Abstract
OBJECTIVE
To compare oral voriconazole with placebo in addition to topical antifungals in the treatment of filamentous fungal keratitis.
DESIGN, SETTING, AND PARTICIPANTS
The Mycotic Ulcer Treatment Trial II (MUTT II), a multicenter, double-masked, placebo-controlled, randomized clinical trial, was conducted in India and Nepal, with 2133 individuals screened for inclusion. Patients with smear-positive filamentous fungal ulcers and visual acuity of 20/400 (logMAR1.3) or worse were randomized to receive oral voriconazole vs oral placebo; all participants received topical antifungal eyedrops. The study was conducted from May 24, 2010, to November 23, 2015. All trial end points were analyzed on an intent-to-treat basis.
INTERVENTIONS
Study participants were randomized to receive oral voriconazole vs oral placebo; a voriconazole loading dose of 400 mg was administered twice daily for 24 hours, followed by a maintenance dose of 200 mg twice daily for 20 days, with dosing altered to weight based during the trial. All participants received topical voriconazole, 1%, and natamycin, 5%.
MAIN OUTCOMES AND MEASURES
The primary outcome of the trial was rate of corneal perforation or the need for therapeutic penetrating keratoplasty (TPK) within 3 months. Secondary outcomes included microbiologic cure at 6 days, rate of re-epithelialization, best-corrected visual acuity and infiltrate and/or scar size at 3 weeks and 3 months, and complication rates associated with voriconazole use.
RESULTS
A total of 2133 patients in India and Nepal with smear-positive ulcers were screened; of the 787 who were eligible, 240 (30.5%) were enrolled. Of the 119 patients (49.6%) in the oral voriconazole treatment group, 65 were male (54.6%), and the median age was 54 years (interquartile range, 42–62 years). Overall, no difference in the rate of corneal perforation or the need for TPK was determined for oral voriconazole vs placebo (hazard ratio, 0.82; 95% CI, 0.57–1.18; P = .29). In prespecified subgroup analyses comparing treatment effects among organism subgroups, there was some suggestion that Fusarium species might have a decreased rate of perforation or TPK in the oral voriconazole-treated arm; however, this was not a statistically significant finding after Holms-Šidák correction for multiple comparisons (effect coefficient, 0.49; 95% CI, 0.26–0.92; P = .03). Patients receiving oral voriconazole experienced a total of 58 adverse events (48.7%) compared with 28 adverse events (23.1%) in the placebo group (P < .001 after Holms-Šidák correction for multiple comparisons).
CONCLUSIONS AND RELEVANCE
There appears to be no benefit to adding oral voriconazole to topical antifungal agents in the treatment of severe filamentous fungal ulcers. All patients in this study were enrolled in India and Nepal; therefore, it is possible that organisms in this region may exhibit characteristics different from those in other regions of the world.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00996736
Fungal corneal ulcers present a therapeutic challenge to clinicians because of their poor outcomes and the sparse evidence to guide treatment.1–7 One study6 estimated that up to 1% of a population in South India would develop a central corneal ulcer and that up to 50% of these ulcers would be fungal. Although fungal ulcers are less common in the United States, they can occur after trauma, with contact lens wear, or after refractive surgery.8,9 An outbreak of Fusarium keratitis among contact lens wearers was related to the ReNu Moisture-Loc contact lens solution (Bausch & Lomb), which was subsequently removed from the market.10
The best treatment strategies for fungal keratitis have not been well characterized. Topical natamycin, a polyene, is the only antifungal agent approved by the US Food and Drug Administration for treatment of fungal keratitis. Voriconazole, a newer generation triazole, has gained popularity as the preferred treatment of fungal keratitis.11,12 In 1 in vitro study,13 voriconazole was the only drug tested to which 100% of fungal isolates commonly implicated in keratitis were susceptible. However, the Mycotic Ulcer Treatment Trial I (MUTT I)14 demonstrated significantly better visual acuity at 3 months in patients with filamentous fungal keratitis randomized to topical natamycin, 5%, vs topical voriconazole, 1%, with fewer adverse events, such as perforation. This finding may be attributable to intermittent drug dosing of topical voriconazole, resulting in intervals of subtherapeutic drug levels. Oral voriconazole exhibits excellent ocular penetration and may provide more consistent drug levels, particularly for deep stromal infections.15,16 In MUTT II, we investigated whether oral voriconazole is a beneficial adjuvant to topical antifungal therapy.
Methods
Trial Design
MUTT II was a National Eye Institute-funded, double-masked, placebo-controlled, randomized clinical trial comparing clinical outcomes in study participants with severe fungal corneal ulcers receiving oral voriconazole vs placebo. The study was conducted from May 24, 2010, to November 23, 2015. All study participants received topical voriconazole, 1%, eyedrops (Vfend IV; Pfizer Inc) reconstituted in sterile water for injection with benzalkonium chloride, 0.01% (Aurolab Inc). Once the results of MUTT I were available,14 topical natamycin, 5%, eyedrops (Natacyn; Alcon) preserved with benzalkonium chloride, 0.01% were added for both arms (May 14,2012). The oral voriconazole and placebo tablets were identical in appearance to ensure masking of patients, physicians, and investigators (Pfizer).
Ethical approval was obtained from the University of California, San Francisco, Committee on Human Research; the Aravind Eye Care System Institutional Review Board, Madurai, India; the Dartmouth-Hitchcock Medical Center Committee for the Protection of Human Subjects, Hanover, New Hampshire; and Nepal Netra Jyoti Sangh, Kathmandu, Nepal. Written informed consent was obtained from all participants, and the trial conformed to the Declaration of Helsinki.17 The Data Safety and Monitoring Committee performed 7 interim reviews for safety, data quality, and trial conduct. Participants did not receive travel funds and free eyeglasses. The trial protocol is available in Supplement 1.
Outcomes
The primary outcome of the trial was rate of perforation or the need for therapeutic penetrating keratoplasty (TPK) within 3 months. Secondary outcomes included best spectacle-corrected visual acuity (BSCVA) at 3 weeks and 3 months, infiltrate and/or scar size at 3 weeks and 3 months, time to re-epithelialization, microbiologic cure at 6 days (±1 day), as well as complications such as endophthalmitis and evisceration.
Study Participants
Enrollment centers included multiple sites of the Aravind Eye Care System (Madurai, Pondicherry, Coimbatore, and Tirunelveli, India), the Lumbini and Bharatpur Eye Hospitals in Nepal, and the University of California, San Francisco. Patients who presented with a smear-positive filamentous fungal corneal ulcer and baseline visual acuity of 20/400 (logMAR 1.3) or worse were screened. After their eligibility was confirmed and they provided written informed consent, patients were randomized to receive oral voriconazole or placebo. Patients were excluded if they had evidence of ocular coinfection, active bilateral ulcers, visual acuity less than 20/200 (logMAR 1) in the fellow eye, corneal scar not easily distinguishable from the current ulcer, impending perforation, known liver disease, pregnancy, weight of less than 40 kg, or age younger than 16 years. On July 9,2014, with approval from the Data Safety and Monitoring Committee, the decision was made to include patients weighing less than 40 kg to improve the rate of enrollment.
Interventions
Eligible patients were randomized to receive oral voriconazole vs placebo. At the beginning of the trial, regardless of weight, patients randomized to receive oral voriconazole were given a loading dose of 400 mg twice daily for 24 hours and then a maintenance dose of 200 mg twice daily for 20 days. As of November 24,2010, after 14 study participants (5.8% of a projected 240) had been enrolled, weight-based dosing was introduced to the protocol in an attempt to reduce adverse effects. If patients weighed 50 kg or more, they were given the original oral voriconazole dose. If they weighed 40 to 49 kg, they received a 300-mg loading dose and a 150-mg twice-daily maintenance dose thereafter, and, if they weighed less than 40 kg, they were not enrolled.18,19 When the inclusion criteria were expanded on July 9, 2014, patients weighing less than 40 kg received a 200-mg loading dose followed by a 100-mg twice-daily maintenance dose.
Topical antifungal agents were applied to the affected eye every hour while the patients were awake for the first week and then every 2 hours while they were awake until 3 weeks after enrollment. The initial protocol called foruse of only topical voriconazole, 1%, to avoid issues of drug antagonism or synergy. However, when the MUTT I trial results became available14 demonstrating that topical natamycin, 5%, was superior to topical voriconazole, 1%, topical natamycin, 5%, was added to both arms in addition to topical voriconazole, 1%. This therapy was initiated after 39 study participants (16.3% of the projected 240) had been enrolled (May 14, 2012). Subsequent adding or changing of topical or oral medications, as well as continuation of the study medication, were at the discretion of the treating physician. Most patients were hospitalized for the first 21 days and the drugs were administered by a health technician who directly observed and recorded that the patient had received the dose. If they were outpatients, the patients were asked about adherence and medication bottles were collected and pills were counted.
Study participants were examined at baseline, every 3 days (±1 day) until re-epithelialization, and at 3 weeks and 3 months after enrollment. A calibrated slitlamp biomicroscope was used to assess the infiltrate and/or scar dimensions and depth, presence of perforation, epithelial defect size after administration of topical fluorescein, hypopyon, and ocular adverse events. Infiltrate and/or scar and epithelial defect were measured according to a protocol adapted from the Herpetic Eye Disease Study.20 Depth of stromal involvement was divided into 3 categories: 0 to 33%; more than 33% to 67%, and more than 67% to 100%. All ophthalmologists were certified to ensure adherence to the study protocol and were masked to treatment arm. The patients were queried regarding serious and nonserious systemic adverse events. Liver function tests were conducted before enrollment and 2 weeks after enrollment. Aspartate aminotransferase and alanine aminotransferase serum levels were monitored for all participants by a nonstudy internist to ensure masking of study personnel.
Best spectacle-corrected visual acuity was recorded at 4 m at enrollment, 3 weeks, and 3 months by a masked refractionist certified for the study using a protocol adapted from the Age-Related Eye Disease Study21 using Early Treatment Diabetic Retinopathy Study (ETDRS) tumbling E charts (charts 2305 and 2305A; Precision Vision). Low vision testing was performed at a distance of 0.5 m. The full protocol details have been previously published.21,22
Microbiological methods used in the MUTT I trial have also been previously published in detail.23 Baseline and 6-day (±1 day) scraping and cultures were obtained from the corneal ulcers of all study participants. A positive fungal smear was defined as fungal elements seen under low-power magnification and reduced light. Positive fungal cultures were defined as growth on any 2 media or moderate to heavy growth on 1 medium.
Statistical Analysis
The sample size was determined based on the primary end point: perforation or the need for TPK within 3 months. Simulation-based analyses estimated that a sample size of240 study participants (120 per arm) would provide 80% power to detect a 15% difference in the 3-month perforation or need for TPK rate between topical antifungal plus oral voriconazole vs topical antifungal alone, with a 2-tailed α value of .05 and approximately 15% loss to follow-up. All trial end points were analyzed on an intent-to-treat basis. Random block sizes of 4, 6, and 8 were generated by site using a random allocation sequence written in R, version 2.1 (R Foundation) (K.J.R and T.C.P.).
Baseline characteristics between the 2 arms were compared using the Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables. The prespecified primary analysis used a Cox proportional hazards regression model to estimate the hazard of perforation or need for TPK associated with oral voriconazole compared with placebo while correcting for baseline infiltrate and/or scar as a fixed effect and study site as a random effect. An identical Cox proportional hazards regression model with an interaction term for organism and treatment arm was used to evaluate the effect of oral voriconazole on the prespecified subgroups of Aspergillus species, Fusarium species, and all other organisms. A Wald test was performed to assess the significance of the interaction between treatment and organism subgroups. Holm-Šidák methods were used to correct for multiple comparisons.24 Additional secondary analyses fit multiple linear regression models to BSCVA measured at 3 weeks and 3 months with covariates for treatment arm and baseline BSCVA. Similarly, multiple linear regression models were used to evaluate 3-week and 3-month infiltrate and/or scar size by treatment arm while correcting for baseline infiltrate and/or scar. Time to re-epithelialization was analyzed using a Coxproportional hazards regression model, with treatment arm and baseline epithelial defect as covariates. The Fisher exact test was used to compare adverse events between the 2 arms.
For missing BSCVA data at 3 weeks or 3 months because of a TPK, we used last observation carried forward or logMAR of 1.7 (Snellen equivalent approximately 20/1000), whichever was worse. If there were missing data for infiltrate and/or scar or epithelial defect size resulting from a TPK, the last observation before TPK was carried forward. Sensitivity analyses for the primary analysis Cox proportional hazards regression model included correcting for baseline infiltrate depth instead of infiltrate and/or scar, correcting for weight, looking only at participants with positive baseline cultures, and excluding those with oral antifungal pretreatment, separately including site as a fixed effect. Sensitivity models for secondary BSCVA analyses included last observation carried forward or logMAR 1.7, whichever was worse for study participants who had perforation but did not receive TPK as well as an analysis with no BSCVA values carried forward after TPK. All analyses were conducted using Stata, version 13 (StataCorp), and were performed from March 2 to 21, 2016.
Results
A total of 2133 patients with smear-positive fungal ulcers were screened between May 24, 2010, and November 23, 2015. Of the 787 who were eligible, 240 were enrolled (30.5%) and randomized to receive oral voriconazole vs oral placebo (Figure). All study participants received both topical antifungals. Enrollment sites included 4 hospitals within the Aravind Eye Care system in India (Madurai, 86 patients [35.8%]; Pondicherry, 39 [16.3%]; Coimbatore, 69 [28.8%]; and Tirunelveli (2 [0.8%]) as well as 2 sites in Nepal (Lumbini Eye Hospital, Bhairahwaw, 41 patients [17.1%]; and Bharatpur Eye Hospital, Bharatpur, 3 [1.3%]). Three-month follow-up data were available for 207 of the 240 patients (86.3%). We found no evidence that loss to follow-up was associated with baseline visual acuity, treatment assignment, or infectious organism. Baseline study participant demographics and clinical characteristics are outlined in Table 1 and isolated organisms are described in Table 2. No major differences were identified between arms.
Figure. CONSORT Diagram.
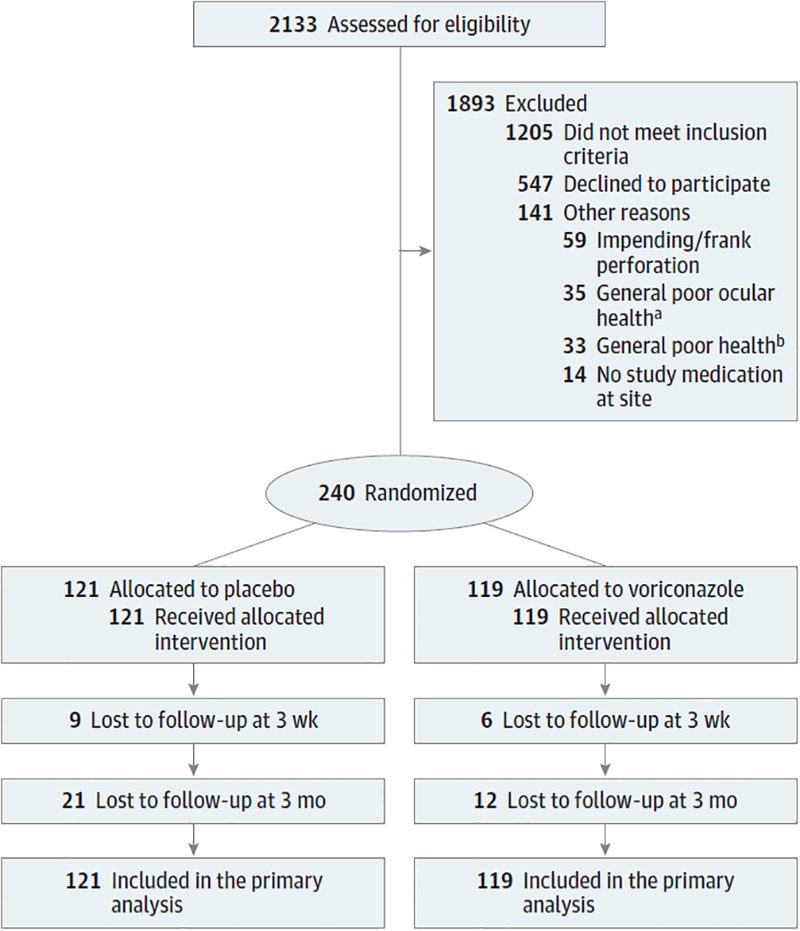
Randomization and treatment of study patients. Available data on patients lost to follow-up were included in the analyses.
a Limbal involvement, endophthalmitis, vitritis, Hansen disease, aphakic bullous keratopathy, monocular, sclera, cataract, glaucoma, neurotrophic ulcer, and uveitis.
b Including diabetes and hypertension.
Table 1.
Baseline Characteristics Between Treatment Groups
| Characteristic | Study Group | |
|---|---|---|
| Placebo (n = 121) | Voriconazole (n = 119) | |
| Sex, No. (%) | ||
| Male | 71 (58.7) | 65 (54.6) |
| Female | 50 (40.3) | 54 (45.4) |
| Age, median (IQR), y | 50 (45–60) | 54 (42–62) |
| Weight, median (IQR), kg | 49 (44–55) | 48 (43–56) |
| Occupation, No. (%) | ||
| Agriculture | 69 (57) | 72 (60.5) |
| Nonagriculturea | 52 (43) | 47 (39.5) |
| Medication use at enrollment, No. (%) | ||
| Topical ocular antifungals | 90 (74.4) | 81 (68.1) |
| Other topical ocular eyedrops | 26 (21.5) | 30 (25.2) |
| Systemic antifungals | 10 (8.3) | 9(7.6) |
| Other systemic agents | 19 (15.7) | 25 (21) |
| Trauma or injury, No. (%)b | ||
| Vegetative matter or wood | 44 (36.4) | 30 (25.2) |
| Metal or otherc | 29 (24) | 34 (28.6) |
| Unknown object | 4(3.3) | 2(1.7) |
| None | 43 (35.5) | 51 (42.9) |
| Affected eye, No. (%)b | ||
| Right | 67 (55.4) | 72 (60.5) |
| Left | 53 (43.8) | 46 (38.7) |
| Visual acuity, median (IQR) | ||
| LogMAR | 1.7 (1.2–1.8) | 1.7 (1.5–1.8) |
| Snellen | CF (20/400, HM) | CF (20/630, HM) |
| Infiltrate and/or scar, median (IQR), mmc | 5.3 (4.6–6.5) | 5.5 (4.4–6.9) |
| Hypopyonb | ||
| No | 33 (27.3) | 32 (26.9) |
| <0.5 mm | 20 (16.5) | 19 (16) |
| ≥0.5 mm | 64 (52.9) | 66 (55.5) |
| Depth, %b | ||
| >0–33 | 35 (28.9) | 24 (20.2) |
| >33–67 | 51 (42.1) | 51 (42.9) |
| >67–100 | 33 (27.3) | 44 (37) |
| Epithelial defect, median (IQR), mmd | 4.7 (3.7–5.9) | 4.6 (3.3–5.8) |
| Duration of symptoms, median (IQR), d | 10 (7–16) | 10 (7–15) |
| Systemic disease, No. (%)e | 11 (9.1) | 15 (12.6) |
Abbreviations: CF, counting fingers; HM, hand motions; IQR, interquartile range.
Includes unemployed or retired.
Some data missing.
Includes dust, battery acid, ash, cement, fingernail, stick, cow or buffalo tail, goat horn, and insect.
Geometric mean of the longest diameter and longest perpendicular to that diameter in millimeters.
Includes diabetes, asthma, and hypertension.
Table 2.
Baseline Microbiologic Culture Results
| Organism | No. (%) | Total (n = 237) | |
|---|---|---|---|
| Placebo (n = 119) | Voriconazole (n = 118) | ||
| Fusarium species | 39 (54.2) | 33 (45.8) | 72 |
| Aspergillus species | 29 (46) | 34 (54) | 63 |
| A flavus | 24 (44.4) | 30 (55.6) | 54 |
| A fumigatus | 3(50) | 3(50) | 6 |
| A niger | 0 | 0 | 0 |
| A terreus | 2 (66.7) | 1 (33.3) | 3 |
| Alternaria species | 0 | 1 (100) | 1 |
| Biopolaris species | 3(75) | 1(25) | 4 |
| Curvularias species | 4 (50) | 4(50) | 8 |
| Exserohilum species | 2 (100) | 0 | 2 |
| Lasiodiplodia species | 1 (50) | 1(50) | 2 |
| Unidentified hyaline | 4(33.3) | 8 (66.7) | 12 |
| Unidentified dematiaceous | 9 (50) | 9(50) | 18 |
| Other species | 5 (45.5) | 6 (54.5) | 11 |
| Fungal culture negative | 23 (52.3) | 21 (47.7) | 44 |
There were a total of 65 perforations, with 30 (46.2%) occurring in the placebo arm and 35 (53.8%) in the oral voriconazole arm. Seventeen (26.2%) of the perforations were managed conservatively. A total of105 TPKs were performed at the discretion of the masked treating ophthalmologist; these included 56 (53.3%) in the placebo group and 49 (46.7%) in the oral voriconazole group. Therapeutic penetrating kerato plasty was performed for impending or frank perforation in 48 study participants (placebo, 23 [41.1%]; oral voriconazole, 25 [51%]) for increasing infiltrate despite current best standard medical therapy or risk of involvement of the corneal limbus in 57 study participants (placebo, 33 [57.9%]; oral voriconazole, 24 [42.1%]).
Overall, there was no evidence of a difference in the rate of perforation or the need for TPK between arms (eFigure in Supplement 2). Cox proportional hazards regression yielded a hazard ratio (HR) of 0.82-fold decreased risk of perforation or need for TPK in the oral voriconazole group compared with the placebo group after controlling for baseline infiltrate and/or scar size and enrollment site, but this finding was not statistically significant (95% CI, 0.57–1.18; P = .29) (Table 3). Sensitivity analyses performed, including controlling for infiltrate depth rather than scar size (HR = 0.90; 95% CI, 0.63–1.29; P = .57), censoring perforation or need for TPK at 3 weeks (HR = 1; 95% CI, 0.64–1.62; P = .93) controlling for weight (HR = 0.84; 95% CI, 0.58–1.20; P = .33), including only patients with baseline culture-positive organisms (HR = 0.73; 95%, CI, 0.50–1.08; P = .12), and excluding patients previously treated with oral antifungal agents (HR = 0.90; 95% CI, 0.61–1.32; P = .47) did not appreciably change the primary outcome.
Table 3.
Prespecified Primary and Secondary Outcomes
| Modela | Result (95% CI)b | P Value |
|---|---|---|
| Primaryanalysis: Coxproportional hazards regression indicating hazard of perforation or need for TPK (n = 237) | ||
| Voriconazole vs placebo | 0.82 (0.57 to 1.18) | .29 |
| Enrollment infiltrate and/or scar | 1.29 (1.15 to 1.45) | <.001 |
| Primary analysis: interaction between treatment and organism subgroup (n = 234) | ||
| Fusarium species: voriconazole vs placebo | 0.49 (0.26 to 0.92) | .03 |
| Aspergillus species: voriconazole vs placebo | 0.78 (0.38 to 1.61) | .50 |
| Other species: voriconazole vs placebo | 1.23 (0.69 to 2.19) | .47 |
| Interaction between organism subgroup and treatment | .15c | |
| Mixed linear regression indicating 3-mo BSCVA (n = 181)d | ||
| Voriconazole vs placebo | −0.02 (−0.18 to 0.14) | .77 |
| Mixed linear regression indicating 3-mo infiltrate or scar (n = 195)d | ||
| Voriconazole vs placebo | −0.16 (−0.49 to 0.17) | .35 |
| Cox proportional hazards regression indicating time to re-epithelialization (n = 238)d | ||
| Voriconazole vs placebo | 0.87 (0.49 to 1.57) | .65 |
Abbreviations: BSCVA, best spectacle-corrected visual acuity; TPK, therapeutic penetrating keratoplasty.
All models corrected for site using random effects.
Cox proportional hazards regression yields a hazard ratio and the mixed linear regressions yield an effect coefficient.
Wald test for interaction.
Controlled for baseline values.
Mean (SD) 3-month visual acuity was logMAR 1.30 (0.61) (Snellen equivalent 20/400) in the placebo arm and logMAR 1.26 (0.61) (Snellen equivalent 20/364) in the oral voriconazole arm. When we assigned a visual acuity value of logMAR 1.7 (Snellen equivalent approximately 20/1000) for participants with TPK or last observation carried forward, whichever was worse, we found no difference in visual acuity between those randomized to oral voriconazole vs placebo after correcting for baseline visual acuity at 3 weeks (predicted BSCVA, −0.04 logMAR; 95% CI, −0.14 to 0.07 logMAR; P = .49) or at 3 months (predicted BSCVA, −0.02 logMAR; 95% CI, −0.18 to 0.14 logMAR; P = .77) (Table 3). A sensitivity analysis with actual BSCVA at 3 months (ie, no BSCVA values carried forward after TPK) also did not detect a difference between arms (predicted BSCVA, −0.06 logMAR; 95% CI, −0.22 to 0.11 logMAR; P = .49).
Multiple linear regression models found no difference in mean 3-week (predicted scar size, 0.02 mm; 95% CI, −0.31 to 0.36 mm; P = .91) or 3-month (predicted scar size, −0.16 mm; 95% CI, −0.49 to 0.17 mm; P = .35) infiltrate and/or scar size among study participants randomized to receive oral voriconazole after controlling for baseline infiltrate and/or scar size. Cox proportional hazards regression yielded 0.87-fold slower time to re-epithelialization with oral voriconazole compared with placebo (mean [SD], 14.89 [7.60] vs 15.16 [7.12]; 95% CI, 0.49–1.57; P = .65) after controlling for baseline epithelial defect size (right censoring 21 days from enrollment). Overall, there was a statistically significant increase in the rate of adverse events among the oral voriconazole vs placebo group (58 [48.7%] vs 28 [23.1%]; P = .001) (Table 4).
Table 4.
Adverse Events by Treatment Group
| Adverse Event | No. (%) | P Valuea | |
|---|---|---|---|
| Placebo (n = 121) | Voriconazole (n = 119) | ||
| AST or ALT elevated to twice ULN | 0 | 10 (8.4) | .003b |
| Visual disturbance | 0 | 5 (4.2) | .03 |
| Nausea, vomiting, diarrhea, and/or stomach pain | 4(3.3) | 10 (8.4) | .11 |
| Dizziness | 1 (0.8) | 5 (4.2) | .12 |
| Endophthalmitis | 1 (0.8) | 3(2.5) | .37 |
| Ulcer not healing after 6 wk of therapy | 2(1.7) | 5 (4.2) | .44 |
| Evisceration | 0 | 1 (0.8) | .50 |
| Myocardial infarction or stroke | 0 | 1 (0.8) | .50 |
| Dermatologic reaction | 0 | 1 (0.8) | .50 |
| Lethargy | 1 (0.8) | 2(1.7) | .62 |
| Increase in hypopyon (>2 mm) | 8 (6.6) | 5 (4.2) | .77 |
| Local allergic reaction | 1 (0.8) | 1 (0.8) | >.99 |
| Headache | 8 (6.6) | 7(5.9) | >.99 |
| Fever | 2(1.7) | 2(1.7) | >.99 |
| Total adverse eventsc | 28 (23.1) | 58 (48.7) | <.001b |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
Fisher exact test.
Statistically significant after Holms-Šidák correction for multiple comparisons.
This was a nonprespecified analysis.
Of the 240 enrolled patients, 193 (80.4%) had culture-positive results at day 1. Of these 193 patients, 51.8% had culture-positive results at day 6 (voriconazole, 50 of 97 [51.5%] and placebo, 50 of 96 [52.1%]). Among Fusarium species, 15 of39 (38.5%) were culture positive inthe placebo arm vs 15 of 33 (45.5%) in the oral voriconazole arm (P = .82). Among Aspergillus species, 24 of 29 (82.8%) were culture positive in the placebo arm compared with 19 of 34 (55.9%) in the oral voriconazole arm (P = .43) and, in all other organisms, 11 of 28 (39.3%) were positive in the placebo arm compared with 16 of 30 (53.3%) in the voriconazole arm (P = .64).
Discussion
In this study, there was no benefit to adding oral voriconazole to topical antifungal eyedrops in the treatment of severe smear-positive, filamentous fungal ulcers. There was no decrease in the rate of perforation or the need for TPK among participants receiving oral voriconazole vs placebo. In addition, we found no difference in 3-month visual acuity, infiltrate and/or scar size, or rate of re-epithelialization among those treated with oral voriconazole vs placebo. In prespecified subgroup analyses, there was some suggestion that Fusarium species might have a decreased rate of perforation or need for TPK in the oral voriconazole-treated arm; however, this finding was not statistically significant after Holms-Šidák correction for multiple comparisons. We observed a statistically significant increase in the risk of adverse events in the oral voriconazole group, including elevation of serum aspartate aminotransferase and/or alanine aminotransferase levels and visual hallucinations, which are well-known adverse effects of the drug.25,26
In vitro evidence27 suggests that fungal isolates from keratitis should be susceptible to voriconazole; however, 3 randomized clinical trials13,14,28 and a recent Cochrane review of the literature2 have concluded that topical natamycin, 5%, is superior to topical voriconazole, 1%, in the treatment of filamentous fungal ulcers.28 There is little information in the literature regarding the value of adding oral antifungal medications, despite the fact that it is a common practice among ophthalmologists. Two case reports29,30 of successful treatment with combined topical, oral, and/or intravenous voriconazole and 1 small randomized clinical trial31 found a possible improvement in time to ulcer healing among patients treated with oral voriconazole compared with oral itraconazole in Aspergillus species. However, a larger placebo-controlled trial32 randomizing patients with severe culture-positive fungal ulcers to receive oral ketoconazole vs placebo plus topical natamycin, 5%, did not find a benefit in the group receiving the oral antifungal.
Limitations to this study include the fact that we enrolled patients with severe ulcers; therefore, it may have been difficult to detect a difference between groups because of the advanced nature of the infection. Several study participants were receiving antifungal treatment before enrollment, which has previously been identified33 as a risk factor for worse outcome; however, these patients were randomized equally to both arms, and most were receiving topical rather than systemic therapy. We used weight-based dosing of oral voriconazole. Efficacy of oral voriconazole may be related to serum concentration, and monitoring a voriconazole trough concentration would have verified that our study participants had the levels necessary to ensure drug efficacy. However, the lowest dose chosen for the smallest patients in the study represented an accepted therapeutic dose for oral voriconazole.26 In addition, most fungal keratitis occurs in regions where voriconazole trough concentration monitoring is not possible; therefore, these results reflect real-life treatment situations. All patients in this study were enrolled in India and Nepal; therefore, it is possible that organisms in this region may exhibit characteristics different from those in other regions of the world. In addition, only small numbers of each organism were represented in this study, which may make it difficult to draw conclusions about the best treatment for each organism. Most of the infections in this study were related to agricultural exposure and not contact lens wear, such as those seen in developed countries. It may be that these differing risk factors and/or genetic factors would modify the interaction between the infectious organism, antifungal medications, and host responses. Our recruitment rate for eligible study participants was 30.5%, which is mostly attributable to the fact that patients at Lumbini in Nepal travel long distances across the Indian border to reach the eye hospital and they were unable or unwilling to commit to hospitalization or follow-up. Finally, we did not assess whether there might be a synergistic, additive, or antagonistic association between natamycin and voriconazole in this trial.
Conclusions
There appears to be no benefit to adding oral voriconazole to topical antifungal agents in the treatment of severe filamentous fungal ulcers. Given the lack of observed benefit combined with the significant increased risk of systemic adverse effects and cost associated with oral voriconazole ($560–$580 for sixty 20-mg tablets http://www.goodrx.com/voriconazole), we cannot recommend the use of adjuvant oral voriconazole for the treatment of severe filamentous fungal keratitis at this time.
Supplementary Material
Key Points.
Question Is oral voriconazole beneficial when added to topical antifungals in the treatment of fungal keratitis?
Findings In this randomized clinical trial of 240 patients in India and Nepal with severe filamentous fungal keratitis, oral voriconazole or placebo was administered in addition to topical antifungal eyedrops. Oral voriconazole did not decrease the rates of perforation or need for therapeutic penetrating keratoplasty, and patients receiving oral voriconazole experienced more adverse events.
Meaning This study shows no benefit to adding oral voriconazole to topical antifungal eyedrops in the treatment of severe filamentous fungal ulcers.
Acknowledgments
Funding/Support: This work was supported by grants U10 EY018573 (Drs Acharya and Lietman) and K23 EY025025 (Dr Rose-Nussbaumer) from the National Eye Institute and grants from That Man May See, the Harper/Inglis Trust, the South Asia Research Foundation, and Research to Prevent Blindness (Drs Acharya and Lietman). Natamycin, 5%, and oral voriconazole were donated by Alcon and Pfizer, respectively.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Ms Ray and Dr Porco had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Prajna, Srinivasan, Das, Oldenburg, McLeod, Zegans, Porco, Acharya, Lietman.
Acquisition, analysis, or interpretation of data: Prajna, Krishnan, Rajaraman, Patel, Srinivasan, Das, Ray, O’Brien, Oldenburg, Zegans, Porco, Lietman, Rose-Nussbaumer.
Drafting of the manuscript: Patel, Rose-Nussbaumer.
Critical revision of the manuscript for important intellectual content: Prajna, Krishnan, Rajaraman, Srinivasan, Das, Ray, O’Brien, Oldenburg, McLeod, Zegans, Porco, Acharya, Lietman.
Statistical analysis: Srinivasan, Ray, Porco, Rose-Nussbaumer.
Obtained funding: Oldenburg, Zegans, Lietman.
Administrative, technical, or material support: Prajna, Krishnan, Rajaraman, Das, Ray, O’Brien, Oldenburg, McLeod, Acharya.
Study supervision: Prajna, Patel, Das, O’Brien, Oldenburg, McLeod, Zegans, Acharya, Lietman.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Group Information: The Mycotic Ulcer Treatment Trial Group—Aravind Eye Hospital, Madurai, Tamil Nadu, India: N. Venkatesh Prajna, MD (principal investigator), Prajna Lalitha, MD, Jeena Mascarenhas, MD, Muthiah Srinivasan, MD, Manoranjan Das, MD, Rajarathinam Karpagam, MSc, Malaiyandi Rajkumar, MSc, S. R. Sumithra, and C. Sundar. Aravind Eye Hospital, Coimbatore, Tamil Nadu, India: Revathi Rajaraman, MD (site director), Anita Raghavan, MD, and P. Manikandan, MPhil. Aravind Eye Hospital, Pondicherry, Tamil Nadu, India: K. Tiruvengada Krishnan, MD(site director) and N. Shivananda, MD. Aravind Eye Hospital, Tirunelveli, Tamil Nadu, India: R. Meenakshi, MD (site director), J. Bharathi, and E. Raja. Bharatpur Eye Hospital, Chitwan, Nepal: Byanju Raghunandan, MD (site director), Kamal Bahadur Khadka, MD, RanjeetShah, MD, and Anju Ligal, MsC. Francis I. Proctor Foundation, University of California, San Francisco: Thomas M. Lietman, MD (principal investigator), Nisha R. Acharya, MD, MS (principal investigator), Stephen D. McLeod, MD, Jennifer Rose-Nussbaumer, MD,John P. Whitcher, MD, MPH, Travis C. Porco, PhD, Salena Lee, OD, Vicky Cevallos, MT(ASCP), Brett L. Shapiro, MD, Catherine E. Oldenburg, MPH, PhD, Kieran S. O’Brien, MPH, and Kevin C. Hong, BA. Lumbini Eye Institute, Bhairahawa, Nepal: Sushila Patel, MD (site director), Salma K. C. Rai, MD, Bel Bahadur Thapa, MD, Binita Bhattarai, MD, Ramesh C. Giri, Abhijeet Sarkar, Santosh Ghimire, Krishna Kunwar, Roji Yadav, Srijana S. Gautam, Sandeep Bashyal, Rojina Begam, and Amar Gautam. Committees—Data and Safety Monitoring Committee: Marian Fisher, PhD (chair), Anthony Aldave, MD, Donald Everett, MA, Jacqueline Glover, PhD, K. Ananda Kannan, MD, Steven Kymes, PhD, and Ivan Schwab, MD. Resource Centers—Coordinating Center, Francis I. Proctor Foundation, University of California, San Francisco: Thomas M. Lietman, MD (principal investigator), Nisha R. Acharya, MD, MS (principal investigator), Stephen D. McLeod, MD, Jennifer Rose-Nussbaumer, MD, John P. Whitcher, MD, MPH, Travis C. Porco, PhD, David Glidden, PhD, Salena Lee, OD, Kathryn Ray, MA, Vicky Cevallos, MT (ASCP), Brett L. Shapiro, MD, Catherine E. Oldenburg, MPH, PhD, Kieran S. O’Brien, MPH, Kevin C. Hong, BA, David Glidden, PhD, and Kathryn Ray, MA. Project Office—National Eye Institute, Rockville, Maryland: Donald Everett, MA. Photography Reading Center—Dartmouth Medical School, Lebanon, New Hampshire: Michael E. Zegans, MD, and Christine M. Kidd, PhD.
REFERENCES
- 1.Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea. 2002;21(6):555–559. [DOI] [PubMed] [Google Scholar]
- 2.FlorCruz NV, Evans JR. Medical interventions for fungal keratitis. Cochrane Database Syst Rev. 2015; (4):CD004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feilmeier MR, Sivaraman KR, Oliva M, Tabin GC, Gurung R. Etiologic diagnosis of corneal ulceration at a tertiary eye center in Kathmandu, Nepal. Cornea. 2010;29(12):1380–1385. [DOI] [PubMed] [Google Scholar]
- 4.Dunlop AA, Wright ED, Howlader SA, et al. Suppurative corneal ulceration in Bangladesh: a study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust NZJ Ophthalmol. 1994;22(2):105–110. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY, Ng TP, Fong KS, Tan DT. Risk factors and clinical outcomes between fungal and bacterial keratitis: a comparative study. CLAOJ. 1997;23(4): 275–281. [PubMed] [Google Scholar]
- 6.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81(11):965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitcher JP, Srinivasan M. Corneal ulceration in the developing world—a silent epidemic. Br J Ophthalmol. 1997;81(8):622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma S, Tuft SJ. Fusarium solani keratitis following LASIKfor myopia. Br J Ophthalmol. 2002; 86(10):1190–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernal MD, Acharya NR, Lietman TM, Strauss EC, McLeod SD, Hwang DG. Outbreak of Fusarium keratitis in soft contact lens wearers in San Francisco. Arch Ophthalmol. 2006;124(7):1051–1053. [DOI] [PubMed] [Google Scholar]
- 10.Yildiz EH, Abdalla YF, Elsahn AF, et al. Update on fungal keratitis from 1999 to 2008. Cornea. 2010;29(12):1406–1411. [DOI] [PubMed] [Google Scholar]
- 11.Hariprasad SM, Mieler WF, Lin TK, Sponsel WE, Graybill JR. Voriconazole in the treatment of fungal eye infections: a review of current literature. Br J Ophthalmol. 2008;92(7):871–878. [DOI] [PubMed] [Google Scholar]
- 12.Loh AR, Hong K, Lee S, Mannis M, Acharya NR. Practice patterns in the management of fungal corneal ulcers. Cornea. 2009;28(8):856–859. [DOI] [PubMed] [Google Scholar]
- 13.Walsh TJ, Pappas P, Winston DJ, et al. ; National Institute of Allergy and Infectious Diseases Mycoses Study Group. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346(4):225–234. [DOI] [PubMed] [Google Scholar]
- 14.Prajna NV, Krishnan T, Mascarenhas J, et al. ; Mycotic Ulcer Treatment Trial Group. The Mycotic Ulcer Treatment Trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiel MA, Zinkernagel AS, Burhenne J, Kaufmann C, Haefeli WE. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob Agents Chemother. 2007;51(1):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Day DM, Head WS, Robinson RD, Clanton JA. Corneal penetration of topical amphotericin B and natamycin. Curr Eye Res. 1986;5(11):877–882. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 18.Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34(5): 563–571. [DOI] [PubMed] [Google Scholar]
- 19.Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. Pharmacokinetics and safety of voriconazole following intravenous-to oral-dose escalation regimens. Antimicrob Agents Chemother. 2002;46(8):2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101(12):1883–1895. [DOI] [PubMed] [Google Scholar]
- 21.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials. 1999;20(6):573–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan M, Mascarenhas J, Rajaraman R, et al. ; Steroids for Corneal Ulcers Trial Group. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012;130(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalitha P, Prajna NV, Oldenburg CE, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea. 2012;31(6):662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 25.Zrenner E, Tomaszewski K, Hamlin J, Layton G, Wood N. Effects of multiple doses of voriconazole on the vision of healthy volunteers: a double-blind, placebo-controlled study. Ophthalmic Res. 2014; 52(1):43–52. [DOI] [PubMed] [Google Scholar]
- 26.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45(7): 649–663. [DOI] [PubMed] [Google Scholar]
- 27.Lalitha P, Sun CQ, Prajna NV, et al. ; Mycotic Ulcer Treatment Trial Group. In vitro susceptibility of filamentous fungal isolates from a corneal ulcer clinical trial. Am J Ophthalmol. 2014;157(2):318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S, Das S, Virdi A, et al. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br J Ophthalmol. 2015;99(9):1190–1195. [DOI] [PubMed] [Google Scholar]
- 29.Hernández Prats C, LlinaresTello F, Burgos San José A, Selva Otaolaurruchi J, Ordovás Baines JP Voriconazole in fungal keratitis caused by Scedosporium apiospermum. Ann Pharmacother. 2004;38(3):414–417. [DOI] [PubMed] [Google Scholar]
- 30.Jones A, Muhtaseb M. Use of voriconazole in fungal keratitis. J Cataract Refract Surg. 2008;34 (2):183–184. [DOI] [PubMed] [Google Scholar]
- 31.Parchand S, Gupta A, Ram J, Gupta N, Chakrabarty A. Voriconazole for fungal corneal ulcers. Ophthalmology. 2012;119(5):1083. [DOI] [PubMed] [Google Scholar]
- 32.Rajaraman R, Bhat P, Vaidee V, et al. Topical5% natamycin with oral ketoconazole in filamentous fungal keratitis: a randomized controlled trial. Asia Pac J Ophthalmol (Phila). 2015;4(3):146–150. [DOI] [PubMed] [Google Scholar]
- 33.Sun CQ, Prajna NV, Krishnan T; Mycotic Ulcer Treatment Trial Group. Effect of pretreatment with antifungal agents on clinical outcomes in fungal keratitis [published online June 21, 2016]. Clin Exp Ophthalmol. doi: 10.1111/ceo.12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


