Abstract
Background
Although a number of serum biomarkers for detection of hepatocellular carcinoma (HCC) have been explored, their exact diagnostic value remains unclear. We aimed to conduct a direct comparison of five representative serum biomarkers for detecting HCC and to derive multi-marker prediction algorithms.
Patients and methods
In total, 846 patients were recruited from three hospitals in China, including 202 HCC patients, 226 liver cirrhosis patients, 215 chronic hepatitis B virus-infected patients, and 203 healthy volunteers. Serum levels of alpha-fetoprotein (AFP), lens culinaris agglutinin-reactive AFP (AFP-L3), des-gamma-carboxyprothrombin (DCP), squamous cell carcinoma antigen, and centromere protein F autoantibody were measured by ELISA. The diagnostic performances of individual biomarkers and multi-marker combinations were evaluated by receiver operating characteristics analysis. The bootstrapping method was adopted to adjust for potential overfitting of all diagnostic indicators.
Results
DCP exhibited the best diagnostic performance, with areas under the curve (AUC) for detecting HCC of 0.82 (95% CI 0.64–0.80) and sensitivity of 65.2% (95% CI 63.3–82.1%) at 90% specificity. Of note, DCP showed similar diagnostic efficacy for detecting AFP-positive and AFP-negative HCC. After a comprehensive search for multi-marker combinations, a two-marker prediction algorithm including AFP and DCP was constructed and yielded an AUC of 0.87 (95% CI 0.68–0.84) for detecting HCC. In addition, the combination showed good ability in discriminating early-stage HCC and decompensated liver cirrhosis, with an AUC of 0.81 (95% CI 0.75–0.86).
Conclusion
DCP could be a complementary biomarker in the early diagnosis of HCC. The constructed multi-marker prediction algorithms could contribute toward distinguishing HCC from non-malignant chronic liver diseases.
Keywords: early detection, liver cirrhosis, prediction model
Introduction
Liver cancer is the sixth most commonly diagnosed cancer worldwide and the second leading cause of cancer-related death.1,2 Hepatocellular carcinoma (HCC) accounts for 70–90% of all new liver cancer cases, largely in association with chronic hepatitis B virus (HBV) infection.1–4 Stage at diagnosis is the most important prognostic factor, with a 5-year overall survival rate of 50–70% at early stages and less than 5% at advanced stages. Therefore, diagnosis of HCC at a curative stage and surveillance of non-malignant chronic liver diseases carrying a risk of HCC are key to improving the poor prognosis of HCC.
Abdominal ultrasonography is the main recommended tool in HCC diagnosis and surveillance.3,5 However, abdominal ultrasonography presents rather limited sensitivity in detecting HCC and is further hampered by inter- and intra-observer variability.6 Alpha-fetoprotein (AFP) is currently the best established serum biomarker for the diagnosis of HCC.7,8 However, the diagnostic performance of AFP in detecting HCC is suboptimal, with sensitivities of 41–65% and specificities of 80–90% at a commonly used cutoff value of 20 ng/mL.9,10 In particular, much lower sensitivity was observed in detecting early-stage HCC and smaller tumors.9 Therefore, the identification of effective and reliable non-invasive biomarkers for diagnosis and surveillance of HCC is urgently needed and is of high clinical and public health relevance.
To date, a number of serum biomarkers carrying diagnostic potential for detecting HCC have been identified,11,12 such as lens culinaris agglutinin-reactive AFP (AFP-L3),13,14 des-gamma carboxyprothrombin (DCP),15,16 squamous cell carcinoma antigen (SCCA),17,18 and centromere protein F autoantibody (anti-CENPF).19 Although these biomarkers have been evaluated in several studies, the diagnostic performance varied greatly across studies, and evidence of a direct comparison of the diagnostic performance of multiple biomarkers in the same population is sparse, especially for Chinese populations.7,8 In addition, promising results were commonly reported in some studies which tried to combine different biomarkers and constructed multi-marker prediction models.7,8,14 However, some results should be interpreted with caution because of a lack of validation (lacking either the use of external independent validation populations or adoption of appropriate internal validation methods), which would lead to significant overfitting regarding the diagnostic performance.
In the present study, we therefore selected five representative biomarkers (AFP, AFP-L3, DCP, SCCA, and anti-CENPF) with diagnostic potential for detecting HCC. Serum levels of the five biomarkers were simultaneously measured in a large sample set (N=846) comprising patients with HCC, liver cirrhosis, chronic HBV infection, and healthy controls, recruited in three hospitals in China. We aimed to conduct a direct comparison of the diagnostic performance of the five biomarkers for detecting HCC and to derive and validate multi-marker algorithms carrying good diagnostic potential in distinguishing HCC from non-malignant chronic liver diseases.
Patients and methods
Study design and population
This study was conducted based on a cross-sectional design. Eight hundred eighty-four eligible participants were consecutively recruited from three hospitals in China (Cancer Hospital of Chinese Academy of Medical Science, Beijing Youan Hospital, and Beijing Friendship Hospital) from November 2013 to December 2014, including 202 HCC patients, 226 patients with liver cirrhosis, 215 patients with chronic HBV infection, and 203 healthy volunteers.
HCC was diagnosed according to the Chinese guidelines of diagnosis and treatment for HCC (2012 version, China).20 Liver cirrhosis was diagnosed according to the guidelines of prevention and treatment for chronic hepatitis jointly proposed by the Chinese Society of Hepatology and the Chinese Society of Infectious Diseases (2015, China),21 and the following diagnostic criteria were adopted: 1) histological or clinical evidence of liver cirrhosis, including definite chronic hepatitis based on clinical manifestation of portal hypertension, diagnosis of liver cirrhosis indicated by ultrasound, and/or computed tomography (CT); 2) evidence of HBV infection; and 3) for patients without histological evidence, at least two of the following criteria should be met: i) presentation of liver cirrhosis changes indicated by imaging techniques such as ultrasound and CT; ii) platelets <100×109/L; iii) serum albumin <35 g/L or prothrombin time >1.3 second; iv) gastroesophageal varices diagnosed by gastroscopy; and v) fibroscan >12.4 kPa (alanine transaminase <5× upper limit of normal). Chronic HBV infection was diagnosed according to the guidelines of the prevention and treatment for chronic hepatitis (2015, China).21 Healthy volunteers were outpatients with normal liver biochemistry, no history of liver disease, and no malignant disease. Patients with heart, lung, or kidney diseases were excluded from the study.
All participants were recruited before receiving any radiotherapy, chemotherapy, or operative treatment and had no other malignant diseases. The tumor stages of HCC patients were defined according to the Union for International Cancer Control (UICC) TNM classification (7th version). TNM stage I was defined as early-stage HCC in our analyses.
The study was approved by the ethics committees of the respective hospitals (Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, the Clinical Research Ethics Committee of Beijing Friendship Hospital, Capital Medical University, and the Ethics Committee of Beijing Youan Hospital of Capital Medical University). Written informed consent was obtained from each participant.
Laboratory analysis
Preparation of serum samples
Blood samples were collected from each participant at the hospital visit. After completion of blood clotting, blood samples were immediately centrifuged at 2000–2500 g for 10 min and stored at −80°C until analyses.
AFP
A commercial ELISA kit was used (CanAg, Fujirebio Dignostics, Göteborg, Sweden). The test was conducted as per the instructions of the kit. In brief, 100 μL antibody solution and 25 μL AFP calibrators were added to each well. Serum samples was then added into the strip wells. The wells were incubated for 1 h at room temperature, and then, each strip was washed six times, followed by the addition of 100 μL tetramethylbenzidine (TMB) horseradish peroxidase (HRP) substrate for 30 min at room temperature. The absorbance at 620 nm was read on the microplate reader SpectraMax M3 (Molecular Devices LLC, Sunnyvale, CA, USA).
SCCA
Serum SCCA levels were determined using the SCCA detection kit (Xeptagen, Marghera, Italy). The test was conducted as per the instructions of the kit. In brief, 100 μL was dispensed per well of standard solution starting from 8 ng/mL and performing in-plate twofold serial dilutions to a final concentration of 0.5 ng/mL. Then, 100 μL of 1:8 diluted samples was added in duplicate with 100 μL dilution buffer as a blank. Samples were incubated for 1 h at room temperature and washed six times. Following this, 100 μL of diluted enzyme-conjugated streptavidin solution was added and incubated for 1 h at room temperature, then washed six times. Then, 100 μL chromogen solution per well was applied. The color was allowed to develop for 10–15 min at room temperature in the dark and then stopped by adding 100 μL of stop solution. The optical density values of each well were measured at 450 nm on the microplate reader SpectraMax M3 (Molecular Devices LLC).
AFP-L3
Serum AFP-L3 levels were determined using the AFP-L3 detection kit (Beijing Rejing Biotechnology Company, Beijing, China). In brief, 400 μL of serum sample was pipet-ted and mixed with 600 μL washing buffer. A column was inserted in a collection tube and centrifuged to equilibrate the column. After this, 600 μL of diluted serum sample was transferred to the column and left to stand at room temperature for 10 min. Then, 1200 μL of washing buffer was added to the column and centrifuged at 3000 rpm for 20 s at room temperature. The flowthrough was discarded and 600 μL elution buffer was added to the column and centrifuged at 3000 rpm for 20 s. Serum AFP-L3 was detected in the collection tube and the ratio of AFP-L3 was calculated using the predefined formula.
DCP
A commercial Lumipulse protein induced by vitamin K absence/antagonist II (PIVKA-II) detection kit (Fujirebio, Tokyo, Japan) was used to determine DCP levels. The test was conducted as per the instructions of the kit. Briefly, the magnetic particle in each test tube was combined with PIVKA-II monoclonal antibody and antiprothrombin polyclone antibody labeled by alkaline phosphatase. The Lumi-pulse G PIVKA-II calibration solution and washing buffer were prepared. The LUMIPULSE G1200 immunochemistry system was used for detection of DCP using a 100 μL serum sample (Fujirebio, Japan).
CENPF autoantibody
Serum autoantibody to CENPF was determined using an in-house CENPF ELISA kit. In brief, 96-well microplates (Nunc, Thermo Fisher Scientific, Waltham, MA, USA) were coated with 100 μL CENP-F antigen (8 μg/mL) and incubated at 4°C overnight. The reaction was blocked with 10% newborn bovine sera (Life Technologies & Invitrogen Corporation, Burlington, Canada). Serum diluted with 10% newborn bovine sera was incubated for 1 h at 37°C. The samples were washed five times, followed by the addition of 100 μL 1:8000 dilution of anti-human immunoglobulin G-peroxidase antibody produced in rabbit (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C. Then, 100 μL of TMB HRP-Substrate (Solarbio Science & Technology, Beijing, China) was added for 10 min at 37°C, and the reaction was stopped by adding 50 μL of stop solution (Solarbio Science & Technology, Beijing, China). The absorbance was read immediately at 450 nm or 620 nm on the microplate reader SpectraMax M3 (Molecular Devices).
Statistical analysis
Study population characteristics were first described and compared between different study groups. The differences in serum levels of the five biomarkers between different groups were examined by chi-square tests. For evaluation of the diagnostic performance of biomarkers, the following diagnosis- related indicators were used: sensitivity (true-positive rate), specificity (true-negative rate), and the area under the receiver operating characteristics (ROC) curve (AUC). For each individual biomarker, a logistic regression model using the original test values was adopted to construct the prediction model. Based on the predicted probabilities derived from the prediction models, AUCs and 95% CIs (calculated based on 2000 bootstrap samples) were calculated and reported, and the differences in AUCs of individual biomarkers were examined by the bootstrap method (2000 bootstrap samples). Moreover, sensitivities of each individual biomarker at cutoffs yielding 90% specificity were calculated. In addition to the apparent estimates of indicators, the .632+ bootstrap method (1000 bootstrap samples with replacement) was applied to adjust for potential overestimation of diagnostic performance.22
Multi-marker algorithms were further explored using logistic regression models based on different biomarker combinations. ROC analyses were further conducted using the approach described above. All the diagnostic-related indicators (e.g., sensitivity, specificity, and AUC) were further adjusted for potential overestimation using the .632+ bootstrap method. The differences in AUCs for different multi-marker algorithms were examined by the bootstrap method (2000 bootstrap samples).
Statistical analyses were performed with the statistical software R version 3.3.2.23 R package “pROC” was employed to perform the ROC analysis. R package “Daim” was used to conduct the .632+ bootstrap analyses. All tests were two sided and p-values of 0.05 or less were considered to be statistically significant.
Results
Overall, 846 participants were recruited in this study, including 202 HCC patients, 226 patients with liver cirrhosis, 215 patients with chronic HBV infection, and 203 healthy volunteers. The characteristics of the study population are shown in Table 1 and Table S1. Overall, more men and older patients were included in the HCC, liver cirrhosis, and chronic HBV infection groups compared to the healthy control group. It was found that 17.3% and 6.9% of the patients in the HCC group and 18.6% and 8.9% of the patients in the liver cirrhosis group also carried HBV infection and hepatitis C virus (HCV) infection, respectively. For the HCC group, 46.5% of the patients were diagnosed at TNM stage I, and 93.6% of the patients had no metastasis. For the liver cirrhosis group, 40.2% (n=91), 29.2% (n=66), and 16.8% (n=38) of the patients had a Child–Pugh score of A, B, and C, respectively. Moreover, 77.9% (n=176) and 22.1% (n=50) of the liver cirrhosis patients were at the decompensated and compensated stage, respectively.
Table 1.
Study population characteristics
| Characteristics | HCC (n=202) | CHB (n=215) | LC (n=226) | HC (n=203) |
|---|---|---|---|---|
| Age (years), mean±SD | 57.2±11.5 | 39.2±11.8 | 50.5±10.4 | 48.2±11.0 |
| Gender, n (%) | ||||
| Male | 170 (84.2) | 134 (62.3) | 161 (71.2) | 97 (47.8) |
| Female | 32 (15.8) | 80 (37.2) | 65 (28.8) | 106 (52.2) |
| Missing | – | 1 (0.5) | – | – |
| HBV infection, n (%) | ||||
| HBV+ | 132 (65.3) | 215 (100.0) | 184 (81.4) | 0 (0) |
| HBV− | 27 (13.3) | 0 (0.0) | 6 (2.7) | 203 (100.0) |
| Missing | 43 (21.3) | 0 (0.0) | 36 (15.9) | 0 (0) |
| HCV infection, n (%) | ||||
| HCV+ | 14 (6.9) | 0 (0) | 2 (8.9) | 0 (0) |
| HCV− | 133 (65.8) | 31 (14.4) | 100 (44.2) | 203 (100.0) |
| Missing | 55 (27.2) | 184 (85.6) | 124 (54.9) | 0 (0) |
| TNM tumor stage, n (%) | ||||
| I | 94 (46.5) | – | – | – |
| >I | 97 (48.0) | – | – | – |
| Missing | 11 (5.4) | – | – | – |
| Child–Pugh, n (%) | ||||
| A | – | – | 91 (40.2) | – |
| B | – | – | 66 (29.2) | – |
| C | – | – | 38 (16.8) | – |
| Missing | – | – | 31 (13.7) | – |
| Metastasis, n (%) | ||||
| Yes | 13 (6.4) | – | – | – |
| No | 189 (93.6) | – | – | – |
Note: “–” = not applicable.
Abbreviations: CHB, chronic hepatitis B virus infection; HBV, hepatitis B virus; HC, healthy control; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LC, liver cirrhosis; TNM, tumor-node-metastasis.
Table S2 presents the associations between the clinicopathological factors and the serum levels of five individual markers in HCC patients. Apart from SCCA, the other four markers, AFP, AFP-L3, DCP, and CENPF, showed significantly higher serum levels in advanced-stage HCCs (TNM stage >I) than in early-stage HCCs (TNM stage I). Other factors, including age, gender, HBV antigens (HBsAg and HBeAg), and HCV infection, were found to have no association with serum levels of the five biomarkers (all p-values<0.05).
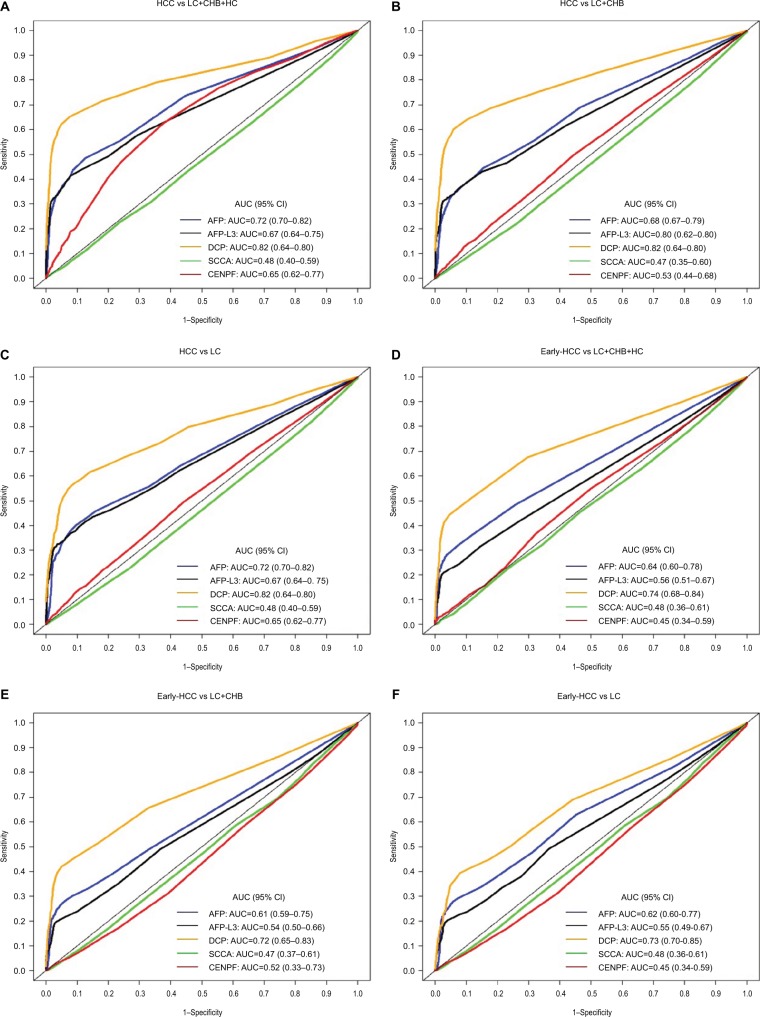
We conducted a direct comparison of the diagnostic performance of the five individual biomarkers in detecting HCC. ROC analyses were conducted based on the logistic regression model and the AUC values of each biomarker in predicting the presence of HCC were calculated. To minimize the potential overestimation, all the diagnostic indicators were further adjusted using the .632+ bootstrap method. (Comparisons between the apparent and .632+ adjusted ROC curves for the five individual biomarkers in discriminating HCC with controls are shown in Figures S2–S6.) The AUCs decreased slightly after adjusting the potential overfitting by the .632+ bootstrap method. For instance, the apparent and .632+ adjusted AUCs (95% CIs) of AFP for discriminating early-stage HCC with liver cirrhosis were 0.74 (0.67–0.79) and 0.62 (0.60–0.77), respectively. Detailed results regarding the comparison of the diagnostic performance of the five biomarkers for detecting HCC are shown in Table 2, Figure 1 and Figure S1. Overall, four of the biomarkers (AFP, AFP-L3, DCP, and CENPF; but not SCCA) presented diagnostic potential in detecting HCC. DCP presented the highest diagnostic value for discriminating HCC versus controls, with a .632+ adjusted AUC of 0.82 (95% CI 0.64–0.80), which is statistically significantly higher than the traditional biomarker AFP (AUC=0.72, 95% CI 0.70–0.82) (p=0.045). When defining cutoffs to yield 90% specificity, the sensitivities of DCP, AFP, AFP-L3, and CENPF in detecting HCC were 65.2% (95% CI 63.3–82.1%), 43.7% (95% CI 36.4–62.3%), 42.6% (95% CI 33.3–53.8%), and 20.4% (95% CI 10.8–42.6%), respectively. When restricting the outcome to early-stage HCC only, the diagnostic performance of all five biomarkers in terms of AUC decreased slightly, but DCP still carried the best diagnostic value, with an AUC of 0.74 (95% CI 0.68–0.84) and sensitivity of 51.0% (95% CI 40.0–75.0%) at 90% specificity. Notably, DCP also presented diagnostic potential in discriminating early-stage HCC versus liver cirrhosis, with an AUC of 0.73 (95% CI 0.70–0.85).
Table 2.
Direct comparison of the diagnostic performance of five markers in detecting HCC
| AFP
|
AFP-L3
|
DCP
|
SCCA
|
CENPF
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b |
| (a) HCC vs LC, CHB, and HC | |||||||||
| 0.72 (0.70–0.82) | 43.7 (36.4–62.3) | 0.67 (0.64–0.75) | 42.6 (33.3–53.8) | 0.82 (0.64–0.80) | 65.2 (63.3–82.1) | 0.48 (0.40–0.59) | 7.5 (0–24.2) | 0.65 (0.62–0.77) | 20.4 (10.8–42.6) |
| (b) HCC vs LC and CHB | |||||||||
| 0.68 (0.67–0.79) | 38.8 (29.4–50.0) | 0.65 (0.62–0.73) | 39.1 (28.3–52.8) | 0.80 (0.62–0.80) | 64.1 (59.7–80.3) | 0.47 (0.35–0.60) | 8.8 (0–31.0) | 0.53 (0.44–0.68) | 14.0 (0–29.0) |
| (c) HCC vs LC | |||||||||
| 0.67 (0.68–0.80) | 40.1 (31.8–57.1) | 0.65 (0.62–0.74) | 40.5 (30.0–53.3) | 0.78 (0.64–0.81) | 57.2 (48.6–75.9) | 0.47 (0.35–0.60) | 8.8 (0–31.0) | 0.52 (0.33–0.73) | 11.8 (0–28.6) |
| (d) Early-stage HCC vs LC, CHB, and HC | |||||||||
| 0.64 (0.60–0.78) | 36.5 (24.2–57.6) | 0.56 (0.51–0.67) | 23.4 (7.7–39.2) | 0.74 (0.68–0.84) | 51.0 (40.0–75.0) | 0.48 (0.36–0.61) | 8.0 (0–24.1) | 0.45 (0.34–0.59) | 6.8 (0–18.2) |
| (e) Early-stage HCC vs LC and CHB | |||||||||
| 0.61 (0.59–0.75) | 30.2 (18.8–43.8) | 0.54 (0.50–0.66) | 22.1 (9.5–38.1) | 0.72 (0.65–0.83) | 45.5 (38.9–72.4) | 0.47 (0.37–0.61) | 7.0 (0–30.8) | 0.52 (0.33–0.73) | 11.8 (0–28.6) |
| (f) Early-stage HCC vs LC | |||||||||
| 0.62 (0.60–0.77) | 30.6 (21.6–53.1) | 0.55 (0.49–0.67) | 22.8 (8.3–40.0) | 0.73 (0.70–0.85) | 48.3 (41.9–76.5) | 0.48 (0.36–0.61) | 8.0 (0–24.1) | 0.45 (0.34–0.59) | 6.8 (0–18.2) |
Notes:
AUC was adjusted for potential overfitting by the .632+ bootstrap method.
.632+ bootstrap adjusted sensitivity at cutoffs yielding 90% specificity.
Abbreviations: AFP, alpha-fetoprotein; AFP-L3, lens culinaris agglutinin-reactive AFP; AUC, area under the curve; CENPF, centromere protein F autoantibody; CHB, chronic hepatitis B virus infection; DCP, des-gamma-carboxyprothrombin; HC, healthy control; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SCCA, squamous cell carcinoma antigen; SEN, sensitivity; SPE, specificity.
Figure 1.
Comparison of .632+ adjusted receiver operating characteristics curves of AFP, AFP-L3, DCP, SCCA, and CENPF for discriminating: (A) HCC vs CHB+LC+HC;
(B) HCC vs CHB+LC; (C) HCC vs CHB; (D) early-stage HCC vs CHB+LC+HC; (E) early-stage HCC vs CHB+LC; and (F) early-stage HCC vs LC.
Abbreviations: AFP, alpha-fetoprotein; AFP-L3, lens culinaris agglutinin-reactive AFP; AUC, area under the curve; CENPF, centromere protein F autoantibody; CHB, chronic hepatitis B virus infection; DCP, des-gamma-carboxyprothrombin; HC, healthy control; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SCCA, squamous cell carcinoma antigen.
We further examined the differences in diagnostic value of the four biomarkers in detecting AFP-positive or AFP-negative patients with HCC, and the results are shown in Table 3. At the commonly used positivity threshold of 20 ng/mL, 99 of the 202 HCC patients (49.0%) were defined as AFP positive, including 38 early-HCC cases (18.8%). Excellent diagnostic performance was observed for AFP-L3 in detecting AFP-positive HCCs, with AUCs higher than 0.90. However, in contrast, AFP-L3 presented no diagnostic value in detecting AFP-negative HCCs. For DCP and CENPF, similar diagnostic values were observed in detecting AFP-negative and AFP-positive HCCs, and the differences were not statistically significant (all p>0.05). For instance, the .632+ adjusted AUCs of DCP in detecting AFP-positive and AFP-negative HCCs versus patients with liver cirrhosis were 0.68 (95% CI 0.62–0.87) and 0.66 (95% CI 0.60–0.81), respectively (p=0.743).
Table 3 (a).
Diagnostic performance of four markers in detecting AFP-positive or AFP-negative patients with HCC
| Marker | HCC vs LC+CHB+HC
|
HCC vs LC+CHB
|
HCC vs LC
|
|||
|---|---|---|---|---|---|---|
| AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | |
| AFP-positivec | ||||||
| AFP-L3 | 0.95 (0.89–0.99) | 89.4 (82.6–100.0) | 0.93 (0.90–0.98) | 85.1 (70.0–100.0) | 0.93 (0.91–0.99) | 87.9 (71.4–100.0) |
| DCP | 0.84 (0.63–0.85) | 65.4 (61.4–88.9) | 0.82 (0.62–0.84) | 64.5 (59.5–87.9) | 0.78 (0.63–0.84) | 59.3 (50.0–83.3) |
| SCCA | 0.46 (0.36–0.61) | 7.8 (0–21.4) | 0.45 (0.31–0.62) | 8.4 (0–29.4) | 0.45 (0.31–0.62) | 8.4 (0–29.4) |
| CENPF | 0.65 (0.62–0.81) | 21.7 (5.9–47.6) | 0.53 (0.40–0.71) | 11.7 (0–29.2) | 0.53 (0.40–0.71) | 11.7 (0–29.2) |
| AFP-negativec | ||||||
| AFP-L3 | 0.48 (0.46–0.53) | 6.7 (0–11.5) | 0.46 (0.44–0.52) | 7.2 (0–10.3) | 0.46 (0.44–0.52) | 7.0 (0–10.7) |
| DCP | 0.78 (0.62–0.81) | 56.3 (48.4–77.8) | 0.76 (0.61–0.82) | 56.6 (48.1–75.8) | 0.73 (0.61–0.81) | 52.1 (41.4–72.5) |
| SCCA | 0.47 (0.36–0.59) | 6.8 (0–27.3) | 0.47 (0.34–0.63) | 7.8 (0–38.1) | 0.47 (0.34–0.63) | 7.8 (0–38.1) |
| CENPF | 0.60 (0.55–0.77) | 20.0 (6.7–41.2) | 0.50 (0.38–0.67) | 12.5 (0–31.6) | 0.50 (0.38–0.67) | 12.5 (0–31.6) |
(b).
Diagnostic performance of four markers in detecting AFP-negative or AFP-positive patients with early-stage HCC
| Marker | Early-HCC vs LC+ CHB+HC
|
Early-HCC vs LC+ CHB
|
Early-HCC vs LC
|
|||
|---|---|---|---|---|---|---|
| AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | |
| AFP-positivec | ||||||
| AFP-L3 | 0.85 (0.61–100.0) | 75.4 (25.0–100.0) | 0.83 (0.62–1) | 74.0 (33.3–100.0) | 0.83 (0.63–1) | 73.7 (33.3–100.0) |
| DCP | 0.72 (0.63–0.90) | 41.4 (26.7–83.4) | 0.70 (0.62–0.88) | 42.6 (28.6–83.3) | 0.68 (0.62–0.87) | 37.1 (25.0–76.5) |
| SCCA | 0.45 (0.33–0.60) | 7.9 (0–22.2) | 0.47 (0.33–0.64) | 7.0 (0–33.3) | 0.47 (0.33–0.64) | 7.0 (0–33.3) |
| CENPF | 0.54 (0.40–0.84) | 12.2 (0–50.0) | 0.44 (0.21–0.73) | 4.9 (0–25.0) | 0.44 (0.21–0.73) | 4.9 (0–25.0) |
| AFP-negativec | ||||||
| AFP-L3 | 0.47 (0.44–0.54) | 9.2 (0–14.3) | 0.46 (0.43–0.53) | 5.4 (0–13.3) | 0.46 (0.43–0.53) | 9.4 (0–13.3) |
| DCP | 0.69 (0.61–0.82) | 48.0 (28.6–70.0) | 0.69 (0.59–0.82) | 39.0 (29.4–68.8) | 0.66 (0.60–0.81) | 36.7 (23.8–64.7) |
| SCCA | 0.49 (0.35–0.62) | 10.8 (0–31.3) | 0.46 (0.35–0.60) | 7.2 (0–33.3) | 0.46 (0.35–0.60) | 7.2 (0–33.3) |
| CENPF | 0.45 (0.30–0.67) | 9.0 (0–22.2) | 0.44 (0.32–0.60) | 6.3 (0–30.0) | 0.44 (0.32–0.60) | 6.3 (0–30.0) |
Note:
AUC was adjusted for potential overfitting by the .632+ bootstrap method.
.632+ bootstrap adjusted sensitivity at cutoffs yielding 90% specificity.
The threshold is 20 ng/mL to define the positivity of AFP.
Abbreviations: AFP, alpha-fetoprotein; AFP-L3, lens culinaris agglutinin-reactive AFP; AUC, area under the curve; CENPF, centromere protein F autoantibody; CHB, chronic hepatitis B virus infection; DCP, des-gamma-carboxyprothrombin; HC, healthy control; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SCCA, squamous cell carcinoma antigen; SEN, sensitivity; SPE, specificity.
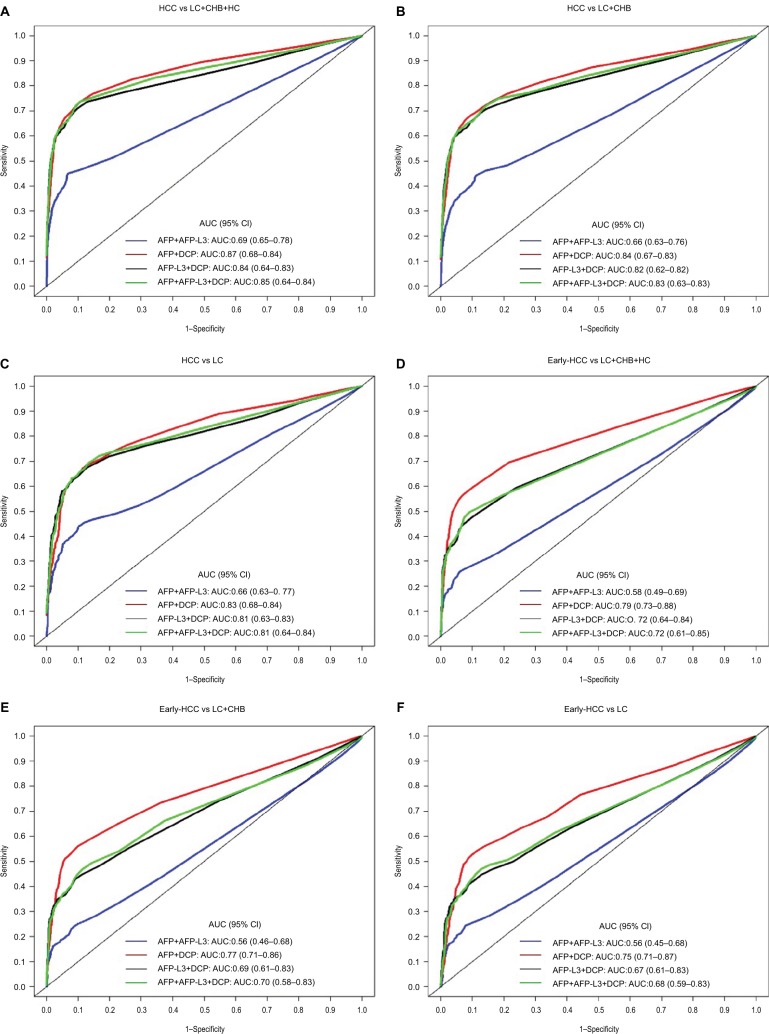
To further enhance the diagnostic performance, a comprehensive search for different biomarker combinations was conducted, and multi-marker prediction algorithms were constructed using logistic regression models. To avoid potential overfitting, all diagnosis-related indicators were further adjusted using the .632+ bootstrap method. Comparisons of the diagnostic performance of the multi-marker algorithms are shown in Table 4 and Table S3 and comparison of the ROC curves of different prediction algorithms in detecting HCC is shown in Figure 2. Overall, for two-marker combinations, a prediction algorithm including AFP and DCP showed the best diagnostic value. The apparent AUC (without correction) of the two-marker algorithm in detecting early-stage HCC was 0.86 (95% CI 0.81–0.90). After correction for potential overfitting, the .632+ adjusted AUC was 0.79 (95% CI 0.73–0.88) and the .632+ adjusted sensitivity was 59.8% (95% CI 46.4–77.4%) at 90% specificity. Further combining AFP-L3 with AFP and DCP did not improve the overall diagnostic performance, but yielded reductions in terms of AUC and sensitivity (Table 4). We further examined the diagnostic performance of the biomarker combinations for discriminating early-stage HCC versus decompensated liver cirrhosis and compensated liver cirrhosis, as shown in Table S4. The combination of AFP and DCP also exhibited good ability in discriminating early-stage HCC versus decompensated liver cirrhosis or compensated liver cirrhosis, with apparent AUCs of 0.81 (95% CI 0.75–0.86) and 0.84 (95% CI 0.78–0.90), respectively, and the difference was not statistically significant (p=0.422).
Table 4(a).
Diagnostic performance of marker combinations in detecting HCC
| Marker | HCC vs LC+CHB +HC
|
HCC vs LC+CHB
|
HCC vs LC
|
|||
|---|---|---|---|---|---|---|
| AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | |
| AFP+AFP-L3 | 0.69 (0.65–0.78) | 46.5 (35.2–57.7) | 0.66 (0.63–0.76) | 40.9 (30.8–53.7) | 0.66 (0.63–0.77) | 43.4 (30.8–55.3) |
| AFP+DCP | 0.87 (0.68–0.84) | 73.8 (63.6–84.2) | 0.84 (0.67–0.83) | 68.2 (59.4–78.5) | 0.83 (0.68–0.84) | 64.2 (53.9–76.6) |
| AFP-L3+DCP | 0.84 (0.64–0.83) | 71.0 (60.4–83.3) | 0.82 (0.62–0.82) | 66.3 (54.5–77.8) | 0.81 (0.63–0.83) | 63.7 (52.8–77.8) |
| AFP+AFP-L3+DCP | 0.85 (0.64–0.84) | 73.7 (60.8–85.1) | 0.83 (0.63–0.83) | 65.7 (54.7–78.6) | 0.81 (0.64–0.84) | 64.5 (52.8–78.7) |
(b).
Diagnostic performance of marker combinations in detecting early-stage HCC
| Marker | Early-stage HCC vs LC+CHB+HC
|
Early-stage HCC vs LC +CHB
|
Early-stage HCC vs LC
|
|||
|---|---|---|---|---|---|---|
| AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | AUC (95% CI)a | SEN at 90% SPE (95% CI)b | |
| AFP+AFP-L3 | 0.58 (0.49–0.69) | 26.8 (10.0–42.9) | 0.56 (0.46–0.68) | 25.0 (9.5–41.7) | 0.56 (0.45–0.68) | 25.7 (7.7–42.1) |
| AFP+DCP | 0.79 (0.73–0.88) | 59.8 (46.4–77.4) | 0.77 (0.71–0.86) | 56.0 (43.2–70.6) | 0.75 (0.71–0.87) | 52.6 (37.0–68.6) |
| AFP-L3+DCP | 0.72 (0.64–0.84) | 47.9 (33.3–72.2) | 0.69 (0.61–0.83) | 42.9 (25.0–64.3) | 0.67 (0.61–0.83) | 42.5 (22.2–63.7) |
| AFP+AFP-L3+DCP | 0.72 (0.61–0.85) | 49.7 (34.8–73.3) | 0.70 (0.58–0.83) | 45.3 (25.0–64.3) | 0.68 (0.59–0.83) | 42.7 (23.1–64.7) |
Note:
AUC was adjusted for potential overfitting by the .632+ bootstrap method.
.632+ bootstrap adjusted sensitivity at cutoffs yielding 90% specificity.
Abbreviations: AFP, alpha-fetoprotein; AFP-L3, lens culinaris agglutinin-reactive AFP; AUC, area under the curve; CHB, chronic hepatitis B virus infection; DCP, des-gamma-carboxyprothrombin; HC, healthy control; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SEN, sensitivity; SPE, specificity.
Figure 2.
Comparison of .632+ adjusted receiver operating characteristics curves of four different multi-marker combinations for discriminating: (A) HCC vs CHB+LC+HC; (B) HCC vs CHB+LC; (C) HCC vs CHB; (D) early-stage HCC vs CHB+LC+HC; (E) early-stage HCC vs CHB+LC; and (F) early-stage HCC vs LC.
Abbreviations: AFP, alpha-fetoprotein; AFP-L3, lens culinaris agglutinin-reactive AFP; AUC, area under the curve; CENPF, centromere protein F autoantibody; CHB, chronic hepatitis B virus infection; DCP, des-gamma-carboxyprothrombin; HC, healthy control; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SCCA, squamous cell carcinoma antigen.
When further combining age and gender with AFP and DCP, the new prediction algorithm showed better diagnostic performance compared to the previous algorithm including AFP and DCP, with a .632+ AUC of 0.88 (95% CI 0.80–0.93) and a sensitivity of 65.4% at 90% specificity in detecting early-stage HCC (Table S5). The comparison of the ROC curves for the two prediction algorithms is shown in Figure S7, and detailed descriptions of the regression equations and optimal probabilities for the two algorithms are shown in Tables S6 and S7.
Discussion
In the present study, serum levels of five biomarkers were simultaneously measured in a large set of samples (N=846). After direct comparison of the diagnostic performance of the five biomarkers, DCP was identified as the best performing biomarker in distinguishing HCC from non-malignant chronic liver diseases, with a .632+ adjusted AUC of 0.82 (95% CI 0.64–0.80) and a .632+ adjusted sensitivity of 65.2% (95% CI 63.3–82.1%) at 90% specificity. We further constructed a prediction algorithm combining AFP and DCP, which presented enhanced diagnostic performance compared to individual biomarkers. The .632+ adjusted AUC of the two-marker algorithm in distinguishing HCC from non-malignant chronic liver diseases was 0.87 (95% CI 0.68–0.84), and the adjusted sensitivity was 73.8% at a specificity of 90%. Notably, the algorithm also showed good ability in discriminating HCC versus liver cirrhosis, with an adjusted AUC of 0.83 (95% CI 0.68–0.84). Furthermore, additionally combining sociodemographic factors of age and gender slightly improved the diagnostic efficacy for detecting HCC.
The definition of the cutoff values directly affects the sensitivity and specificity of the respective biomarkers. In our study, the cutoffs were defined in such a way as to yield the same specificity in order to make a fair comparison of sensitivities for the five biomarkers. The sensitivities were further corrected for potential overestimation using the state-of-the-art bootstrap method. Therefore, the diagnostic performance of the respective biomarkers may be underestimated compared to other published results. Comparisons between the apparent and the .632+ adjusted ROC curves for all five biomarkers are also provided in Figures S2–S6.
DCP, also known as PIVKA-II, has been proposed as a potential serological biomarker for HCC detection in a variety of studies.15,16,24,25 In a meta-analysis summarizing 12 studies, a pooled sensitivity of 71% (95% CI 68.0–73.0%) and a pooled specificity of 84.0% (95% CI 83.0–86.0%) were reported,15 which are similar to the results of our study. Of note, our analyses additionally showed that DCP had a similar diagnostic efficacy in detecting AFP-negative or AFP-positive patients with HCC, indicating that DCP could be potentially used as a complement biomarker of AFP in the diagnosis of HCC. With regard to the laboratory methods of detecting DCP, techniques such as ELISA have been developed and widely used in previous studies.8,26,27 There fore, the combination of DCP and AFP could be potentially implemented in a cost-efficient manner when translated into clinical application.
Our results of the low sensitivity of AFP-L3 in detecting AFP-negative HCC cases were in line with previous studies.13,28 These findings indicated that AFP-L3 may have limited utilization as an independent diagnostic biomarker for HCC, given the relatively high proportion of HCC patients who could be missed by AFP. Evidence on whether combining AFP-L3 and AFP could yield better diagnostic performance than single biomarkers is still conflicting. Although enhanced diagnostic performance for the combination of AFP-L3 and AFP was reported in some studies,29,30 this was not observed in our study. Therefore, the role of AFP-L3 in the diagnosis of HCC should be further evaluated in future studies.
In our study, SCCA was found to show no diagnostic value in the early detection of HCC, which was in line with a previous study conducted by Soyemi and colleagues.17 In a meta-analysis including 12 studies, the pooled sensitivity and specificity of SCCA in detecting HCC were 59% and 76%, respectively.18 However, strong heterogeneity existed owing to the different sample sizes, the different study designs, and the various cutoffs adopted in different studies. Further large prospective studies are required to rigorously evaluate the diagnostic accuracy of SCCA in detecting HCC.
In our previous study, CENPF autoantibody showed potential diagnostic value for early HCC and the majority of early HCC cases with negative AFP were positive for autoantibody to CENPF. Furthermore, the combination of autoantibody to CENPF with AFP improved the ability to diagnose HCC at an early stage, with an AUC of 0.882, or with a sensitivity of 86.7% and a specificity of 68.8%.19 However, in the present study, the performance of CENPF autoantibody showed a lower AUC value in distinguishing HCC from controls, probably as a result of the different controls used and autoimmune diseases which may exist in the cases with liver cirrhosis and hepatitis.
Some biomarkers examined in our study were reported to have a prognostic value in previous studies.26,31,32 For instance, the levels of AFP, AFP-L3, and DCP usually increase as HCC progresses, i.e., with the increase in the size and number of lesions and progression to portal vein invasion.33,34 Moreover, elevated levels of biomarkers were associated with a higher rate of recurrence and poorer survival rate, as reported in previous studies.32 Owing to the limitation of the cross-sectional design, the prognostic value of the individual biomarkers and multi-marker combinations cannot be assessed in our study. However, the exact prognostic value of the multi-marker combinations for predicting progression and survival of HCC patients deserves to be further studied in future investigations.
It should be noted that the diagnostic performance of all five individual biomarkers still does not meet the requirement for HCC screening, given the suboptimal sensitivity for early-stage HCC. Exploration of novel effective biomarkers and the construction of multi-marker combinations are possible ways to further enhance the diagnostic performance in the future. Although promising results regarding multi-marker prediction models were frequently reported in many studies, few of them were independently validated or used rigorous statistical methods to adjust for potential overestimation of diagnostic indicators, as done in our study.24,29,30 For example, in a study by Ertle and colleagues,24 the AUC for the prediction model including AFP and DCP in detecting HCC was reported to be 0.91 using a sample set of 164 HCC and 422 controls, but no external validation or interval validation was adopted.
Patients with liver cirrhosis carry a great risk for the development of HCC, particularly patients at the decompen-sated stage.35–37 The identification and surveillance of patients with liver cirrhosis at high risk of HCC are highly clinically relevant. Current guidelines from the American Association for the Study of Liver Disease (AASLD) and the European Association for the Study of the Liver recommend surveillance of cirrhotic patients with ultrasound with or without AFP every 6–12 months. However, evidence suggested that ultrasound was suboptimal for detecting early-stage HCC and AFP provided no additional benefit to ultrasound.38–41 Our results showed that combining DCP with AFP improved diagnostic efficacy in distinguishing early-stage HCC with liver cirrhosis, indicating that the combination could be considered as a complementary tool for liver cirrhosis surveillance. However, important issues such as surveillance interval and cost-effectiveness of surveillance strategies need to be further explored in future studies.
To our knowledge, our study is the first to conduct a direct comparison of the diagnostic performance of five recently recognized biomarkers in the diagnosis of HCC using a large sample set from China. Specific strengths and limitations deserve careful consideration when interpreting our study. Strengths include that the five biomarkers were simultaneously measured in a large sample set. We also adopted state-of-the-art methods to adjust for potential overestimation of the diagnostic indicator for the single and multi-marker prediction models. The limitations include that the diagnostic performance of biomarkers in detecting HCC with different tumor sizes or tumor differentiation status was not evaluated in our current analysis, and this should be addressed in further studies. Moreover, since the diagnostic performance of the constructed prediction algorithms is still not optimal regarding early diagnosis of HCC, further improvement by combination with other promising biomarkers or established risk prediction models (such as the Child–Pugh–Turcotte score and the model for end-stage liver disease score) would be highly recommended.
In summary, DCP could be used as a complementary biomarker in the diagnosis of HCC and to improve the identification of patients with AFP-negative HCC. The constructed multi-marker prediction algorithms would contribute toward distinguishing HCC from non-malignant chronic liver diseases and may be useful for the surveillance of cirrhosis for HCC.
Acknowledgments
We thank Xiaojin Li, Anjian Xu, Xiaomin He, and Yongle Wu for their contribution to the participant recruitment and specimen collection. We also thank the participants who participated in our study, and Dr. Prudence Carr for the English proof-reading. This study was funded in part by the National Natural Science Foundation of China (grant number 81071973), Capital Foundation of Medical Developments (grant numbers 2014-1-2181, 2016-2-2025), and State Key Projects Specialized on Infectious Diseases (grant numbers 2017ZX10201201-006-003, 2017ZX10201201-007-002, 2017ZX10203202-004-007).
Footnotes
Disclosure
The authors report no potential conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends – an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Poon D, Anderson BO, Chen LT, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10(11):1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 4.Shariff MI, Cox IJ, Gomaa AI, et al. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3(4):353–367. doi: 10.1586/egh.09.35. [DOI] [PubMed] [Google Scholar]
- 5.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122(6):1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juarez-Hernández E, Motola-Kuba D, Chávez-Tapia NC, Uribe M, Barbero Becerra V. Biomarkers in hepatocellular carcinoma: an overview. Expert Rev Gastroenterol Hepatol. 2017;11(6):549–558. doi: 10.1080/17474124.2017.1311785. [DOI] [PubMed] [Google Scholar]
- 8.Lou J, Zhang L, Lv S, Zhang C, Jiang S. Biomarkers for hepatocellular carcinoma. Biomark Cancer. 2017;9:1–9. doi: 10.1177/1179299X16684640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101(3):524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 10.Witjes CD, van Aalten SM, Steyerberg EW, et al. Recently introduced biomarkers for screening of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. 2013;7(1):59–64. doi: 10.1007/s12072-012-9374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39(4):410–433. doi: 10.1053/j.seminoncol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Van Hees S, Michielsen P, Vanwolleghem T. Circulating predictive and diagnostic biomarkers for hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2016;22(37):8271–8282. doi: 10.3748/wjg.v22.i37.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leerapun A, Suravarapu SV, Bida JP, et al. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol. 2007;5(3):394–402. doi: 10.1016/j.cgh.2006.12.005. quiz 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caviglia GP, Abate ML, Petrini E, Gaia S, Rizzetto M, Smedile A. Highly sensitive alpha-fetoprotein, Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxyprothrombin for hepatocellular carcinoma detection. Hepatol Res. 2016;46(3):E130–E135. doi: 10.1111/hepr.12544. [DOI] [PubMed] [Google Scholar]
- 15.Zhu R, Yang J, Xu L, et al. Diagnostic performance of des-γ-carboxy prothrombin for hepatocellular carcinoma: a meta-analysis. Gastroenterol Res Pract. 2014;2014:529314. doi: 10.1155/2014/529314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvas-cular invasion. J Hepatol. 2015;62(4):848–854. doi: 10.1016/j.jhep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Soyemi OM, Otegbayo JA, Ola SO, Akere A, Soyemi T. Comparative diagnostic efficacy of serum squamous cell carcinoma antigen in hepatocellular carcinoma. BMC Res Notes. 2012;5:403. doi: 10.1186/1756-0500-5-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Shao C, Zhou Q, Zhu Y, Zhu J, Tu C. Diagnostic accuracy of serum squamous cell carcinoma antigen and squamous cell carcinoma antigen-immunoglobulin M for hepatocellular carcinoma: a meta-analysis. Mol Clin Oncol. 2015;3(5):1165–1171. doi: 10.3892/mco.2015.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong Y, Long J, Li H, et al. An analysis of immunoreactive signatures in early stage hepatocellular carcinoma. EBioMedicine. 2015;2(5):438–446. doi: 10.1016/j.ebiom.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health of the People’s Republic of China Diagnosis, management, and treatment of hepatocellular carcinoma (V2011) Journal of Clinical Hepatology. 2011;11:1141–1159. [Google Scholar]
- 21.Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Chinese Journal of Hepatology. 2015;23:888–905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efron B, Tibshirani R. Improvements on cross-validation: the 632+ bootstrap method. J Am Stat Assoc. 1997;92(438):548–560. [Google Scholar]
- 23.R Core Team [homepage on the Internet] R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. [Accessed February 05, 2018]. Available from: http://www.R-project.org/ [Google Scholar]
- 24.Ertle JM, Heider D, Wichert M, et al. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87(2):121–131. doi: 10.1159/000346080. [DOI] [PubMed] [Google Scholar]
- 25.Yu R, Ding S, Tan W, et al. Performance of protein induced by vitamin K absence or antagonist-II (PIVKA-II) for hepatocellular carcinoma screening in Chinese population. Hepat Mon. 2015;15(7):e28806. doi: 10.5812/hepatmon.28806v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Rhim H, Kim YS, Kang TW, Song KD. Post-ablation des-gamma-carboxy prothrombin level predicts prognosis in hepatitis B-related hepatocellular carcinoma. Liver Int. 2016;36(4):580–587. doi: 10.1111/liv.12991. [DOI] [PubMed] [Google Scholar]
- 27.Ji J, Wang H, Li Y, et al. Diagnostic evaluation of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatitis B virus-related hepa-tocellular carcinoma in China: a large-scale, multicentre study. PLoS One. 2016;11(4):e0153227. doi: 10.1371/journal.pone.0153227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu B, Tian X, Sun J, Meng X. Evaluation of individual and combined applications of serum biomarkers for diagnosis of hepatocellular carcinoma: a meta-analysis. Int J Mol Sci. 2013;14(12):23559–23580. doi: 10.3390/ijms141223559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim TS, Kim do Y, Han KH, et al. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol. 2016;51(3):344–353. doi: 10.3109/00365521.2015.1082190. [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, Jang JY, Jeong SW, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 2017;96(11):e5811. doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song P, Tang Q, Feng X, Tang W. Biomarkers: evaluation of clinical utility in surveillance and early diagnosis for hepatocellular carcinoma. Scand J Clin Lab Invest Suppl. 2016;245:S70–S76. doi: 10.1080/00365513.2016.1210328. [DOI] [PubMed] [Google Scholar]
- 32.Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer. 2015;4(2):126–136. doi: 10.1159/000367735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto K, Imamura H, Matsuyama Y, et al. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16(10):2795–2804. doi: 10.1245/s10434-009-0618-y. [DOI] [PubMed] [Google Scholar]
- 34.Tada T, Kumada T, Toyoda H, et al. Relationship between Lens culinaris agglutinin-reactive alpha-fetoprotein and pathologic features of hepatocellular carcinoma. Liver Int. 2005;25(4):848–853. doi: 10.1111/j.1478-3231.2005.01111.x. [DOI] [PubMed] [Google Scholar]
- 35.West J, Card TR, Aithal GP, Fleming KM. Risk of hepatocellular carcinoma among individuals with different aetiologies of cirrhosis: a population-based cohort study. Aliment Pharmacol Ther. 2017;45(7):983–990. doi: 10.1111/apt.13961. [DOI] [PubMed] [Google Scholar]
- 36.Mair RD, Valenzuela A, Ha NB, et al. Incidence of hepatocellular carcinoma among US patients with cirrhosis of viral or nonviral etiologies. Clin Gastroenterol Hepatol. 2012;10(12):1412–1417. doi: 10.1016/j.cgh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velázquez RF, Rodríguez M, Navascués CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37(3):520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 38.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52(1):132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography – a randomised study. Aliment Pharmacol Ther. 2013;38(3):303–312. doi: 10.1111/apt.12370. [DOI] [PubMed] [Google Scholar]
- 40.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(1):169–177. doi: 10.1111/apt.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snowberger N, Chinnakotla S, Lepe RM, et al. Alpha fetoprotein, ultrasound, computerized tomography and magnetic resonance imaging for detection of hepatocellular carcinoma in patients with advanced cirrhosis. Aliment Pharmacol Ther. 2007;26(9):1187–1194. doi: 10.1111/j.1365-2036.2007.03498.x. [DOI] [PubMed] [Google Scholar]