Abstract
Whether IL-17A has pathogenic and/or protective roles in the gut mucosa is controversial and few studies have analyzed specific cell populations for protective functions within the inflamed colonic tissue. Here we provide evidence for IL-17A dependent regulation of the tight junction protein occludin during epithelial injury that limits excessive permeability and maintains barrier integrity. Analysis of epithelial cells showed that in the absence of Act-1 signaling, the protective effect of IL-17A was abrogated and inflammation was enhanced. We demonstrate that following acute intestinal injury, IL-23R+ RORγt+ γδ T cells in the colonic lamina propria are the primary producers of early, gut-protective IL-17A, over other cell populations such as memory Th17 cells and ILC3. This production of IL-17A was IL-23 independent, leaving protective IL-17 intact in the absence of IL-23. These results suggest that IL-17 producing resident γδ T cells are important for the maintenance, and protectionof epithelial barriers in the intestinal mucosa.
Introduction
IL-17A is the hallmark cytokine of the T-helper (Th17) subset of CD4+ T cells, which have been implicated as the primary pathogenic population in a range of autoimmune disorders (Langrish et al., 2005; Louten et al., 2009; Sallusto and Lanzavecchia, 2009). IL-23 was shown to promote the terminal differentiation and expansion of Th17 effector cells and is thought to orchestrate chronicity and severity in disease models such as experimental autoimmune encephomyelitis (EAE) and inflammatory bowel disease (IBD) (McGeachy et al., 2009; Park et al., 2005; Yen et al., 2006). Specifically in the case of Crohn’s Disease (CD), single nucleotide polymorphisms in the IL-23R locus are associated with disease susceptibility (Duerr et al., 2006), and patients with active CD present with increased numbers of IL-23 and IL-17 expressing cells in the gut lamina propria (Hölttä et al., 2008).
However, increasing evidence suggests opposing effects of the cytokines IL-23 and IL-17 in their contribution to intestinal immunopathology. Murine models of innate (Buonocore et al., 2010) and T cell (Ahern et al., 2010; Yen et al., 2006) driven colitis demonstrate an inflammatory role for IL-23, and neutralizing IL-23 had a protective effect. The efficacy of neutralizing IL-23 clinically has also shown some promise. Administration of Ustekinumab, a monoclonal antibody against the p40 shared subunit of IL-12 and IL-23, showed positive responses in CD patients resistant to anti-tumor necrosis factor (TNF) treatment (Sandborn et al., 2012), and recent data from a Phase 2a study for MEDI2070 – a monoclonal antibody against the p19 subunit of IL-23 – showed great promise in treating TNFα therapy resistant CD patients (Sands et al., 2015) 42.4% of the patients on MEDI2070 showed a clinical response and reduced inflammatory scores compared to 10% in the placebo group and treatment did not increase adverse events, suggesting a more favorable benefit-risk profile compared with the inhibition of both IL-12 and IL-23 through sequestration of p40. In contrast to IL-23, various murine models of colitis suggest a protective role for IL-17A. IL-17A neutralization increased tissue damage in a dextran sodium sulfate (DSS) model of IBD (Ogawa et al., 2004), and IL-17A or IL-17 receptor alpha (RA) deficient T cells resulted in exacerbated colitis when transferred into RAG-1 deficient recipients (O’Connor et al., 2009). Importantly, phase II clinical trials with Secukinumab, targeting IL-17A (Hueber et al., 2012), or Brodalumab, targeting IL-17RA (Targan et al., 2012), was ineffective in treating CD and resulted in either higher rates of adverse events or worsening of CD respectively While the data clearly do not show any efficacy for neutralizing IL-17A or IL-17RA in CD, the current understanding of the mechanism of IL-17 mediated protective effects in both mouse and man is lacking.
Given the suggested role of IL-17A in maintaining barrier function of epithelial tissues (Kinugasa et al., 2000), we were interested in addressing the protective effects of IL-17A on preserving epithelial integrity in a DSS model of acute colonic injury. We demonstrate that IL-17A promoted epithelial barrier function by regulating the cellular localization of the tight junction protein occludin during DSS mediated injury and protected the mice from excessive gut permeability. In addition, our data show that IL-17A can mediate these protective effects by signaling through Act-1 on epithelial cells. We examined the production of IL-17 and IL-22 by multiple adaptive and innate cell populations following intestinal insult and interestingly, we found that the initial source of tissue-protective IL-17A is primarily a tissue resident IL-23R+ γδ T cell as opposed to CD4+ αβ T cell or innate lymphoid cell (ILC), while the major source of IL-22 was from mucosal ILC3. Unexpectedly, the colonic tissue resident γδ T cells did not require IL-23 signaling for the production of IL-17A and mice deficient for IL-23R were protected from the increased barrier dysfunction after DSS treatment that is seen Il-17 −/− mice. Importantly, neutralizing IL-23 dampens adaptive and innate cell activation, while maintaining the production of protective IL-17A during epithelial injury. Taken together, these data provide a mechanism by which IL-17A producing innate cells help protect the epithelial barrier against excessive permeability after injury.
Results
Gut epithelial integrity is compromised in the absence of IL-17 during injury
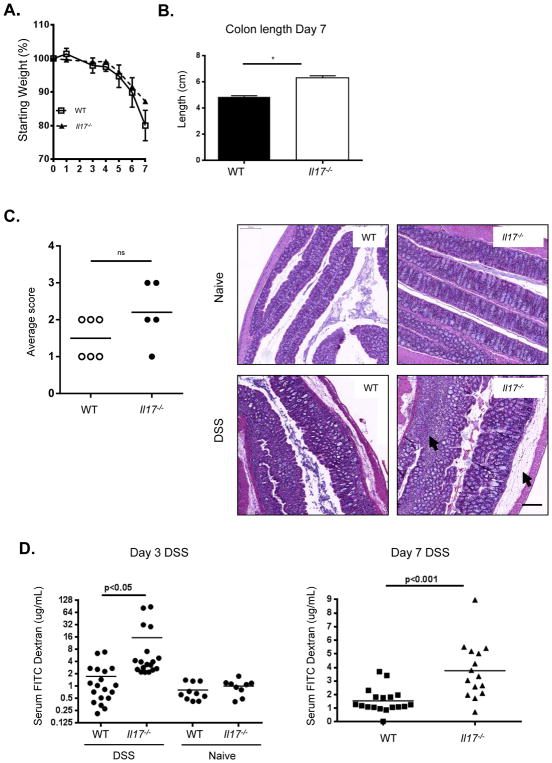
In order to investigate the role of IL-17 in disease progression after gut mucosal injury, we administered DSS in the drinking water of either WT or Il-17−/− mice for 7 days. Mice were monitored for signs and symptoms of injury severity and scored according to weight loss, consistency of stool, rectal bleeding, and colon length. Overall weight loss and colon length were comparable between WT and Il-17−/− mice after 7 days of DSS treatment (Figures 1A and B). Upon gross physical and histological examination, more severe pathology was observed in the Il-17−/− colons compared to WT colons (Figure 1C). While both groups of mice exhibited significant epithelial disruption and the presence of abscesses, severity of disease in the absence of IL-17 was further characterized by enhanced bleeding into the mucosal lumen (Figure 1C) as well as increased edema and cellular infiltrate into the submucosal layer (Figures 1D and Figure S1). To further characterize the damage to the epithelial layer, permeability was quantified by orally administering FITC-dextran to mice on day 3 and day 7 of DSS treatment and measuring the amount present in the serum (Figure 1E). While baseline permeability in naïve mice was similar between WT and Il-17−/− mice, the diffusion of FITC-dextran across the epithelium after DSS treatment was significantly increased in colons from Il-17−/− mice compared to those from WT mice at both time points, suggesting that IL-17 deficiency reduced the ability to maintain appropriate barrier function during DSS-induced injury. Together, these data suggest that IL-17 may provide protection against excessive permeability after mucosal injury. To test if the increased permeability from IL-17 KO mice observed in DSS-induced injury was specific to the model, we neutralized IL-17 in another mouse model of colitis, the T cell transfer model. Anti-IL-17A antibodies did not have any impact on weight loss throughout the course of the disease as compared to controls, but the mice exhibited increased FITC dextran permeabilization at a relatively early time point between three and four weeks after T cell transfer (Figure S2A and B). Interestingly, neutralization of IL-23R had a protective effect on the mice, clearly differentiating IL-17 from IL-23 in colitis.
Figure 1. Il-17−/− mice suffer worse epithelial injury and enhanced gut permeability after DSS administration.
(A) Weight loss of WT (squares), Il-17−/− (triangles) mice over time during DSS treatment, representative data from 3 independent experiments, n=5–7/group, mean ± SEM. (B) Colon length at day 7, combined data from 3 experiments, mean ± SEM. *p<0.05, **p<0.001 (C) Il-17−/− colons exhibit increased bleeding into lumen, representative colons from 3 experiments, n=4–7/group. (D) Pathology scores of disease severity. H&E of colons reveal enhanced edema and lymphocytic infiltrate in Il-17−/− colons (arrows) after DSS, 20x magnification shown. (E) Detection of FITC-dextran in plasma showing increased colon permeability in Il-17−/− over WT after 3 and 7 days of DSS, representative data from 2–3 experiments, or combined data from 3 experiments (day 7), means indicated, *p<0.01. All statistics generated using the one-way ANOVA, with Tukey’s Multiple Comaprisons.
Altered sub-cellular localization of the tight junction protein occludin in the absence of IL-17A following intestinal injury
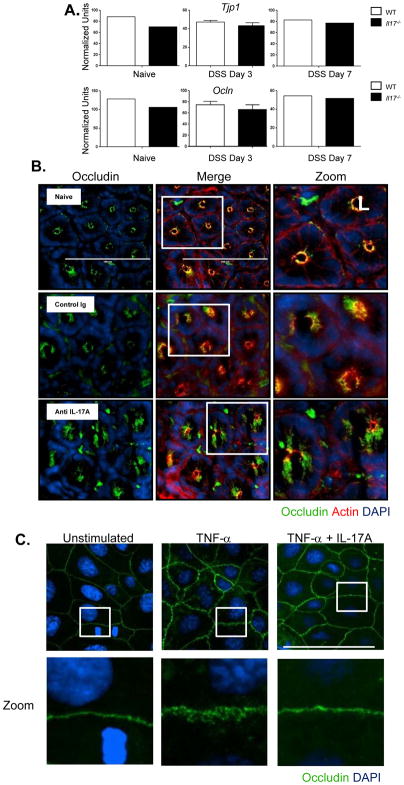
An important component of maintaining intestinal barrier function is the formation and maintenance of tight junctions between epithelial cells. The tight junction complex, composed of proteins including claudins, occludins, and zonula occludens 1 (ZO-1), is a critical structure in regulating intestinal permeability and the epithelial paracellular pathway (Turner, 2009). As IL-17A deficient mice exhibited increased intestinal permeability following gut injury, we hypothesized that the epithelial tight junctions would be compromised in these animals. Transcripts of claudin-1, ZO-1 and occludin did not show any differences between WT and Il-17−/− mice at baseline, and while expression was modulated at day 3 after DSS, IL-17 deficiency did not impact ZO-1 or occludin (Figures 2A) expression. While transcript levels of occludin were similar between WT and Il-17 −/− colon tissue samples, altered intra-cellular localization could explain the increase in permeability and loss of barrier function. Tight junction disruption including internalization of occludin from the tight junction has previously been demonstrated to contribute to increased intestinal permeability (Clayburgh et al., 2005). Cross sectional images of the colonic crypts of epithelial cells in naïve mice showed clear staining of occludin colocalized with f-actin on the apical surface of the cell (Figure S3 and Figure 2B). In contrast to naïve or control Ig treated mice, in animals treated with anti-IL-17A, the colon epithelial cells showed diffuse occludin staining which appeared to extend into cytoplasm as well as a loss of co-localization with f actin. These results demonstrate that while occludin message and protein expression did not seem to be altered with IL-17A neutralization, its cellular localization and ultimately tight junction function was impacted. To determine if IL-17 had a direct impact on occludin subcellular localization, we turned to an in vitro system using the human epithelial colon adenocarcinoma line, Caco-2. TNFα levels are markedly increased in mouse models of IBD as well as CD patients, and TNFα has been shown to disrupt tight junctions and increase epithelial barrier permeability in Caco-2 cells (Ma et al., 2004), Indeed, the addition of TNFα into the culture of Caco-2 monolayers resulted in altered cellular localization for occludin, leading to a disorganized staining pattern that extended into the cytoplasm (Figure 2C). This TNFα mediated disruption of occludin localization was dramatically decreased in the presence of IL-17A. These results show that IL-17A can directly regulate TNFα mediated disruption of tight junction proteins in Caco-2 cells and these data complement the effects of neutralizing IL-17A observed in vivo.
Figure 2. Dysregulation of occludin cellular localization in the absence of IL-17A following DSS induced injury.
(A) Colonic tissues were analyzed by RT PCR for the tight junction proteins ZO-1 and occludin mRNA at day 3 and day 7 after DSS treatment. No difference in ZO-1 or occludin message between WT or Il-17 −/− mice. (B) C57BL/6 mice were subcutaneously administered with either control IgG or anti-IL-17A antibody at 20mg/kg 2 days before DSS treatment. Immunofluorescence images of occludin (green), f-actin (red), DNA (blue) of distal colon segments 3 days after DSS. The fluorescent images depict cross sections of the intestinal crypts in the distal colon with the apical surface of the cell oriented toward the lumen (L). The third column represents a magnified image from the white box in the second column. (C) Caco-2 cells were plated on Poly-L-lysine coated coverslips and treated with recombinant human TNFα (10ng/mL) or TNFα (10ng/mL) + recombinant human IL-17A (10ng/mL) and cultured for 24 hrs. Immunofluorescence images of occludin (green) and DNA (blue). The bottom row represents a magnified image from the corresponding white box in the first row.
IL-17 signals through an Act-1 pathway in epithelial cells to maintain barrier integrity
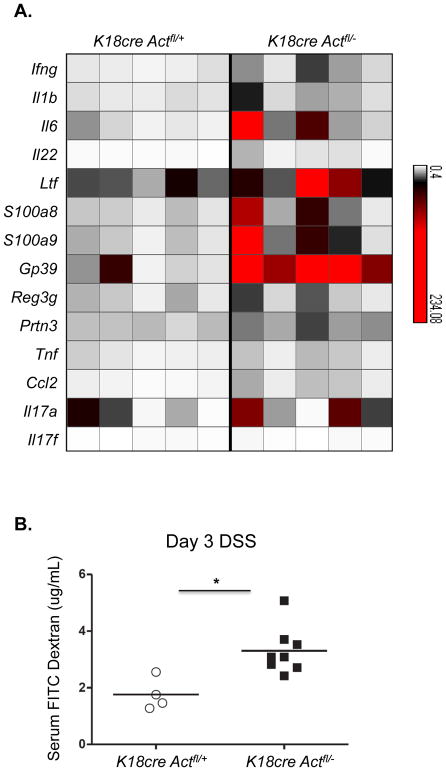
Act-1 is an essential adaptor protein used by IL-17 to mediate downstream cytokine signaling during an inflammatory response (Qian et al., 2002, 2007). We would therefore hypothesize that if IL-17 is helping to maintain epithelial integrity during DSS-colitis, we would expect that in the absence of the ability to signal through Act-1, gut epithelium would exhibit enhanced pathology after treatment with DSS. Act-1 was originally cloned as an NFκB activator, but it has also been shown to negatively regulate B cell function and humoral responses (Qian et al., 2004). In order to test the specific contribution of IL-17 signaling on epithelial cells, we utilized a mouse with a conditional deletion of Act-1 driven by an epithelial specifc protein K18, and thus leaving Act-1 signaling intact in B cells., We induced DSS-colitis in control mice (K18CreAct1fl/+) and epithelial-specific Act-1-deficient mice (K18CreAct1fl/−) and real-time PCR was performed on colonic tissue from both groups in order to examine changes in the overall inflammatory response in the absence of IL-17 mediated Act-1 signaling in epithelial cells (Figure 3A). Upon analysis, message levels for many inflammatory markers were upregulated in the K18CreAct1fl/− mice compared to control mice. These data suggest that the Act-1 signaling pathway plays a role in IL-17-dependent maintenance of epithelial barriers by regulating the extent of inflammation during DSS induced colitis. In addition, we found that mice with the epithelial-specific deficiency in Act-1 exhibited significantly increased gut permeability at day 3 after DSS-induced colitis, as measured by the presence of FITC-dextran in the serum (Figure 3B). Together, these data provide one possible mechanism by which IL-17 signaling directly on epithelial cells maintains mucosal integrity and functional epithelial tight junction.
Figure 3. Enhanced inflammatory signature in the absence of IL-17-induced Act-1 signaling in epithelial cells.
(A) Epithelial cells from the distal colons of control or K18CreAct1fl/− DSS-treated mice were analyzed by RT-PCR. Message levels of inflammatory genes are represented in a heat map as fold change over levels in naïve mice. Each column represents an individual mouse; n=5/group. (B) Detection of FITC-dextran in plasma after 3 days of DSS-treatment revealing increased permeability in epithelial specific Act-1 deficient mice. One of two experiments is shown; *p value = 0.01.
Early IL-17 in the intestinal lamina propria is produced by a RAG and RORγt dependent population
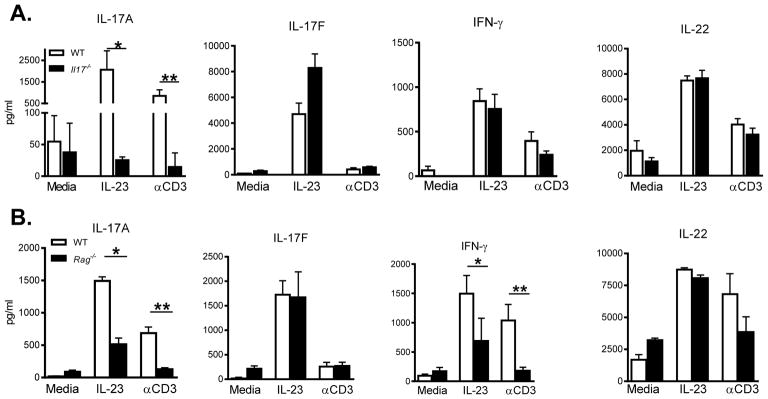
In order to elucidate changes in proinflammatory cytokine expression in the absence of IL-17, we next analyzed colon samples from both WT and Il-17−/− mice at 3 days after DSS administration by RT-PCR. Message levels for IFNγ, IL-22, IL-23p19 and TNFα were comparable between the two groups (Figure S4). Since epithelial cells are capable of producing immune activating cytokines, whole tissue preparations can dilute the ability to detect lymphocyte-specific defects by RT-PCR analysis. Therefore, by isolating and directly re-stimulating colonic lamina propria lymphocytes (cLPL) from mice after DSS administration, we are better able to determine the cytokines most affected locally by the absence of IL-17A. After isolation from the colon, cells were stimulated overnight with either αCD3 or IL-23 and supernatants were analyzed for cytokine protein. WT cells produced abundant amounts of IL-17A, IL-17F, IL-22 and IFNγ in response to both stimuli (Figure 4A). Surprisingly, supernatants from cultures of cells deficient in IL-17A had no inherent defect in overall cytokine production as all protein levels were similar to WT mice, with the exception of IL-17A. Taken together, these data support the conclusion that the production of other cytokines by cLPL cells is independent of the ability to produce IL-17A and suggest that the increased pathology in the colon of Il-17−/− mice is due primarily to the absence of IL-17A.
Figure 4. Cytokine production by colonic lamina propria cells following DSS-induced injury.
Colonic LPL cells were restimulated with media alone, IL-23 or αCD3 overnight and supernatant was analyzed by ELISA or luminex to determine levels of IL-17A, IL-17F, IFNγ and IL-22. (A) Cytokine protein levels detected in supernatants of cLPL isolated from either WT (open bars) and Il-17−/− (closed bars) mice, *p<0.016; **p=0.007; (B) WT and Rag−/− mice, (IL-17A *p=0.0001;**p<0.005), (IFNγ *p<0.048, **p<0.006).
We next sought to identify the primary source of IL-17 by comparing WT and Rag1−/− mice responses to DSS administration. We found that compared to WT cLPL cultures, RAG1 deficient cLPL cells produced less IL-17A and IFNγ in response to IL-23 or αCD3 stimulation (Figure 4B), suggesting that both of these cytokines are mainly secreted by a RAG1 dependent population. In contrast, IL-23, but not αCD3, induced IL-17F and IL-22 production in Rag1−/− mice similar to the levels observed in WT mice (Figure 4B). These data indicate that the induction of IL-17F and IL-22 is RAG-1 independent. These data clearly show that a population of IL-23 and CD3 responsive T lymphocytes, and not ILCs, is contributing to early IL-17A production and is acting as the primary source of IL-17A in our model.
We have previously shown that production of IL-17A by CD4+ T cells is dependent on the transcription factor RORγt (Ivanov et al., 2006). We sought to determine if production of IL-17A by CD3+ lymphocytes was dependent on RORγt, by treating Rorc −/− mice with DSS for 3 days and examining the cytokine production in culture supernatants from isolated cLPL cells. The absence of RORγt not only abrogated IL-17A and IL-17F production in response to αCD3 as expected, but also significantly reduced IL-22 production after TCR ligation (Figure S5). Strikingly, production of all three cytokines in response to IL-23 stimulation was also severely defective in cLPL cultures from RORγt-deficient mice after DSS treatment. Interestingly, IFNγ production was also slightly reduced in response to IL-23 stimulation in Rorc −/− cell cultures compared to WT (Figure S5). Together these data provide evidence that RORγt is required for the trans-activation of IL-23 signaling and optimal production of IL-17A, IL-17F, and IL-22. Importantly, the results provided here implicate ‘innate’ IL-17 and IL-22 secreting populations in the gut that require RORγt for their effector function and contribute to gut protection after injury.
γδ T cells are the primary source of early IL-17A production
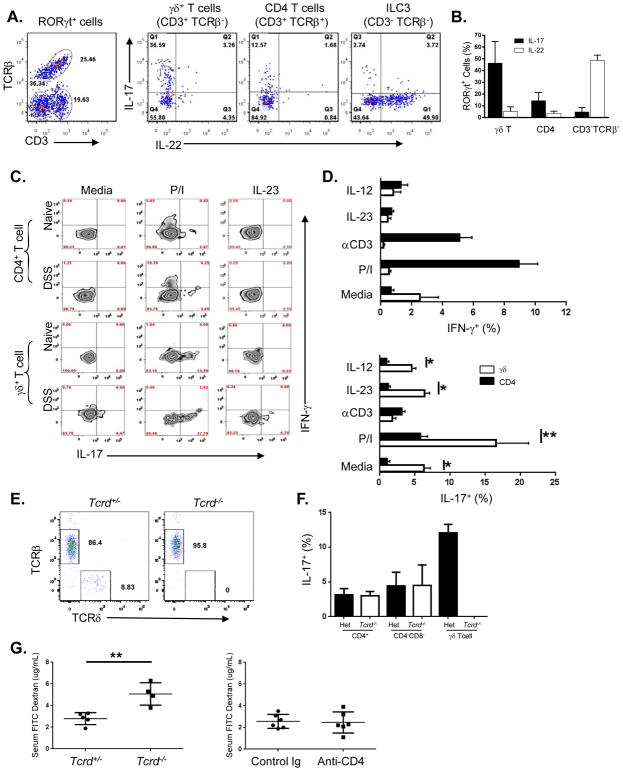
‘Innate’ intestinal populations including ILC3 and tissue resident γδ T cells are early sources of both IL-17 and IL-22 (Martin et al., 2009; Spits et al., 2013). In order to identify the specific contribution of the various RORγt expressing population to IL-17 and IL-22 production, we isolated cLPL cells from Rorc(gt)-GfpTG mice after DSS administration and used surface marker expression to phenotype the different populations. Using GFP expression to detect RORγt expressing cells, we identified three major RORγt+ populations in the colon after DSS treatment: CD3+ γδ T cells, CD4+ αβ T cells and CD3− ILC3 cells (Figure 5A). Intracellular staining for IL-17A and IL-22 revealed that γδ-T cells were the major source of IL-17A in the lamina propria after DSS-induced injury, with over 30% of cells expressing the cytokine (Figures 5A and B) and very few IL-22 producing γδ-Tcells. In contrast to γδ-T cells, ILC3 were the predominant source of IL-22 with approximately 50% of the population staining positive for this cytokine, and very few cells were positive for IL-17. CD4+ T cells only had a minor contribution to IL-17 and IL-22 at this early time point. These data suggest that IL-17A and IL-22 are initially produced by distinct innate cell populations in the gut lamina propria in response to injury. In addition, we clearly demonstrated that γδ T cells are the primary IL-17A producing population. Thus, given their ability to rapidly respond to proteins and inflammatory cytokines (Cua and Tato, 2010), γδ T cells, as opposed to ILC3 are likely to be important in maintaining epithelial integrity in our model through the secretion of IL-17.
Figure 5. Different subsets of cLPL cells produce either IL-17 or IL-22 after restimulation.
(A) Dot plots of the three major RORγt expressing populations that were identified from bulk cLPL cells isolated after 3 days of DSS. Intracellular cytokine analysis of cLPL cells showing IL-17 vs IL-22 production for each population after 3 hrs of restimulation in vitro. Representative dot plots are shown. (B) Percentage of RORγt-GFP+ cells expressing either IL-17 or IL-22 by population, combined data from two experiments is shown, means ± SD. (C) Detection of intracellular IL-17 (x-axis) and IFNγ (y-axis) in cLPL from either naïve or day 3-DSS treated mice after 3–4 hours restimulation ex vivo. Representative zebra plots show either a CD4+ T cell gate (top two rows) or a γδ+ T cell gate (bottom two rows). (D) Bar graph showing percentage of either CD4+ or γδ T cells from DSS treated mice that are positive for IFNγ (top) and IL-17 (bottom), combined data from 2 experiments, with means ± SEM (*p<0.0001; **p<0.04). Two-tailed, Students t test performed. (E) Colon LPL cells from Tcrd +/− and Tcrd −/− mice gated on live, CD3+Thy1+ cells. (F) Colon LPL cells as in (E), stimulated with PMA/ionomycin and gated on the populations indicated. (G) Detection of serum FITC dextran after 3 days of 2.5% DSS in drinking water in the indicated mice.
In general, distinct subsets of γδ T cells exist and have unique function and homing capabilities, including the ability to produce either IL-17A or IFNγ in response to a variety of stimuli (Martin et al., 2009; Ribot et al., 2009). We next determined the capacity of γδ T cells in the mucosal lamina propria to also produce IFNγ after injury induction in comparison to CD4+ αβ T cells. When naïve cLPL cells were isolated and stimulated ex vivo with either PMA and ionomycin or IL-23 we found that while CD4+ T cells were the largest IFNγ producing population, it was γδ T cells that again were the major IL-17A producers (Figure 5C). In addition, after DSS treatment the γδ T cells were still the major source of IL-17A protein, exhibiting a much larger percentage of cytokine positive cells after DSS induced injury (Figure 5C). Our experiments also revealed that lamina propria γδ T cells were more responsive to IL-23 re-stimulation and produced significantly more IL-17A than CD4+ cLPL cells after DSS treatment (Figure 5D). While γδ T cells were the major producers of IL-17A in the colon lamina propria, a minor population of CD4+ T cells was also positive for this cytokine ex vivo, particularly after PMA and ionomycin stimulation. To test the specific contribution of γδ T cells in barrier function during acute DSS injury, we utilized Tcrd KO mice for our studies. We did not find any γδ T cells in the colon lamina propria of Tcrd KO mice (Figure 5E), and did not observe any compensatory increase in the frequency of IL-17 producing CD4+ T cells or CD4-CD8- double negative T cells (Figure 5F). Consistent with the protective role for γδ T cells during DSS injury, Tcrd KO mice exhibited increased FITC dextran permeability (Figure 5G). Finally, we did not observe a protective role for CD4+ T cells as their depletion in vivo did not impact gut permeability (Figure 5G). These data provide evidence that γδ T cell populations in the gut lamina propria are readily able to produce IL-17A protein upon activation, and become quickly responsive to IL-23 stimulation. These ‘innate’ T lymphocytes are therefore, the early source of tissue-protective IL-17A during gut epithelial injury.
IL-17 production by γδ T cells is independent of IL-23 signaling
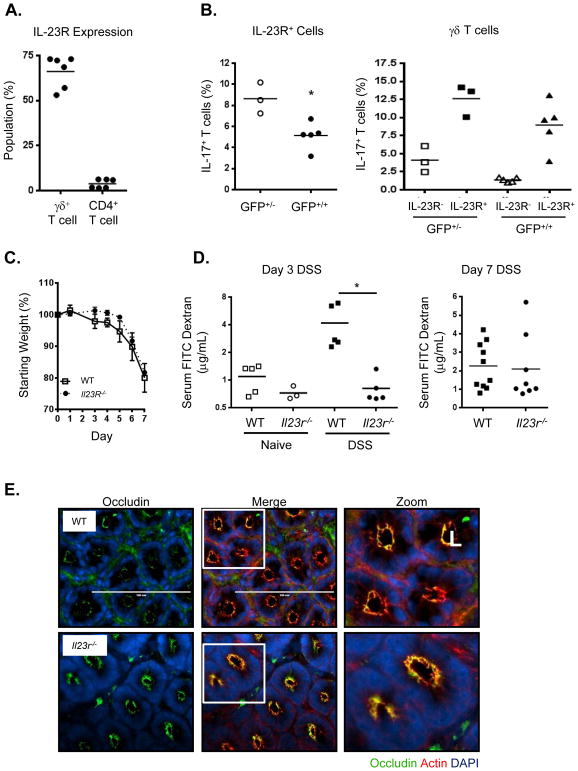
Having shown that the tissue resident RORγt+ γδ T cells are potent producers of IL-17 in response to IL-23, we wanted to determine if IL-23-signaling was required for the early IL-17 production during injury. To do this we made use of the Il23r-Gfp homozygous knock-in mice which allowed us to compare IL-17 production in the presence or absence of IL23R expression. GFP+ cells isolated from Il-23r-Gfp+/− and Il23r-Gfp+/+ littermates after DSS administration were restimulated ex vivo and compared for IL-17 production. Significantly less IL-17 production was observed in the GFP+ cLPLs from Il23r-Gfp+/+ than from Il-23r-Gfp+/− (Figure 6A), indicating that IL-23 does contribute to a portion of the overall IL-17 production early after injury. While there was a significant reduction in the number of γδ T cells in the colon in the absence of IL-23R-signaling, the proportion of IL-17+ cells was comparable between strains (Figure 6B). In contrast, no difference was found in either the proportion of CD4+ cells present in the colon or in the percentage of IFNγ+ cells whether or not IL-23R was present (Figures S6A and B). We hypothesized that the IL-23 independent γδ T cells would still be able to produce protective IL-17 and confer barrier function in the absence of IL-23 during DSS injury. While both WT and Il-23r −/− mice rapidly lost weight (Figure 6C) and exhibited epithelial disruption and the presence of abscesses, Il-23r −/− mice did not exhibit a decrease in barrier function after administration of DSS at both day 3 and day 7 (Figure 6D). Serum FITC dextran levels were surprisingly lower in Il-23r −/− mice than in WT animals at day 3. In support of the maintenance of barrier function, Il23r −/− mouse colon epithelial cells did not exhibit cytoplasmic localization of occludin, but had a staining pattern similar to WT epithelial cells in the crypts (Figure 6E). These data suggest that the early dysregulation of barrier function during DSS is dependent on the absence of IL-17, but not IL-23. IL-23 and IL23R signaling has previously been shown to contribute to inflammation in a DSS model of colitis (Cox et al., 2012). The authors propose that both IL-17 and IL-22 synergize to induce inflammatory cytokines and chemokines resulting in neutrophil infiltration. We did not observe a dramatic protective effect in IL23R deficient animals following DSS injury in terms of weight loss and gut inflammation, and this may be due to variations in the flora between animal facility to facility. However, barrier function in IL-23R deficient mice was comparable to WT mice, if not enhanced at early time points in a T cell transfer model of IBD, we show that in contrast to neutralizing IL-17A alone, targeting IL-23 preserves protective IL-17A produced by IL-23R-independent colonic γδ T cells which promotes maintenance of epithelial barrier function.
Figure 6. IL23R deficient mice do not exhibit increased gut permeability after DSS treatment.
(A) IL-23R expression in different lamina propria lymphocyte populations as determined by Il-23r-Gfp reporter using Il-23r-Gfp+/− mice, combined data from two experiements. (B) IL-17 production is decreased overall in the absence of IL-23R signaling as shown by comparison of Il-23r-Gfp+/− (GFP+/−) (IL-23R present) and Il-23r-Gfp+/+ (GFP+/+ )(IL-23R absent) mice for all GFP+ cLPL (left panel) or γδ T cells only (right panel). (C) Weight loss of WT (squares), Il-23r −/− (circles) mice over time during DSS treatment, representative data from 3 independent experiments, n=5–7/group, mean ± SEM. (D) Detection of FITC-dextran in serum showing increased colon permeability in WT over Il23r −/− mice after 3 and 7 days of DSS, representative data from 2–3 experiments, or combined data from 2 experiments (day 7), means indicated, *p<0.01. All statistics generated using the one-way ANOVA, with Tukey’s Multiple Comaprisons. (E) Immunofluorescence images of occludin (green), f-actin (red), DNA (blue) of distal colon segments from WT or Il23r −/− mice 3 days after DSS. The fluorescent images depict cross sections of the intestinal crypts in the distal colon with the apical surface of the cell oriented toward the lumen (L). The third column represents a magnified image from the white box in the second column.
Discussion
IL-17 promotes the expression of proteins in mucosal tissues that are involved with barrier function during an assault (Kao et al., 2004; Kinugasa et al., 2000; Ogawa et al., 2004). Some of these proteins, such as cellular tight-junction protein, ZO-1, become de-localized early after DSS treatment and this is thought to be a marker of epithelial injury (Poritz et al., 2007). Additionally, E-cadherin is an adhesion molecule involved in the polarity of epithelial cells and along with claudin proteins are required for maintenance of paracellular permeability in mucosal tissue (Balda and Matter, 2008). In addition to these studies, our data show that the absence of IL-17A results in increased epithelial injury and compromised barrier function after DSS treatment. We show that IL-17A regulates the cellular localization of the tight junction protein occludin in the crypts of colon epithelial cells during injury, and IL-17 dependent activation of Act-1 in epithelial cells prevents excess inflammation after DSS. Mice with a conditional deletion of Act-1 in epithelial cells showed an increase in gut permeability after DSS, reinforcing the protective role for IL-17 in barrier function. Interestingly, TNFα mediated barrier disruption in Caco-2 monolayers was reversed with the addition of recombinant IL-17A, suggesting a direct role for IL-17 and IL-17 signaling on epithelial cells in the regulation of occludin. These data demonstrate one potential mechanism by which IL-17A signaling through Act-1 supports barrier function, by maintaining occludin localization at tight junctions during DSS injury.
IL-17 has generally been classified as a pathogenic cytokine in a variety of inflammatory diseases. While IL-17 and IL-17 signaling was previously shown to contribute to pathogenicity in a DSS model of colitis (Qian et al., 2007), it is important to note that the data we present suggest a protective role for IL-17A during an acute model of gut injury. While chronic and long term production of IL-17 mediates inflammatory effects and neutrophil recruitment into the gut, we demonstrate that early, innate sources of IL-17 is a key player in controlling epithelial permeability in an acute model of injury. While the protective role of IL-17A has been discussed in the literature, few studies have analyzed specific IL-17A producing cell populations for their functional significance within the inflamed tissue. Our results involving the gut mucosa concur with previously published work using lung and skin models in which subsets of γδ T cells are important for barrier protection (D’Souza et al., 1997; Jameson et al., 2002; Nakasone et al., 2007). In addition to their protective role in the lung and skin, we provide evidence in this study that γδ T cell derived IL-17A in the colonic lamina propria also provide protection within the colon during DSS injury. We show that colonic γδ T cells are the primary producers of gut-protective IL-17A and are dependent on RORγt, but not IL-23 for their function. RORγt+ TH17 cells are also characterized by the production of IL-17. However, in our animal housing conditions, we found that CD4+ TH17 cells make up a very minor subset of IL-17-producing cells at 3 days post DSS treatment. In contrast to a subset of gut γδT cells that produced constitutive IL-17, CD4+ T cells required PMA/ionomycin stimulation ex vivo to detect IL-17, which may not be representative of their production in vivo. Further confirming the minor contribution of CD4+ T cell derived IL-17, depleting CD4+ T cells did not have an impact on FITC dextran permeability, while Tcrd KO mice showed a clear increase in gut leakiness. The Tcrd−/− study combined with the CD4+ T cell depletion experiment demonstrate the critical function of IL-17 producing γδT cells in protecting barrier surfaces. Our analysis of colonic tissue after early injury confirms the existence of a protective role for γδ T cell-derived IL-17A in the colon that works to limit permeability of the epithelial barrier in order to reduce common complications of mucosal injury, such as excessive edema and anemia.
γδ T cells are categorized according to their differential TCR expression and have distinct functions and homing capabilities. Specific subsets of γδ T cells are able to produce either IL-17A or IFNγ and become activated in the absence of antigen-specific activation (Martin et al., 2009; Ribot et al., 2009). In our DSS injury model, gut homing γδ T cells produce significantly more IL-17A than IFNγ, both constitutively and after the occurrence of an injury. Due to constitutive expression of the IL-23R on γδ T cells as well as other innate cLPL populations, IL-23 is likely to contribute to the overall IL-17 production by these cells. However, γδ T cells seem to be less dependent on IL-23, as receptor deficiency does not significantly abrogate their ability to produce IL-17 after mucosal injury. Thus, it is IL-17 and not IL-23 that is primarily responsible for the observed protective effect in the gut.
There is also a requirement for the Th17 associated-transcription factor RORγt in cLPL populations to secrete both IL-22 and IL-17F in response to re-stimulation with either IL-23 or αCD3. Interestingly, our data shows that IL-22 production, often associated with IL-17-secreting cells, is independent of the ability to produce IL-17A (Liang et al., 2006; Veldhoen et al., 2008). Colonic LPL isolated from Il-17 −/− mice and re-stimulated with IL-23 after injury lead to normal IL-22 production despite the absence of IL-17. It is tempting to speculate whether the balance between IL-17 and IL-22 can mediate their protective or pathogenic effects on the epithelium. This interplay between IL-17 and IL-22 has previously been demonstrated in lung epithelial cells (Sonnenberg et al., 2010). However, further work will need to be done to determine if the pathogenic or protective effect of IL-22 in the gut epithelium can be regulated depending on the presence or absence of IL-17A. While IL-22 is generally thought to be protective at mucosal surfaces, in certain circumstances IL-22 is known to promote inflammation. IL-22 has been shown to stimulate epithelial cell production of chemokines which recruit inflammatory myeloid cells including CXCL1, CXCL2, and CXCL5 (Eken et al., 2013), as well as the proinflammatory cytokine IL-18 (Muñoz et al., 2015). Thus, uncontrolled production of IL-22 in concert with other cytokines may contribute to inflammation. We demonstrate that neutralizing IL-23R signaling in a T cell transfer model of colitis was efficacious in limiting disease, even when treated at later time points, while neutralizing IL-17 recapitulated many of the similar phenotypes we observed in the DSS model of colitis, including increased gut permeability and weight loss. In contrast to IL-23, these two different mouse models of gut damage and human clinical trials consistently demonstrate a protective role for IL-17.
While IL-23 is an important cytokine for driving IL-17 responses, we show that the early protective IL-17A is independent of IL-23, and that the absence of IL-23 signaling does not lead to excessive barrier dysfunction during DSS injury. These findings provide a potential explanation of the dramatic differences between neutralizing IL-23 and IL-17A in DSS injury and epithelial permeability. While there was a significant decrease in the frequency of IL-17 producing cells in IL23R deficient animals, IL-23 independent production of protective IL-17 was still intact in the tissue resident γδ population. We propose that neutralizing IL-23 minimizes tissue inflammation, T cell and myeloid cell activation while leaving the protective function of IL-17 from γδ T cells intact. This early IL-17 production by γδ T cells in our model establishes this population as a major player in limiting inflammation-induced damage in gut epithelium after an injury through an Act1-dependent mechanism of protection. It is notable that γδ T IEL cells can also stimulate mucosal healing after 6 days of DSS treatment and recruit macrophages to injured areas to prevent commensal penetration after damage (Ismail et al., 2009; Pull et al., 2005). Therefore, it is clear from these studies that there exist multiple mechanisms by which γδ T cells and their secretion of IL-17A are important for the maintenance, protection and repair of epithelial barriers in intestinal mucosa.
Recently, a clinical study tested the efficacy of an anti-IL-17A monoclonal antibody in treatment of Crohn’s Disease (CD). Strikingly, blockade of IL-17A in these patients exacerbated their disease and was characterized by an increase in inflammatory markers over baseline (Hueber et al., 2012). Furthermore, for a subset of patients taking the drug, 44% developed additional infections, while none in the placebo group had an adverse event related to infection (Hueber et al., 2012). Similar results were obtained in a separate study targeting IL-17RA (Targan et al., 2012), further confirming the deleterious effects of neutralizing IL-17A or IL-17 signaling for the treatment of CD. Although elimination of IL-17A has been efficacious in other inflammatory disorders such as psoriasis, its failure in the treatment of CD is yet another cautionary tale of the pleiotropic effects of cytokines used as therapeutics (Shen and Durum, 2010). These clinical studies are a strong endorsement of the protective effects of IL-17A in human intestinal mucosa, and provide further support for the data presented within this paper.
Experimental Procedures
Mice
Il-17−/−, Rorc−/−, Rorc(γt)-GfpTG, Il-23r-Gfp, Tcrd−/− were derived as previously described and bred and housed within micro isolator caging units at MRL (Awasthi et al., 2009; Ivanov et al., 2006; Lochner et al., 2008; Nakae et al., 2002) Rag1−/− and WT C57Bl/6 or littermate controls were used as age and sex matched controls where indicated (Jackson Laboratories, Bar Harbor, ME). K18CreAct1fl/− were provided by Xiaoxia Li and are described previously (Qian et al., 2007; Swaidani et al., 2009) All animal procedures were approved by the Institutional Animal Care and Use Committee of Merck Research Laboratories in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care.
DSS and FITC-Dextran Administration
Mice were given 3.5% DSS in drinking water for 3 days before lamina propria cells were isolated from the colons for in vitro culture or a 1cm length of the distal colon was snap frozen for taqman analysis. Alternatively, DSS drinking water was administered for 7 days and mice were monitored for weight loss and signs of disease. RNA isolation and real-time PCR was performed as previously described (Fehniger et al., 1999; McGeachy et al., 2009). Mice were gavaged with FITC-Dextran (4kDa, Sigma Aldrich) as previously described (Dawson et al., 2009) 3 hours prior to fluorometric analysis of FITC fluorescence in plasma.
T cell-driven colitis
Spleen cells from B/6 were processed through 100 μm nylon filters and purified for CD4 using magnetic bead separation (Myltenyi). CD3+ CD4+ CD25− CD45RBhigh T cells were sorted with FACS Aria (BD). The cells (3x105) were injected intravenously, and mice were monitored and weighed for 5 weeks post injection. At the study endpoint, mice were killed and bled and intestines were collected for analysis. Anti IL23R (Merck, mouse IgG1) and anti IL-17A (Merck, mouse IgG1) and isotype control (Merck, mouse IgG1) were administered s.c. at day 14 after transfer at a dose of 50 mpk, and once weekly until 42 days post transfer.
Histology
Dissected colons were fixed in 10% NBF before embedding in paraffin. Hematoxylin and eosin staining was performed on sections of tissue for grading of pathology. All images were acquired using the Zeiss Mirax Midi scanner with a 20x objective.
Immunofluorescence Microscopy
Distal colons were flushed with PBS, embedded in Tissue-Tek O.C.T. compound (SAKURA Finetechnical Company, Ltd., Tokyo, Japan) in cryomolds and snap frozen in liquid nitrogen for cryosectioning. Cryosections were prepared on a Leica Cryostat (Leica Microsystems, Wetzlar, Germany) at −21° C in 5μm thickness. Sections were mounted on glass slides, fixed in 100% ethanol at 4° C for 30 minutes followed by 3 minute of −20° C acetone fixation at room temperature. The slides were washed in PBS and blocked in FBS and rabbit serum. The tissue sections were stained with a monoclonal occludin antibody OC-3F10 (Life Technologies) at 4° C overnight. After washing in PBS, the sections were stained with a rabbit anti-mouse IgG Alexa Fluor 488 conjugated secondary antibody (Life Technologies) and Alexa Fluor 546 Phalloidin (Life Technologies) for 60 minutes at room temperature. The tissue sections were treated with ProLong Mountant with DAPI (Life Technologies) and covered with a coverslip. Fluorescence was visualized on the EVOS FL Cell Imaging System (Life Technologies) at 40X magnification.
Colonic lamina propria cell isolation
Colons were removed from naïve and DSS treated mice and epithelial cells were stripped by incubating in a 37° water bath in cell dissociation solution made with HBSS (BioWhittaker), 5mM EDTA (Invitrogen), and 10mM HEPES (Life Technologies). Supernatant with IEL and epithelial cells was discarded and colonic tissue was then incubated in a digestion cocktail containing HBSS, 10% FCS (Hyclone Laboratories, Logan, Utah), 1mg/ml collagenase type IV, 0.5 mg/ml DNase I and 0.5mg/ml dispase (all from Sigma-Aldrich) in a 37° water bath. Digested tissue was processed through a 70μm filter and washed before lymphocytes were separated using a percoll gradient (GEHealthcare, Upsala, Sweden) and resuspended in complete RPMI (Mediatech Inc, Manassas, VA) supplemented with 10% FCS (Hyclone), 1% HEPES, 50μM 2-mercaptoethanol (Invitrogen), 1% sodium pyruvate, and penicillin and streptomycin (both from Mediatech).
Cell Culture
Colonic lamina propria cells were isolated as above and put into single cell suspensions at 1 or 2 x106 cells/ml. Cells were plated for a final volume of 200μl and restimulated with media alone, 40 ng/ml of IL-23 (DNAX), 10ng/ml of IL-12 (DNAX) or 4μg/ml of αCD3 (BioXcell) for 20 hours; or with PMA and ionomycin for 3 hours. Caco-2 [Caco2] (ATCC HTB-37) were cultured in Eagle’s Minimum Essential Medium in 20% FBS following ATCC instructions.
Flow cytometry
For intracellular cytokine staining cells were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin (both Sigma-Aldrich) in the presence of Golgi-plug (BD Biosciences) for 4 hours in complete medium. Surface staining was then performed in the presence of Fc-blocking antibodies (2.4G2, BD) and using αCD3 (500A2, BD), αCD4 (RMU-5, BD), αTCRβ (H57-597, BD), α–γδTCR (UC7-13D5, eBioscience; or GL3, BD) and α-NKp46 (29A1.4, eBioscience). Cells were then fixed and permeablized using cytofix-cytoperm kit (BD) as directed before intracellular staining using antibodies against IFNγ, IL-22 and IL-17A (all from BD). All samples were collected using Canto II (BD) and data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
RNA Isolation
For gene expression analysis, RNA was isolated by homogenizing colons into RNA STAT-60 (Tel-Test, Friendswood, TX, USA) using a polytron homogenizer, then extracting total RNA according to the manufacturer’s instructions. After isopropanol precipitation, total RNA was re-extracted with phenol:chloroform:isoamyl alcohol (25:24:1) (Sigma-Aldrich, St. Louis, MO) using phase-lock light tubes (5 Prime, Thermo Fisher Scientific Inc., Pittsburgh, PA). For DSS treated samples: total RNA was isolated using RNeasy Midi method (Qiagen, Valencia, CA), where tissue was first homogenized into RLT Buffer plus beta-Mercaptoethanol using a polytron homogenizer, and following manufacturer’s instructions. Total RNA was then re-cleaned over Rneasy Mini columns (Qiagen) following manufacturer’s protocol.
Real-time quantitative PCR
DNase-treated total RNA was reverse-transcribed using QuantiTect Reverse Transcription (Qiagen, Valencia, CA) according to manufacturer’s instructions. Primers were designed using Primer Express (Applied Biosystems, Life Technologies, Foster City, CA) or obtained commercially from Applied Biosystems (Foster City, CA). Gene specific preamplification was done on 10 ng cDNA per Fluidigm Biomark manufacturer’s instructions (Fluidigm, Foster City). Real-time quantitative PCR was then done on the Fluidigm Biomark using one of two different chemistries. For the first chemistry, two gene-specific unlabelled primers were utilized at 400 nM with Taqman Gene Expression Master Mix plus EvaGreen. For the second chemistry, two unlabelled primers at 900 nM each were used with 250 nM of FAM-labeled probe (Applied Biosystems, Life Technologies, Foster City, CA) with Taqman Universal PCR Master Mix with UNG. Samples and primers were run on 96.96 Array(s) per manufacturer’s instructions (Fluidigm). The absence of genomic DNA contamination was confirmed using primers that recognize genomic region of the CD4 promoter. Ubiquitin levels were measured in a separate reaction and used to normalize the data by the Δ-Δ Ct method. (Using the mean cycle threshold value for ubiquitin and the gene of interest for each sample, the equation 1.8 ^ (Ct ubiquitin minus Ct gene of interest) x 104 was used to obtain the normalized values.)
Luminex
Plasma cytokine levels were measured using Luminex system (Millipore Corporation). Plasma samples were collected and spun at 6000 rpm for 15 min at 4°C and . was collected and stored at −80°C analysis.
Supplementary Material
Acknowledgments
The authors thank Dan Littman and Vijay Kuchroo for the generous gifts of gene deficient and transgenic mice, respectively. We also thank Mandy McGeachy and Katia Boniface for reading of the manuscript and for helpful discussion.
Footnotes
Author Contributions
Conceptualization, C.M.T. and D.J.C.; Methodology, J.S.L., C.M.T., and D.J.C., Investigation, J.S.L., C.M.T., B.S., F.G., C.C., Y.C., W.M.B., M.J., K.C., G.A., T.K.M., Writing – Original Draft, J.S.L., C.M.T., D.J.C., Writing – Review & Editing, J.S.L., D.J.C., Resources, X.L., Supervision, D.J.C.
References
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 Drives Intestinal Inflammation through Direct Activity on T Cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Riol-Blanco L, Jäger A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677–3682. doi: 10.1242/jcs.023887. [DOI] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ota N, Leonard J, Roose-Girma M, Diehl L, Ouyang W, Ghilardi N. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5:99–109. doi: 10.1038/mi.2011.54. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- Dawson PA, Huxley S, Gardiner B, Tran T, McAuley JL, Grimmond S, McGuckin MA, Markovich D. Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic NaS1 null mouse. Gut. 2009;58:910–919. doi: 10.1136/gut.2007.147595. [DOI] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken a, Singh aK, Treuting PM, Oukka M. IL-23R(+) innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 2013;00:1–12. doi: 10.1038/mi.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- Hölttä V, Klemetti P, Sipponen T, Kociubinski G, Westerholm-Ormio M, Salo H, Räsänen L, Kolho KL, Färkkilä M, Savilahti E, et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175–1184. doi: 10.1002/ibd.20475. [DOI] [PubMed] [Google Scholar]
- Hueber S, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, GFB, DYM, MK, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;12:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182:3047–3054. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123:1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-Producing γδ T Cells Selectively Expand in Response to Pathogen Products and Environmental Signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M, Eidenschenk C, Ota N, Wong K, Lohmann U, Kühl AA, Wang X, Manzanillo P, Li Y, Rutz S, et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity. 2015;42:321–331. doi: 10.1016/j.immuni.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in Il-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, Uezu K, Nakamura K, Higa F, Ishikawa H, O’Brien RL, et al. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 2007;9:251–258. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- O’Connor W, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the Tight Junction Protein ZO-1 in Dextran Sulfate Sodium Induced Colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Zhao Z, Jiang Z, Li X. Role of NF kappa B activator Act1 in CD40-mediated signaling in epithelial cells. Proc Natl Acad Sci U S A. 2002;99:9386–9391. doi: 10.1073/pnas.142294499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, Omori SA, Rickert RC, Scott M, et al. Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity. 2004;21:575–587. doi: 10.1016/j.immuni.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- Ribot JC, DeBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009;11:620–624. doi: 10.1016/j.micinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Gasink C, Gao LL, Blank Ma, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- Sands BE, Chen J, Penney M, Newbold P, RF, van der Merwe R, Patra K, Pulkstenis E, Drappa J, Gasser RA., Jr OP025. A randomized, double-blind placebo-controlled phase 2a induction study of MEDI2070 (anti-p19 antibody) in patients with active Crohn’s disease who have failed anti-TNF antibody therapy. J Crohn’s Colitis. 2015;9:S15–S16. [Google Scholar]
- Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: New light on inflammatory bowel disease. Neurochemical Research. 2010:940–946. doi: 10.1007/s11064-009-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan SR, Feagan BG, Vermeire S, Panaccione R, Melmed GY, Blosch C, Newmark R, Zhang N, Chon Y, Lin SL, et al. Mo2083 A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Tolerability, and Efficacy of AMG 827 in Subjects With Moderate to Severe Crohn’s Disease. Gastroenterology. 2012;143:e26. [Google Scholar]
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.