Abstract
IMPORTANCE
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal disease with no known cause. Case studies primarily of athletes and several case-control studies have suggested that high levels of strenuous physical activity (PA) may increase the risk for ALS. This relationship has yet to be evaluated among women in population-based cohort studies.
OBJECTIVE
To evaluate the relationship between PA and risk for ALS mortality in a large cohort of postmenopausal women.
DESIGN, SETTING, AND PARTICIPANTS
The Women’s Health Initiative (WHI) enrolled 161 809 postmenopausal women, aged 50 to 79 years (mean [SD] age, 63.6 [7.24] years), between 1993 and 1998 into either a clinical trial or an observational study at 40 clinical research centers across the United States. We conducted a cohort study from November 2014 to September 2015 using baseline and mortality data during an average of 9.6 years of follow-up from the entire WHI cohort, through September 1, 2013 (with 1.1% lost to follow-up), to address whether there is a relationship between PA and ALS mortality.
EXPOSURES
The WHI assessed frequency and duration of mild, moderate, and strenuous PA at baseline via self-administered questionnaire.
MAIN OUTCOMES AND MEASURES
Underlying cause of death from ALS collected from death certificates.
RESULTS
The WHI enrolled 161 809 women, of whom 165 died of ALS; women who died of ALS were older (median age, 66 years; interquartile range, 61-69 years) compared with the total WHI study population (median age, 63 years; interquartile range, 57-69 years). Age-adjusted ALS mortality rates varied from 7.4 (95% CI, 5.5-9.9)/100 000 person-years for no strenuous PA to 10.6 (95% CI, 5.6-20.0)/100 000 person-years for strenuous PA 3 or more days per week (P = .07). Adjusted for age and body mass index (calculated as weight in kilograms divided by height in meters squared), the odds ratio for death from ALS for participants with strenuous PA 3 or more days per week compared with no reported strenuous PA was 1.56 (95% CI, 1.02-2.37; P = .04).
CONCLUSIONS AND RELEVANCE
To our knowledge, this is the first cohort study to report an increased risk for ALS mortality associated with strenuous PA in postmenopausal women. The association between strenuous PA and ALS risk observed does not compromise the overall benefit of strenuous PA for total mortality, coronary heart disease, and breast cancer reported in other WHI investigations, but it may provide an important clue to the etiology of ALS, if replicated by other studies.
Amyotrophic lateral sclerosis (ALS) is the most common form of degenerative motor neuron disease in adulthood,1 with an estimated incidence between 1 and 2 cases per 100 000 persons per year.2,3 The incidence of ALS rises with increasing age, particularly among men, with a mean age at onset of approximately 55 to 58 years.4,5 The disease is typically rapidly progressive and ultimately fatal, with a median survival of approximately 2 to 4 years after onset.1
The specific cause of ALS is unknown.6 Approximately 5% to 10% of cases are familial and characterized by an autosomal mode of inheritance.7–11 Expansion of hexanucleotide repeat sequences in the noncoding region of C9orf72 is the most common genetic cause of familial ALS and is present in about 10% of sporadic cases.12,13 The remaining majority of sporadic cases have no single identifiable causative agent. Cigarette smoking14,15 and other environmental and occupational exposures16–19 have been reported as increasing risk for ALS. Increased risk for ALS mortality has also been observed in men with a history of military service.20,21
Recent research has suggested a link between strenuous physical activity (PA) and ALS onset. Early investigations noted higher rates of death from ALS among professional athletes than in the general population.22–24 Only a few population-based studies have investigated the potential relationship between strenuous PA or past participation in athletics and the occurrence of ALS, yielding inconsistent results.25,26 Some case-control studies have provided moderate evidence for an increased incidence of ALS later in life related to leisure PA and sports participation,18,27–29 while other studies have reported no association between the volume of leisure PA and ALS.30
To date, no large population-based longitudinal study has enrolled enough women, has collected information on strenuous PA, and has provided enough follow-up time to assess risk for death from ALS. We used data from more than 161 000 women in the Women’s Health Initiative (WHI)31 to evaluate the relationship between strenuous PA and risk of ALS mortality. We hypothesized that frequency, duration, and energy expenditure of strenuous PA are associated with an increased risk for ALS mortality. As a secondary evaluation, we examined relationships between light and moderate intensity PA to determine whether there may be an intensity effect for PA and the risk for death from ALS.
Methods
The WHI recruited 161 809 postmenopausal women aged 50 to 79 years between 1993 and 1998 at 40 clinical centers across the United States into 1 or more arms of the clinical trial component or into the observational study.31 We conducted a cohort study from November 2014 to September 2015 using baseline and mortality data from all women enrolled in the WHI, regardless of the study component. The primary end point was underlying cause of death from ALS reported on death certificates. Mortality follow-up has been practically complete, with only about 1.1% lost to follow-up. Information on 165 deaths in which ALS was the underlying cause was available for the current study based on follow-up to September 1, 2013. Institutional review board approval was obtained at each clinical center, overseen by the WHI coordinating center. All participants provided written informed consent.
A self-administered personal habits questionnaire ascertained usual PA, exercise, and lifestyle information at the time of WHI enrollment. Physical activities and exercise were classified as mild, moderate (described as not exhausting), or strenuous or very hard (described as “work[ing] up a sweat and your heart beats fast”), with examples given for each category. Activity frequency within each category was reported in days per week and duration was estimated in 20-minute increments (<20, 20-39, 40-59, and ≥60 minutes). Metabolic equivalent task (MET) scores assigned to each category from a compendium of PAs32 were used to calculate energy expenditure in MET-hours per week in each category from reported frequency and duration (using the midpoint of the 20-minute intervals). These summary variables have been used in several analyses of the WHI cohort.33–35 The frequency, duration, and energy expenditure within each intensity of PA were considered individually in examining the relationship between PA and ALS mortality. An additional question asked participants to indicate whether they usually did strenuous or very hard exercise 3 or more days per week at ages 18, 35, and 50 years.
The PA questions have been tested for reliability and validity in the WHI population.36,37 The test-retest reliability for PA variables, including strenuous PA, reported as a weighted κ coefficient ranged from 0.67 to 0.71.38,39 The interclass correlation coefficient for total energy expenditure for recreational activity was 0.77. Sixty-three women completed both the 7-day PA recall and the WHI personal habits questionnaires and wore an accelerometer for 1 week. Both questionnaires correlated well (r = 0.73) with the accelerometer. The WHI measure of PA had comparable validity, sensitivity, and measurement bias compared with the 7-day PA questionnaire.37
The entire WHI cohort (n = 161 809) was used for the analyses. Descriptive statistics characterized the study population. Categorical variables are presented as frequency and percentage and continuous variables are reported as mean (standard deviation) or, if the distribution was skewed, median (interquartile range). Two-sample t tests compared baseline PA variables between WHI participants who died of ALS and the remaining cohort. Age-adjusted mortality rates and their 95% confidence intervals were calculated using direct method with the entire WHI population as the standard population. To quantify hazard ratios (HRs) and 95% confidence intervals for ALS mortality, we used Cox proportional hazards models adjusted for potential confounders. Sensitivity analysis was conducted excluding women who died of ALS within 2 years of WHI baseline (n = 4). Analyses were performed with SAS version 9.4 statistical software (SAS Institute, Inc). All tests were 2-sided at α = .05.
Results
The WHI study has included 165 women with ALS as the underlying cause of death as of the 2013 follow-up. Among these women, the mean (SD) follow-up time to death or censor was 9.59 (4.0) years (range, 0.87-17 years). The distribution of baseline characteristics and unadjusted percentage of deaths from ALS for the WHI cohort are presented in Table 1. Women who died of ALS had a median age of 66 years (interquartile range, 61-69 years) at study entry, were predominantly non-Hispanic white (n = 145 [87.9%]), and were not employed at the time of enrollment (n = 101 [61.2%]). Baseline characteristics associated with ALS mortality in this sample included increased age (P < .001), lower body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) (P = .04), not having diabetes (P = .03), and marital status of never married and married compared with divorced, separated, or widowed (P = .04). Reported cardiovascular disease and cancer at baseline were not significantly related to the risk of ALS. Risk was slightly higher among women who reported arthritis, but there was no observed relationship with joint pain, generally associated with decreased PA, or with depression score (data not shown).
Table 1.
Frequency of Death From ALS by Baseline Characteristics in the Total Women’s Health Initiative Cohort
| Characteristic | Total, No. (%) | Death From ALS, No. (% Among Total) | P Value |
|---|---|---|---|
| Age, y | |||
| 50-59 | 53558 (33.1) | 30 (0.06) | <.001 |
| 60-69 | 72587 (44.9) | 97 (0.13) | |
| 70-79 | 35664 (22.0) | 38 (0.11) | |
| Race/ethnicity | |||
| Black | 14627 (9.1) | 14 (0.10) | .02 |
| White | 133534 (82.7) | 145 (0.11) | |
| Other | 13232 (8.2) | 4 (0.03) | |
| Region | |||
| Northeast | 36914 (22.8) | 44 (0.12) | .70 |
| South | 41919 (25.9) | 40 (0.10) | |
| Midwest | 35563 (22.0) | 34 (0.10) | |
| West | 47413 (29.3) | 47 (0.10) | |
| Education | |||
| High school or less | 36261 (22.6) | 33 (0.09) | .64 |
| Some college | 60903 (37.9) | 61 (0.10) | |
| College graduate or higher | 63410 (39.5) | 70 (0.11) | |
| BMI | |||
| <18.5 | 1397 (0.9) | 0 | .04 |
| 18.6-24.9 | 54934 (34.2) | 61 (0.11) | |
| 25.0-29.9 | 55666 (34.7) | 69 (0.12) | |
| 30.0-34.5 | 29743 (18.5) | 23 (0.08) | |
| 35.0-39.9 | 12185 (7.6) | 7 (0.06) | |
| ≥40.0 | 6439 (4.0) | 2 (0.03) | |
| Diabetes | |||
| No | 152082 (94.1) | 162 (0.11) | .03 |
| Yes | 9618 (5.9) | 3 (0.03) | |
| Smoking | |||
| Never | 81430 (51.0) | 83 (0.10) | .85 |
| Past | 67110 (42.0) | 66 (0.10) | |
| Current | 11143 (7.0) | 13 (0.12) | |
| Physicalactivity, total MET-h/wk | |||
| <2.5 | 39421 (25.5) | 34 (0.09) | .03a |
| 2.5 to <18.25 | 77232 (50.0) | 68 (0.09) | |
| ≥18.25 | 37684 (24.4) | 52 (0.14) | |
| Annual income, $ | |||
| <35000 | 62099 (38.4) | 63 (0.10) | .42 |
| 35 000 to <75 000 | 60855 (37.6) | 64 (0.10) | |
| >75 000 | 27970 (17.3) | 32 (0.11) | |
| Do not know | 10885 (6.7) | 6 (0.06) | |
| Marital status | |||
| Never married | 7095 (4.4) | 10 (0.14) | .04 |
| Divorced or separated | 25803 (16.0) | 16 (0.06) | |
| Widowed | 27881 (17.3) | 25 (0.09) | |
| Married | 97638 (60.6) | 113 (0.12) | |
| Marriage-like relation | 2620 (1.6) | 0 |
Abbreviations: ALS, amyotrophic lateral sclerosis; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MET, metabolic equivalent task.
P value for strenuous physical activity was adjusted for age.
Baseline characteristics of women and reported frequency and energy expenditure of strenuous PA for the entire WHI cohort are shown in eTable 1 and the eFigure in the Supplement. Overall, 12.6% of women reported strenuous PA on 3 or more days per week and 70.8% reported no strenuous PA. There was an elevated percentage of deaths from ALS with increasing frequency of strenuous PA in the WHI, with percentage of death from ALS varying from 0.09% for 0 days per week to 0.16% for 4 days per week (P for trend = .06) (Table 2). Age-adjusted ALS mortality rates varied from 7.4 (95% CI, 5.5-9.9)/100 000 person-years for women who reported no strenuous PA to 10.6 (95% CI, 5.6-20.0)/100 000 person-years for women who did strenuous PA on 3 or more days per week (P = .07).
Table 2.
Relationship of Strenuous PA to Risk of Death From ALS
| Strenuous PA Variable | Total, No. | Death From ALS, No. (%) | Age-Adjusted P for Trend |
|---|---|---|---|
| Episodes, No./wk | |||
| 0 | 114623 | 101 (0.09) | .06 |
| 1 | 7694 | 8 (0.10) | |
| 2 | 10262 | 12 (0.12) | |
| 3 | 11956 | 18 (0.15) | |
| 4 | 3859 | 6 (0.16) | |
| ≥5 | 4585 | 4 (0.09) | |
| Duration/episode, min | |||
| <20 | 6035 | 7 (0.12) | .29 |
| 20-39 | 13 209 | 13 (0.10) | |
| 40-59 | 9176 | 14 (0.15) | |
| ≥60 | 9225 | 14 (0.15) |
Abbreviations: ALS, amyotrophic lateral sclerosis; PA, physical activity.
Women who died of ALS compared with those who did not die of ALS had greater mean (SD) minutes of strenuous PA per week (39.1 [75.3] vs 29.3 [68.3] min/wk, respectively; age-adjusted P = .04) and energy expenditure from strenuous PA (4.6 [8.8] vs 3.4 [8.0] MET-h/wk, respectively; age-adjusted P = .03) (Table 3). There was no association between increasing duration of strenuous PA per episode at baseline and death from ALS (P for trend = .29) (Table 2). There were no significant associations between frequency or duration of either mild or moderate PA and ALS mortality (eTable 2 in the Supplement).
Table 3.
Strenuous PA Among Women With and Without Death From ALS in the Women’s Health Initiative
| Strenuous PA Variable | Mean (SD) | Age-Adjusted P Value | |
|---|---|---|---|
| Women Without Death FromALS | Women With Death FromALS | ||
| Episodes, No./wk | 0.7 (1.4) | 0.9 (1.5) | .06 |
| Duration, min/wk | 29.3 (68.3) | 39.1 (75.3) | .04 |
| Energy expenditure, MET-h/wk | 3.4 (8.0) | 4.6 (8.8) | .03 |
Abbreviations: ALS, amyotrophic lateral sclerosis; MET, metabolic equivalenttask; PA, physical activity.
Women also self-reported information on strenuous PA 3 or more days per week at ages 18, 35, and 50 years (Figure 1). About half (50.6%) of the women with eventual death from ALS reported that they had performed strenuous PA 3 or more days per week at age 50 years, with a similar percentage at age 35 years (43.4%) and at age 18 years (46.8%). The HR for ALS mortality with strenuous PA 3 or more days per week compared with no strenuous PA at age 50 years was 1.58 (95% CI, 1.16-2.15), with a similar, although attenuated, association at baseline. However, there was no association between strenuous PA at ages 18 or 35 years and risk of ALS mortality.
Figure 1. Age-Adjusted Hazard Ratios for Amyotrophic Lateral Sclerosis Mortality in Women With 3 or More Days per Week of Strenuous Physical Activity vs None at Baseline and Ages 50, 35, and 18 Years.
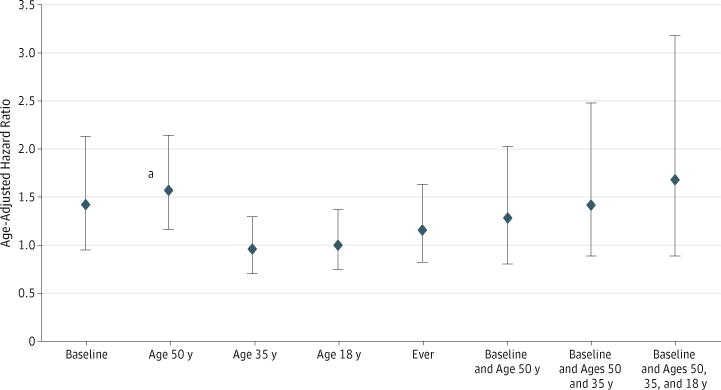
Error bars indicate 95% confidence intervals.
aP < .05.
In post hoc analysis, the association of strenuous PA with ALS mortality by baseline characteristics listed in Table 1 was further evaluated by comparing age-adjusted rates of death from ALS dichotomized into 3 or more days per week of strenuous PA vs no strenuous PA (eTable 3 in the Supplement). Age-adjusted rates of death from ALS were higher for those who participated in strenuous PA 3 or more days per week vs no strenuous PA for practically all categories except some college, hypertension, and high cholesterol levels. The age-adjusted rates of death from ALS for strenuous PA 3 or more days per week by baseline cigarette smoking, BMI, and diabetes status are presented in Figure 2. Smoking status was defined as never (<100 cigarettes/lifetime), past (≥100 cigarettes/lifetime and no use in the previous 6 months), and current (any cigarette use in the previous 6 months). Current smoking was relatively rare in the WHI (7.0%), and only 13 deaths from ALS occurred among current smokers. Age-adjusted rates of death from ALS had a nonsignificant increase across smoking categories. The association between strenuous PA and ALS mortality was primarily among women who were past cigarette smokers (Figure 2), with an age-adjusted HR of 2.34 (95% CI, 1.30-4.18) for ALS mortality with strenuous PA on 3 or more days per week compared with no strenuous PA among these women.
Figure 2. Age-Adjusted Amyotrophic Lateral Sclerosis (ALS) Mortality Rates for Women With 3 or More Days per Week of Strenuous Physical Activity (PA) vs None by Baseline Body Mass Index (BMI), Diabetes, and Smoking Status.
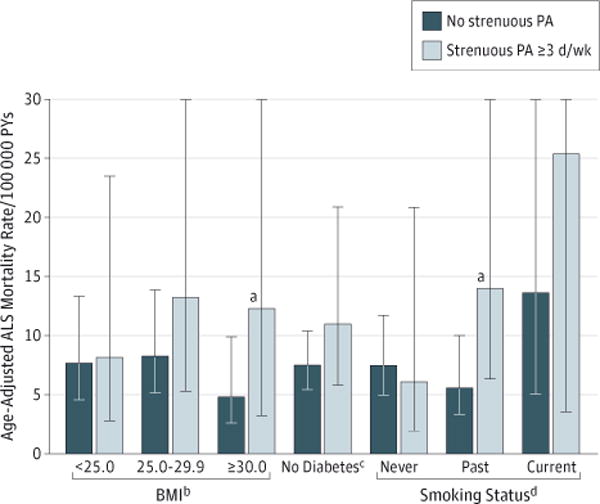
PYs indicates person-years; error bars, 95% confidence intervals.
aP < .05 for difference in age-adjusted death rates.
bCalculated as weight in kilograms divided by height in meters squared.
cThere were only 3 deaths from ALS among women with diabetes, with no deaths from ALS among diabetic women who performed strenuous PA on 3 or more days per week; age-adjusted mortality rates and 95% confidence intervals could not be calculated by level of strenuous PA.
dSmoking status was defined as never (≥100 cigarettes/lifetime), past (100 cigarettes/lifetime and no use within the past 6 months), or current (any use within the past 6 months).
Body mass index was inversely related to death from ALS (Table 1). In stratified analysis, the age-adjusted HR was 2.47 (95% CI, 1.01-6.07) for ALS mortality with strenuous PA on 3 or more days per week compared with none among women with a BMI of 30.0 or higher, and the relationship among women with a BMI lower than 25.0 or between 25.0 and 29.9 was nonsignificant (Figure 2). In a logistic regression model adjusted for age and BMI, the odds ratio for death from ALS was 1.56 (95% CI, 1.02-2.37; P = .04) for women with 3 or more days per week of strenuous PA compared with no reported strenuous PA.
Diabetes status was also associated with ALS mortality (Table 1), with women reporting no diabetes at baseline having higher rates of ALS mortality. In stratified analysis (Figure 2), the age-adjusted HR for death from ALS among nondiabetic women with 3 or more days per week of strenuous PA vs none was 1.46 (95% CI, 0.96-2.21). The few deaths from ALS among diabetic women (n = 3) and no deaths from ALS among diabetic women who performed strenuous PA on 3 or more days per week prohibited calculation of an HR for this subgroup.
In Cox proportional hazards models (Table 4), there was a nonsignificant trend for increasing hazard for death from ALS with increasing duration of strenuous PA (P = .11) when adjusted for education, cigarette smoking, region, race/ethnicity, BMI, and age. For duration of strenuous PA for 40 minutes or longer compared with no strenuous PA, the HR for ALS mortality was 1.64 (95% CI, 1.07-2.51). In a similar adjusted model, the HR was 1.65 (95% CI, 0.99-2.73) for ALS mortality with 3 or more days per week of strenuous PA compared with no reported strenuous PA at baseline.
Table 4.
Cox Regression Model for HRs for Deaths From Amyotrophic Lateral Sclerosis in the Women’s Health Initiativea
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Age | 1.05 (1.02-1.07) | <.001 |
| BMI | 0.98 (0.95-1.01) | .14 |
| Race/ethnicity | ||
| Black | 1.22 (0.66-2.25) | .10 |
| Other | 0.31 (0.10-0.97) | |
| White | 1 [Reference] | |
| Region | ||
| Midwest | 0.93 (0.58-1.50) | .95 |
| Northeast | 1.06 (0.68-1.66) | |
| South | 0.96 (0.60-1.52) | |
| West | 1 [Reference] | |
| Smoking status | ||
| Current | 1.68 (0.92-3.06) | .22 |
| Past | 1.02 (0.72-1.44) | |
| Never | 1 [Reference] | |
| Education | ||
| High school or less | 1 [Reference] | .93 |
| College graduate or higher | 0.94 (0.60-1.46) | |
| Some college | 0.91 (0.58-1.43) | |
| Duration of strenuous PA per episode, mina | ||
| None | 1 [Reference] | .11 |
| <20 | 1.36 (0.63-2.92) | |
| 20-39 | 0.86 (0.45-1.65) | |
| ≥40 | 1.64 (1.07-2.51) | |
| Frequency of strenuous PA, d/wka | ||
| 0 | 1 [Reference] | .28 |
| 1-2 | 1.18 (0.72-1.94) | |
| 3 | 1.65 (0.99-2.73) | |
| ≥4 | 1.06 (0.51-2.20) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; PA, physical activity.
Duration and frequency variables were tested in separate adjusted models with similar HR estimates for remaining covariates.
Discussion
The identification of modifiable risk factors for ALS is a promising approach to minimize the burden of this disease and to begin generating hypotheses as to the etiology, especially in sporadic cases. To our knowledge, this is the first attempt to describe the relationship between PA and risk for ALS mortality in a cohort of postmenopausal women. The results of this WHI follow-up study support a possible direct association between strenuous PA, but not less intense PA, and risk of death from ALS that was independent of age, education, BMI, and smoking.
The largest population-based case-control study of lifetime PA and the risk of ALS was conducted recently by Huisman et al27 in the Netherlands. A total of 636 cases and 2166 controls were identified who completed a semistructured questionnaire on lifelong occupations, sport activity, and active recreational hobbies. The ALS cases had significantly higher levels of leisure PA compared with controls, similar to the WHI findings. No significant effects were seen for cases and controls for organized strenuous PA such as marathons or triathlons or in occupational activity.
What might be the pathophysiology of strenuous PA and ALS risk? Harwood et al25 suggested a relationship between oxidative stress and ALS. Increased metabolism during strenuous exercise may increase oxidative stress possibly associated with abnormal mitochondrial activity, potentially resulting in an increase in reactive oxygen species and neuronal cell damage in genetically susceptible individuals. Longstreth et al40 also proposed that intense motor nerve firing during strenuous PA may cause glutamate toxic effects. The stronger positive association of strenuous PA and risk of death from ALS among former smokers in the WHI may be consistent with a cumulative effect of oxidative stress from cigarette smoking14 and increased amounts of strenuous PA following smoking cessation, eg, to prevent weight gain or as part of overall behavioral changes. Individuals who lack adequate antioxidant response perhaps from genetic abnormalities or dietary deficiencies may be at increased risk, consistent with the recent observation of a substantially decreased risk of ALS associated with increased intake of ω-3 polyunsaturated fatty acids.41 The suggested compound effect of oxidative stress from cigarette smoking and PA and inadequate antioxidant response supports the idea of a long preclinical phase of ALS similar to other neurodegenerative diseases, such as Alzheimer and Parkinson diseases.42
Associations between BMI and diabetes and risk for death from ALS were also observed in this sample. The lowest incidence of ALS was observed among women with a baseline BMI of 30.0 or higher, and very few deaths from ALS occurred among women with diabetes. One large longitudinal study that evaluated motor neuron disease, primarily ALS, among 1.3 million women in England found an inverse association of BMI and ALS risk.43 However, the investigators did not include measures of PA. A very large population-based case-control study evaluated 3650 ALS cases from the Danish registry between 1982 and 2009 and 365 000 controls matched on age and sex,44 finding a strong inverse association of both diabetes and obesity for patients with ALS who were aged 40 years or older. Another recent population-based case-control study in the Netherlands found that lower presymptomatic BMI was associated with ALS, increased energy intake, and fat consumption,45 adjusted for lifetime PA. These 4 large studies, including the WHI, are consistent in demonstrating the inverse association of BMI years before diagnosis of ALS and contribute to recent evidence that whole-body and cellular metabolism may play a role in the development of ALS.46
Any adverse association of strenuous PA and risk of death from ALS should be evaluated in the context of the known benefits of PA in the WHI34–36,47,48 and many other studies.49–52 Previous studies of PA and health outcomes in the WHI reported that higher PA levels were associated with a reduced risk of breast cancer,35 ovarian cancer,47 cardiovascular disease,36 and improved survival in women with incident breast cancer.34 The HR for increased risk for ALS mortality with strenuous PA in the WHI, even in this low-activity population, was similar in magnitude to the increased HR for total mortality, coronary heart disease incidence, and breast cancer incidence from no strenuous PA in the WHI during the same period. Therefore, even if substantiated by other studies, these findings should not affect the recommendations for increasing strenuous PA according to national guidelines.53
The low incidence of ALS restricts longitudinal studies of specific risk factors and ALS to very large cohorts. The WHI cohort has relatively complete mortality follow-up, information from participants about strenuous PA at baseline and ages 18, 35, and 50 years, and information on many other factors; therefore, it provided a unique opportunity to evaluate the possible association of strenuous PA and ALS. Even so, there are important limitations to the WHI data set.
The WHI was not designed to study ALS. Therefore, we depended on death certificates for underlying cause of death. It is possible that some women in the WHI with incident ALS who died were coded to another underlying cause of death, such as pneumonia and respiratory failure, thereby reducing the total number of incident deaths from ALS. Our estimated rate of ALS based on age of the cohort is consistent with the literature.2,4,5 It is thus unlikely that any missing deaths from ALS, eg, coded to another cause of death, substantially biased the observed associations with PA.
The prevalence of strenuous PA 3 or more days per week in the WHI cohort was only 13%, thus reducing the power of the study despite the large sample size. The information on PA was collected from a self-administered questionnaire, which can introduce recall bias, particularly for reporting PA levels at ages 18, 35, and 50 years. Duration of strenuous PA was reported in 20-minute intervals, with a maximum of 1 hour or more. This may lead to underestimation of total energy expenditure from strenuous PA for those who reported exercising for at least 1 hour at a time. However, the strenuous PA measure was strongly related to the risk of other diseases (such as breast cancer and cardiovascular disease) and total mortality, was repeatable over time, and was correlated with accelerometer measures.34–39,47,48
The analyses investigating the associations between other baseline characteristics and ALS mortality by strenuous PA were conducted post hoc, with limited exposure data collected at one time. Therefore, it is not possible to fully separate the influence of the primary exposure of strenuous PA on BMI, diabetes status, smoking, etc, and vice versa. The observed relationships between BMI, diabetes, and smoking and ALS mortality should thus be interpreted cautiously. It is also possible that other unmeasured factors explain the association between PA and death from ALS.
Conclusions
This study raises an interesting question about whether there may be a subpopulation of individuals more susceptible to adverse effects of strenuous PA or whether there are combinations of environmental exposures, such as tobacco smoke, unique occupations, genetic variations, or health states (ie, diabetes and BMI), and strenuous PA that increase risk for ALS. The results of this study, if replicated, may provide a clue for further etiological research into ALS and the effects of strenuous PA on neurological function. Additional longitudinal studies, especially in populations that are enriched with more physically active participants than the WHI, have more detailed measures of exposures, and include better evaluation of patients with ALS, are necessary to further test the relationship between strenuous PA and ALS mortality.
Supplementary Material
Key Points.
Question
Is strenuous physical activity a risk factor for amyotrophic lateral sclerosis mortality in postmenopausal women?
Findings
In this cohort study of 161 809 women enrolled in the Women’s Health Initiative, there were 165 deaths from amyotrophic lateral sclerosis through 2013. Women who self-reported strenuous physical activity on 3 or more days per week at baseline had significant 56% increased odds for amyotrophic lateral sclerosis mortality, adjusted for age and body mass index.
Meaning
If replicated by other studies, the observed association between strenuous physical activity and amyotrophic lateral sclerosis mortality provides a clue to the etiology of the disease.
Acknowledgments
Funding/Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Dr Eaglehouse is supported by grant T32CA186783 from the National Cancer Institute, National Institutes of Health.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaneurology.com
Author Contributions: Drs Chang and Kuller had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Eaglehouse, Talbott, Kuller.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Eaglehouse, Talbott, Kuller.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Talbott, Chang, Kuller.
Administrative, technical, or material support: Eaglehouse, Talbott.
Study supervision: Kuller.
Conflict of Interest Disclosures: None reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Previous Presentation: This work was presented in part at Scientific Sessions 2015 of the American Heart Association; November 8, 2015; Orlando, Florida.
Contributor Information
Yvonne L. Eaglehouse, Division of Cancer Prevention and Population Science, Cancer Institute, University of Pittsburgh, Pittsburgh, Pennsylvania; Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania.
Evelyn O. Talbott, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania.
Yuefang Chang, Department of Neurological Surgery, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Lewis H. Kuller, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.Borasio GD, Miller RG. Clinical characteristics and management of ALS. Semin Neurol. 2001;21(2):155–166. doi: 10.1055/s-2001-15268. [DOI] [PubMed] [Google Scholar]
- 2.Guidetti D, Bondavalli M, Sabadini R, et al. Epidemiological survey of amyotrophic lateral sclerosis in the province of Reggio Emilia, Italy: influence of environmental exposure to lead. Neuroepidemiology. 1996;15(6):301–312. doi: 10.1159/000109920. [DOI] [PubMed] [Google Scholar]
- 3.Kondo K. Epidemiology of motor neuron disease. In: Leigh PN, Swash M, editors. Motor Neuron Disease: Biology and Management. New York, NY: Springer; 1995. pp. 19–33. [Google Scholar]
- 4.Mehta P, Antao V, Kaye W, et al. Prevalence of amyotrophic lateral sclerosis: United States, 2010-2011. MMWR Surveill Summ. 2014;63(suppl 7):1–14. [PubMed] [Google Scholar]
- 5.Gubbay SS, Kahana E, Zilber N, Cooper G, Pintov S, Leibowitz Y. Amyotrophic lateral sclerosis: a study of its presentation and prognosis. J Neurol. 1985;232(5):295–300. doi: 10.1007/BF00313868. [DOI] [PubMed] [Google Scholar]
- 6.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(suppl 1):S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 7.Figlewicz DA, McInnis MG, Goto J, et al. Identification of flanking markers for the familial amyotrophic lateral sclerosis gene ALS1 on chromosome 21. J Neurol Sci. 1994;124(suppl):90–95. doi: 10.1016/0022-510x(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin RL, Vajda A, Hardiman O. Heritability of amyotrophic lateral sclerosis: insights from disparate numbers. JAMA Neurol. 2015;72(8):857–858. doi: 10.1001/jamaneurol.2014.4049. [DOI] [PubMed] [Google Scholar]
- 9.Rosen DR, Sapp P, O’Regan J, et al. Genetic linkage analysis of familial amyotrophic lateral sclerosis using human chromosome 21 microsatellite DNA markers. Am J Med Genet. 1994;51(1):61–69. doi: 10.1002/ajmg.1320510114. [DOI] [PubMed] [Google Scholar]
- 10.Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 11.Chiò A, Calvo A, Mazzini L, et al. PARALS Extensive genetics of ALS: a population-based study in Italy. Neurology. 2012;79(19):1983–1989. doi: 10.1212/WNL.0b013e3182735d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne S, Elamin M, Bede P, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. 2012;11(3):232–240. doi: 10.1016/S1474-4422(12)70014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geevasinga N, Menon P, Nicholson GA, et al. Cortical function in asymptomatic carriers and patients with c9orf72 amyotrophic lateral sclerosis. JAMA Neurol. 2015;72(11):1268–1274. doi: 10.1001/jamaneurol.2015.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong SW, Huisman MH, Sutedja NA, et al. Smoking, alcohol consumption, and the risk of amyotrophic lateral sclerosis: a population-based study. Am J Epidemiol. 2012;176(3):233–239. doi: 10.1093/aje/kws015. [DOI] [PubMed] [Google Scholar]
- 15.Armon C. Smoking may be considered an established risk factor for sporadic ALS. Neurology. 2009;73(20):1693–1698. doi: 10.1212/WNL.0b013e3181c1df48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek AM, Barchowsky A, Bowser R, et al. Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environ Pollut. 2015;197:181–186. doi: 10.1016/j.envpol.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Malek AM, Barchowsky A, Bowser R, et al. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neurodegener Dis. 2014;14(1):31–38. doi: 10.1159/000355344. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, Su FC, Callaghan BC, Goutman SA, Batterman SA, Feldman EL. Environmental risk factors and amyotrophic lateral sclerosis (ALS): a case-control study of ALS in Michigan. PLoS One. 2014;9(6):e101186. doi: 10.1371/journal.pone.0101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisskopf MG, McCullough ML, Morozova N, Calle EE, Thun MJ, Ascherio A. Prospective study of occupation and amyotrophic lateral sclerosis mortality. Am J Epidemiol. 2005;162(12):1146–1152. doi: 10.1093/aje/kwi343. [DOI] [PubMed] [Google Scholar]
- 20.Weisskopf MG, Cudkowicz ME, Johnson N. Military service and amyotrophic lateral sclerosis in a population-based cohort. Epidemiology. 2015;26(6):831–838. doi: 10.1097/EDE.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beard JD, Kamel F. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis etiology and survival. Epidemiol Rev. 2015;37:55–70. doi: 10.1093/epirev/mxu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiò A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(pt 3):472–476. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- 23.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis M, Gordon PH. Lou Gehrig, Rawhide, and 1938. Neurology. 2007;68(8):615–618. doi: 10.1212/01.wnl.0000254623.04219.aa. [DOI] [PubMed] [Google Scholar]
- 25.Harwood CA, McDermott CJ, Shaw PJ. Physical activity as an exogenous risk factor in motor neuron disease (MND): a review of the evidence. Amyotroph Lateral Scler. 2009;10(4):191–204. doi: 10.1080/17482960802549739. [DOI] [PubMed] [Google Scholar]
- 26.Hamidou B, Couratier P, Besançon C, Nicol M, Preux PM, Marin B. Epidemiological evidence that physical activity is not a risk factor for ALS. Eur J Epidemiol. 2014;29(7):459–475. doi: 10.1007/s10654-014-9923-2. [DOI] [PubMed] [Google Scholar]
- 27.Huisman MH, Seelen M, de Jong SW, et al. Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(9):976–981. doi: 10.1136/jnnp-2012-304724. [DOI] [PubMed] [Google Scholar]
- 28.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. 2002;59(5):773–775. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]
- 29.Strickland D, Smith SA, Dolliff G, Goldman L, Roelofs RI. Physical activity, trauma, and ALS: a case-control study. Acta Neurol Scand. 1996;94(1):45–50. doi: 10.1111/j.1600-0404.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 30.Pupillo E, Messina P, Giussani G, et al. EURALS Consortium Physical activity and amyotrophic lateral sclerosis: a European population-based case-control study. Ann Neurol. 2014;75(5):708–716. doi: 10.1002/ana.24150. [DOI] [PubMed] [Google Scholar]
- 31.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 33.Chomistek AK, Manson JE, Stefanick ML, et al. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. J Am Coll Cardiol. 2013;61(23):2346–2354. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin ML, McTiernan A, Manson JE, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the Women’s Health Initiative. Cancer Prev Res (Phila) 2011;4(4):522–529. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McTiernan A, Kooperberg C, White E, et al. Women’s Health Initiative Cohort Study Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA. 2003;290(10):1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 36.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 37.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31(2):193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 38.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 suppl):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen HQ, Herting JR, Kohen R, et al. Recreational physical activity in postmenopausal women is stable over 8 years of follow-up. J Phys Act Health. 2013;10(5):656–668. doi: 10.1123/jpah.10.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Hypotheses to explain the association between vigorous physical activity and amyotrophic lateral sclerosis. Med Hypotheses. 1991;34(2):144–148. doi: 10.1016/0306-9877(91)90183-y. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald KC, O’Reilly EJ, Falcone GJ, et al. Dietary ω-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol. 2014;71(9):1102–1110. doi: 10.1001/jamaneurol.2014.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisen A, Kiernan M, Mitsumoto H, Swash M. Amyotrophic lateral sclerosis: a long preclinical period? J Neurol Neurosurg Psychiatry. 2014;85(11):1232–1238. doi: 10.1136/jnnp-2013-307135. [DOI] [PubMed] [Google Scholar]
- 43.Doyle P, Brown A, Beral V, Reeves G, Green J. Incidence of and risk factors for motor neurone disease in UK women: a prospective study. BMC Neurol. 2012;12:25. doi: 10.1186/1471-2377-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kioumourtzoglou MA, Rotem RS, Seals RM, Gredal O, Hansen J, Weisskopf MG. Diabetes mellitus, obesity, and diagnosis of amyotrophic lateral sclerosis: a population-based study. JAMA Neurol. 2015;72(8):905–911. doi: 10.1001/jamaneurol.2015.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huisman MH, Seelen M, van Doormaal PT, et al. Effect of presymptomatic body mass index and consumption of fat and alcohol on amyotrophic lateral sclerosis. JAMA Neurol. 2015;72(10):1155–1162. doi: 10.1001/jamaneurol.2015.1584. [DOI] [PubMed] [Google Scholar]
- 46.Ngo ST, Steyn FJ. The interplay between metabolic homeostasis and neurodegeneration: insights into the neurometabolic nature of amyotrophic lateral sclerosis. Cell Regen (Lond) 2015;4(1):5. doi: 10.1186/s13619-015-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y, Chlebowski R, LaMonte MJ, et al. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: results from the Women’s Health Initiative. Gynecol Oncol. 2014;133(1):4–10. doi: 10.1016/j.ygyno.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seguin R, Buchner DM, Liu J, et al. Sedentary behavior and mortality in older women: the Women’s Health Initiative. Am J Prev Med. 2014;46(2):122–135. doi: 10.1016/j.amepre.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gebel K, Ding D, Chey T, Stamatakis E, Brown WJ, Bauman AE. Effect of moderate to vigorous physical activity on all-cause mortality in middle-aged and older Australians. JAMA Intern Med. 2015;175(6):970–977. doi: 10.1001/jamainternmed.2015.0541. [DOI] [PubMed] [Google Scholar]
- 51.US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Dept of Health & Human Services, Public Health Service, Centers for Disease Control & Prevention, National Center for Chronic Disease Prevention & Health Promotion; 1996. [Google Scholar]
- 52.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, Lancet Physical Activity Series Working Group Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.US Department of Health and Human Services. Physical Activity Guidelines for Americans. Washington, DC: US Dept of Health & Human Services; 2008. 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


