Abstract
Emerging technologies are now giving us unprecedented access to manipulate brain circuits, shedding new light on treatments for amblyopia. This research is identifying key circuit elements that control brain plasticity and highlight potential therapeutic targets to promote rewiring in the visual system during and beyond early life. Here, we explore how such recent advancements may guide future pharmacological, genetic, and behavioral approaches to treat amblyopia. We will discuss how animal research, which allows us to probe and tap into the underlying circuit and synaptic mechanisms, should best be used to guide therapeutic strategies. Uncovering cellular and molecular pathways that can be safely targeted to promote recovery may pave the way for effective new amblyopia treatments across the lifespan.
Keywords: Critical period, Light deprivation, Monocular deprivation, Neuromodulatory systems, Environmental enrichment, Dark exposure, E/I balance
New molecular/pharmacological and genetic approaches
Novel experimental approaches in neuroscience have recently identified promising molecular, pharmacological, and genetic avenues for amblyopia therapy. The common goal of these therapies is to harness the brain’s inherent ability to restructure itself by tapping into specific brain circuits and cellular mechanisms that promote plasticity. Such targets include, for example, excitatory and inhibitory synaptic components, neuromodulators, and epigenetic regulators. Commonly used pharmacological agents, transcranial direct current and magnetic stimulation (tDCS/TMS), and behavioral therapies may act through these or other cellular pathways yet to be discovered. Understanding the precise cellular mechanisms that promote circuit changes in experimental animals promises to guide new therapeutic approaches for treating amblyopia in humans.
Targeting inhibitory and excitatory synapses
Monocular visual deprivation during a critical period in early life remodels excitatory synapses extensively, inducing a rapid loss of dendritic spines and elimination of many axonal branches of geniculocortical afferents serving the deprived eye (Antonini & Stryker, 1993; Mataga et al., 2004). These losses of input are followed by a progressive expansion of axons and potentiation of responses from the open eye (Antonini et al., 1999; Frenkel & Bear, 2004). Because these modifications are assumed to underlie the development of amblyopia, excitatory synapses represent strong candidate targets for its treatment. Indeed, recent reports have revealed that changes in the levels of the excitatory postsynaptic density protein PSD-95 govern the duration of the critical period for ocular dominance (OD) plasticity in the visual cortex, independent of changes in inhibitory circuits (Huang et al., 2015). PSD-95 expression increases in the visual cortex during the critical period for OD plasticity and promotes the progressive maturation of the so-called “silent” synapses that contain only NMDA-type glutamate receptors and that lack AMPA receptors. Genetic loss of PSD-95 function leads to the persistence of silent synapses, allowing the juvenile form of OD plasticity to be maintained lifelong. Strikingly, using a viral gene silencing approach to reduce PSD-95 in the visual cortex of adult mice rejuvenates excitatory synapses by reinstating silent synapses like those in the immature cortex and reopens a critical period for visual cortical plasticity (Huang et al., 2015).
Converging studies also point to intracortical inhibitory synapses as key regulatory sites of critical period plasticity (reviewed in Takesian & Hensch, 2013; see also Hensch & Quinlan, this volume). Reducing the inhibitory synapse function by intracortical microperfusion of a GABA synthesis inhibitor or GABAA receptor antagonist can enhance plasticity in the rodent visual cortex during adulthood (Harauzov et al., 2010). However, this manipulation does not produce plasticity like that in the critical period, where responses in the deprived eye are dramatically reduced. Instead, it accelerates or enhances the adult form of plasticity seen in rodents, which increases the response to the open fellow eye with little or no effect on the deprived-eye responses. In contrast, transplantation of specific types of embryonic inhibitory neurons into a postnatal visual cortex creates a second critical period of OD plasticity that follows the end of the normal one and is of similar duration (Southwell et al., 2010; Tang et al., 2014). The most prominent feature of this second critical period is the reduction of deprived-eye responses, exactly as in the normal critical period. Future work is needed to elucidate how the interplay of the excitatory and inhibitory synaptic function across cortical cell types may control cortical network plasticity.
Targeting neuromodulatory systems
Evidence has accumulated that neuromodulatory systems are also key targets for inducing plasticity to improve amblyopia. Neuromodulators such as serotonin and acetylcholine are released in the visual cortex from projections arising from the raphe nuclei and basal forebrain. These inputs are normally activated by salient stimuli and specific behavioral states, such as reward acquisition, punishment, and exercise (Fu et al., 2014; Hangya et al., 2015). However, these neuromodulatory systems can also be pharmacologically targeted by drugs commonly used to treat depression, such as selective serotonin reuptake inhibitors (SSRIs), or Alzheimer’s disease, such as cholinesterase inhibitors. Interestingly, it has been found that these pharmacological agents promote recovery from amblyopia in rodent models. For example, chronic treatment with SSRIs to enhance serotonergic signaling reopens a period of plasticity in the visual cortex of adult amblyopic rats, allowing for the recovery of visual acuity (Maya Vetencourt et al., 2008). Likewise, boosting acetylcholine signaling with a cholinesterase inhibitor enables recovery from amblyopia in the adult visual cortex (Morishita et al., 2010). How do neuromodulators act within visual cortical circuits? Recent studies have uncovered a specific set of cortical inhibitory neurons that respond robustly to neuromodulators to enhance cortical plasticity (Letzkus et al., 2011; Pi et al., 2013; Fu et al., 2014; Fu et al., 2015). These GABAergic cells reside in the outermost layers of the cortex and are identified by the selective expression of the vasoactive intestinal peptide (VIP). Optogenetic activation of VIP cells directly drives plasticity in the primary visual cortex of the adult mouse (Fu et al., 2015). VIP cells are thought to augment cortical activity and plasticity through inhibition of other cortical GABAergic interneurons (Letzkus et al., 2011; Pfeffer et al., 2013; Pi et al., 2013; Donato et al., 2013; Fu et al., 2014; Fu et al., 2015). Other cell types in the visual cortex may also have a role in the plasticity induced by neuromodulators in adult mice. Pairing acetylcholine release in the visual cortex with specific visual stimuli enhances stimulus-selective responses of the cortical neurons, by engaging astrocyte-dependent strengthening of excitatory synapses (Chen et al., 2012).
Ongoing clinical trials with drugs targeting these neuromodulatory systems highlight this approach as a promising avenue for amblyopia treatment in adult patient populations. SSRI treatment has been shown to augment visually-evoked potentials (VEPs) in normal human subjects (Normann et al., 2007). In a few adult patients with amblyopia, SSRI (citalopram) enhanced visual acuity improvements when combined with two weeks of occlusion therapy, but effects in the population were not significantly different from placebo. (Thompson et al., 2014). Another study pairing SSRIs with video game training demonstrated that while video games improved visual acuity, no added value of the SSRI treatment was observed (Uusitalo, 2013). It is possible that such behavioral and pharmacological manipulations reach a ceiling effect if they engage similar neuromodulatory pathways. Likewise, an ongoing clinical study at Boston Children’s Hospital is using donepezil, a cholinesterase inhibitor that is typically used to treat Alzheimer’s disease, to boost cholinergic signaling and recover vision in amblyopic patients (T. Hensch, personal communication).
Targeting epigenetic regulation using histone deacetylase (HDAC) inhibitors
Brain circuits respond to environmental signals via dynamic changes in DNA methylation and histone modifications that control gene transcription (Fagiolini et al., 2009). Visual stimulation during early life induces histone acetylation in the mouse visual cortex, but the same stimulation in adulthood has little effect. This age-related decline in the capacity for experience-dependent regulation of histone acetylation makes it a candidate to underlie the developmental reduction in visual cortical plasticity (Putignano et al., 2007). In fact, increasing histone acetylation by inhibition of HDACs can reinstate plasticity in the adult visual cortex of rodents to allow recovery from amblyopia (Putignano et al., 2007; Silingardi et al., 2010). Thus, HDAC inhibitors may represent yet another class of drugs with the potential to improve visual acuity beyond early life.
The HDAC inhibitor valproate (VPA) has already been found in a clinical study to reopen a period of plasticity to learn absolute pitch (Gervain et al., 2013). Absolute pitch, the ability to produce or identify a musical pitch without a reference sound, is possessed by only about 0.01% of the general population and acquired during a critical period in early life. Generally, absolute pitch is learned through musical training before 6 years of age and is rarely, or perhaps never, acquired during adulthood (Van Hedger et al., 2015). However, administration of VPA, a commonly used mood stabilizer, opened a window of opportunity for adults to learn absolute pitch. These findings suggest that epigenetic actions of VPA may reset cortical circuitry to allow for juvenile-like plasticity. Future work will be required to reveal the cellular and circuit mechanisms underlying HDAC inhibitors such as VPA.
Transcranial direct current stimulation (tDCS)/transcranial magnetic stimulation (TMS)
Pharmacological interventions aimed to stimulate cortical plasticity pose risks of side effects. Therefore, there is a great deal of interest in discovering novel and less invasive alternatives to activate endogenous plasticity mechanisms. One promising strategy is to use transcranial direct current or magnetic stimulation, noninvasive brain stimulation techniques that can transiently alter neural excitability in targeted brain regions. Ongoing work is attempting to exploit this technique in adult patients with amblyopia to open a brief window of opportunity to improve visual function (B. Thompson, personal communication). A recent study found that a single session of tDCS can temporarily increase VEPs and contrast sensitivity driven by amblyopic eyes of adult patients (Ding et al., 2016), paving the way for future studies that will combine this stimulation technique with visual training for long-term improvements. Intriguingly, tDCS may increase excitability by a reduction in GABAergic inhibition (Stagg et al., 2009), a mechanism known to regulate adult visual cortical plasticity. It should be mentioned, however, that these current/magnetic stimulation protocols may have a problem of pathway specificity. It is, therefore, essential that measures be taken to ensure that changes are restricted to target circuits.
Combining pharmacological interventions and behavioral training
Across both human and animal studies, it is evident that pharmacological intervention alone is not generally sufficient for successful treatment of amblyopia. Instead, the research strongly supports the need to combine a pharmacological approach with personalized behavioral training, with the goal of targeting plasticity within specific brain regions or specific cortical circuits. Interestingly, VPA treatment improved absolute pitch, but not other measures of auditory function (T. Hensch, personal communication), suggesting that VPA may not induce widespread effects, but may instead induce focal plasticity in response to targeted training paradigms. In the mouse visual system, recovery of closed-eye responses following long-term monocular deprivation is preferentially enhanced to the particular visual stimuli presented during VIP cell activation induced by running (Kaneko & Stryker, 2014). Can these pharmacological approaches be used to shorten or enhance behavioral treatment? The next section will discuss promising behavioral treatments that could be used alone or in combination with pharmacological approaches.
Environmental and behavioral treatments
In an attempt to find novel noninvasive methods of stimulating visual plasticity in adulthood, a number of behavioral interventions have emerged that may help to treat amblyopia in humans. These include manipulations of the environment, such as exposure to an enriched environment, or complete visual deprivation, both of which reactivate robust plasticity in the visual cortex. Similarly, engaging humans and animals in voluntary physical exercise (e.g., running wheels) and visuomotor tasks has led to remarkable increases in neuronal plasticity. Finally, novel vision training paradigms may result in more practical, less expensive therapies, and faster recovery. Here, we highlight some of these novel treatments and discuss their success in both animals and humans.
Environmental enrichment (EE)/running
It is now evident that increased levels of environmental stimulation have a profound impact on experience-dependent plasticity within both the developing and adult brain. Experimental animals are generally raised in small cages with only their littermates, strongly limiting social interactions and physical exercise. Recent studies have found significant effects of environmentally-enriched cages in which the animals are housed in large groups, have access to running wheels, and are exposed to a complex environment that elicits social and exploratory behaviors. Notably, adult amblyopic rats housed in enriched environments recovered from long-term monocular deprivation (Sale et al., 2007). Moreover, raising mice in an enriched environment extended the critical period for a juvenile form of OD plasticity into adulthood (Greifzu et al., 2014) and allowed an adult form of OD plasticity to persist even throughout life (Greifzu et al., 2016). Remarkably, placing standard-cage-reared mice into an enriched environment as adults restored OD plasticity, apparently rejuvenating the visual cortex (Greifzu et al., 2014; Greifzu et al., 2016). These effects of EE on plasticity seem largely to be due to the reduction of GABAergic inhibition to juvenile levels (Greifzu et al., 2014) accompanied by decreased perineuronal nets in the visual cortex (Sale et al., 2007). These studies highlight EE as a noninvasive means of harnessing known plasticity mechanisms (reduced intracortical inhibition) to promote visual recovery. Surprisingly, the use of just one of the components of EE has recently been shown to preserve plasticity to older ages, namely a running wheel allows adult mice raised in standard cages to express OD-plasticity into adulthood (Kalogeraki et al., 2014). Furthermore, even short-term running, just during the 7-day monocular deprivation period, restored OD-plasticity to adult standard cage-raised mice. Notably, it is important to distinguish the extension into adult life or the enhancement of the effects of deprivation (e.g., MD) from the enhancement of recovery of the visual function, because the underlying mechanisms may be different.
However, the human environment is generally much more ‘enriched’ than that of any experimental animal, raising the question of whether EE in rodents may be translated into a treatment protocol for humans. Social interactions, novelty, exercise, and engagement of the visuomotor systems are all components of EE that may engage distinct brain regions, circuits, and cellular mechanisms. Researchers using animal models or human subjects should strive toward identifying the key aspects of an EE that can be implemented to promote plasticity.
Dark exposure
It has long been known that dark rearing from birth retains the visual cortex in an immature state and prolongs the critical period for OD plasticity (e.g., Cynader, 1983; Mower et al., 1985; Fox et al., 1991). Recent work has shown that periods of total darkness that eliminate all visually-driven activity can reactivate robust plasticity in the adult visual cortex of rats (He et al., 2006; He et al., 2007) and mice (Stodieck et al., 2014), and in juvenile kittens (Duffy & Mitchell, 2013). The ability to promote synaptic plasticity in the adult visual cortex through dark exposure was predicted by the BCM sliding threshold theory of synaptic plasticity, which predicts that the loss of patterned visual experience would lower the value of the synaptic modification threshold and enable recovery of weakened deprived-eye inputs (Cooper & Bear, 2012). Indeed, dark exposure in adulthood stimulates the expression of the NMDA receptor, a molecular switch known to lower the threshold for synaptic modification (Yashiro et al., 2005; He et al., 2006; Philpot et al., 2007).
The plasticity that is reactivated by dark exposure can be harnessed to promote the recovery from amblyopia. For example, rats rendered amblyopic by chronic monocular deprivation initiated at eye opening recovered visual acuity when 10 days of dark exposure in young adulthood (P70–100) were followed by binocular vision or reverse occlusion (He et al., 2007). Remarkably, short-term dark exposure (10 days) can reinstate visual cortical plasticity even in very old mice (P535; Stodieck et al., 2014). However, the effects of dark exposure on adult plasticity may vary across species; while 10 days of darkness enhanced OD plasticity in juvenile kittens, this treatment failed to restore OD plasticity in adult cats (Duffy et al., 2016). The recovery from amblyopia following dark exposure was rapid, occurring in kittens within just a week after removal from the darkness (Duffy & Mitchell, 2013). Importantly, repetitive performance of a visual task following dark exposure further improved the recovery of acuity, but delaying the visual stimulation for several weeks following the dark exposure prevented the recovery of visual acuity (Eaton et al., 2016). This finding suggests that a period of darkness opens a limited window of plasticity during which the cortex is more receptive to visual training and may guide the design of new therapies.
It may be puzzling that manipulations that on the surface appear to be very different—putting animals either in complete darkness or in an enriched environment—both stimulate robust plasticity in the adult cortex. It is possible that distinct neural mechanisms lead to a common outcome, possibly through a common substrate. A reduction in inhibitory synaptic transmission by dark exposure may be a feature in common with EE. As with environmental enrichment (Greifzu et al., 2014), dark exposure may work through a rejuvenation of intracortical inhibition, including a reduction of the excitatory drive onto fast-spiking interneurons (Huang et al., 2010; Gu et al., 2016) and a decrease in the number of parvalbumin-expressing inhibitory cells and surrounding peri-neuronal nets (Stodieck et al., 2014). Indeed, the adult recovery from long-term monocular deprivation can be stimulated by a reduction in the activity of inhibitory neurons (Kaneko & Stryker, 2014). Dark exposure also reduces neurofilament protein levels, which is hypothesized to destabilize the neuronal cytoskeleton (Duffy & Mitchell, 2013), and promotes the recovery of thalamocortical synaptic transmission and the density of dendritic spines on pyramidal neurons in the primary visual cortex (Montey & Quinlan, 2011).
An exciting pilot study is now evaluating whether adult amblyopic humans will also recover visual function following a brief period in complete darkness (B. Backus & E. Quinlan, personal communication). Preliminary results suggest that 5 days of darkness is well tolerated, with no reports of anxiety or changes in physical health. An ongoing study is now evaluating the effects of 10 days of darkness on adult amblyopic patients. To exploit the transient period of plasticity immediately following darkness, the patients will undergo intensive visual training in the weeks following the dark exposure. The practicality of using this protocol in humans as a treatment for amblyopia must be addressed, as studies in rodents and kittens suggested that shorter periods of dark exposure are ineffective, and even brief periods of light exposure prevented the effects (He et al., 2007; Mitchell et al., 2016). Thus, access to completely dark environments for a sufficient duration of time with proper support to guarantee safety and well-being may pose challenges for widespread use. Interestingly, visual deprivation for several days by binocular intravitreal injections of the pufferfish toxin tetrodotoxin promotes similar fast visual recovery in amblyopic cats (Fong et al., 2016), raising the possibility of developing novel pharmacological techniques to transiently block vision, if adequate safety measures can reliably be ensured. Blindfolding may offer another solution—one study suggested that blindfolding normally sighted adults for 5 days leads to rapid changes in experience-dependent functional neural connectivity (Merabet et al., 2008). However, it has been shown in amblyopic kittens that binocular visual deprivation by lid-suture does not recapitulate the recovery-promoting effects of darkness (Duffy et al., 2015). If it is established that 10 days of complete darkness promotes visual recovery in amblyopic humans, future studies in both humans and animal models may identify methods to shorten, segment, or facilitate binocular occlusion. A further understanding of the neural mechanisms underlying dark exposure may allow us to predict which combination of treatments will induce robust visual cortical plasticity. Dark exposure offers a novel and promising noninvasive approach for the recovery of vision.
Exercise and visuomotor engagement
Recent research links physical activity to profound adult plasticity in the visual cortex, providing another promising behavioral intervention for recovery from amblyopia. Remarkably, allowing adult mice to run on a treadmill potently enhances visual cortical activity (Niell & Stryker, 2010) and promotes recovery of vision following monocular deprivation (Kaneko & Stryker, 2014). Moreover, visual stimulation or running alone did not improve visual function, suggesting that plasticity is facilitated only in activated neural circuits during running (Kaneko & Stryker, 2014). Recent studies have identified the key cellular mechanism underlying the effects of locomotion—VIP cells (Fu et al., 2014). In fact, genetic silencing of VIP cells in amblyopic adult mice prevents the recovery of the visual function by running, suggesting that the activity of these cells is necessary for the enhanced plasticity (Fu et al., 2015).
The potential of physical activity to promote amblyopic recovery has caught the attention of the clinical field. Adult subjects who intermittently cycled on a stationary bicycle while watching a movie showed enhanced effects of transient eye patching compared to those subjects who watched the movie while sitting still (Lunghi & Sale, 2015). Moreover, tasks that directly engage both visual and motor circuits have achieved great success in reversing amblyopia. For example, recovery from amblyopia is expedited by tasks requiring coordination of hand and eye movements, such as having patients manipulate objects during visual training (reviewed in Daw, 2013). Patients with amblyopia exhibit impairments in oculomotor performance, including saccadic eye movements (Niechwiej-Szwedo et al., 2010; Niechwiej-Szwedo et al., 2012; McKee et al., 2016; Perdziak et al., 2016), smooth pursuit (Raashid et al., 2016), fixation stability (González et al., 2012; Chung et al., 2015), hand-eye coordination (Niechwiej-Szwedo et al., 2011, 2014), and execution of grasping movements (Grant et al., 2007; Suttle et al., 2011). Therefore, targeting visuomotor circuits during treatment may help to alleviate some of these deficits.
Novel visual training procedures
There is no doubt that conventional patch therapy, directed toward improving the visual function of the amblyopic eye while occluding the fellow eye, is effective in the majority of cases if initiated at the appropriate age. However, it is not always effective, and it has additional limitations: patching interferes with binocular input, which can lead to poor binocular outcome, and it often has poor compliance (reviewed in Birch, 2012; Hess & Thompson, 2015). When the patch therapy is ineffective, there is no consensus on effective treatment. Since the 1970s, a number of alternative procedures, many inspired by the animal research literature, have been proposed. None of these have attained the degree of acceptance that would make them a new standard of care. We note a number of them here not as an endorsement but to foster a critical examination of the relationship between laboratory research findings and approaches to therapy for human patients.
Some alternative strategies use binocular exposure approaches to treat amblyopia. For example, a set of training paradigms called “monocular fixation in a binocular field” (MFBF), in which both eyes remain open while a vision task is accomplished by a single eye, have been reported to have some success (Brock, 1963; Cohen, 1981). One MFBF procedure places a red filter over the dominant eye such that both eyes receive light, but only the amblyopic eye can see the markings of a red pen used to perform various tasks (reviewed in Daw, 2013). The Cambridge Vision Stimulator treatment combined a short-term occlusion, visual stimulation using contours of all orientations, and training exercises, although efficacy was not demonstrated in a randomized clinical trial (Campbell et al., 1978). Indeed, there are many reports of successful results of active vision therapy with only minimal amounts of occlusion (reviewed in Garzia, 1987). Some reports suggest that this approach may be particularly successful in older patients; for example, a group of older children and adult patients with anisometropic amblyopia achieved long-lasting improvements in visual acuity and a binocular function following a treatment that combined active vision therapy with occlusion of only 2–5 h per day, although the role, if any, of active vision was not demonstrated using a control group (Wick et al., 1992).
Video game therapy has emerged as a form of active vision training aimed at improving both acuity and stereopsis in both amblyopic children and adults (reviewed in Hess & Thompson, 2015; Levi et al., 2015). Anaglyphic video games were developed for vision therapy more than 30 years ago (Press, 1981) and used as an MFBF approach for the treatment of amblyopia (Ludlam, 1992). The clinical finding that suppression—a reduced contribution of the amblyopic eye during binocular viewing—is an important part of the amblyopia syndrome along with a greater understanding of the biological underpinnings of amblyopia has spurred new dichoptic approaches to promote “binocular rebalancing”. These approaches may reactivate latent binocular pathways by reducing inhibitory interactions, boosting attenuated excitatory function, and/or shifting the synaptic modification threshold in favor of potentiation (reviewed in Hess & Thompson, 2015). For example, recent studies have found that both amblyopic preschool children and adults can show enhanced improvements in visual and motor function by playing dichoptic iPad or iPod games (Hess et al., 2011; Birch et al., 2015; Vedamurthy et al., 2015; Webber et al., 2016). Since the lack of binocular function is a key risk factor for persistent amblyopia, the development of new binocular training strategies may have a substantial impact on improving acuity and recovering stereopsis (reviewed in Levi et al., 2015). Unfortunately, recent randomized clinical trials have failed to provide evidence that video games are any better than patching in older children or that they improve binocular function (Holmes et al., 2016; Kelly et al., 2016). Future work will be required to elucidate how monocular and dichoptic experience contribute to amblyopia recovery across distinct patient populations.
Elaborate visual training regimes have been shown in some cases by others (Paul Harris, unpublished observation) to improve vision in amblyopic humans but are expensive and time intensive. They have not yet been adopted widely because of a lack of controlled trials that demonstrate both efficacy and safety. Therefore, there is a continued need to seek novel, faster training approaches. For example, a study in adult amblyopic macaques suggested that implementing a ‘global’ training paradigm may lead to visual improvements that generalize beyond the trained stimulus (Kiorpes & Mangal, 2015). Moreover, training strategies that engage attentional and emotional processes, including movies and action video games, appear in some reports to be particularly successful (reviewed in Levi & Li, 2009, and in Bavelier et al., 2010). Such training is likely to stimulate neuromodulatory systems that promote plasticity and to activate circuits encoding higher-order visual functions that are impaired in patients with amblyopia (reviewed in Kiorpes, 2006).
Use of animal models to find treatments for amblyopia
With the advent of new technologies in neuroscience, the field continues to rely heavily on animal models to uncover the neural mechanisms underlying human pathologies such as amblyopia. The mouse in particular has emerged as an ideal model for taking advantage of genetic, optogenetic, physiological, and imaging tools that allow experimenters to label, manipulate, and monitor specific cell types with great precision. Nevertheless, it is not yet clear whether rodent models are appropriate for the study of amblyopia and its treatment in humans.
One potential limitation of mice for the study of amblyopia is that the mouse does not have a fovea, and therefore the entire mouse retina resembles the peripheral retina of the primate (Naarendorp et al., 2010). Although amblyopia was first described as a deficit in foveal vision, amblyopic deficits have also been detected in the visual field periphery (Irene Gottlob, personal communication). In fact, patients with amblyopia have been shown to exhibit decreased motion and contrast detection through the amblyopic eye (Katz et al., 1984; Levi et al., 1984). Thus, the absence of foveal vision may not exclude the mouse a priori as a model to study human amblyopia. Furthermore, the mouse primary visual cortex exhibits binocular integration and disparity selectivity that support depth perception (Scholl et al., 2013), potentially allowing the use of mice to study the loss of stereoscopic vision associated with amblyopia.
Another major consideration when evaluating the effectiveness of potential amblyopia treatments in mice is that the common forms of human amblyopia are not generally studied in the mouse. Basic research on amblyopia in rodents has focused almost exclusively on monocular deprivation. However, human patients with amblyopia exhibit various types of amblyopia that are generally classified as anisometropic, strabismic, and deprivation amblyopia. Deprivation amblyopia is the least common form of amblyopia, accounting for less than 5 per cent of cases (Holmes & Clarke 2006). Can we get closer to the human condition? Experiments in monkeys have employed more subtle forms of deprivation such as anisometropia and strabismus, revealing reduced binocularity and poorer spatial resolution in V1 neurons driven by the amblyopic eye (Movshon et al., 1987; Kiorpes et al., 1998). In mice, one possibility is to take advantage of the available genetic models to identify genetic ocular defects that better approach the common human forms of amblyopia (Engle, 2007). However, while exploring new experimental models of amblyopia, it is also important to take advantage of the tremendous progress over the past 50 years in our understanding of monocular deprivation in mice as a premier model for understanding critical periods for cortical plasticity and the underlying regulating factors.
Assessing outcomes of amblyopia treatment
What is the best test to assess amblyopia and amblyopia recovery in experimental models? In animal models, amblyopia is generally assessed using grating acuity. Animals are presented with drifting gratings at increasing spatial frequencies to determine optomotor, behavioral, or neural thresholds. Grating acuity is a strong measure of the degraded visual function associated with amblyopia, particularly deprivation amblyopia, and corresponds to measures of Snellen (optotype) acuity in humans (Levi & Klein, 1982). Thus, grating acuity is an appropriate and relatively easy way to assess acuity in animals. However, it should be noted that grating and optotype acuity are not equivalent. This may be particularly important for the detection of crowding effects, which are marked in amblyopia. Moreover, not all grating tasks reveal the same acuity. In particular, using the visual water task in rodents, a visual discrimination task based on reinforcement learning (Prusky et al., 2000; Prusky & Douglas, 2004), allows measurement of good perceptual thresholds of visual acuity.
In addition to assessing acuity as a recovery measure, it will be important to extend our evaluation to other visual deficits associated with amblyopia. For example, the loss of stereoptic depth perception may have the greatest impact on the quality of life for the amblyopic patient, impairing the ability on visuomotor tasks and limiting career options (Levi et al. 2015). Moreover, adults who lack stereopsis tend to be refractory to therapy. Individuals with amblyopia, particularly strabismus, also exhibit oculomotor deficits (McKee et al., 2016) and other visuomotor deficits during the performance of fine motor tasks (Grant & Moseley, 2011). Thus, developing novel methods of measuring trajectories for the recovery of binocular vision and visuomotor ability in both experimental animal models and humans may provide valuable insights into the success of future amblyopia treatments.
Finally, there is a need to evaluate the effectiveness of amblyopia treatments on higher brain regions. Deficits in vision function associated with amblyopia may result from changes in extrastriate regions instead of or as well as the primary visual cortex (Schröder et al., 2002; reviewed in Kiorpes & McKee, 1999). Few studies assess the extent of impairment or recovery outside of the primary visual cortex. Using less V1-centric metrics to assess recovery of the function by tapping into the higher-order dorsal and ventral streams will provide further insights into novel treatments. Studies outside of purely visual regions also have the potential to illuminate deficits in unexpected pathways, such as visuomotor and cross-modal connections (for example, visual–tactile interactions; Niechwiej-Szwedo et al., 2016). So far, the study of the neural underpinnings of amblyopia and the molecular factors that promote recovery from amblyopia has been largely restricted to V1. Future work that determines whether other brain regions show critical periods and plasticity mechanisms that are similar to those observed in V1 will better inform us how and when to treat amblyopia.
Toward identifying common pathways to recovery
Novel molecular, pharmacological, and behavioral treatments are emerging to harness the brain’s plasticity mechanisms for the recovery from amblyopia (see Fig. 1). Ongoing challenges will be to determine how the various behavioral manipulations engage plasticity factors and how these plasticity factors interact within the cortical networks to promote the desired rewiring. A number of distinct candidate plasticity factors have been identified, and ongoing work will address whether these factors operate independently or as components of a common mechanism. For example, exercise and video games may engage neuromodulatory systems to activate specific types of cortical inhibitory interneurons known to control plasticity. Identifying “hub” circuits, cells, or molecular mechanisms that promote visual plasticity promises to lead to better-targeted treatment strategies that should mitigate side effects and accelerate recovery from amblyopia in human patients.
Fig. 1.
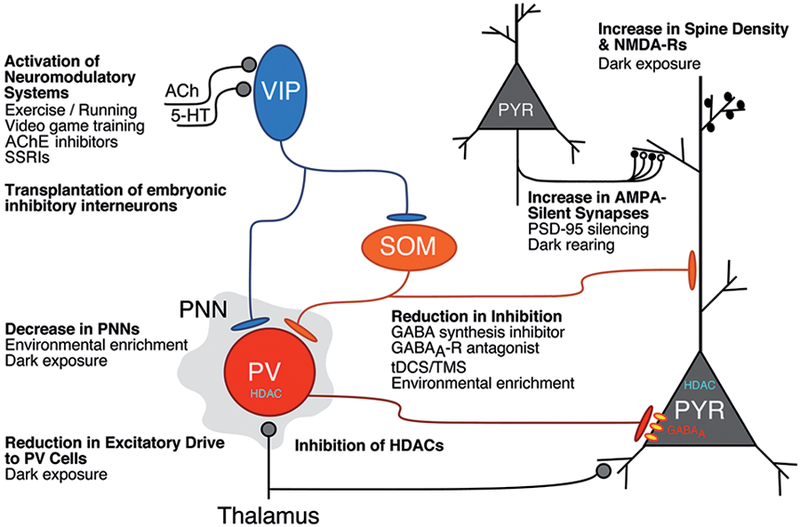
Cellular and circuit mechanisms underlying molecular, pharmacological, and environmental approaches to increase plasticity in the visual cortex. Release of neuromodulators in the visual cortex, such as acetylcholine (ACh) or serotonin (5-HT = 5-hydroxytryptophan), activate VIP (vasoactive intestinal polypeptide) inhibitory cells that promote cortical activity and plasticity by inhibiting other inhibitory interneuron subtypes, PV (parvalbumin), and SOM (somatostatin) cells (Letzkus et al., 2011; Pi et al., 2013; Fu et al., 2014; Kaneko & Stryker, 2014; Fu et al., 2015). These neuromodulatory systems can be activated by pharmacological treatments such as cholinesterase (AChE) inhibitors (Morishita et al., 2010) or selective serotonin reuptake inhibitors (SSRIs; Maya Vetencourt et al., 2008; Thompson et al., 2014) or behavioral therapies such as exercise (Fu et al., 2015; Lunghi & Sale, 2015) and video game training (Bavelier et al., 2010). Transplantation of embryonic inhibitory neurons into the postnatal visual cortex induces a second critical period of OD plasticity after the normal one (Southwell et al., 2010; Tang et al., 2014; Isstas et al., 2017). Plasticity is also enhanced in the adult visual cortex by decreasing perineuronal nets that predominantly enwrap the PV cells by pharmacological (Pizzorusso et al., 2002) or behavioral interventions, such as environmental enrichment (Sale et al., 2007) or dark exposure (Stodieck et al., 2014). A reduction in the excitatory drive to PV cells (Huang et al., 2010; Gu et al., 2016) and an increase in spine density and NMDA-Rs (Yashiro et al., 2005; He et al., 2006; Philpot et al., 2007; Montey & Quinlan, 2011) may also contribute to the enhanced plasticity that occurs with dark exposure. Various manipulations that reduce inhibitory synaptic function have been found to enhance visual cortical plasticity, including drugs that inhibit GABA synthesis or GABAA receptors (Harauzov et al., 2010), tDCS/TMS (Stagg et al., 2009), and environmental enrichment (Greifzu et al., 2014). Inhibition of HDACs can also reinstate plasticity in the adult visual cortex to allow recovery from amblyopia (Putignano et al., 2007; Silingardi et al., 2010). Finally, an increase in AMPA-silent synapses (white synaptic boutons) underlies the heightened plasticity following knock-out or virus-mediated gene silencing of PSD-95 (Huang et al., 2015). Silent synapses also persist in the adult visual cortex in dark-reared mice (Funahashi et al., 2013). The figure is modified from the one presented by Takao Hensch at the Lasker/IRRF Initiative on Amblyopia workshops. Woods Hole, MA, July/August, 2015.
Recommendations
The emergence of novel genetic, imaging, and electrophysio-logical tools to probe circuit and cellular mechanisms is shedding light on new targets to promote plasticity in visual circuits. Pharmacological agents, transcranial direct current or magnetic stimulation (tDCS/TMS), and behavioral therapies may harness these plasticity mechanisms for amblyopia treatment. A focus on understanding how such treatments engage precise circuit, cellular, and molecular mechanisms will provide insights into focused strategies to promote recovery.
Novel visual training paradigms that exploit our increased understanding of the biological underpinnings of amblyopia recovery are needed. Future work should continue to seek training strategies that are tailored to the individual patient to engage attentional, emotional, and visuomotor circuits for faster and more effective recovery.
The use of animal models such as mice to study treatments for human amblyopia presents numerous challenges because of differences in both visual function and visual cortical circuits between species. Furthermore, mouse research has been largely confined to studying deprivation amblyopia, the least common form of human amblyopia. New experimental procedures mimicking the more common forms of human amblyopia in mice and other animal models would enhance progress for the treatment of amblyopia in humans. Nevertheless, the knowledge gained over the past 50 years of animal research, with mice as the premier model of the last decade, should continue to inform our understanding of critical periods and their underlying cellular and molecular mechanisms.
Grating acuity is a reliable measure of amblyopia that can be readily assessed in both animal models and humans. However, developing novel methods of measuring trajectories for recovery of other visual functions, including binocular vision and visuomotor ability, is likely to improve the success of amblyopia treatments in humans. Moreover, future work should be directed at determining whether brain regions outside of the primary visual cortex can additionally be targeted to promote recovery from amblyopia.
Acknowledgments
The authors would like to thank Anne Takesian,* who served as scribe for this discussion section. We would also like to thank all discussion group participants: Yuzo Chino, Nigel Daw, Kevin Duffy, Simon Grant, Paul Harris, Takao Hensch, Suzanne McKee, Ewa Niechwiej-Szwedo, Elizabeth Quinlan, Anu Sharma, Paul Sieving.
Footnotes
FM Kirby Neurobiology Center, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
References
- Antonini A, Fagiolini M & Stryker MP (1999). Anatomical correlates of functional plasticity in mouse visual cortex. Journal of Neuroscience 19, 4388–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A & Stryker MP (1993). Rapid remodeling of axonal arbors in the visual cortex. Science 260, 1819–1821. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y & Hensch TK (2010). Removing brakes on adult brain plasticity: From molecular to behavioral interventions. Journal of Neuroscience 30, 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE (2012). Amblyopia and binocular vision. Progress in Retinal and Eye Research 33, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Li SL, Jost RM, Morale SE, De La Cruz A, Stager D, Dao L & Stager DR (2015). Binocular iPad treatment for amblyopia in preschool children. Journal of AAPOS 19, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock FW (1963). New methods for testing binocular control. Journal of the American Optometric Association 34, 443–450. [Google Scholar]
- Campbell FW, Hess RF, Watson PG & Banks R (1978). Preliminary results of a physiologically based treatment of amblyopia. British Journal of Ophthalmology 62, 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C & Sur M (2012). Nucleus basalis enabled stimulus specific plasticity in the visual cortex is mediated by astrocytes. Proceedings of the National Academy of Sciences of the United States of America 109, E2832–E2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST, Kumar G, Li RW & Levi DM (2015). Characteristics of fixational eye movements in amblyopia: Limitations on fixation stability and acuity? Vision Research 114, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH (1981). Monocular fixation in a binocular field. Journal of the American Optometric Association 52, 801–806. [PubMed] [Google Scholar]
- Cooper LN & Bear MF (2012). The BCM theory of synapse modification at 30: Interaction of theory with experiment. Nature Reviews Neuroscience 13, 798–810. [DOI] [PubMed] [Google Scholar]
- Cynader M (1983). Prolonged sensitivity to monocular deprivation in dark-reared cats: Effects of age and visual exposure. Brain Research 284, 155–164. [DOI] [PubMed] [Google Scholar]
- Daw NW (2013). Visual Development (2nd ed.). New York: Springer. [Google Scholar]
- Ding Z, Li J, Spiegel DP, Chen Z, Chan L, Luo G, Yuan J, Deng D, Yu M & Thompson B (2016). The effect of transcranial direct current stimulation on contrast sensitivity and visual evoked potential amplitude in adults with amblyopia. Scientific Reports 6, 19280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Rompani SB & Caroni P (2013). Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276. [DOI] [PubMed] [Google Scholar]
- Duffy KR, Bukhamseen DH, Smithen MJ & Mitchell DE (2015). Binocular eyelid closure promotes anatomical but not behavioural recovery from monocular deprivation. Vision Research 114, 151–160. [DOI] [PubMed] [Google Scholar]
- Duffy KR, Lingley AJ, Holman KD & Mitchell DE (2016). Susceptibility to monocular deprivation following immersion in darkness either late into or beyond the critical period. Journal of Comparative Neurology 524, 2643–2653. [DOI] [PubMed] [Google Scholar]
- Duffy KR & Mitchell DE (2013). Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Current Biology 23, 382–386. [DOI] [PubMed] [Google Scholar]
- Eaton NC, Sheehan HM & Quinlan EM (2016). Optimization of visual training for full recovery from severe amblyopia. Learning & Memory 23, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle EC (2007). Genetic basis of congenital strabismus. Archives of Ophthalmology 125, 189–195. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL & Champagne FA (2009). Epigenetic influences on brain development and plasticity. Current Opinion in Neurobiology 19, 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong M-F., Mitchell DE, Duffy KR & Bear MF (2016). Rapid recovery from the effects of early monocular deprivation is enabled by temporary inactivation of the retinas. Proceedings of the National Academy of Sciences of the United States of America 113, 14139–14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Daw N, Sato H & Czepita D (1991). Dark-rearing delays the loss of NMDA-receptor function in kitten visual cortex. Nature 350, 342–344. [DOI] [PubMed] [Google Scholar]
- Frenkel MY & Bear MF (2004). How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron 44, 917–923. [DOI] [PubMed] [Google Scholar]
- Fu Y, Kaneko M, Tang Y, Alvarez-Buylla A & Stryker MP (2015). A cortical disinhibitory circuit for enhancing adult plasticity. eLife e05558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Daracy DP, Nicoll RA, Huang ZJ & Stryker MP (2014). A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi R, Maruyama T, Yoshimura Y & Komatsu Y (2013). Silent synapses persist into adulthood in layer 2/3 pyramidal neurons of visual cortex in dark-reared mice. Journal of Neurophysiology 109, 2064–2076. [DOI] [PubMed] [Google Scholar]
- Garzia RP (1987). Efficacy of vision therapy in amblyopia: A literature review. American Journal of Optometry and Physiological Optics 64, 393–404. [DOI] [PubMed] [Google Scholar]
- Gervain J, Vines BW, Chen LM, Seo RJ, Hensch TK, Werker JF & Young AH (2013). Valproate reopens critical-period learning of absolute pitch. Frontiers in Systems Neuroscience 7, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González EG, Wong AM, Niechwiej-Szwedo E, Tarita-Nistor F & Steinbach MJ (2012). Eye position stability in amblyopia and in normal binocular vision. Investigative Ophthalmology & Visual Science 53, 5386–5394. [DOI] [PubMed] [Google Scholar]
- Grant S, Melmoth DR, Morgan MJ & Finlay AL (2007). Prehension deficits in amblyopia. Investigative Ophthalmology & Visual Science 48, 1139–1148. [DOI] [PubMed] [Google Scholar]
- Grant S & Moseley MJ (2011). Amblyopia and real-world visuomotor tasks. Strabismus 19, 119–128. [DOI] [PubMed] [Google Scholar]
- Greifzu F, Kalogeraki E & Löwel S (2016). Environmental enrichment preserved lifelong ocular dominance plasticity, but did not improve visual abilities. Neurobiology of Aging 41, 130–137. [DOI] [PubMed] [Google Scholar]
- Greifzu F, Pielecka-Fortuna J, Kalogeraki E, Krempler K, Favaro PD, Schlüter OM & Löwel S (2014). Environmental enrichment extends ocular dominance plasticity into adulthood and protects from stroke-induced impairments of plasticity. Proceedings of the National Academy of Sciences of the United States of America 111, 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Tran T, Murase S, Borrell A, Kirkwood A & Quinlan EM (2016). Neuregulin-dependent regulation of fast-spiking interneuron excitability controls the timing of the critical period. Journal of Neuroscience 36, 10285–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Ranade SP, Lorenc M & Kepecs A (2015). Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell 162, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, Dicristo G, Pasquale RD, Cancedda L, Pizzorusso T, Viegi A, Berardi N & Maffei L (2010). Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. Journal of Neuroscience 30, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W & Quinlan EM (2006). Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. Journal of Neuroscience 26, 2951–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K & Quinlan EM (2007). Experiencedependent recovery of vision following chronic deprivation amblyopia. Nature Neuroscience 10, 1134–1136. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B & Thompson B (2011). Restoration of binocular vision in amblyopia. Strabismus 19, 110–118. [DOI] [PubMed] [Google Scholar]
- Hess RF & Thompson B (2015). Amblyopia and the binocular approach to its therapy. Vision Research 114, 4–16. [DOI] [PubMed] [Google Scholar]
- Holmes JM & Clarke MP (2006). Amblyopia. Lancet 367, 1343–1351. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Manh VM, Lazar EL, Beck RW, Birch EE, Kraker RT, Crouch ER, Erzurum SA, Khuddus N, Summers AI, Wallace DK & Pediatric Eye Disease Investigator Group (2016). Effect of a binocular iPad game vs. part-time patching in children aged 5 to 12 years with amblyopia: A randomized clinical trial. JAMA Ophthalmology 134, 1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Gu Y, Quinlan EM & Kirkwood A (2010). A refractory period for rejuvenating GABAergic synaptic transmission and ocular dominance plasticity with dark exposure. Journal of Neuroscience 30, 16636–16642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Stodieck SK, Goetze B, Cui L, Wong MH, Wenzel C, Hosand L, Dong Y, Löwel S & Schlüter OM (2015). Progressive maturation of silent synapses governs the duration of a critical period. Proceedings of the National Academy of Sciences of the United States of America 112, E3131–E3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isstas M, Teichert M, Bolz J & Lehmann K (2017). Embryonic interneurons from the medial, but not the caudal ganglionic eminence trigger ocular dominance plasticity in adult mice. Brain Structure and Function 222, 539–547. [DOI] [PubMed] [Google Scholar]
- Kalogeraki E, Greifzu F, Haack F & Löwel S (2014). Voluntary physical exercise promotes ocular dominance plasticity in adult mouse primary visual cortex. Journal of Neuroscience 34, 15476–15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M & Stryker M (2014). Sensory experience during locomotion promotes recovery of function in adult visual cortex. eLife 3, e02798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LM, Levi DM & Bedell HE (1984). Central and peripheral contrast sensitivity in amblyopia with varying field size. Documenta Ophthalmologica 58, 351–373. [DOI] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, Dao L, Beauchamp CL, Leffler JN & Birch EE (2016). Binocular iPad game vs. patching for treatment of amblyopia in children: A randomized clinical trial. JAMA Ophthalmology 134, 1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L (2006). Visual processing in amblyopia: Animal studies. Strabismus 14, 3–10. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O’Keefe LP, Cavanaugh JR & Movshon JA (1998). Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. Journal of Neuroscience 18, 6411–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L & Mangal P (2015). “Global” visual training and extent of transfer in amblyopic macaque monkeys. Journal of Vision 15, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L & McKee SP (1999). Neural mechanisms underlying amblyopia. Current Opinion in Neurobiology 9, 480–486. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C & Lüthi A (2011). A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331. [DOI] [PubMed] [Google Scholar]
- Levi DM & Klein S (1982). Hyperacuity and amblyopia. Nature 298, 268–270. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA & Aitsebaomo P (1984). Detection and discrimination of the direction of motion in central and peripheral vision of normal and amblyopic observers. Vision Research 24, 789–800. [DOI] [PubMed] [Google Scholar]
- Levi DM, Knill DC & Bavelier D (2015). Stereopsis and amblyopia: A mini-review. Vision Research 114, 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM & Li RW (2009). Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Research 49, 2535–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlam WM (1992). The use of the opti-mum system I computerized VT in treating strabismus and amblyopia In Computers and Vision Therapy Programs, ed. Press LJ, pp. 37–40. Santa Ana: Optometric Extension Program. [Google Scholar]
- Lunghi C & Sale A (2015). A cycling lane for brain rewiring. Current Biology 25, R1122–R1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Mizaguchi Y & Hensch TK (2004). Experiencedependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 44, 1031–1041. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E & Maffei L (2008). The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 18, 385–388. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Schor CM & Movshon JA (2016). Saccadic latency in amblyopia. Journal of Vision 16, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Hamilton R, Schlaug G, Swisher JD, Kiriakopoulos ET, Pitskel NB, Kauffman T & Pascual-Leone A (2008). Rapid and reversible recruitment of early visual cortex for touch. PLos One 3, e3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE, MacNeill K, Crowder NA, Holman K & Duffy KR (2016). Recovery of visual functions in amblyopic animals following brief exposure to total darkness. Journal of Physiology 594, 149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montey KL & Quinlan EM (2011). Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nature Communications 2, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N & Hensch TK (2010). Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330, 1238–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L & Boothe RG (1987). Effects of early unilateral blur on the macaque’s visual system. III. Physiological observations. Journal of Neuroscience 7, 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD, Caplan CJ, Christen WG & Duffy FH (1985). Dark rearing prolongs physiological but not anatomical plasticity of the cat visual cortex. Journal of Comparative Neurology 235, 448–466. [DOI] [PubMed] [Google Scholar]
- Naarendorp F, Esdaille TM, Banden SM, Andrews-Labenski J, Gross OP & Pugh EN Jr. (2010). Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. Journal of Neuroscience 30, 12495–12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Chandrakumar M, Goltz HC & Wong AM (2012). Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior, I: Saccadic eye movements. Investigative Ophthalmology & Visual Science 53, 7458–7468. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Chin J, Wolfe PJ, Popovich C & Staines WR (2016). Abnormal visual experience during development alters the early stages of visual-tactile integration. Behavioural Brain Research 304, 111–119. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji ZA & Wong AM (2010). Effects of anisometropic amblyopia on visuomotor behavior, I: Saccadic eye movements. Investigative Ophthalmology & Visual Science 51, 6348–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji Z & Wong AM (2011). Effects of anisometropic amblyopia on visuomotor behavior, III: Temporal eye-hand coordination during reaching. Investigative Ophthalmology & Visual Science 52, 5853–5861. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Goltz HC, Chandrakumar M & Wong AM (2014). Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior: III. Temporal eye-hand coordination during reaching. Investigative Ophthalmology & Visual Science 55, 7831–7838. [DOI] [PubMed] [Google Scholar]
- Niell CM & Stryker MP (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann C, Schitz D, Fürmaier A, Doing C & Bach M (2007). Long-term plasticity of visual evoked potentials in humans is altered in major depression. Biological Psychiatry 62, 373–380. [DOI] [PubMed] [Google Scholar]
- Perdziak M, Witkowska DK, Gryncewicz W & Ober JK (2016). Not only amblyopic but also dominant eye in subjects with strabismus show increased saccadic latency. Journal of Vision 16, 12. [DOI] [PubMed] [Google Scholar]
- Pfeffer C, Xue M, He M, Huang Z & Scanziani M (2013). Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nature Neuroscience 16, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Cho KK & Bear MF (2007). Obligatory role of NR2A for metaplasticity in visual cortex. Neuron 53, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ & Kepecs A (2013). Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW & Maffei L (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Press LJ (1981). Electronic games and strabismic therapy. Journal of Optometry and Visual Development 12, 35–39. [Google Scholar]
- Prusky GT & Douglas RM (2004). Characterization of mouse cortical spatial vision. Vision Research 44, 3411–3418. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW & Douglas RM (2000). Behavioral assessment of visual acuity in mice and rats. Vision Research 40, 2201–2209. [DOI] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L & Pizzorusso T (2007). Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron 53, 747–759. [DOI] [PubMed] [Google Scholar]
- Raashid RA, Liu IZ, Blakeman A, Goltz HC & Wong AM (2016). The initiation of smooth pursuit is delayed in anisometropic amblyopia. Investigative Ophthalmology & Visual Science 57, 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R & Maffei L (2007). Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nature Neuroscience 10, 679–681. [DOI] [PubMed] [Google Scholar]
- Scholl B, Burge J & Priebe NJ (2013). Binocular integration and disparity selectivity in mouse primary visual cortex. Journal of Neurophysiology 109, 3013–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder JH, Fries P, Roelfsema PR, Singer W & Engel AK (2002). Ocular dominance in extrastriate cortex of strabismic amblyopic cats. Vision Research 42, 29–39. [DOI] [PubMed] [Google Scholar]
- Silingardi D, Scali M, Belluomini G & Pizzorusso T (2010). Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. European Journal of Neuroscience 31, 2185–2192. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP & Gandhi SP (2010). Cortical plasticity induced by inhibitory neuron transplantation. Science 327, 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM & Johansen-Berg H (2009). Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. Journal of Neuroscience 29, 5202–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodieck SK, Greifzu F, Goetze B, Schmidt KF & Löwel S (2014). Brief dark exposure restored ocular dominance plasticity in aging mice and after a cortical stroke. Experimental Gerontology 60, 1–11. [DOI] [PubMed] [Google Scholar]
- Suttle CM, Melmoth DR, Finlay AL, Sloper JJ & Grant S (2011). Eye-hand coordination skills in children with and without amblyopia. Investigative Ophthalmology & Visual Science 52, 1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE & Hensch TK (2013). Balancing plasticity/stability across brain development. Progress in Brain Research 207, 3–34. [DOI] [PubMed] [Google Scholar]
- Tang Y, Stryker MP, Alvarez-Buylla A & Espinosa JS (2014). Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proceedings of the National Academy of Sciences of the United States of America 111, 18339–18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Lagas AK, Stinear CM, Byblow WD, Russel BR & Kydd RR (2014). The use of selective serotonin reuptake inhibitors to treat amblyopia in adulthood. Investigative Ophthalmology & Visual Science 55, 801.24408972 [Google Scholar]
- Uusitalo H (2013). Hermo pharma reports topline data with HER-801 from clinical study in adult amblyopia. Available at: http://evaluategroup.com/Universal/View.aspx?type=Story&id=453937 (accessed September 21, 2016).
- Van Hedger SC, Heald LM, Koch R & Nusbaum HC (2015). Auditory working memory predicts individual differences in absolute pitch learning. Cognition 140, 95–110. [DOI] [PubMed] [Google Scholar]
- Vedamurthy I, Nahum M, Huang SJ, Zheng F, Bayliss J, Bavelier D & Levi DM (2015). A dichoptic custom-made action video game as a treatment for adult amblyopia. Vision Research 114, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber AL, Wood JM & Thompson B (2016). Fine motor skills of children with amblyopia improve following binocular treatment. Investigative Ophthalmology & Visual Science 57, 4713–4720. [DOI] [PubMed] [Google Scholar]
- Wick B, Wingard M, Cotter S & Scheiman M (1992). Anisometropic amblyopia: Is the patient ever too old to treat? Optometry and Vision Science 69, 866–878. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Corlew R & Philpot BD (2005). Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. Journal of Neuroscience 25, 11684–11692. [DOI] [PMC free article] [PubMed] [Google Scholar]


