]. Further studies revealed that interferon regulatory factor 2 (IRF2), a potent transcriptional repressor of ISGs [76], is expressed specifically in the uterine LE and represses activity of IFN-stimulated response element (ISRE)-containing promoters [72,77] (Figure 1). Indeed, all components of the ISGF3 transcription factor complex (STAT1, STAT2, and IRF9) and most classical ISGs (B2M, GBP2, IFI27, IFIT1, ISG15, MIC, and OAS) contain one or more ISRE in their promoters. Thus, constitutive expression of IRF2 in the LE was hypothesized to restrict induction of most classical ISGs by IFNT in the stroma and GE of the uterus [78]. In endometrial LE during pregnancy, the lack of ISG induction and silencing of ISGs, such as MHC and B2M, may be a critical mechanism preventing immune rejection of the semi-allogeneic conceptus [73]. Although Type I IFNs are involved in both innate and adaptive responses of the immune system, little information is available on the role of IFNT in the immunology of the uterus and pregnancy.
Figure 1.
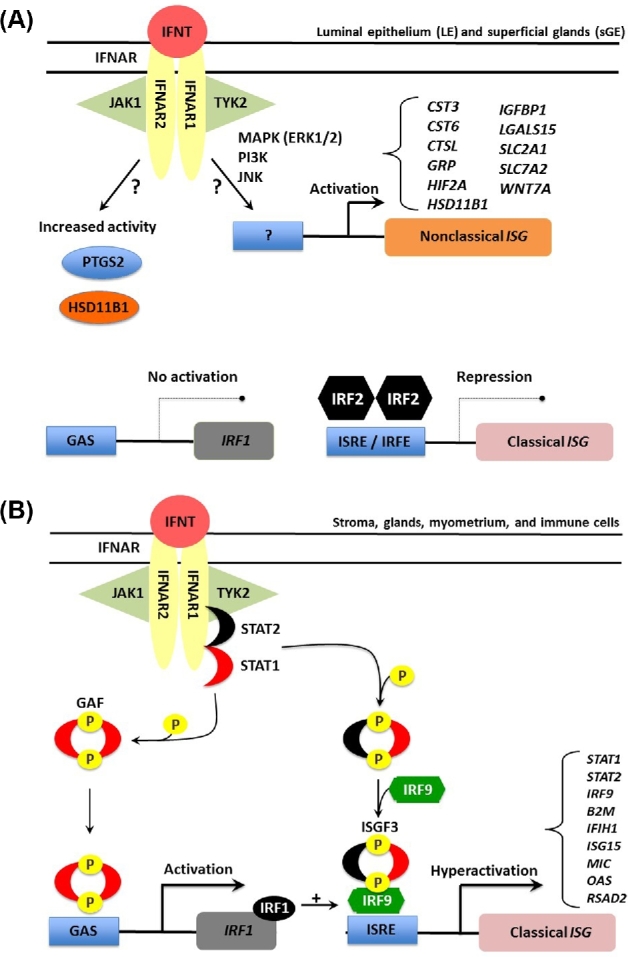
Schematic illustrating the current working hypothesis on IFNT signaling in endometrial epithelia and stroma of the ovine uterus. IFNT, produced in large amounts by the developing conceptus, binds to the Type I IFN receptor (IFNAR) present on cells of the ovine endometrium. In the cells of the endometrial luminal epithelium (LE) and superficial glands (sGE), IFNT induces IRF2 which prevents uterine LE and sGE from expressing classical ISGs such as STAT1, STAT2, and IRF9 (A). IRF2, a potent and stable repressor of transcription of genes in the nucleus, increases in abundance during early pregnancy only in uterine LE and sGE. The presence of IRF2 (interferon regulatory factor 2) inhibits binding of IRF1 to the promoter region of ISRE-containing target genes through direct competitive binding of IRF2 to ISRE and subsequent coactivator repulsion. IFNT can stimulate the transcription of a number of nonclassical IFNT-stimulated genes (ISG) as well as increase the activity of certain intracellular enzymes (prostaglandin synthase 2, PTGS2 and HSD11B1, hydroxysteroid 11-beta dehydrogenase). However, the noncanonical signaling pathways mediating these effects of IFNT in the LE/sGE are largely unknown. In cells of the stroma and middle to deep glands (B), IFNT-mediated association of the IFNAR subunits facilitates the cross-phosphorylation and activation of two Janus kinases, Tyk2 and JAK1, which in turn phosphorylate the receptor and creates a docking site for signal transducer and activator of transcription 2 (STAT2). STAT2 is then phosphorylated, thus creating a docking site for STAT1 that is then phosphorylated. STAT1 and STAT2 are then released from the receptor and can form two transcription factor complexes. Interferon-stimulated gene factor 3 (ISGF3), formed by association of the STAT1-2 heterodimer with IRF9 in the cytoplasm, translocates to the nucleus, and transactivates genes containing an ISRE, such as STAT1, STAT2, and IRF9. Gamma activation factor (GAF) is formed by binding of STAT1 homodimers, which translocates to the nucleus and transactivate genes containing a gamma activation sequence (GAS) element(s), such as IRF1. IRF1 can also bind and transactivate ISRE-containing genes. The simultaneous induction of STAT2 and IRF9 gene expression by IFNT appears to shift transcription factor formation from GAF towards predominantly ISGF3. Therefore, IFNT activation of the JAK-STAT signal transduction pathway allows for constant formation of ISGF3 and GAF transcription factor complexes and hyperactivation of ISG expression.
A significant challenge is to determine which of the classical ISGs induced in the endometrium by IFNT have a biological role in conceptus elongation and/or establishment of pregnancy. CXCL10 (chemokine (C-X-C motif) ligand 10 or IP-10) is one classical ISG that stimulates trophectoderm growth and adhesion in vivo [79,80]. Other chemokines, such as CXCL12 in sheep [81] and CCL8 and CCL11 in cattle [82], may also have a role in conceptus–endometrial interactions. Thus, it is likely that other classical ISGs have biological roles in conceptus elongation and implantation in ruminants, particularly since IFNT has embryotrophic effects mediated by the endometrium [83,84].
Nonclassical IFNT-stimulated genes in the endometrium and the role of progesterone
A number of approaches, including microarray analysis of human U3A (STAT1 null) cells and ovine endometrium and in situ hybridization analysis, were used to discover IFNT-regulated specific genes in the uterine LE during pregnancy [85–89]. In sheep, IFNT was found to stimulate expression of a number genes [cystatin 3 (CST3), CST6, cathepsin L (CTSL), gastrin releasing peptide (GRP), hydroxysteroid dehydrogenase 11 B2 (HSD11B1), insulin-like growth factor binding protein 1 (IGFBP1), galectin 15 (LGALS15), solute carrier family 2 member 1 (SLC2A1), solute carrier family 5 member 11 (SLC5A11), solute carrier family 7, member 2] in the endometrial LE and(or) GE that have biological activities potentially important for conceptus elongation and implantation [50,57] (Table 1). Of note, none of those genes are classical ISGs induced by other Type I IFNs. Thus, they are “nonclassical” IFNT-stimulated genes. Further, those genes are first induced by progesterone in the endometrial epithelia before IFNT can stimulate their expression. Many nonclassical IFNT-stimulated genes identified to date have biological activities that implicate them in trophectoderm (proliferation, migration, attachment and (or) adhesion, and nutrient transport) [50,90–93]. For example, knockdown of an amino acid transporter (SLC7A1) in the conceptus trophectoderm [94] and inhibition of HSD11B1 activity in utero or in the conceptus trophectoderm compromised conceptus elongation in sheep [84,95]. In cattle, IFNT actions on the endometrium are not as well understood in terms of nonclassical IFNT-stimulated genes, but recent transcriptomic studies have started to uncover them [58,59,96–98].
Table 1.
Effects of ovarian progesterone (P4) and intrauterine infusion of IFNT or prostaglandins (PGs) on elongation- and implantation-related genes expressed in the endometrial epithelia of the ovine uterus.1
| Gene symbol and general function | P4 | IFNT |
|---|---|---|
| Transporters of glucose | ||
| SLC2A1 | ↑ | + |
| SLC2A5 | n.d. | n.e. |
| SLC2A12 | n.d. | + |
| SLC5A1 | ↑ | + |
| SLC5A11 | ↑ | + |
| Transporters of amino acids | ||
| SLC1A5 | n.d. | n.d. |
| SLC7A2 | ↑ | + |
| Cell proliferation, migration, attachment | ||
| GRP | ↑ | + |
| IGFBP1 | ↑ | + |
| LGALS15 | ↑ | ++ |
| SPP1 | ↑ | + |
| Proteases and their inhibitors | ||
| CTSL1 | ↑ | ++ |
| CST3 | ↑ | + |
| CST6 | ↑ | + |
| Intracellular enzymes | ||
| HSD11B1 | ↑ | + |
| PTGS2 | ↑ | n.e.2 |
| Transcription factors | ||
| HIF1A | ↑ | + |
| HIF2A | ↑ | + |
1Effect of hormone or factor denoted as induction (↑), stimulation (+), no effect (n.e.), decrease (−), or not determined (n.d.).
2No effect (n.e.) on gene expression, but increases PTGS2 activity.
SLC2A1, Solute carrier family 2, facilitated glucose transporter member 1; SLC2A5, Solute carrier family 2, facilitated glucose transporter member 5; SLC2A12, Solute carrier family 2, facilitated glucose transporter member 12; SLC1A5, Solute carrier family 5, facilitated glucose transporter member 5; SLC5A11, Solute carrier family 2, facilitated glucose transporter member 11; SLC1A5, Neutral amino acid transporter B(0); SLC7A2, Low affinity cationic amino acid transporter 2; GRP, gastrin releasing peptide; IGFBP1, insulin-like growth factor binding protein 1; LGALS15, galectin 15; SPP1, secreted phosphoprotein 1; CTSL1, cathepsin L; CST3, cystatin 3; CST6, cystatin 6; HSD11B1, hydroxysteroid 11-beta dehydrogenase type 1; PTGS2, prostaglandin synthase 2; HIF1A, hypoxia inducible factor 1 alpha; HIF2A, hypoxia inducible factor 2 alpha.
Critical signaling components of the JAK-STAT signaling system (STAT1, STAT2, IRF9) are not expressed in endometrial LE [72]. Thus, it was reasoned that IFNT regulates expression of genes in endometrial LE of the ovine uterus using a noncanonical, STAT1-independent signaling pathway. Indeed, other Type I IFNs utilize noncanonical mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) cascades [99]. In ovine endometrium and LE cells in vitro, IFNT activates distinct JAK, epidermal growth factor receptor, MAPK (ERK1/2), PI3K-AKT, and(or) Jun N-terminal kinase (JNK) signaling modules to regulate expression of PGE2 receptors [100,101]. Additionally, PTGS2-derived prostaglandins and HSD11B1-derived cortisol are part of the noncanonical pathway of IFNT action on the endometrium in sheep [102–104]. Available evidence supports the idea that combinatorial paracrine effects of progesterone and IFNT, via canonical and noncanonical signaling pathways, stimulates biological processes in the endometrium that regulate conceptus elongation for establishment of pregnancy. Future investigations should focus on understanding the biological roles of classical and nonclassical IFNT-stimulated genes as well as genes downregulated by IFNT in pregnancy establishment.
Transport of arginine into the pregnant uterus and its roles in conceptus development
Arginine is an abundant amino acid in the uterine fluid and conceptus, and its concentrations increase progressively during the first half of gestation [105–107]. Progesterone plays an important role in inducing the expression of arginine transporters in the placenta and uterus [4,92]. Arginine is required for the synthesis of many biologically active molecules, including nitric oxide (NO), polyamines (putrescine, spermidine, and spermine), creatine, and agmatine [108]. Of those substances, NO and polyamines stimulate cell proliferation and migration, cellular remodeling, angiogenesis, and dilation of blood vessels to increase blood flow, whereas creatine is essential to neurological and skeletal muscle development [109]. Furthermore, arginine activates the mechanistic target of rapamycin (MTOR) cell signaling pathway to promote protein synthesis and tissue remodeling [110], and also stimulates expression of IFNT by conceptus trophectoderm [111]. Thus, arginine is a nutritionally essential amino acid for gestating, lactating, and young mammals.
Arginine synthesis
Although diets provide arginine, at least 50% of its daily requirement in gestating mammals (e.g. pigs and sheep) is met through endogenous synthesis [112,113]. Arginine is synthesized from glutamine, glutamate, and proline via the intestinal-renal axis in most mammals, including cattle, sheep, and pigs [114]. For example, the porcine fetal small intestine can synthesize citrulline (the immediate precursor of arginine) from glutamine, glutamate, and proline beginning at Day 30 of gestation and the capacity of this synthetic pathway increases progressively between Days 30 and 114 (term) of pregnancy [115]. Similarly, this synthetic pathway is detected in the ovine fetal small intestine on Day 40 of gestation and becomes more active with advancing gestation [106]. Arginase activity is nearly absent from the fetal intestine, thereby maximizing the release of citrulline and arginine from the gut [114]. Much evidence shows that endogenous synthesis of arginine is insufficient under certain physiological conditions, such as pregnancy, lactation and neonatal growth in livestock, rodents, and humans [112,115,116]. This necessitates maternal dietary supplementation of arginine to ensure optimal survival, growth and development of embryos and fetuses.
Arginine catabolism
Homeostasis of arginine in animals is regulated not only by its synthesis but also by its catabolism. Owing to the extensive catabolism of arginine by arginase in the small intestine of postweaning mammals, only about 60% of dietary arginine enters the portal circulation of pregnant gilts [117]. The quantitatively major products of intestinal arginine degradation include ornithine and proline, which are released into the portal circulation. In ruminants, dietary protein and nonprotein nitrogen (including free arginine) are converted by ruminal microbes into microbial protein, which enters the abomasum and small intestine for digestion to release arginine. Approximately 8% of arginine in the portal vein is extracted by the liver where it is used for synthesis of protein and urea [117], and nearly 90% of arginine in the portal vein can escape the liver to be available for extrahepatic organs.
Transport of arginine is the first step in its utilization by cells, including those in the placenta and uterus, since arginine is not taken up in a significant quantity from extracellular fluid by simple diffusion. This is essential for transporting arginine across the cell membrane of the conceptus that lacks de novo synthesis of arginine [118]. As a cationic amino acid, arginine shares the same transporters with lysine, ornithine, and histidine. System y+, which was the first transport system identified as a cationic amino acid transporter (CAT), is selective for cationic amino acids and is Na+-independent [119]. Three different CAT genes (SLC7A1, SLC7A2, and SLC7A3) have been cloned, which encode for four homologous proteins cationic amino acid transporter (CAT) (CAT-1, CAT-2A, CAT-2B, and CAT-3), respectively, in animal tissues including the uterus and placenta [120]. In addition, systems bo,+ and Bo,+, which are expressed in mouse blastocysts [121], can transport neutral amino acids as well as cationic amino acids [122]. These two systems are distinguished by their dependence on Na+ in that system Bo,+ is Na+-dependent, while system bo,+ is Na+-independent. The fourth transport system named y+L has high affinity for both neutral and cationic amino acids [119]. Transport of arginine and other cationic amino acids through this system is Na+-independent and its apparent affinity for neutral amino acids decreases dramatically when Na+ in the medium is replaced with K+. To avoid an imbalance among basic amino acids, the level of dietary arginine supplementation for mammals (e.g. pigs) should not be greater than 2.0% on an as-fed basis (90% dry matter) [123].
There are multiple pathways in cells for arginine utilization, as noted previously. In mammalian cells, the arginine that is not used for protein synthesis enters catabolic pathways initiated by arginase, arginine: glycine amidinotransferase, arginine decarboxylase, and nitric oxide (NO) synthase. These pathways are used to synthesize ornithine, creatine, agmatine, and NO, respectively [109,124,125]. In the ovine conceptus, agmatine production from arginine is an alternative pathway for polyamine synthesis (Figure 2) that is essential for embryonic survival and development [125–127]. At present, little is known about quantitative aspects of arginine catabolism in gestating mammals. Based on our published work with nongestating swine [115], we estimated that the small intestine and extraintestinal tissues can catabolize: (a) 153 and 365 mg arginine/(kg BW day), respectively, in the 64-kg pig fed a basal diet containing 1.35% arginine without arginine supplementation; and (b) 405 and 743 mg arginine/(kg BW day) in the 67-kg pig fed the basal diet supplemented with 2% arginine. Based on the daily excretion of creatinine, homoarginine, agmatine, and NOx in the urine, creatine synthesis is the major pathway for arginine catabolism in pigs supplemented with 0, 315 and 630 mg Arg/(kg BW d) [108]. Although the liver and kidney can synthesize homoarginine from Arg and lysine, this pathway is quantitatively minor for arginine utilization in the animals. Based on the production of creatine + homoarginine + agmatine + NOx via the nonarginase pathway, the arginase pathway (calculated as rates of arginine catabolism via arginase and nonarginase pathways minus rates of arginine catabolism via the nonarginase pathway) contributed to 76%, 82%, and 85% of arginine catabolized in extraintestinal tissues of the 65-kg pig supplemented with 0, 315, and 630 mg Arg/(kg BW day), respectively [108]. Rates of urinary excretion of arginine are negligible in comparison with dietary arginine intake, indicating that dietary arginine undergoes extensive degradation in mammals [108]. Knowledge of arginine metabolism in mammals is essential to designing effective means to improve conceptus growth, development, and survival.
Figure 2.
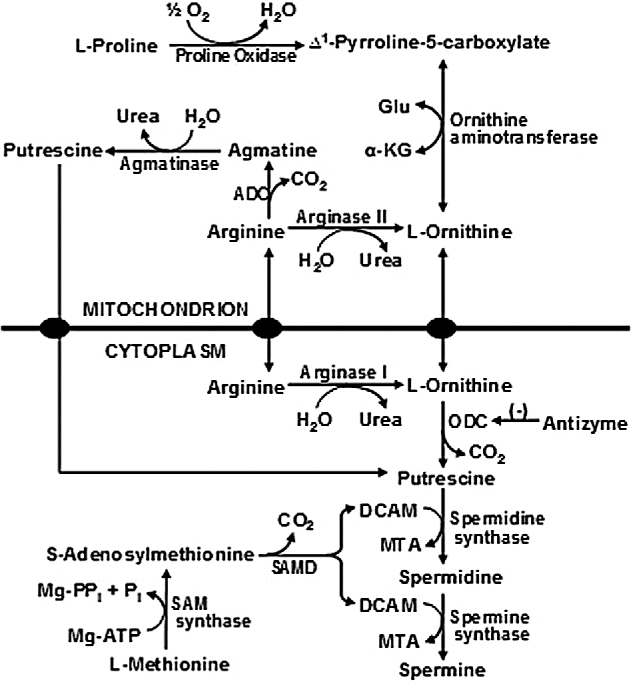
Synthesis of polyamines from arginine in the mammalian conceptus. Arginine is hydrolyzed to ornithine plus urea by arginase I and arginase II in many cell types (possibly except for porcine placentae and neonatal small intestine). Synthesis of putrescine from ornithine is catalyzed by ornithine decarboxylase (a cytosolic enzyme) in all cell types. In placental mitochondria of sheep, arginine may be decarboxylated to form agmatine by arginine decarboxylase (ADC) and agmatine is then converted into putrescine by agmatinase. DCAM, decarboxylated 5-adenosylmethionine; α-KG, α-ketoglutarate; MTA, methylthioadenosine; SAMD, S-adenosyl-methionine decarboxylase; SAM, S-adenosylmethionine. Adapted from Wu et al. [213].
Beneficial effects of dietary arginine on pregnancy outcomes in livestock mammals
Strong experimental evidence indicates beneficial effects of dietary arginine supplementation on embryonic/fetal survival and growth in mammals. For example, Mateo et al. [128] found that dietary supplementation with 0.83% arginine (as 1.0% arginine-HCl; 16.6 g arginine/sow per day) between Days 30 and 114 of gestation increased the number of live-born piglets by 2 per litter and litter birth weight by 24% [128]. In addition, supplementing 1% arginine to the barley-based diet of sows with high ovulation rates between Days 14 and 28 of gestation enhanced the number of fetuses on Day 70 by three per litter, as well as the ratio of secondary-to-primary muscle fibers in fetal pigs [129]. Likewise, dietary supplementation of gilts with 1% arginine for 16 days, beginning on Day 17 of gestation, enhanced placental weight by 16% and the number of live-born piglets per litter by 1.2 at farrowing [113]. Furthermore, dietary supplementation with 1% arginine between Days 25 and 80 of gestation increased the birth weight of piglets by 10%, while markedly reducing the percentage of piglets with a birth weight of <0.85 and <1.0 kg by 47% and 33%, respectively, in gilts giving birth to ≤14 piglets per litter [130]. Collectively, sufficient provision of dietary arginine is essential to maximize reproductive performance in swine [131].
Besides swine, intravenous administration of arginine can improve embryonic survival in pregnant sheep [132]. Likewise, intravenous administration of arginine prevents intrauterine growth restriction (IUGR) in underfed ewes [133]. Specifically, beginning on Day 28 of gestation, Suffolk ewes were fed a diet providing 100% or 50% (underfed) of NRC nutrient requirements and, between Day 60 of gestation and parturition, underfed ewes received intravenous infusions of saline or 155 μmol L-arginine per kg body weight three times daily, whereas control ewes received saline. The birth weights of lambs from saline-infused underfed ewes were 23% lower than those of lambs from control-fed dams [133]. The administration of arginine to underfed ewes increased concentrations of arginine in maternal plasma (69%), fetal brown adipose tissue mass (48%), and birth weights of lambs by 21%, as compared to saline-infused underfed ewes [134]. Similar results were observed for diet-induced obese ewes [135]. Furthermore, intravenous administration of arginine (345 μmol arginine-HCl per kg BW three times daily) between Days 100 and 121 of gestation reduced the percentage of lambs born dead by 23%, increased the percentage of lambs born alive by 59%, and enhanced birth weights of quadruplets by 23%, without affecting maternal body weight [136].
Transport of glucose into the pregnant uterus: roles of glucose and fructose in conceptus development
Glucose Transporter 1 (GLUT1/SLC2A1)
The energy substrate for mammalian conceptuses switches from pyruvate to glucose at the blastocyst stage in concert with increases in expression of uterine glucose transport proteins. In ovine uteri, total recoverable glucose increases 12-fold between Days 10 and 15 of pregnancy, but not the estrous cycle [137]. Correspondingly, GLUT1 mRNA and protein in ovine uterine LE/sGE increase 7.2-fold between Days 10 and 14 of pregnancy and remain elevated thereafter, whereas GLUT3 is expressed only in conceptus trophectoderm. In ovariectomized ewes, GLUT1 mRNA increased 4.2-fold in response to P4 and an additional 2.1-fold in response to IFNT.
Fructose is detected in uterine flushings of pregnant gilts as early as Day 12 and is present in significantly greater quantities than glucose in allantoic fluid and fetal blood (see 138). Additionally, fructose is undetectable in blood of pregnant gilts and sows [139]. White et al. [140] reported that fructose injected intraperitoneally into an in utero fetus does not cross the placenta or get converted into glucose; however, glucose, injected in the same manner, can cross the placenta and be converted to fructose. Thus, the placenta is the site of fructose production and the fructose synthesized from glucose is a mechanism whereby conceptuses may sequester a hexose sugar from the mother (see 138).
In pigs, glucose is transported from maternal blood across the uterine LE and conceptus trophectoderm/chorion, and into the vascular supply of the conceptus by solute carrier transporters SLC2A1, SLC2A3, and SLC2A4 [141]. Fructose is synthesized from glucose via the polyol pathway in the placenta with glucose and NADPH being converted to sorbitol and NADP+ by aldose reductase (AKR1B1), then sorbitol and NAD+ are converted to fructose and NADH, respectively, by sorbitol dehydrogenase (SORD). Sorbitol is very abundant in placentae and fetal fluids of sheep, cows and humans, but particularly abundant in human placentae during early pregnancy [142,143]. SORD has been localized to trophectoderm of bovine conceptuses [144].
Steinhauser et al. [141] reported that conceptuses of pigs contain higher concentrations of fructose than glucose and that fructose is not detected in maternal blood. Fructose is synthesized from glucose via AKR1B1 and SORD, and transported across cell membranes by solute carriers SLC2A5 and SLC2A8 for conversion to fructose-1-phosphate (F-1-P) by ketohexokinase (KHK). The expression of SLC2A8, SLC2A5, AKR1B1, SORD, and KHK mRNAs and proteins were analyzed using quantitative PCR and immunohistochemistry or in situ hybridization in endometria and placentae of cyclic and pregnant gilts. Progesterone upregulated SLC2A8 protein in uterine LE and GE during the peri-implantation period of pregnancy, but expression was limited to placental chorion and blood vessels after Day 30 of pregnancy in pigs. Progesterone increased expression of SLC2A5 mRNA in uterine LE and GE after implantation, and the chorion expressed SLC2A5 between Days 30 and 85 of gestation. AKR1B1 and SORD proteins were localized to uterine LE during the peri-implantation period, but expression switched to the chorion by Day 20 and was maintained there through Day 85. Uterine expression of AKR1B1 mRNA was downregulated by estrogen. KHK protein localized to trophectoderm/chorion throughout gestation. These results indicate the conversion of glucose to fructose and the expression of fructose transports at the uterine-placental interface of pigs. The shift in expression from uterine LE to chorion during pregnancy suggests that free-floating conceptuses are supported by fructose synthesized by the uterus, but after implantation, the chorion is self-sufficient for fructose synthesis and transport.
After its synthesis, fructose can be transported across cell membranes by SLC2A5, a low-affinity, high-capacity transporter for fructose, but not glucose, and by SLC2A8 that has a high affinity for glucose, but can also transport fructose [145]. SLC2A8 is necessary for decidualization of the uterus in mice during implantation of blastocysts, most likely due to its ability to transport glucose into the differentiating stromal cells, but SLC2A5 has not been detected in human or rodent endometria or placentae [146,147]. Because humans and rodents do not have fructogenic placentae, the differences in expression of these particular transporters are to be expected.
Once fructose enters a cell, it is phosphorylated by either KHK to produce the metabolite F-1-P or by hexokinase-I or -II to produce F-6-P [148]. F-1-P is converted to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate (DHAP) for entry into the glycolytic pathway or it can act as a signaling molecule to induce NFkB activation and subsequent production of cytokines [148,149]. In contrast, F-6-P is likely metabolized via other pathways such as the hexosamine pathway [138].
Fructose and hypoxia
It is of interest that the naked mole-rat can live in low oxygen (5%) for hours and even without oxygen for up to 18 min. Naked mole-rats switch from glucose to fructose as an energy source for anaerobic respiration, and this may be relevant to ungulate and cetacean conceptuses that exist in utero in a hypoxic environment rich in fructose. Park et al. [150] reported that laboratory mice die in an environment lacking oxygen within 20 s, whereas the mole rats live in the same oxygen-free environment for up to 18 min. Naked mole-rats lacking oxygen switched metabolism to anaerobic metabolism of fructose to lactate in the brain and increased expression of glucose transporter SLC2A1. Tissues, especially the brain of the naked mole rats, took in glucose via the glucose transporter SLC2A1 and KHK converted the glucose to F-1-P more rapidly than hexokinase converted glucose to F-6-P by hexokinase. F-1-P is transported via SLC2A5 to other tissues in which it is metabolized to triose sugars (DHAP; glyceraldehyde-3-PO4, 3PGA), as well as pyruvate and lactate. Compared to control naked mole-rats, those under complete anoxia at 30 min had increases in DHAP (more than 3-fold), 3PGA (about 6-fold), lactate (about 4-fold), pyruvate (about 2-fold), and citrate (about 2-fold). Citrate inhibits phosphofructokinase, but not ketohexokinase; therefore, anaerobic metabolism of glucose and fructose is independent of a negative feedback mechanism. Aerobic respiration allows for 1 mol of glucose to yield 30 mol of ATPs, but anaerobic respiration yields only 2 mol ATP per 1 mol glucose. However, via the Cori Cycle, lactate can be converted to pyruvate and pyruvate can, via gluconeogenesis, be used for synthesis of glucose, and allow anaerobic respiration to continue indefinitely.
Synthesis of hyaluronic acid from fructose and glucose via the hexosamine biosynthetic pathway
Hyaluronic acid (HA), a glycosaminoglycan synthesized via the hexosamine biosynthesis pathway, affects angiogenesis and other aspects of the placenta during pregnancy [151]. In a porcine trophectoderm cell line, glucose and fructose are equivalent in stimulating the synthesis of HA in a GFPT1-dependent manner [138]. The metabolism of fructose via the hexosamine pathway leads to the synthesis of glycosaminoglycans, e.g., HA, uridine diphosphate-N-acetyl glucosamine, and uridine diphosphate-N-acetyl galactosamine that are involved in the synthesis of glycolipids, glycosaminoglycans, and proteoglycans critical to functions of cells and tissues. Note that glutamine, another substrate for hexosamine synthesis, is particularly abundant in the allanotic fluid of sheep (e.g., 25 mM on Day 60 of gestation) and pigs (e.g., 2 mM on Day 40 of gestation). HA and hyaluronidase increase in the uterine lumen of pigs in response to progesterone [152] and stimulate angiogenesis, morphogenesis, and tissue remodeling of the placenta as reported for the human placenta [151,153]. The accumulation of HA, also known as Wharton's Jelly, occurs in the placentae of most mammals and localizes to the umbilical cord primarily, but to a lesser extent to placental blood vessels [154]. HA is an abundant component of the extracellular matrix that (1) provides for hydration and hydrodynamics; (2) stimulates proliferation, migration, and adhesion of cells; (3) supports blood vessels and fragments of HA stimulate angiogenesis; (4) plays a role in innate immunity; and (5) supports mesenchymal cells; and (6) provides a niche for stem cells such as hematopoietic stem cells [155]. Angiogenesis is critical to conceptus development in all species, and available results indicate that fructose is used for synthesis of glycosaminoglycans such as HA that support angiogenesis, particularly in the placenta.
Glucose, fructose, and mechanistic target of rapamycin cell signaling in sheep
Research focused on IUGR and adult onset of metabolic disease in humans, use sheep as a model but fail to account for the role of fructose which is the major hexose sugar in the ovine conceptus [138]. In ewes, the maximum concentrations of glucose in allantoic fluid are about 1.1 mM between Days 35 and 140 of pregnancy, while concentrations of fructose are between 11.1 and 33 mM [156]. Therefore, fructose is exerting maximum effects on proliferation of ovine trophectoderm cells at molar concentrations well below those in allantoic fluid [157]. Fructose induces proliferation of an ovine trophectoderm cell line via activation of the AKT-tuberous sclerosis complex 2 (TSC2)-MTOR signaling cascade. Activation of this cascade is mediated by O-GlcNAcylation from UDP-N-acetylglucosamine (UDP-GlcNAc), a primary product of the hexosamine biosynthesis pathway.
Novel results from studies using a pig trophectoderm cell line revealed that fructose is actively involved in stimulating proliferation and mRNA translation via activation of MTOR cell signaling, and synthesis of glycosaminoglycans, specifically HA, via the hexosamine biosynthetic pathway [138]. Ovine trophectoderm cells were then used to assess effects of glucose and fructose on MTOR Complex 1 (MTORC1) that includes the regulatory-associated protein of MTOR (Raptor), mammalian LST8/G-protein β-subunit like protein (mLST8/GβL) and their AKT1 substrate 1, proline-rich (AKT1S1) and dep domain-containing protein 6 (DEPDC6) that is involved with nutrient and energy sensing as well as and protein synthesis and is responsive to insulin, growth factors, serum, phosphatidic acid, amino acids, and oxidative stress [157]. As for pig trophectoderm cells, glucose and fructose were equivalent in stimulating proliferation of ovine trophectoderm cells via the hexosamine biosynthetic pathway. In the presence of azaserine (an inhibitor of GFPT1), neither glucose nor fructose induced proliferation of ovine trophectoderm cells. Fructose and glucose require GFPT1 activity for conversion of glucose-6-phosphate (G-6-P) and fructose-6-phosphate (F-6-P) to glucosamine-6-phosphate (GlcN-6-P). Inhibition of GFPT1 in ovine trophectoderm cells treated with glucose or fructose decreased production of GlcN-6-P to basal control levels. The abundance of total (t-MTOR) and phosphorylated MTOR (p-MTOR) in ovine trophectoderm cells increased significantly at 0.5, 1, 24, 48, and 96 h of incubation with glucose or fructose. This effect was decreased significantly in the presence of 2 μM azaserine which reduced stimulatory effects of glucose and fructose to phosphorylate TSC2 to basal levels. Thus, UDP-GlcNAc stimulates proliferation of ovine trophectoderm cells via activation of the AKT-TSC2-MTOR cell signaling cascade. Collectively, available results indicate that fructose and glucose may increase cellular functions by activating integrated cell signaling pathways affecting proliferation of both ovine and porcine trophectoderm cell lines. Thus, fructose and glucose can be metabolized via the nonoxidative hexosamine biosynthesis pathway to GlcN-6-P by GFPT1, increase O-GlcNAcylation of cellular proteins/enzymes by OGT, and then activate the Akt-TSC2-MTOR cell signaling cascade to stimulate proliferation of ovine trophectoderm cells.
Fructose, glucose, and one-carbon metabolism
The pathway for glycolysis involves glucose and fructose (e.g., F-6-P and fructose 1, 6 bisphosphate) that can be metabolized to 3-phosphoglycerate. The 3-phosphoglycerate dehydrogenase (PHGDH) enzyme initiates the branching pathway from glycolysis for serine biosynthesis [158,159]. The 3-phosphoglycerate is converted to 3-phosphohydroxypyruvate which then undergoes transamination, possibly with glutamate, followed by phosphate ester hydrolysis. This pathway is known as serineogenesis [160].
Serine is the principal source of one-carbon groups in humans (see 160). This involves both cytosolic and mitochondrial pathways for production of N5, N10-methylene-tetrahydrofolate. In mitochondria, N5, N10-methylene-tetrahydrofolate is converted to formate which is released to the cytosol where it is incorporated into N10-formyl-tetrahydrofolate. N10-formyl-tetrahydrofolate is used directly for purine synthesis or further reduced to provide one-carbon groups for the production of thymidylate and methyl groups for synthetic, regulatory, and epigenetic methylation reactions.
Fructose is metabolized via the hexosamine biosynthesis pathway and activates mTOR cell signaling [157] and MTOR induces expression of activation transcription factor 4 (ATF4) which stimulates serinogenesis [159]. Serine may become a substrate for one-carbon metabolism in both the cytosol and the mitochondria. Experiments in humans with labeled serine revealed that the mitochondrial pathway is quite active [161]. In mitochondria, there is conversion of serine and glycine to formate which is released into the cytoplasm and nucleus for cellular events required for growth and development of the conceptus. The concentrations of formate in plasma and amniotic fluid of ovine conceptuses (191 and 296 μM, respectively) are greater than in maternal plasma (33 μM). Serine, a primary substrate for 1C metabolism, is also more abundant in fetal plasma (0.9 mM, Day 120 of gestation), and amniotic fluid (0.9 mM, Day 120 of gestation) and allantoic fluid (11.5 mM, Day 120 of gestation) than maternal plasma (0.06 mM, Day 120 of gestation) [106]. Dimethylglycine, a choline metabolite, is 30 times higher in plasma from fetal lambs than ewes [160]. Purine biosynthesis can provide nucleotide precursors for production of ATP for energy metabolism and GTP critical for cell signaling [162] as well as purines for nucleic acid synthesis. The partnership between purinosomes (a multienzyme complex for de novo purine biosynthesis) and mitochondria provides 1C units generated by the mitochondrial conversion of serine to formate for incorporation into the purine ring during de novo biosynthesis. Colocalization and physical linkage between purinosomes and mitochondria is inhibited by rapamycin, an inhibitor of MTOR [162]. Further, formylglycinamidine ribonucleotide synthase and adenylosuccinate lyase, core protein components of purinosomes, are less abundant in mitochondria of HeLa cells (immortal human cervical cancer cell line) treated with rapamycin. Thus, mTOR has a role in linking purinosomes and mitochondria.
Fructose stimulates proliferation of ovine trophectoderm cells via MTOR cell signaling and MTOR upregulates production of purines and one-carbon groups, particularly formate, in both normal and cancer cells [158]. Thus, we propose that fructose enhances purine and formate production by the ovine placenta in an MTOR-dependent manner [160,161]. Formate is produced by folate-mediated 1C metabolism for the synthesis of purines and thymidine and the provision of methyl groups for transmethylation reactions. Purines are required for the synthesis of nucleotides and nucleic acids, while thymidine is required for the synthesis of DNA. Methyl groups are required for the synthesis of key molecules such as phosphatidylcholine and creatine, and for methylation of cytosine residues in DNA required for normal development of fetal and neonatal animals [163].
Tetrahydrofolate (THF) is the primary intracellular carrier of 1C units. The provision of methyl groups via the reduction of N5,N10-methylene-THF to N5-methyl-THF by NADPH is catalyzed by methylenetetrahydrofolate reductase, followed by the transfer of the methyl group from N5-methyl-THF to homocysteine by methionine synthase to form methionine. The latter may be incorporated into SAM (S-adenosylmethionine), the principal biological methylating agent by methionine adenosyltransferase (see 164). Methionine synthase is one of only two mammalian enzymes known to require vitamin B12 for synthesis of their prosthetic groups. Methionine is used for the synthesis of SAM. The human genome contains more than 200 methyltransferases that use SAM to methylate a variety of acceptors, including DNA, RNA, and proteins, regulating gene expression, mRNA processing, and protein function. In addition, methyltransferases are responsible for the synthesis of key molecules such as creatine, phosphatidylcholine, and epinephrine and for detoxification of inorganic arsenic ions [163].
Folate metabolism
Folate metabolism may play a key role in the reduction of NADP to NADPH [165], which is critically important in growing and developing tissues such as the placentae and embryos/fetuses of conceptuses during pregnancy. However, quantitatively, the concentration of folate in the fetal plasma is low but the pentose cycle activity in trophectoderm cells is particularly high to generate NADPH. A role of folate in conceptus NADPH provision remains to be determined. Low folate status is a risk factor for development of neural tube defects such as spina bifida and anencephaly [165]. The precise mechanism of action of folate is not understood. Formate is produced primarily in mitochondria from serine, glycine, and two intermediates of choline catabolism, sarcosine, and dimethylglycine. Mitochondrial N10-formyl-THF gives rise to formate which leaves the mitochondrion and is incorporated into the cytosolic pool of N10-formyl-THF which may be used for purine synthesis [166]. Cytosolic N10-formyl-THF may also be reduced to N5, N10-methylene-THF and then to N5-methyl-THF. In addition, the formyl group of N10-formyl-THF may be oxidized to CO2 as an exit for excess one-carbon groups from folate metabolism [167]. Formate also derives from other metabolic reactions which do not require THF, such as methanol metabolism, phytanic acid oxidation, tryptophan catabolism, and cholesterol synthesis [164]. Although intermediates in 1C metabolism occur primarily within cells, formate is abundant in fetal plasma.
Linking MTOR and one-carbon metabolism
The MTORC1 cell signaling pathway can stimulate serinogenesis by increasing expression of ATF4 which increases expression of PHGDH, phosphoserine aminotransferase, phosphoserine phosphatase, and mitochondrial serine hydroxymethyltranferase (SHMT2) for synthesis of serine and then glycine and formate. Methylenetetrahydrofolate dehydrogenase 2 is a key enzyme in the mitochondrial tetrahydrofolate cycle in both embryonic and cancer cells [159]. The following scenario may allow metabolism of fructose by the ovine placenta: (1) hexokinase in the ovine placenta converts glucose to F-6-P; (2) F-6-P is metabolized via the hexosamine biosynthesis pathway to UDP-GlcNAc for glycylation of TSC2 to activate MTOR that stimulates proliferation of tropectoderm/chorion cells; (3) MTOR activates ATF4 and downstream genes for serinogenesis for synthesis of purines, thymidine and SAM; and (4) fructose is metabolized to 3-phosphoglycerate for synthesis of serine and glycine for 1 C metabolism [167–169]. Knowledge gleaned from understanding these pathways is essential to understanding common and differential pathways for metabolism of glucose and fructose in the placenta and embryos/fetuses to mitigate IUGR.
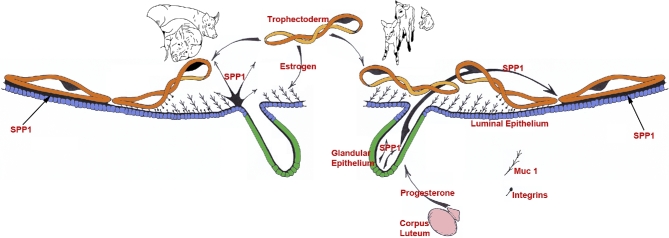
Secreted phosphoprotein 1: roles in implantation and placentation (Figure 3)
Figure 3.
Expression, regulation, and proposed functions of SPP1 produced by the uterine epithelia of pregnant pigs and sheep. Center to left: as porcine conceptuses (trophectoderm) elongate they secrete estrogens for pregnancy recognition. These estrogens also induce the synthesis and secretion of secreted phosphoprotein 1 (SPP1 or osteopontin [OPN]) from the uterine LE directly adjacent to the conceptus undergoing implantation. The implantation cascade is initiated when progesterone from CL downregulates Muc 1 on the surface of uterine LE. This exposes integrins on the uterine LE and conceptus trophectoderm cell surfaces for interaction with SPP1, and likely other ECM proteins, to mediate adhesion of conceptus trophectoderm to uterine LE for implantation. Center to right: as the lifespan of the CL is extended due to the actions of IFNT secretion from elongating ovine conceptuses they secrete progesterone. Progesterone then induces the synthesis and secretion of SPP1 from the uterine GE. The implantation cascade is initiated with downregulation of mucin 1 (Muc 1) (the regulatory mechanism remains to be identified) on the uterine LE surface to expose integrins on the LE and conceptus trophectoderm cell surfaces for interaction with SPP1 to mediate adhesion of conceptus trophectoderm to uterine LE for implantation.
The implantation cascade
Implantation and placentation are critical events in pregnancy. Implantation failure during the first 3 weeks of pregnancy is a major cause of infertility in all mammals [170,171]. Implantation begins when the apical surfaces of uterine LE and conceptus (embryo and associated placental membranes) trophectoderm cells adhere to each other [172–174]. The events of implantation can be characterized as an adhesion cascade and are highly synchronized, requiring reciprocal secretory, and physical interactions between a developmentally competent conceptus and the uterine endometrium during a restricted period of the uterine cycle termed the “window of receptivity.” These initial interactions between apical surfaces of uterine LE and conceptus Tr occur sequentially and include nonadhesive or precontact, apposition, and firm adhesion, and conclude with formation of a placenta that supports fetal–placental development throughout pregnancy [175,176]. The term “implantation” is somewhat of a misnomer for pigs and sheep; nevertheless, it refers to the initial stages of placentation in livestock species. Pigs demonstrate true epitheliochorial placentation in which there is no displacement or invasion of the maternal tissues and the conceptus remains within the uterine lumen throughout gestation [177]. In contrast to the pig, sheep demonstrate synepitheliochorial placentation in which limited fusion of conceptus trophectoderm with uterine LE occurs. Two morphologically and functionally distinct cell types, i.e., mononucleate trophectoderm cells and binucleate cells (BNCs), are present in the trophectoderm of ruminant placentae. BNCs migrate and fuse with individual uterine LE cells to form trinucleate syncytial cells, thereby assimilating the LE. The syncytia of sheep subsequently enlarge through continued BNC migration and fusion with uterine LE cells to form syncytial plaques [178].
A significant conceptual advance regarding the adhesion cascade of implantation was made by multiple research groups in the early 1990s. They reported the unique expression of integrins at the apical surface of uterine LE cells [179–181]. This advance was coupled with emerging reports that Mucin-1 (Muc-1), a cell-surface glycoprotein expressed at the apical surface of human and mouse uterine LE cells downregulated during implantation [182–184]. The emerging concept that conceptus attachment for implantation first requires loss of antiadhesive molecules in the glycocalyx of uterine LE, comprised largely of mucins, that sterically inhibits attachment was subsequently observed in other species. This results in “unmasking” of molecules, including selectins and galectins, which contribute to initial attachment of conceptus Tr to uterine LE. These low-affinity contacts are then replaced by a repertoire of adhesive interactions between integrins and maternal extracellular matrix (ECM) molecules which are the dominant contributors to stable adhesion at implantation. Motivated by this concept, studies to examine the implantation cascade using pigs and sheep as animal models were initiated at Texas A&M University. It was anticipated that significant advances in our understanding of the adhesion cascade of implantation could be made through exploitation of the process of conceptus elongation followed by the protracted and incremental attachment of conceptus Tr to uterine LE that is observed during implantation in livestock species. Between 1996 and 2001, it was established in pigs and sheep that Muc-1 expression is downregulated in the uterine LE, and multiple integrin receptors are expressed at the apical surfaces of conceptus Tr and uterine LE during implantation [185–189]. These integrins bind to the ECM molecule, secreted phosphoprotein 1 (SPP1) or osteopontin (OPN), and potentially other ECM proteins and molecules at the maternal conceptus interface.
SPP1 is an adhesion molecule well suited to mediate conceptus–uterine interactions during the peri-implantation period of pregnancy
SPP1 is an acidic ECM protein member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family of proteins that share a GRGDS amino acid sequence that binds to transmembrane integrins [190]. It is a multifunctional protein expressed by cells from a large variety of tissues and involved in many physiological and pathological processes. Notably, this protein in the SIBLING family has been independently identified as a protein associated with metastatic cancers (2ar), as an ECM protein of bones and teeth (OPN, SPP1), as a cytokine produced by activated lymphocytes and macrophages (early T-cell activation factor 1 [Eta-1]), and as a major constituent of the uterus and placenta during pregnancy [188,191–193]. SPP1 has been reported to (1) stimulate cell-cell adhesion; (2) increase cell-ECM communication; (3) promote cell migration; (4) decrease cell death; (5) stimulate immunoglobulin production; (6) induce changes in phosphorylation of focal adhesion (FA) kinase and paxillin; (7) stimulate phosphotidylinositol 3΄-kinase activity; (8) alter intracellular calcium levels; and (9) promote calcium phosphate deposition [189,194–197]. Uterine expression of OPN/2ar (SPP1) was first observed in the endometrial tissue of pregnant mice [198], and later in the uterine GE of women [199,200]. Its apical localization in the secretory epithelial cells and accumulation within the luminal spaces of multiple organs indicated that SPP1, secreted by epithelia including uterine epithelia, binds integrins on luminal surfaces to affect communication between the surface epithelium and the external environment [200]. It was first proposed that SPP1 and integrins may be involved in trophoblast-endometrial interactions during the initial attachment phase of implantation when it was noted that the αvβ3 and α4β1 integrin heterodimers, which bind to SPP1, are present at the apical surface of uterine LE cells during the window of implantation in women [201].
SPP1 is an adhesion molecule for implantation in pigs (reviewed in Johnson et al. [202]) (see Figure 3)
SPP1 expression in uterine LE increases markedly during the peri-implantation period of pigs, but is never observed in uterine LE during the estrous cycle. SPP1 mRNA is initially induced by conceptus estrogens in discrete regions of the LE juxtaposed to the conceptus just prior to implantation on Day 13, then expands to the entire LE by Day 20 when firm adhesion of conceptus trophectoderm to uterine LE occurs [203]. However, SPP1 mRNA is not present in pig conceptuses [202,204]. In contrast to mRNA, SPP1 protein is abundant along the apical surfaces of uterine LE and Tr/chorion, but only in areas of direct contact between the uterus and conceptus [202,204]. SPP1 levels remain high at this interface throughout pregnancy [204], as do multiple integrin subunits that potentially form heterodimeric receptors that bind OPN [174,185].
Affinity chromatography and immunoprecipitation experiments were performed to test whether αv, α4, α5, β1, β3, β5, and β6 expressed by porcine trophectoderm cells (pTr2) and porcine uterine LE cells (pUE), directly bind SPP1. Detergent extracts of surface-biotinylated pTr2 and pUE cells were incubated with SPP1-Sepharose and the proteins that bound to SPP1 were eluted with EDTA to chelate cations and release the bound integrins. To identify these integrins, immunoprecipitation assays were performed using antibodies that successfully immunoprecipitated integrin subunits from pig and sheep uterine epithelial cell lysates. SPP1 directly bound the αvβ6 integrin heterodimer on pig trophectoderm cells and αvβ3 on pig uterine LE cells [204]. SPP1 binding promoted dose- and integrin-dependent attachment of pig trophectoderm and pig uterine epithelial cells, and stimulated haptotactic migration of pig trophectoderm cells, i.e., cells migrated directionally along a physical gradient of immobilized SPP1 [205]. Further, immunofluorescence staining revealed that both SPP1 and αv integrin subunit localized to the apical surface of cells at the interface between uterine LE and conceptus trophectoderm on Day 20 of pregnancy. The αv integrin subunit staining pattern revealed large aggregates at the junction between Tr and uterine LE, suggesting the formation of SPP1-induced in vivo FAs at the apical surfaces of both conceptus trophectoderm and uterine LE that facilitate conceptus attachment to the uterus for implantation [205]. However, these FAs were no longer observed by Day 50 of gestation, suggesting that as implantation transitions into stable placentation, the endometrial epithelial-chorion bilayer develops complex folds to increase interdigitation of uterine and placental tissues, thereby providing a flexible, 3-dimensional (3D) structure that dissipates shear stress at the uterine-placental interface [206]. Without substantial physical forces to drive formation of FAs, there is an eventual loss of FAs at the uterine-placental interface. The β3 subunit appeared in aggregates on the apical surface of LE cells, but not trophectoderm cells, fitting with affinity chromatography data indicating direct binding of αvβ3 on pUE cells to SPP1 [205]. Finally, SPP1-coated microspheres revealed colocalization of the αv integrin subunit and talin (an essential mediator of integrin activation) to FAs at the apical domain of pTr2 cells in vitro [204], and knockdown of the αv integrin subunit in pTr2 cells by siRNA reduced pTr2 attachment to SPP1 [206]. Those results indicate that SPP1 binds integrins to stimulate substrate rigidity and cytoskeletal tension-induced FA assembly, attachment, and cytoskeletal force-driven migration of pTr2 cells to promote conceptus implantation in pigs.
SPP1 is an adhesion molecule for implantation in sheep (reviewed in Johnson et al. [202]) (see Figure 3)
The temporal and spatial expression of SPP1 in the uteri and placentae of sheep is similar to that previously described for the pig with two exceptions: (1) unlike in the pig, SPP1 is not expressed by uterine LE, but rather is expressed by uterine GE, and (2) although large SPP1-induced FAs assemble during the peri-implantation period of pigs, they are not observed at the uterine-placental interface until later stages of pregnancy in sheep.
In contrast to pigs, SPP1 is not synthesized by ovine uterine LE, but is nonetheless a component of histotroph due to secretion from the uterine GE into the uterine lumen of pregnant ewes as early as Day 13. It is not secreted by uterine GE of cyclic ewes [187,207], whereas SPP1 mRNA is detected in some uterine GE by Day 13, and is present in the majority of GE by Day 19 of gestation [207]. Progesterone induces expression of SPP1 in the uterine glands; and induction is associated with a loss of progesterone receptor in uterine GE. Analysis of uterine flushings from pregnant ewes has identified a 45-kDa fragment of SPP1 with greater binding affinity for αvβ3 integrin receptor than native 70 kDa SPP1 [188,207–209]. This 45 kDa fragment promotes greater cell attachment than the full-length 70-kDa protein [210]. Comparison of the spatial distribution of SPP1 mRNA and protein by in situ hybridization and immunofluorescence analyses of cyclic and pregnant ovine uterine sections has provided significant insight into the physiology of uterine SPP1 during pregnancy. SPP1 mRNA increases in the uterine GE during the peri-implantation period; however, it is not present in LE or conceptus Tr [207]. In contrast, the immunoreactive 45-kDa SPP1 protein is present at the apical surfaces of uterine LE and GE, and on conceptus Tr where the integrin subunits αv, α4, α5, β1, β3, and β5 could contribute to the assembly of several SPP1 receptors including αvβ3, αvβ1, αvβ5, α4β1, and α5β1 heterodimers which are expressed constitutively on the apical surfaces of conceptus Tr and uterine LE cells [187,188]. These results indicate that SPP1 is a component of histotroph secreted from uterine GE into the uterine lumen of pregnant ewes in response to progesterone, and that SPP1 binds integrin receptors expressed on uterine LE and conceptus Tr.
Affinity chromatography and immunoprecipitation experiments, similar to those described previously for pigs, determined whether αv, α4, α5, β1, β3, β5, and β6 integrins expressed by ovine Tr cells (oTr1) directly bind SPP1. Effective immunoprecipitation of labeled oTr1 integrins occurred with antibodies to αv and β3 integrin subunits, as well as an antibody to the integrin αvβ3 heterodimer. Antibody to the αv integrin subunit also precipitated a β chain, presumed to be the β3 integrin subunit, as an antibody to the β3 integrin subunit precipitated an α chain at the same relative size as the bands precipitated by an antibody to the αvβ3 heterodimer. Thus, the αvβ3 integrin on oTr1 cells binds SPP1 [211]. SPP1 binding to the αvβ3 integrin receptor induced in vitro FA assembly, a prerequisite for adhesion and migration of ovine trophectoderm cells, through activation of (1) P70S6K via crosstalk between FRAP1/MTOR and MAPK pathways; (2) MTOR, PI3K, MAPK3/MAPK1 (Erk1/2) and MAPK14 (p38) signaling to stimulate migration of ovine trophectoderm cells; and (3) FA assembly and myosin II motor activity to induce migration of ovine trophectoderm cells [211]. Collectively, those results indicate that SPP1 binds αvβ3 integrin receptor to activate cell signaling pathways that act in concert to mediate adhesion, migration, and cytoskeletal remodeling of conceptus trophectoderm cells essential for expansion and elongation of conceptuses and their attachment to uterine LE for implantation.
Focal adhesions, the hallmark of activated integrins, are prominent structures of cells grown in culture; however, they are rarely observed in vivo. It is noteworthy that large aggregations of FA-associated proteins that have been interpreted to be extraordinary in vivo FAs are present at the uterine-placental interface of sheep [212]. By Day 40 of pregnancy in sheep, the punctate apical surface staining of integrin receptor subunits identified in uterine LE and conceptus Tr is replaced by large aggregates of αv, α4, β1, and β5 subunits in interplacentomal uterine LE and conceptus Tr/chorion [188]. Integrin aggregates are detectable only in the gravid uterine horn of unilaterally pregnant sheep, demonstrating a requirement for attachment of conceptus Tr to uterine LE and for integrin aggregates to increase in number and size through Day 120 of pregnancy. In some regions of the interplacentomal interface, greater subunit aggregation was seen on the uterine side; in other regions it was predominant on the placental side; whereas in some others, both uterine and placental epithelia exhibited prominent FAs. However, by Day 120 of pregnancy, extensive FAs were seen along most of the uterine-placental interface [212]. The placentomes, which provide hematotrophic support to the fetus and placenta, exhibited diffuse immunoreactivity for these integrins compared with interplacentomal regions possibly due to extensive folding at this interplacentomal interface and the 3D nature of the ECM within placentomes. It is noteworthy that interplacentomal endometrial stroma, only within the gravid horns of unilaterally pregnant sheep, also exhibited robust but punctate immunostaining αv and β3 integrins and ECM proteins including the native 70 kDa (rather than the 45-kDa fragment of SPP1), fibronectin, vitronectin and several other members of the SIBLING family (unpublished results) beginning around Day 40 of pregnancy and increasing through Day 120. Stromal cells in this same tissue compartment of the gravid horn also exhibited upregulation of smooth muscle actin, desmin, and vimentin indicative of myofibroblast differentiation. These stromal/myofibroblasts are surrounded by a connective tissue matrix that is more strain shielded due to crosslinking of ECM in three dimensions compared to the complex forces focused at maternal conceptus interface [212]. These results indicate that FA assembly at the uterine-placental interface and within placentomes and stromal compartments reflects dynamic adaptation to increasing forces caused by the growing conceptus. Cooperative binding of multiple integrins to SPP1 deposited at the uterine-placental interface forms a strong adhesive mosaic to maintain a tight connection and increased tensile strength and signaling activity between uterine and placental surfaces along regions of epitheliochorial placentation in sheep.
Summary
This review of research at Texas A&M University highlights the value of excellent collaborations among the coauthors of this paper in the conduct of research to advance understanding of the roles of IFNT, SPP1, CSH2, GH1, amino acids, and glucose in mechanisms central to the establishment of pregnancy and the maintenance of a successful pregnancy. Ongoing research continues to clarify the roles of IFNT related to pregnancy in ruminants, but also its potential as a therapeutic molecule for treatment of inflammatory diseases such as obesity and diabetes.
Conflict of Interest: The authors have declared that no conflict of interest exists.
Footnotes
Grant Support: Funding for the research described in this review was supported by grants from (1) USDA (91-37203-6548, 92-02755, 91-37203-6548, 98-01983, 00-32503-9137, 2001-35203-02166); (2) USDA-CSREES NRI CGP (2006-00863, 2008-00547, 2016-67015-24958, 2015-06857); (3) USDA CSREES AFRI (2009-01722, 2011-67015-20028, 2014-67015-21770, 2015-67015-23276; (4) NIH (HD32534) and (5) BARD Grant OEP 9604563. The authors thank current and former graduate students and postdoctoral fellows who worked with us at Texas A&M University between 1992 and 2017.
References
- 1. Schramm W, Bovaird L, Glew ME, Schramm G, McCracken JA. Corpus luteum regression induced by ultra-low pulses of prostaglandin F2 alpha. Prostaglandins 1983; 26:347–364. [DOI] [PubMed] [Google Scholar]
- 2. Ott TL, Zhou Y, Mirando MA, Stevens C, Harney JP, Ogle TF, Bazer FW. Changes in progesterone and oestrogen receptor mRNA and protein during maternal recognition of pregnancy and luteolysis in ewes. J Mol Endocrinol 1993; 10:171–183. [DOI] [PubMed] [Google Scholar]
- 3. Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod 1995; 53:1527–1543. [DOI] [PubMed] [Google Scholar]
- 4. Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod 2010; 16:135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fincher KB, Bazer FW, Hansen PJ, Thatcher WW, Roberts RM. Proteins secreted by the sheep conceptus suppress induction of uterine prostaglandin F2a release by oestradiol and oxytocin. Reproduction 1986; 76:425–433. [DOI] [PubMed] [Google Scholar]
- 6. Vallet JL, Gross TS, Fliss MFV, Bazer FW. Effects of pregnancy, oxytocin, ovine trophoblast protein-1 and their interactions on endometrial production of prostaglandin F2α in vitro in perifusion chambers. Prostaglandins 1989; 38:113–124. [DOI] [PubMed] [Google Scholar]
- 7. Vallet JL, Bazer FW, Fliss MF, Thatcher WW. The effect of ovine conceptus secretory proteins and purified ovine trophoblast protein-one on interestrous interval and plasma concentrations of prostaglandins F2α, E and 13,14-dihydro-15-keto-prostaglandin F2α in cyclic ewes. Reproduction 1988; 84:493–504. [DOI] [PubMed] [Google Scholar]
- 8. Mirando MA, Ott TL, Harney JP, Bazer FW. Ovine trophoblast protein-one inhibits development of endometrial responsiveness to oxytocin in ewes. Biol Reprod 1990; 43:1070–1078. [DOI] [PubMed] [Google Scholar]
- 9. Ott TL, Van Heeke G, Hostetler CE, Schalue TK, Olmsted JJ, Johnson HM, Bazer FW. Intrauterine injection of recombinant ovine interferon-tau extends the interestrous interval in sheep. Theriogenology 1993; 40:757–769. [DOI] [PubMed] [Google Scholar]
- 10. Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW. Ovine Interferon-τ regulates expression of endometrial receptors for estrogen and oxytocin, but not progesterone. Biol Reprod 1995; 53:732–745. [DOI] [PubMed] [Google Scholar]
- 11. Spencer TE, Ing NH, Ott TL, Mayes JS, Becker WS, Watson GH, Mirando MA, Bazer FW. Intrauterine injection of ovine interferon-τ (IFN-τ) alters oestrogen and oxytocin receptor expression in the endometrium of cyclic ewes. J Mol Endocrinol 1995; 15:203–220. [DOI] [PubMed] [Google Scholar]
- 12. Spencer TE, Mirando MA, Ogle TF, Bazer FW. Ovine interferon-tau inhibits estrogen receptor up-regulation and estrogen-induced luteolysis in cyclic ewes. Endocrinology 1995; 136:4932–4944. [DOI] [PubMed] [Google Scholar]
- 13. Spencer TE, Bazer FW. Ovine interferon tau suppresses transcription of the estrogen receptor and oxytocin receptor genes in the ovine endometrium. Endocrinology 1996; 137:1144–1147. [DOI] [PubMed] [Google Scholar]
- 14. Spencer TE, Ott TL, Bazer FW. Expression of interferon regulatory factors one and two in the ovine endometrium: Effects of pregnancy and ovine interferon tau. Biol Reprod 1998; 58:1154–1162. [DOI] [PubMed] [Google Scholar]
- 15. Choi Y, Johnson GA, Burghardt RC, Berghman LR, Joyce MM, Taylor KM, Stewart MD, Bazer FW, Spencer TE. Interferon regulatory factor two restricts expression of interferon-stimulated genes to the endometrial stroma and glandular epithelium of the ovine uterus. Biol Reprod 2001; 65:1038–1049. [DOI] [PubMed] [Google Scholar]
- 16. Fleming JGW, Bazer FW, Johnson GA, Choi YS, Spencer TE. Cloning of the ovine estrogen receptor alpha gene and regulation by interferon tau. Endocrinology 2001;142:2879–2887. [DOI] [PubMed] [Google Scholar]
- 17. Fleming JGW, Spencer TE, Safe SH, Bazer FW. The ovine uterine oxytocin receptor gene: Regulation of expression by estradiol and role of interferon tau signaling for establishment of pregnancy in ruminants. Endocrinology 2006; 147:899–911.16254027 [Google Scholar]
- 18. Newton GR, Ott TL, Woldesenbet S, Shelton AH, Bazer FW. Biochemical and immunological properties of related small ruminant trophoblast interferons. Theriogenology 1996; 46:703–716. [DOI] [PubMed] [Google Scholar]
- 19. Knickerbocker JJ, Thatcher WW, Bazer FW, Drost M, Barron DH, Fincher KB, Roberts RM. Proteins secreted by Day-16 to -18 bovine conceptuses extend corpus luteum function in cows. Reproduction 1986; 77:381–391. [DOI] [PubMed] [Google Scholar]
- 20. Helmer SD, Hansen PJ, Anthony RV, Thatcher WW, Bazer FW, Roberts RM. Identification of bovine trophoblast protein-1, a secretory protein immunologically related to ovine trophoblast protein-1. Reproduction 1987; 79:83–91. [DOI] [PubMed] [Google Scholar]
- 21. Helmer SD, Hansen PJ, Thatcher WW, Johnson JW, Bazer FW. Intrauterine infusion of highly enriched bovine trophoblast protein-1 complex exerts an antiluteolytic effect to extend corpus luteum lifespan in cyclic cattle. Reproduction 1989; 87:89–101. [DOI] [PubMed] [Google Scholar]
- 22. Thatcher WW, Hansen PJ, Gross TS, Helmer SD, Plante C, Bazer FW. Antiluteolytic effects of bovine trophoblast protein-1. J Reprod Fertil 1989; 37:91–99. [PubMed] [Google Scholar]
- 23. Meyer MD, Drost M, Ott TL, Bazer FW, Badinga L, Li J, Roberts RM, Hansen PJ, Thatcher WW. Recombinant bovine and ovine interferon tau extend corpus luteum lifespan and reduce uterine secretion of prostaglandin F2α in cattle. J Dairy Sci 1995; 78:1921–1931. [DOI] [PubMed] [Google Scholar]
- 24. Tysseling KA, Thatcher WW, Bazer FW, Hansen PJ, Mirando MA. Mechanisms regulating prostaglandin F2α secretion from bovine endometrium. J Dairy Sci 1998; 81:382–389. [DOI] [PubMed] [Google Scholar]
- 25. Chen C, Spencer TE, Bazer FW. Fibroblast Growth Factor-10: A Stromal Mediator of Epithelial Functionin the Ovine Uterus. Biol Reprod 2000; 63:959–966. [DOI] [PubMed] [Google Scholar]
- 26. Chen C, Spencer TE, Bazer FW. Expression of hepatocyte growth factor and its receptor c-met in the ovine uterus. Biol Reprod 2000; 62:1844–1850. [DOI] [PubMed] [Google Scholar]
- 27. Satterfield MC, Hayashi K, Song G, Black SG, Bazer FW, Spencer TE. Progesterone regulates FGF10, MET, IGFBP1, and IGFBP3 in the endometrium of the ovine uterus. Biol Reprod 2008; 79:1226–1236. [DOI] [PubMed] [Google Scholar]
- 28. Ka H, Spencer TE, Johnson GA, Bazer FW. Keratinocyte growth factor: Expression by endometrial epithelia of the porcine uterus. Biol Reprod 2000; 62:1772–1778. [DOI] [PubMed] [Google Scholar]
- 29. Ka H, Jaeger LA, Johnson GA, Spencer TE, Bazer FW. Keratinocyte growth factor in up-regulated in the porcine uterine endometrium and functions in trophectoderm cell proliferation and differentiation. Endocrinology 2001; 142:2303–2310. [DOI] [PubMed] [Google Scholar]
- 30. Ka H, Al-Ramadan S, Johnson GA, Jaeger LA, Burghardt RC, Spencer TE, Bazer FW. Regulation of fibroblast growth factor-7 expression by progesterone and estradiol-17β and their receptors in the porcine endometrium. Biol Reprod 2007; 77:172–180. [DOI] [PubMed] [Google Scholar]
- 31. Spencer TE, Ott TL, Gertler A, Gootwine E, Bazer FW. Effects of recombinant ovine interferon tau, placental lactogen and growth hormone on the ovine uterus. Biol Reprod 1999; 61:1409–1418. [DOI] [PubMed] [Google Scholar]
- 32. Stewart DM, Johnson GA, Gray CA, Schuler LA, Burghardt RC, Joyce MM, Bazer FW, Spencer TE. Prolactin receptor and uterine milk protein expression in the ovine uterus during the estrous cycle and early pregnancy. Biol Reprod 2000; 62:1779–1789. [DOI] [PubMed] [Google Scholar]
- 33. Stewart DM, Johnson GA, Vyhlidal CA, Burghardt RC, Safe SH, Yu-Lee LY, Bazer FW, Spencer TE. Interferon-tau activates multiple signal transducer and activator of transcription proteins and has complex effects on interferon-responsive gene transcription in ovine endometrial epithelial cells. Endocrinology 2001; 142:98–107 [DOI] [PubMed] [Google Scholar]
- 34. Stewart D, Johnson GA, Vyhlidal CA, Burghardt RC, Safe SH, Yu-Lee L-y, Bazer FW, Spencer TE. Interferon tau activates multiple STAT proteins and has complex effects on interferon-responsive gene transcription in ovine endometrial epithelial cells. Endocrinology 2001; 142:1786–1794. [DOI] [PubMed] [Google Scholar]
- 35. Noel S, Herman A, Johnson GA, Gray CA, Stewart MD, Bazer FW, Gertler A, Spencer TE. Ovine placental lactogen specifically binds to endometrial glands of the ovine uterus. Biol Reprod 2003; 68:772–780. [DOI] [PubMed] [Google Scholar]
- 36. Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Fetal-maternal interactions during the establishment of pregnancy in ruminants. Reproduction 2007; 64:379–396. [DOI] [PubMed] [Google Scholar]
- 37. Martin C, Pessemesse L, de la Llosa-Hermier MP, Martal J, Djiane J, Charlier M. Interferon-{tau} upregulates prolactin receptor mRNA in the ovine endometrium during the peri-implantation period. Reproduction 2004; 128:99–105. [DOI] [PubMed] [Google Scholar]
- 38. Imakawa K, Anthony RV, Kazemi M, Marotti KR, Polites HG, Roberts RM. Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature 1987; 330:377–379. [DOI] [PubMed] [Google Scholar]
- 39. Roberts RM. Interferon-tau. Nature 1993; 362:583–583. [DOI] [PubMed] [Google Scholar]
- 40. Pontzer CH, Torres BA, Vallet JL, Bazer FW, Johnson HM. Antiviral activity of the pregnancy recognition hormone ovine trophoblast protein-1. Biochem Biophys Res Commun 1988; 152:801–807. [DOI] [PubMed] [Google Scholar]
- 41. Pontzer CH, Ott TL, Bazer FW, Johnson HM. Localization of an antiviral site on the pregnancy recognition hormone, ovine trophoblast protein 1. Proc Natl Acad Sci 1990; 87:5945–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pontzer CH, Bazer FW, Johnson HM. Antiproliferative activity of a pregnancy recognition hormone, ovine trophoblast protein-1. Cancer Res 1991; 51:19–26. [PubMed] [Google Scholar]
- 43. Jarpe MA, Pontzer CH, Ott TL, Bazer FW, Johnson HM. Predicted structural motif of interferon tau. Protein Eng Des Sel 1994; 7:863–867. [DOI] [PubMed] [Google Scholar]
- 44. Pontzer CH, Ott TL, Bazer FW, Johnson HM. Structure/function studies with interferon tau: evidence for multiple active sites. J Interferon Res 1994; 14:133–141. [DOI] [PubMed] [Google Scholar]
- 45. Ott TL, Heeke GV, Johnson HM, Bazer FW. Cloning and expression in S. cerevisiae of a synthetic gene for the pregnancy recognition hormone ovine trophoblast protein-1: Purification and antiviral activity. J Interferon Res 1991; 11:357–364. [DOI] [PubMed] [Google Scholar]
- 46. VanHeeke G, Ott TL, Strauss A, Ammaturo D, Bazer FW. High yield expression and secretion of the pregnancy recognition hormone ovine interferon-τ by Pichia pastoris. J Interferon Res 1996; 16:119–126. [DOI] [PubMed] [Google Scholar]
- 47. Bazer FW, Spencer TE, Johnson GA. Interferons and uterine receptivity. Semin Reprod Med 2009; 27:090–102. [DOI] [PubMed] [Google Scholar]
- 48. Hansen TR, Austin KJ, Perry DJ, Pru JK, Teixeira MG, Johnson GA. Mechanism of action of interferon-tau in the uterus during early pregnancy. J Reprod Fertil Suppl 1999; 54:329–339. [PubMed] [Google Scholar]
- 49. Hansen TR, Henkes LK, Ashley RL, Bott RC, Antoniazzi AQ, Han H. Endocrine actions of interferon-tau in ruminants. Soc Reprod Fertil Suppl 2010; 67:325–340. [DOI] [PubMed] [Google Scholar]
- 50. Spencer TE, Sandra O, Wolf E. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction 2008; 135:165–179. [DOI] [PubMed] [Google Scholar]
- 51. Hansen TR, Kazemi M, Keisler DH, Malathy PV, Imakawa K, Roberts RM. Complex binding of the embryonic interferon, ovine trophoblast protein-1, to endometrial receptors. J Interferon Res 1989; 9:215–225. [DOI] [PubMed] [Google Scholar]
- 52. Rosenfeld CS, Han CS, Alexenko AP, Spencer TE, Roberts RM. Expression of interferon receptor subunits, IFNAR1 and IFNAR2, in the ovine uterus. Biol Reprod 2002; 67:847–853. [DOI] [PubMed] [Google Scholar]
- 53. Wang XL, Wang K, Han GC, Zeng SM. A potential autocrine role for interferon tau in ovine trophectoderm. Reprod Dom Anim 2013; 48:819–825. [DOI] [PubMed] [Google Scholar]
- 54. Brooks K, Spencer TE. Biological roles of interferon tau (IFNT) and Type I IFN receptors in elongation of the ovine conceptus. Biol Reprod 2015; 92(2):47. [DOI] [PubMed] [Google Scholar]
- 55. Banerjee P, Fazleabas AT. Extragonadal actions of chorionic gonadotropin. Rev Endocr Metab Disord 2011; 12:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spencer TE, Hansen TR. Implantation and establishment of pregnancy in ruminants. Adv Anat Embryol Cell Biol 2015; 216:105–135. [DOI] [PubMed] [Google Scholar]
- 57. Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Fetal-maternal interactions during the establishment of pregnancy in ruminants. Soc Reprod Fertil Suppl 2007; 64:379–396. [DOI] [PubMed] [Google Scholar]
- 58. Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, Okumu LA, McGettigan PA, Mehta JP, McBride R, O’Gaora P, Roche JF et al. Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod 2011; 85:144–156. [DOI] [PubMed] [Google Scholar]
- 59. Bauersachs S, Ulbrich SE, Reichenbach HD, Reichenbach M, Buttner M, Meyer HH, Spencer TE, Minten M, Sax G, Winter G, Wolf E. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod 2012; 86:46. [DOI] [PubMed] [Google Scholar]
- 60. Forde N, Carter F, Fair T, Crowe MA, Evans AC, Spencer TE, Bazer FW, McBride R, Boland MP, O’Gaora P, Lonergan P, Roche JF. Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol Reprod 2009; 81:784–794. [DOI] [PubMed] [Google Scholar]
- 61. Forde N, Lonergan P. Transcriptomic analysis of the bovine endometrium: What is required to establish uterine receptivity to implantation in cattle? J Reprod Dev 2012; 58:189–195. [DOI] [PubMed] [Google Scholar]
- 62. Bauersachs S, Ulbrich SE, Gross K, Schmidt SE, Meyer HH, Wenigerkind H, Vermehren M, Sinowatz F, Blum H, Wolf E. Embryo-induced transcriptome changes in bovine endometrium reveal species-specific and common molecular markers of uterine receptivity. Reproduction 2006; 132:319–331. [DOI] [PubMed] [Google Scholar]
- 63. Cerri RL, Thompson IM, Kim IH, Ealy AD, Hansen PJ, Staples CR, Li JL, Santos JE, Thatcher WW. Effects of lactation and pregnancy on gene expression of endometrium of Holstein cows at day 17 of the estrous cycle or pregnancy. J Dairy Sci 2012; 95:5657–5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mansouri-Attia N, Aubert J, Reinaud P, Giraud-Delville C, Taghouti G, Galio L, Everts RE, Degrelle S, Richard C, Hue I, Yang X, Tian XC et al. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol Genomics 2009; 39:14–27. [DOI] [PubMed] [Google Scholar]
- 65. Biase FH, Rabel C, Guillomot M, Hue I, Andropolis K, Olmstead CA, Oliveira R, Wallace R, Le Bourhis D, Richard C, Campion E, Chaulot-Talmon A et al. Massive dysregulation of genes involved in cell signaling and placental development in cloned cattle conceptus and maternal endometrium. Proc Natl Acad Sci USA 2016; 113:14492–14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 2014; 32:513–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Johnson GA, Spencer TE, Hansen TR, Austin KJ, Burghardt RC, Bazer FW. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol Reprod 1999; 61:312–318. [DOI] [PubMed] [Google Scholar]
- 68. Johnson GA, Spencer TE, Burghardt RC, Joyce MM, Bazer FW. Interferon-tau and progesterone regulate ubiquitin cross-reactive protein expression in the ovine uterus. Biol Reprod 2000; 62:622–627. [DOI] [PubMed] [Google Scholar]
- 69. Johnson GA, Austin KJ, Collins AM, Murdoch WJ, Hansen TR. Endometrial ISG17 mRNA and a related mRNA are induced by interferon-tau and localized to glandular epithelial and stromal cells from pregnant cows. Endocrine 1999; 10:243–252. [DOI] [PubMed] [Google Scholar]
- 70. Austin KJ, Carr AL, Pru JK, Hearne CE, George EL, Belden EL, Hansen TR. Localization of ISG15 and conjugated proteins in bovine endometrium using immunohistochemistry and electron microscopy. Endocrinology 2004; 145:967–975. [DOI] [PubMed] [Google Scholar]
- 71. Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem 1998; 67:227–264. [DOI] [PubMed] [Google Scholar]
- 72. Choi Y, Johnson GA, Burghardt RC, Berghman LR, Joyce MM, Taylor KM, Stewart MD, Bazer FW, Spencer TE. Interferon regulatory factor-two restricts expression of interferon-stimulated genes to the endometrial stroma and glandular epithelium of the ovine uterus. Biol Reprod 2001; 65:1038–1049. [DOI] [PubMed] [Google Scholar]
- 73. Choi Y, Johnson GA, Spencer TE, Bazer FW. Pregnancy and interferon tau regulate MHC class I and beta-2-microglobulin expression in the ovine uterus. Biol Reprod 2003; 68:1703–1710. [DOI] [PubMed] [Google Scholar]
- 74. Johnson GA, Stewart MD, Gray CA, Choi Y, Burghardt RC, Yu-Lee LY, Bazer FW, Spencer TE. Effects of the estrous cycle, pregnancy, and interferon tau on 2',5'-oligoadenylate synthetase expression in the ovine uterus. Biol Reprod 2001; 64:1392–1399. [DOI] [PubMed] [Google Scholar]
- 75. Song G, Bazer FW, Spencer TE. Pregnancy and interferon tau regulate RSAD2 and IFIH1 expression in the ovine uterus. Reproduction 2007; 133:285–295. [DOI] [PubMed] [Google Scholar]
- 76. Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. Pregnancy and interferon tau regulate RSAD2 and IFIH1 expression in the ovine uterus. Annu Rev Immunol 2001; 19:623–655. [DOI] [PubMed] [Google Scholar]
- 77. Spencer TE, Ott TL, Bazer FW. Expression of interferon regulatory factors one and two in the ovine endometrium: effects of pregnancy and ovine interferon tau. Biol Reprod 1998; 58:1154–1162. [DOI] [PubMed] [Google Scholar]
- 78. Dorniak P, Bazer FW, Spencer TE. Biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci 2012; 91:1627–1638. [DOI] [PubMed] [Google Scholar]
- 79. Nagaoka K, Nojima H, Watanabe F, Chang KT, Christenson RK, Sakai S, Imakawa K. Regulation of blastocyst migration, apposition, and initial adhesion by a chemokine, interferon gamma-inducible protein 10 kDa (IP-10), during early gestation. J Biol Chem 2003; 278:29048–29056. [DOI] [PubMed] [Google Scholar]
- 80. Nagaoka K, Sakai A, Nojima H, Suda Y, Yokomizo Y, Imakawa K, Sakai S, Christenson RK. A chemokine, interferon (IFN)-gamma-inducible protein 10 kDa, is stimulated by IFN-tau and recruits immune cells in the ovine endometrium. Biol Reprod 2003; 68:1413–1421. [DOI] [PubMed] [Google Scholar]
- 81. Ashley RL, Antoniazzi AQ, Anthony RV, Hansen TR. The chemokine receptor CXCR4 and its ligand CXCL12 are activated during implantation and placentation in sheep. Reprod Biol Endocrinol 2011; 9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sakumoto R, Hayashi KG, Fujii S, Kanahara H, Hosoe M, Furusawa T, Kizaki K. Possible roles of CC- and CXC-chemokines in regulating bovine endometrial function during early pregnancy. Int J Mol Sci 2017; 18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brooks K, Spencer TE. Biological roles of interferon tau (IFNT) and type I IFN receptors in elongation of the ovine conceptus. Biol Reprod 2015; 92:47. [DOI] [PubMed] [Google Scholar]
- 84. Dorniak P, Welsh TH Jr., Bazer FW, Spencer TE. Cortisol and interferon tau regulation of endometrial function and conceptus development in female sheep. Endocrinology 2013; 154:931–941. [DOI] [PubMed] [Google Scholar]
- 85. Song G, Spencer TE, Bazer FW. Cathepsins in the ovine uterus: regulation by pregnancy, progesterone, and interferon tau. Endocrinology 2005; 146:4825–4833. [DOI] [PubMed] [Google Scholar]
- 86. Kim S, Choi Y, Bazer FW, Spencer TE. Identification of genes in the ovine endometrium regulated by interferon tau independent of signal transducer and activator of transcription. Endocrinology 2003; 144:5203–5214. [DOI] [PubMed] [Google Scholar]
- 87. Gray CA, Abbey CA, Beremand PD, Choi Y, Farmer JL, Adelson DL, Thomas TL, Bazer FW, Spencer TE. Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol Reprod 2006; 74:383–394. [DOI] [PubMed] [Google Scholar]
- 88. Song G, Spencer TE, Bazer FW. Progesterone and interferon tau regulate cystatin C (CST3) in the endometrium. Endocrinology 2006; 147:3478–3483. [DOI] [PubMed] [Google Scholar]
- 89. Satterfield MC, Bazer FW, Spencer TE. Progesterone regulation of preimplantation conceptus growth and galectin 15 (LGALS15) in the ovine uterus. Biol Reprod 2006; 75:289–296. [DOI] [PubMed] [Google Scholar]
- 90. Spencer TE, Burghardt RC, Johnson GA, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Anim Reprod Sci 2004; 82–83:537–550. [DOI] [PubMed] [Google Scholar]
- 91. Bazer FW, Spencer TE, Johnson GA, Burghardt RC. Uterine receptivity to implantation of blastocysts in mammals. Front Biosci 2011; 3:745–767. [DOI] [PubMed] [Google Scholar]
- 92. Bazer FW, Kim J, Ka H, Johnson GA, Wu G, Song G. Select nutrients in the uterine lumen of sheep and pigs affect conceptus development. J Reprod Dev 2012; 58:180–188. [DOI] [PubMed] [Google Scholar]
- 93. Dorniak P, Bazer FW, Spencer TE. Physiology and endocrinology symposium: Biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci 2013; 91:1627–1638. [DOI] [PubMed] [Google Scholar]
- 94. Wang X, Frank JW, Little DR, Dunlap KA, Satterfield MC, Burghardt RC, Hansen TR, Wu G, Bazer FW. Functional role of arginine during the peri-implantation period of pregnancy. I. Consequences of loss of function of arginine transporter SLC7A1 mRNA in ovine conceptus trophectoderm. FASEB J 2014; 28:2852–2863. [DOI] [PubMed] [Google Scholar]
- 95. Brooks K, Burns G, Spencer TE. Biological roles of hydroxysteroid (11-Beta) dehydrogenase 1 (HSD11B1), HSD11B2, and glucocorticoid receptor (NR3C1) in sheep conceptus elongation. Biol Reprod 2015; 93(2):38. [DOI] [PubMed] [Google Scholar]
- 96. Forde N, Duffy GB, McGettigan PA, Browne JA, Mehta JP, Kelly AK, Mansouri-Attia N, Sandra O, Loftus BJ, Crowe MA, Fair T, Roche JF et al. Evidence for an early endometrial response to pregnancy in cattle: both dependent upon and independent of interferon tau. Physiol Genomics 2012; 44:799–810. [DOI] [PubMed] [Google Scholar]
- 97. Bauersachs S, Wolf E. Uterine responses to the preattachment embryo in domestic ungulates: recognition of pregnancy and preparation for implantation. Annu Rev Anim Biosci 2015; 3:489–511. [DOI] [PubMed] [Google Scholar]
- 98. Lonergan P, Forde N. Maternal-embryo interaction leading up to the initiation of implantation of pregnancy in cattle. Animal 2014; 8(s1):64–69. [DOI] [PubMed] [Google Scholar]
- 99. Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005; 5:375–386. [DOI] [PubMed] [Google Scholar]
- 100. Banu SK, Lee J, Stephen SD, Nithy TK, Arosh JA. Interferon tau regulates PGF2alpha release from the ovine endometrial epithelial cells via activation of novel JAK/EGFR/ERK/EGR-1 pathways. Mol Endocrinol 2010; 24:2315–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lee J, Banu SK, Nithy TK, Stanley JA, Arosh JA. Early pregnancy induced expression of prostaglandin E2 receptors EP2 and EP4 in the ovine endometrium and regulated by interferon tau through multiple cell signaling pathways. Mol Cell Endocrinol 2012; 348:211–223. [DOI] [PubMed] [Google Scholar]
- 102. Dorniak P, Bazer FW, Spencer TE. Prostaglandins regulate conceptus elongation and mediate effects of interferon tau on the ovine uterine endometrium. Biol Reprod 2011; 84:1119–1127. [DOI] [PubMed] [Google Scholar]
- 103. Dorniak P, Welsh TH Jr, Bazer FW, Spencer TE. Endometrial HSD11B1 and cortisol regeneration in the ovine uterus: effects of pregnancy, interferon tau, and prostaglandins. Biol Reprod 2012; 86:124. [DOI] [PubMed] [Google Scholar]
- 104. Brooks K, Burns G, Spencer TE. Biological roles of hydroxysteroid (11-Beta) dehydrogenase 1 (HSD11B1), HSD11B2, and glucocorticoid receptor (NR3C1) in Sheep conceptus elongation. Biol Reprod 2015; 93:38. [DOI] [PubMed] [Google Scholar]
- 105. Gao HJ, Wu G, Spencer TE, Johnson GA, Li X, Bazer FW. Select nutrients in the ovine uterine lumen: I. Amino acids, glucose and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod 2009; 80:86–93 [DOI] [PubMed] [Google Scholar]
- 106. Kwon H, Spencer TE, Bazer FW, Wu G. Developmental changes of amino acids in ovine fetal fluids. Biol Reprod 2003; 68:1813–1820. [DOI] [PubMed] [Google Scholar]
- 107. Wu G, Bazer FW, Tuo W, Flynn SP. Unusual abundance of arginine and ornithine in porcine allantoic fluid. Biol Reprod 1996; 54:1261–1265. [DOI] [PubMed] [Google Scholar]
- 108. Wu ZL, Hou YQ, Hu SD, Bazer FW, Meininger CJ, McNeal CJ, Wu G. Catabolism and safety of supplemental L-arginine in animals. Amino Acids 2016; 48:1541–1552. [DOI] [PubMed] [Google Scholar]
- 109. Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009; 37:153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bazer FW, Johnson GA, Wu G. Amino acids and conceptus development during the peri-implantation period of pregnancy. Adv Exp Med Biol 2015; 843:23–52. [DOI] [PubMed] [Google Scholar]
- 111. Kim JY, Burghardt RC, Wu G, Johnson GA, Spencer TE, Bazer FW. Select nutrients in the ovine uterine lumen: IX. Differential effects of arginine, leucine, glutamine and glucose on interferon tau, orinithine decarboxylase and nitric oxide synthase in the ovine conceptus. Biol Reprod 2011; 84:1139–1147. [DOI] [PubMed] [Google Scholar]
- 112. Hou YQ, Yao K, Yin YL, Wu G. Endogenous synthesis of amino acids limits growth, lactation, and reproduction in animals. Adv Nutr 2016; 7:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE. Impacts of amino acid nutrition on pregnancy outcome in pigs: Mechanisms and implications for swine production. J Anim Sci 2010; 88: E195–E204. [DOI] [PubMed] [Google Scholar]
- 114. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 1998; 336:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dekaney CM, Wu G, Jaeger LA. Gene expression and activity of enzymes in the arginine biosynthetic pathway in porcine fetal small intestine. Pediatr Res 2003; 53:274–280. [DOI] [PubMed] [Google Scholar]
- 116. Hou YQ, Yin YL, Wu G. Dietary essentiality of “nutritionally nonessential amino acids” for animals and humans. Exp Biol Med (Maywood) 2015; 240:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wu G, Bazer FW, Cudd TA, Jobgen WS, Kim SW, Lassala A, Li P, Matis JH, Meininger CJ, Spencer TE. Pharmacokinetics and safety of arginine supplementation in animals. J Nutr 2007; 137:1673S–1680S. [DOI] [PubMed] [Google Scholar]
- 118. Wang XQ, Frank JW, Little DR, Dunlap KA, Satterfiled MC, Burghardt RC, Hansen TR, Wu G, Bazer FW. Functional role of arginine during the peri-implantation period of pregnancy. I. Consequences of loss of function of arginine transporter SLC7A1 mRNA in ovine conceptus trophectoderm. FASEB J 2014; 28:2852–2863. [DOI] [PubMed] [Google Scholar]
- 119. Devés R, Boyd CA. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev 1998; 78:487–545. [DOI] [PubMed] [Google Scholar]
- 120. Gao HJ, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen: III. Expression of cationic amino acid transporters in ovine uterus and peri-implantation conceptuses. Biol Reprod 2009; 80:602–609. [DOI] [PubMed] [Google Scholar]
- 121. Van Winkle LJ, Christensen HN, Campione AL. Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J Biol Chem 1985; 260:12118–12123. [PubMed] [Google Scholar]
- 122. Van Winkle LJ, Campione AL, Gorman JM. Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J Biol Chem 1988; 263:3150–3163. [PubMed] [Google Scholar]
- 123. Gao KG, Jiang ZY, Lin YC, Zheng C, Zhou G, Chen F, Yang L, Wu G. Dietary L-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 2012; 42:2207–2214. [DOI] [PubMed] [Google Scholar]
- 124. Gao HJ, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen: V. Nitric oxide synthase, GTP cyclohydrolase and ornithine decarboxylase in ovine uteri and peri-implantation conceptuses. Biol Reprod 2009; 81:67–76. [DOI] [PubMed] [Google Scholar]
- 125. Wang XQ, Burghardt RC, Romero JJ, Hansen TR, Wu G, Bazer FW. Functional roles of arginine during the peri-implantation period of pregnancy. III. Arginine stimulates proliferation and interferon tau production by ovine trophectoderm cells via nitric oxide and polyamine-TSC2-MTOR signaling pathways. Biol Reprod 2015; 75:1–17. [DOI] [PubMed] [Google Scholar]
- 126. Lefèvre PL, Palin MF, Chen G, Turecki G, Murphy BD. Polyamines are implicated in the emergence of the embryo from obligate diapause. Endocrinology 2011; 152:1627–1639. [DOI] [PubMed] [Google Scholar]
- 127. Wang XQ, Ying W, Dunlap KA, Lin G, Satterfield MC, Burghardt RC, Wu G, Bazer FW. Arginine decarboxylase and agmatinase: An alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod 2014; 84:1–15. [DOI] [PubMed] [Google Scholar]
- 128. Mateo RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J Nutr 2007; 137:652–656. [DOI] [PubMed] [Google Scholar]
- 129. Bérard J, Bee G. Effects of dietary L-arginine supplementation to gilts during early gestation on foetal survival, growth and myofiber formation. Animal 2010; 4:1680–1687. [DOI] [PubMed] [Google Scholar]
- 130. Dallanora D, Marcon J, Walter MP, Biondo N, Bernardi ML, Wentz I, Bortolozzo FP. Effect of dietary amino acid supplementation during gestation on placental efficiency and litter birth weight in gestating gilts. Livestock Sci 2017; 197:30–35. [Google Scholar]
- 131. Wu G, Bazer FW, Johnson GA, Herring C, Seo H, Dai ZL, Wang JJ, Wu ZL, Wang XL. Functional amino acids in the development of the pig placenta. Mol Reprod Dev 2017; 84:870–882. [DOI] [PubMed] [Google Scholar]
- 132. Luther JS, Windorski EJ, Schauer CS, Kirsch JD. Impacts of L-arginine on ovarian function and reproductive performance in ewes. J Anim Sci 2008; 86(E-Suppl. 2):ii [Google Scholar]
- 133. Lassala A, Bazer FW, Cudd TA, Datta S, Keisler DH, Satterfield MC, Spencer TE, Wu G. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J Nutr 2010; 140:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Satterfield MC, Dunlap KA, Keisler DH, Bazer FW, Wu G. Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino Acids 2013; 45:489–499. [DOI] [PubMed] [Google Scholar]
- 135. Satterfield MC, Dunlap KA, Keisler DH, Bazer FW, Wu G. Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids 2012; 43:1593–1603. [DOI] [PubMed] [Google Scholar]
- 136. Lassala A, Bazer FW, Cudd TA, Datta S, Keisler DH, Satterfield MC, Spencer TE, Wu G. Parenteral administration of L-Arginine enhances fetal survival and growth in sheep carrying multiple fetuses. J Nutr 2011; 141:849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gao H, Wu G, Spencer TE, Johnson GA, Li X, Bazer FW. Select nutrients in the ovine uterine lumen: I. Amino acids, glucose and ions in uterine lumenal fluid of cyclic and pregnant ewes. Biol Reprod 2009; 80:86–93. [DOI] [PubMed] [Google Scholar]
- 138. Kim J, Song G, Wu G, Bazer FW. Functional roles of fructose. Proc Natl Acad Sci USA 2012; 109:E1619–E1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Père MC. Maternal and fetal blood levels of glucose, lactate, fructose, and insulin in the conscious pig. J Anim Sci 1995;73:2994–2999. [DOI] [PubMed] [Google Scholar]
- 140. White CE, Piper EL, Noland PR, Daniels LB. Fructose utilization for nucleic acid synthesis in the fetal pig. J Anim Sci 1982; 55:73–76. [DOI] [PubMed] [Google Scholar]
- 141. Steinhauser CB, Landers M, Myatt L, Burghardt RC, Vallet JL, Bazer FW, Johnson GA. Fructose synthesis and transport at the uterine-placental interface of pigs: cell-specific localization of SLC2A5, SLC2A8, and components of the polyol pathway. Biol Reprod 2016; 95:108–108. [DOI] [PubMed] [Google Scholar]
- 142. Jauniaux E, Hempstock J, Teng C, Battaglia FC, Burton GJ. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: maintenance of redox potential in a low oxygen environment. J Clin Endocrinol Metab 2005; 90:1171–1175. [DOI] [PubMed] [Google Scholar]
- 143. Brusati V, Józwik M, Józwik M, Teng C, Paolini C, Marconi AM, Battaglia FC. Fetal and maternal non-glucose carbohydrates and polyols concentrations in normal human pregnancies at term. Pediatr Res 2005; 58:700–704. [DOI] [PubMed] [Google Scholar]
- 144. Rama F, Castellano MA, Germino NI, Micucci M, Ohanian C. Histochemical location of ketose-reductase in the placenta and fetal tissues. J Anat 1973;114:109–113. [PMC free article] [PubMed] [Google Scholar]
- 145. Debosch BJ, Chen Z, Saben JL, Finck BN, Moley KH. Glucose transporter 8 (GLUT8) mediates fructose-induced de novo lipogenesis and macrosteatosis. J Biol Chem 2014; 289:10989–10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kim ST, Moley KH. Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium. Biol Reprod 2009; 81:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Frolova AI, Moley KH. Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology 2011; 152:2123–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Mandava N, Quiroz-Mercado H. Current knowledge and trends in age-related macular degeneration. Retina 2013; 33:1487–1502. [DOI] [PubMed] [Google Scholar]
- 149. Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, Tanabe K, Andres-Hernando A, Orlicky DJ, Finol E, Inaba S, Li N et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol 2014; 25:2526–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Park TJ, Reznick J, Peterson BL, Blass G, Omerbašić D, Bennett NC, Kuich PHJL, Zasada C, Browe BM, Hamann W, Applegate DT, Radke MH et al. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 2017; 356:307–311. [DOI] [PubMed] [Google Scholar]
- 151. West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985; 228:1324–1326. [DOI] [PubMed] [Google Scholar]
- 152. Ashworth CJ, Fliss MF, Bazer FW. Evidence for steroid control of a putative angiogenic factor in the porcine uterus. J Endocrinol 1990;125:15–19. [DOI] [PubMed] [Google Scholar]
- 153. Ponting JM, Kumar S. Isolation and characterisation of a hyaluronan binding protein, hyaluronectin, from human placenta and its colocalisation with hyaluronan. J Anat 1995; 186:131–142. [PMC free article] [PubMed] [Google Scholar]
- 154. Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton's Jelly form neurons and glia. Stem Cells 2003; 21:50–60. [DOI] [PubMed] [Google Scholar]
- 155. Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's Jelly of the human umbilical cord. Stem Cells 2004; 22:1330–1337. [DOI] [PubMed] [Google Scholar]
- 156. Bazer FW, Spencer TE, Thatcher WW. Growth and development of the ovine conceptus. J Anim Sci 2012; 90:159–170. [DOI] [PubMed] [Google Scholar]
- 157. Wang X, Li D, Wu G, Bazer FW. Functional roles of fructose: crosstalk between O-linked glycosylation and phosphorylation of Akt-TSC2-MTOR cell signaling cascade in ovine trophectoderm cells. Biol Reprod 2016; 95(5):102–102. [DOI] [PubMed] [Google Scholar]
- 158. Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, Barrett FG et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 2011; 476:346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 2016; 351: 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Washburn SE, Caudill MA, Malysheva O, MacFarlane AJ, Behan NA, Harnett B, MacMillan L, Pongnopparat T, Brosnan JT, Brosnan ME. Formate metabolism in fetal and neonatal sheep. Am J Physiol Endocrinol Metab 2015; 308:E921–E927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Brosnan ME, Brosnan JT. The role of dietary creatine. Amino Acids 201648:1785–1791. [DOI] [PubMed] [Google Scholar]
- 162. French JB, Jones SA, Deng H, Pedley AM, Kim D, Chan CY, Hu H, Pugh RL, Zhao H, Zhang Y, Huang TJ, Fang Y et al. Spatial colocalization and functional link of purinosomes with mitochondria. Science 2016; 351:733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Howe CG, Niedzwiecki MM, Hall MN, Liu X, Ilievski V, Slavkovich V, Alam S, Siddique AB, Graziano JH, Gamble MV. Folate and cobalamin modify associations between S-adenosylmethionine and methylated arsenic metabolites in arsenic-exposed Bangladeshi adults. J Nutr 2014; 144:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lamarre SG, Morrow G, Macmillan L, Brosnan ME, Brosnan JT. Formate: an essential metabolite, a biomarker, or more? Clin Chem Lab Med 2013; 51:571–578. [DOI] [PubMed] [Google Scholar]
- 165. Shields DC, Kirke PN, Mills JL, Ramsbottom D, Molloy AM, Burke H, Weir DG, Scott JM, Whitehead AS. The “thermolabile” variant of methylenetetrahydrofolate reductase and neural tube defects: an evaluation of genetic risk and the relative importance of the genotypes of the embryo and the mother. Am J Hum Genet 1999; 64:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pike ST, Rajendra R, Artzt K, Appling DR. Mitochondrial C1-tetrahydrofolate synthase (MTHFD1L) supports the flow of mitochondrial one-carbon units into the methyl cycle in embryos. J Biol Chem 2010; 29:4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Krebs HA, Hems R, Tyler B. The regulation of folate and methionine metabolism. Biochem J 1976; 158:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Butkinaree C, Park K, Hart GW. O-linked beta-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta 2010;1800:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Varma V, Boros LG, Nolen GT, Chang CW, Wabitsch M, Beger RD, Kaput J. Fructose alters intermediary metabolism of glucose in human adipocytes and diverts glucose to serine oxidation in the one-carbon cycle energy producing pathway. Metabolites 2015; 5:364–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Flint APF, Saunders PTK, Ziecik AJ. Blastocyst-endometrium interactions and their significance in embryonic mortality. In: Cole DJA, Foxcroft GR (eds.), Control of Pig Reproduction. London: Butterworth Scientific; 1982:253–275. [Google Scholar]
- 171. Jainudeen MR, Hafez ESE. Reproductive failure in females. In: Hafez ESE. (ed.), Reproduction in Farm Animals. Philadelphia: Lea and Febiger; 1987:399–422. [Google Scholar]
- 172. Denker HW. Implantation: a cell biological paradox. J Exp Zool 1993; 26: 6541–6558. [DOI] [PubMed] [Google Scholar]
- 173. Aplin JD. Adhesion molecules in implantation. Rev Reprod 1997; 2:84–93. [DOI] [PubMed] [Google Scholar]
- 174. Burghardt RC, Johnson GA, Jaeger LA, Ka H, Garlow JE, Spencer TE, Bazer FW. Integrins and extracellular matrix proteins at the maternal-fetal interface in domestic animals. Cells Tissues Organs 2002; 172:202–217. [DOI] [PubMed] [Google Scholar]
- 175. Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science 1994; 266:1508–1518. [DOI] [PubMed] [Google Scholar]
- 176. Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol 2000; 223:217–237. [DOI] [PubMed] [Google Scholar]
- 177. Dantzer V. Electron microscopy of the initial stages of placentation in the pig. Anat Embryol 1985; 172:281–293. [DOI] [PubMed] [Google Scholar]
- 178. Wooding FB. Role of binucleate cells in fetomaternal cell fusion at implantation in the sheep. Am J Anat 1984; 170(2):233–250. [DOI] [PubMed] [Google Scholar]
- 179. Lessey Ba, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest 1992; 90:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tabibzadeh S. Patterns of expression of integrin molecules in human endometrium throughout the menstrual cycle. Hum Reprod 1992; 7:876–882. [DOI] [PubMed] [Google Scholar]
- 181. Klentzeris LD, Bulmer JN, Tredosiewicz LK, Morrison L, Cooke ID. Infertility: Beta-1 integrin cell adhesion molecules in the endometrium of fertile and infertile women. Hum Reprod 1993; 8:1223–1230. [DOI] [PubMed] [Google Scholar]
- 182. Braga VMM, Gendler SJ. Modulation of Muc-1 mucin expression in the mouse uterus during the estrus cycle, early pregnancy and placentation. J Cell Sci 1993; 105:397–405. [DOI] [PubMed] [Google Scholar]
- 183. Aplin JD, Seif MW, Graham RA, Hey NA, Behzad F, Campbell S. The endometrial cell surface and implantation. Expression of the polymorphic mucin MUC-1 and adhesion molecules during the endometrial cycle. Ann NY Acad Sci 1994; 30:103–121. [DOI] [PubMed] [Google Scholar]
- 184. Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Day SK, Carson DD. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 1995; 136:3639–3647. [DOI] [PubMed] [Google Scholar]
- 185. Bowen JA, Bazer FW, Burghardt RC. Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod 1996; 55:1098–1106. [DOI] [PubMed] [Google Scholar]
- 186. Burghardt RC, Bowen JA, Newton GR, Bazer FW. Extracellular matrix and the implantation cascade in pigs. J Reprod Fertil Suppl 1997; 52:151–164. [PubMed] [Google Scholar]
- 187. Johnson GA, Burghardt RC, Spencer TE, Newton GR, Ott TL, Bazer FW. Ovine osteopontin II. Osteopontin and alpha(v)beta(3) integrin expression in the uterus and conceptus during the peri-implantation period. Biol Reprod 1999; 61:892–899. [DOI] [PubMed] [Google Scholar]
- 188. Johnson GA, Bazer FW, Jaeger LA, Ka H, Garlow JE, Pfarrer C, Spencer TE, Burghardt RC. Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol Reprod 2001; 65:820–828. [DOI] [PubMed] [Google Scholar]
- 189. Johnson GA, Burghardt RC, Bazer FW, Spencer TE. Osteopontin: roles in implantation and placentation. Biol Reprod 2003; 69:1458–1471. [DOI] [PubMed] [Google Scholar]
- 190. Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun 2001; 280:460–465. [DOI] [PubMed] [Google Scholar]
- 191. Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 1979; 16:885–893. [DOI] [PubMed] [Google Scholar]
- 192. Franzen A, Heinegard D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J 1985; 232:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Patarca R, Freeman GJ, Singh RP, Wei FY, Durfee T, Blattner F, Ragnier DC, Kozak CA, Mock BA, Morse HC III, Jerrells TR, Cantor H. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med 1989; 170:145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. FASEB J 1993; 7:1475–1482. [PubMed] [Google Scholar]
- 195. Butler WT, Ridall AL, McKee MD. Osteopontin. In: Bilezikian JP, Raisz LG, Rodan GA (eds). Principles of Bone Biology. New York: Academic Press; 1996:167–181. [Google Scholar]
- 196. Weber GF, Cantor H. The immunology of Eta-1/osteopontin. Cytokine Growth Factor Rev 1996; 7:241–248. [DOI] [PubMed] [Google Scholar]
- 197. Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med 2000; 11:279–303. [DOI] [PubMed] [Google Scholar]
- 198. Nomura S, Wills AJ, Edwards DR, Heath JK, Hogan BLM. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol 1988; 106:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Young MF, Kerr JM, Termine JD, Wewer UM, Wang MG, McBride OW, Fisher LW. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics 1990; 7:491–502. [DOI] [PubMed] [Google Scholar]
- 200. Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, Dvorak HF, Senger DR. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell 1992; 3:1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril 1994; 62:497–506. [PubMed] [Google Scholar]
- 202. Johnson GA, Burghardt RC, Bazer FW. Osteopontin: A leading candidate adhesion molecule for implantation in pigs and sheep. J Anim Sci Biotechnol 2014; 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. White FJ, Ross JW, Joyce MM, Geisert RD, Burghardt RC, Johnson GA. Steroid regulation of cell specific secreted phosphoprotein 1 (osteopontin) expression in the pregnant porcine uterus. Biol Reprod 2005; 73:1294–1301. [DOI] [PubMed] [Google Scholar]
- 204. Garlow JE, Ka H, Johnson GA, Burghardt RC, Jaeger LA, Bazer FW. Analysis of osteopontin at the maternal-placental interface in pigs. Biol Reprod 2002; 66:718–725. [DOI] [PubMed] [Google Scholar]
- 205. Erikson DW, Burghardt RC, Bayless KJ, Johnson GA. Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alphavbeta6 on porcine trophectoderm cells and integrin alphavbeta3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration. Biol Reprod 2009; 81:814–825. [DOI] [PubMed] [Google Scholar]
- 206. Frank JW, Seo H, Burghardt RC, Bayless KJ, Johnson GA. ITGAV (alpha v integrins) bind SPP1 (osteopontin) to support trophoblast cell adhesion. Reproduction 2017; 153:695–706. [DOI] [PubMed] [Google Scholar]
- 207. Johnson GA, Spencer TE, Burghardt RC, Bazer FW. Ovine osteopontin. I. Cloning and expression of mRNA in the uterus during the peri-implantation period. Biol Reprod 1999; 61:884–891. [DOI] [PubMed] [Google Scholar]
- 208. Johnson GA, Spencer TE, Burghardt RC, Taylor KM, Gray CA, Bazer FW. Progesterone modulation of osteopontin gene expression in the ovine uterus. Biol Reprod 2000; 62:1315–1321. [DOI] [PubMed] [Google Scholar]
- 209. Senger DR, Perruzzi CA. Cell migration promoted by a potent GRGDS-containing thrombin-cleavage fragment of osteopontin. Biochim Biophys Acta 1996; 1314:13–24. [DOI] [PubMed] [Google Scholar]
- 210. Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 2001; 276:28261–28267. [DOI] [PubMed] [Google Scholar]
- 211. Kim J, Erikson DW, Burghardt RC, Spencer TE, Wu G, Bayless KJ, Johnson GA, Bazer FW. Secreted phosphoprotein 1 binds integrins to initiate multiple cell signaling pathways, including FRAP1/mTOR, to support attachment and force-generated migration of trophectoderm cells. Matrix Biol 2010; 29:369–382. [DOI] [PubMed] [Google Scholar]
- 212. Burghardt RC, Burghardt JR, Taylor JD II, Reeder AT, Nguyen BT, Spencer TE, Johnson GA. Enhanced focal adhesion assembly reflects increased mechanosensation and mechanotransduction at maternal-conceptus interface and uterine wall during ovine pregnancy. Reproduction 2009; 137:567–582. [DOI] [PubMed] [Google Scholar]
- 213. Wu G, Bazer FW, Satterfield MC, Li X, Wang X, Johnson GA, Burghardt RC, Dai Z, Wang J, Wu Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 2013; 45:241–256. [DOI] [PubMed] [Google Scholar]