Abstract
Obesity and tobacco smoking represent the largest challenges to public health, but the causal relationship between nicotine and obesity is poorly understood. Nicotine suppresses body weight gain, a factor impacting smoking initiation and the failure to quit, particularly among obese smokers. The impact of nicotine on body weight regulation in obesity-prone and obesity-resistant populations consuming densely caloric diets is unknown. In the current experiment, body weight gain of adult male rats maintained on a high energy diet (31.8% kcal from fat) distributed into obesity-prone (OP), obesity-resistant (OR) and an intermediate group, which was placed on standard rodent chow (Chow). These rats were surgically implanted with intravenous catheters and allowed to self-administer nicotine (0 or 60 μg/kg/infusion, a standard self-administration dose) in 1-h sessions for 20 consecutive days. Self-administered nicotine significantly suppressed body weight gain but not food intake in OP and Chow rats. Self-administered nicotine had no effect on body weight gain in OR rats. These data suggest that: 1) OR rats are also resistant to nicotine-induced suppression of body weight gain; and 2) nicotine may reduce levels of obesity in a subset of smokers prone to obesity.
Keywords: Obesity phenotypes, Nicotine, Dietary intake, Drugs
1. Introduction
Tobacco smoke and obesity represent the largest causes of preventable deaths worldwide [1]. Over the past 35 years in the United States, rates of cigarette smoking have slowly declined, as the rates of obesity have dramatically increased [2]. Abstinence from smoking is typically accompanied by weight gain [3,4] and research suggests that smoking cessation is in part responsible for the drastic increase in the rates of overweight in the United States [5]. The relationship between smoking and body weight regulation, particularly among the obese population, is poorly understood. Research on the effect of smoking on BMI among obese smokers has resulted in conflicting results [6,7], and is complicated by reliance on self-report data or the challenges of prospective studies. The negative health consequences of smoking are more severe among the obese population [8,9] and the relationship between smoking and obesity requires more attention.
Although research has consistently demonstrated that higher BMI is associated with higher rates of smoking [10], the caual relationship between obesity and nicotine exposure is unclear. Whether chronic nicotine exposure via cigarette smoke prevents the development of obesity in smokers that would otherwise be obese is unknown [9]. It is difficult to assess whether nicotine causes changes in body weight regulation in human smokers; animal models may provide a better opportunity to evaluate this hypothesis. Previous research has demonstrated that large doses of subcutaneous nicotine suppress body weight and food intake in obese rodents [11,12], but the impact of nicotine on body weight and feeding behavior in obesity has not been studied in an animal model of nicotine self-administration.
Outbred rats remain lean when fed chow, but when maintained on a diet modeling the nutritional content of Westernized societies, body weight gain separates into distinct groups: a subset of obesity-prone (OP) and obesity-resistant (OR) rats [13]. OP and OR rats are considered among the best animal models of diet-induced obesity and recapitulate many key features of the human condition. The current experiment evaluated the impact of self-administered nicotine on body weight gain and food intake in OP, OR, and chow-fed rats. Results demonstrated that self-administered nicotine suppressed body weight gain in chow-fed and OP rats without suppression of daily food intake. OR rats were insensitive to the weight-suppressive effects of self-administered nicotine.
2. Methods
2.1. Subjects
Male Sprague-Dawley rats (n = 60; Charles River, Kingston, NY) weighing 275–300 g upon arrival were housed individually in hanging-wire cages on a reverse light-dark 12:12-h cycle (lights off at 0700 h) in a temperature-controlled facility (between 68 and 70 °F). Upon arrival, rats had free access to high energy diet (HED; Research Diets D12266B, New Brunswick, NJ; 31.8% kcal from fat, 25.2% kcal from sucrose, 4.41 kcal/g) and water, unless noted otherwise. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.2. Drugs
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in 0.9% saline; doses are expressed as free base [14,15]. Infusion duration was adjusted daily to account for body weight gain.
2.3. Classification of body weight phenotype groups
Body weight was monitored daily while all rats had free access to HED for two weeks. At the end of this two-week period, the twenty rats that gained the most weight were assigned to OP and the twenty rats that gained the least weight were assigned to OR. The middle tertile gained an intermediate amount of weight and were placed on chow (Purina 5001; 3.36 kcal/g) on Day 15 as a diet control, and will be referred to as the Chow group (n = 20). Rats in the Chow group had at least five days of chow exposure before behavioral procedures. Rats assigned to OP and OR groups remained on HED maintenance. Average body weight for each group on the first day of experimentation was as follows: Chow = 423.3 ± 13.3 g; OP = 445.4 ± 18.2 g; OR = 392.7 ± 21.1 g.
2.4. Procedures
2.4.1. Surgery
After two weeks of diet exposure and phenotype group classification, rats were anesthetized with isoflurane (2–3% in 100% O2) and implanted with catheters into the right jugular vein [14,16]. Rats were allowed to recover for 5–6 days before self-administration procedures, during which catheters were flushed daily with 0.1 ml heparinized saline (30 U/ml) containing timentin (66.67 mg/ml) and streptokinase (8333 U/ml). Catheters were flushed with 0.1 ml heparinized saline (10 U/ml) and heparinized saline (30 U/ml) containing Timentin (66.67 mg/ml) prior to and following the self-administration sessions, respectively.
2.4.2. Self-administration
Self-administration occurred in 38 operant chambers (Med-Associates) [15,17]. Rats were assigned to drug group (0 or 60 μg/kg/infusion nicotine), counterbalanced by body weight within phenotype group (n = 20/phenotype, n = 10 receiving saline or nicotine within phenotype). One nose-poke hole was assigned as active, resulting in the delivery of an i.v. infusion after fulfilling the fixed-ratio (FR) 2 response requirement. The other nose-poke hole was inactive; responses at this nose-poke portal were recorded but had no consequence. Active and inactive nose-poke holes were randomly assigned (left or right). Infusions were accompanied by a 15-sec cue light illuminated above the active nose-poke portal and an unsignaled 1-min timeout, where responses were recorded but had no scheduled consequence. Self-administration occurred in 20 consecutive 1-h daily sessions. Sessions began 1–2 h following the onset of the dark cycle, depending on cohort order. Each phenotype group was equally represented in each cohort, and time of day of self-administration session had no impact on dependent measurements.
Rats included in analyses passed a patency test, which required displaying physical signs of ataxia within 5-s of intravenous injections of methohexital (5 mg/kg). Final sample size following patency testing is as follows: Chow saline, n = 10; Chow nicotine, n = 9; OP saline, n = 9; OP nicotine, n = 9; OR saline, n = 10, OR nicotine, n = 7.
2.4.3. Food intake measurements
Food intake measurements, accounting for spillage, were taken every fifth day of experimentation over 24 h. Measurements occurred while the rat was out of the home cage, during operant sessions to minimize disruption of food intake. Unlike most self-administration experiments [18], these rats had unrestricted access to food, with the exception of the 1-h self-administration session.
2.5. Statistics
Statistical analyses were performed using SPSS. Comparisons between drug group, phenotype, and session (self-administration experiments, every fifth day) or day (feeding experiments) were analyzed by mixed-design and repeated measures ANOVA tests. Planned comparisons between drug groups within each phenotype were analyzed using one-way ANOVA. The data were Greenhouse-Geisser corrected where Mauchly’s Sphericity tests were significant. The α-level for all tests was set at 0.05.
3. Results
3.1. High energy diet exposure suppressed nicotine self-administration
Nicotine groups acquired stable behavior, taking more infusions than saline controls (p b 0.001). Within the nicotine groups, there was a significant effect of phenotype (p = 0.042). Chow rats self-administered more nicotine compared to rats fed HED (p = 0.011), though this did not reach significance when separated into OP and OR rats (Supplemental Fig. 1).
3.2. Self-administered nicotine suppressed body weight gain in OP and Chow, but not OR rats
Self-administered nicotine suppressed BW gain in OP and Chow rats compared to intravenous infusions of saline (Fig. 1). There was a significant main effect of day (p < 0.001); day*phenotype (p < 0.001); day*drug (p < 0.001); and three-way interaction (p = 0.011). Within phenotype, self-administered nicotine significantly suppressed BW gain in Chow rats on all days tested (ps < 0.018; Fig. 1a & b) and in OP rats on Days 5, 10, 15, and 20 (ps < 0.016; Fig. 1a & c). There was no significant impact of nicotine on weight gain in OR rats (Fig. 1a & d).
Fig. 1.
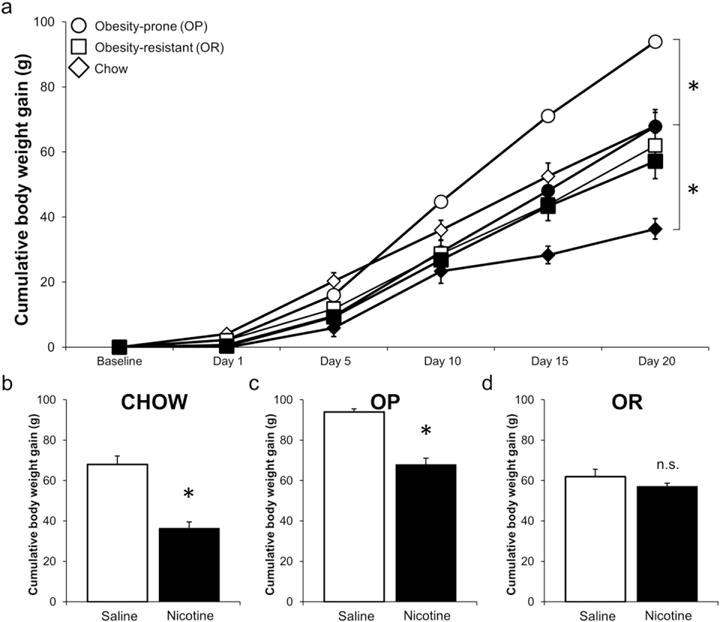
Self-administered nicotine (60 μg/kg/infusion) suppressed body weight gain in OP and Chow rats, but not OR rats (a). Open symbols indicates saline, and filled indicate nicotine groups. For clarity, bar graphs demonstrate suppression of body weight gain in Chow (b), OP (c), and lack of suppression in OR (d) groups after 20 days of self-administration. Data expressed as means ± SEM. *Indicate p < 0.05, between 0 and 60 μg/kg/infusion nicotine within phenotype group.
3.3. Self-administered nicotine had no impact on food intake
There was no significant effect of self-administered nicotine on 24 h food intake at any measurement, analyzed as kcal as a percentage of BW (Fig. 2 shows data from the final 24 h of the experiment only, for clarity). There was no effect of day, phenotype, drug, or interaction term. There was no impact of drug on any day when tested within phenotype. Food intake data were transformed to correct for BW as caloric consumption increased significantly in the saline groups over the 20 days of experimentation as BW increased (p = 0.001), and to control for potential between subject differences. When food intake was expressed as kcal, there was a significant effect of day (p = 0.005), but no significant effect of drug, phenotype, or interaction.
Fig. 2.
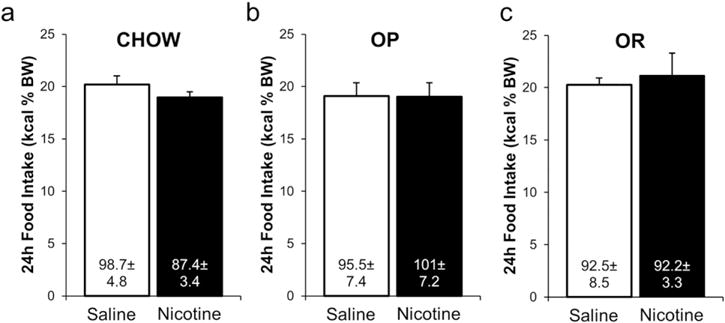
Self-administered nicotine (60 μg/kg/infusion) did not impact 24 h food intake, expressed as kcal as a percentage of BW to account for the between subjects design of the experiment, in Chow (a), OP (b), or OR (c) rats after 20 days of self-administration, when nicotine intake was maximal in all groups. Data expressed as means ± SEM. Values within each bar are mean 24 h kcal consumed on Day 20 of the experiment ± SEM.
4. Discussion
The current data are the first to evaluate the impact of self-administered nicotine on BW in a model of human diet-induced obesity and demonstrate that rats resistant to the development of obesity are resistant to nicotine-induced suppression of BW gain. Many smokers cite weight regulation as a primary reason for smoking initiation and the ability to quit [3]. However, these data suggest that among smokers consuming a typical Westernized diet, a subset may be resistant to nicotine-induced weight-suppression. Therefore, expectations of weight suppression among many weight-concerned smokers may be unfounded.
In the current data, suppression of BW gain by nicotine in OP and Chow rats occurred independent of food intake, replicating previous results [15] and aligning with feeding data from human smokers. We have previously demonstrated that nicotine intake is negatively correlated with BW gain in adult male rats fed standard rodent chow [15]. The magnitude of weight reduction was greater in Chow than OP rats, possibly due to higher total nicotine consumption in Chow rats. Importantly, the lack of BW suppression seen in OR rats was not accompanied by a compensatory increase in food intake. Weight gain in OP nicotine rats was comparable to both lean (Chow and OR saline groups), suggesting that nicotine exposure may prevent the development of obesity. This aligns with a report in humans demonstrating that obese smokers lose weight compared to normal weight smokers during smoking [19]. It is unclear why rats resistant to the development of obesity are also resistant to the weight-suppressive effects of nicotine. It is possible that nicotine acts to suppress BW through similar mechanisms as those resulting in the anti-obesity phenotype in OR rats. Therefore, in OR rats, nicotine cannot act to potentiate reduced weight gain. Furthering our understanding of nicotine-induced BW suppression may lead to insights on resistance to diet-induced obesity.
The notion that OP and OR rats have differential responses to psychostimulants is not new. Selectively bred OP rats are more sensitive to the anorectic effects of D-amphetamine compared to selectively bred OR rats when fed chow, but this difference is occluded with HED maintenance [20]. Similarly, chow-maintained selectively bred OP rats are more sensitive to cocaine-induced locomotor sensitization than chow-maintained OR rats [21,22], but this difference is not present in outbred HED-maintained OP and OR rats [21]. It is possible that in the current experiments, OP rats were more sensitive to the locomotor effects of nicotine, potentially contributing to the suppression of BW. However, previous reports suggest that the differential effect of psychostimulants on behaviors related to BW regulation is blocked with HED-maintenance, which is at odds with the current data.
The current experiment was not designed to test whether the impact of self-administered nicotine on BW regulation pre-exists a manipulation of diet, although it provides some clues. It is likely that the extremes of HED-induced BW gain within the Chow group are more OP-like or OR-like. If the impact of nicotine on BW relies solely on the polygenetic predisposition to develop obesity, and not the combination of genetic and environmental factors, then rats that gained the least weight on HED in the Chow group should be resistant to the weight-suppressive effects of nicotine. However, there was no relationship between weight gain during HED maintenance and the impact of nicotine on weight gain in the Chow group (data not shown). This suggests that resistance to nicotine-induced BW suppression requires consumption of a densely caloric diet. Examining the impact of nicotine on body weight regulation in selectively bred obesity-prone and –resistant rats would remove this confound.
The weight gain in saline and nicotine Chow groups reported here is similar to what is expected in chow-fed rats without previous HED consumption [15], though the exclusion of a chow group without HED exposure in the current design limits our interpretation. It is possible that prior maintenance on HED could impact weight gain when returned to chow. Future work may include a group that remains HED-naïve as a control, or utilize selectively bred obesity-prone and – resistant rats, though these rats are no longer commercially available.
Self-administered nicotine in extended access 23-h sessions has been demonstrated to suppress responding for chow pellets [23,24], but not responding for sucrose [23]. Smokers experience intermittent increases in blood and brain nicotine levels, which can be modeled using 1-h or 23-h access protocols. There are several advantages to the use of 1-h nicotine self-administration sessions in the study of body weight regulation [15]. The robust impact of nicotine on body weight when self-administered over 1-h provides some clues about potential mechanisms of action by which nicotine acts to suppress body weight. As the half-life of nicotine is about 1 h in the rat [25], a metabolite of nicotine with a half-life lasting several hours, such as cotinine, may act to suppress body weight. Alternatively, nicotine may activate downstream pathways that have long lasting effects on body weight regulation, such as increased brown adipose tissue activity.
Nicotine consumption was higher in Chow rats compared to rats maintained on HED, independent of susceptibility to diet-induced obesity, which may suggest that HED-exposure reduces drug-seeking be-haviors. One report supports this idea, demonstrating that HED-exposure impairs cocaine-seeking behaviors [26]. In contrast, it is possible that a prior exposure to HED may increase drug-seeking behaviors. Early life [27] and unpredictable [28] exposure to high-energy diets have been shown to increase drug-seeking when switched to chow. Therefore, it is possible that the behavior observed in the current Chow group is increased in comparison to HED-groups due to the short HED-exposure period. Nevertheless, these self-administration data are at odds with the observation that smokers with obesity smoke more cigarettes per day than normal weight smokers [19]. Future experiments testing the impact of obesity on self-administration across a full dose response curve may provide more insight to this issue.
The results of this experiment provide new insight into the understanding of the interaction between nicotine and obesity and demonstrate that: 1) obesity-resistant rats are also resistant to nicotine-induced suppression of body weight gain; and 2) nicotine may prevent or reduce levels of obesity in a subset of smokers. These data highlight the importance of considering obesity-prone and –resistant rats as separate populations and suggest that expectations of weight regulation by smoking may be unfounded in many weight-concerned smokers.
Supplementary Material
Acknowledgments
Funding source
Research reported in this publication was supported by internal funding at the University of Pittsburgh.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.physbeh.2017.02.007.
References
- 1.C. Centers for Disease and Prevention. Vital signs: current cigarette smoking among adults aged ≥18 years with mental illness - United States. MMWR Morb Mortal Wkly Rep. 2013;62:81–87. (2009-2011) [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoli M, Picciotto MR. Nicotinic regulation of energy homeostasis. Nicotine Tob Res. 2012;14:1270–1290. doi: 10.1093/ntr/nts159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Troiano RP, Pamuk ER, Kuczmarski RJ, Campbell SM. The influence of smoking cessation on the prevalence of overweight in the United States. N Engl J Med. 1995;333:1165–1170. doi: 10.1056/NEJM199511023331801. [DOI] [PubMed] [Google Scholar]
- 6.Fidler JA, West R, Van Jaarsveld CH, Jarvis MJ, Wardle J. Does smoking in adolescence affect body mass index, waist or height? Findings from a longitudinal study, Addiction. 2007;102:1493–1501. doi: 10.1111/j.1360-0443.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper TV, Klesges RC, Robinson LA, Zbikowski SM. A prospective evaluation of the relationships between smoking dosage and body mass index in an adolescent, biracial cohort. Addict Behav. 2003;28:501–512. doi: 10.1016/s0306-4603(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 8.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 9.Rupprecht LE, Donny EC, Sved AF. Obese Smokers as a Potential Subpopulation of Risk in Tobacco Reduction Policy. Yale J Biol Med. 2015 [PMC free article] [PubMed] [Google Scholar]
- 10.Chiolero A, Peytremann-Bridevaux I, Paccaud F. Associations between obesity and health conditions may be overestimated if self-reported body mass index is used. Obes Rev. 2007;8:373–374. doi: 10.1111/j.1467-789X.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 11.Mangubat M, Lutfy K, Lee ML, Pulido L, Stout D, Davis R, et al. Effect of nicotine on body composition in mice. J Endocrinol. 2012;212:317–326. doi: 10.1530/JOE-11-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seoane-Collazo P, Morentin PB Martinez de, Ferno J, Dieguez C, Nogueiras R, Lopez M. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology. 2014;155:1679–1689. doi: 10.1210/en.2013-1839. [DOI] [PubMed] [Google Scholar]
- 13.Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. Am J Phys. 1989;256:R766–R771. doi: 10.1152/ajpregu.1989.256.3.R766. [DOI] [PubMed] [Google Scholar]
- 14.Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. 2013;15:1918–1925. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rupprecht LE, Smith TT, Donny EC, Sved AF. Self-administered nicotine suppresses body weight gain independent of food intake in male rats. Nicotine Tob Res. 2015;18:1869–1876. doi: 10.1093/ntr/ntw113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Low-dose nicotine self-administration is reduced in adult male rats naive to high doses of nicotine: implications for nicotine product standards. Exp Clin Psychopharmacol. 2014;22:453–459. doi: 10.1037/a0037396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith TT, Schaff MB, Rupprecht LE, Schassburger RL, Buffalari DM, Murphy SE, et al. Effects of MAO inhibition and a combination of minor alkaloids, beta-carbolines, and acetaldehyde on nicotine self-administration in adult male rats. Drug Alcohol Depend. 2015;155:243–252. doi: 10.1016/j.drugalcdep.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Behavioral mechanisms underlying nicotine reinforcement. Curr Top Behav Neurosci. 2015;24:19–53. doi: 10.1007/978-3-319-13482-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldheer S, Yingst J, Zhu J, Foulds J. Ten-year weight gain in smokers who quit, smokers who continued smoking and never smokers in the United States, NHANES 2003-2012. Int J Obes. 2015;39:1727–1732. doi: 10.1038/ijo.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenza M, Steardo L, Cottone P, Sabino V. Diet-induced obesity and diet-resistant rats: differences in the rewarding and anorectic effects of D-amphetamine. Psychopharmacology. 2015;232:3215–3226. doi: 10.1007/s00213-015-3981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oginsky MF, Maust JD, Corthell JT, Ferrario CR. Enhanced cocaine-induced locomotor sensitization and intrinsic excitability of NAc medium spiny neurons in adult but not in adolescent rats susceptible to diet-induced obesity. Psychopharmacology (Berlin) 2015;233:773–784. doi: 10.1007/s00213-015-4157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vollbrecht PJ, Nobile CW, Chadderdon AM, Jutkiewicz EM, Ferrario CR. Preexisting differences in motivation for food and sensitivity to cocaine-induced locomotion in obesity-prone rats. Physiol Behav. 2015;152:151–160. doi: 10.1016/j.physbeh.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunney PE, Burroughs D, Hernandez C, LeSage MG. The effects of nicotine self-administration and withdrawal on concurrently available chow and sucrose intake in adult male rats. Physiol Behav. 2015;154:49–59. doi: 10.1016/j.physbeh.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- 25.Adir J, Miller RP, Rotenberg KS. Disposition of nicotine in the rat after intravenous administration. Res Commun Chem Pathol Pharmacol. 1976;13:173–183. [PubMed] [Google Scholar]
- 26.Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacol Biochem Behav. 2007;88:89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morganstern I, Lukatskaya O, Moon SH, Guo WR, Shaji J, Karatayev O, et al. Stimulation of nicotine reward and central cholinergic activity in Sprague-Dawley rats exposed perinatally to a fat-rich diet. Psychopharmacology. 2013;230:509–524. doi: 10.1007/s00213-013-3178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behav Neurosci. 2011;125:930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


