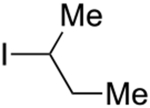
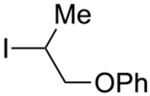
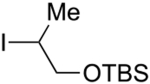
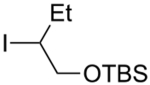
Abstract
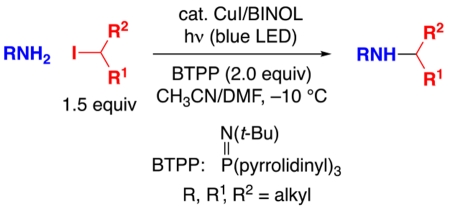
Although the alkylation of an amine by an alkyl halide serves as a “textbook example” of a nucleophilic substitution reaction, the selective mono-alkylation of aliphatic amines by unactivated, hindered halides persists as a largely unsolved challenge in organic synthesis. We report herein that primary aliphatic amines can be cleanly mono-alkylated by unactivated secondary alkyl iodides in the presence of visible light and a copper catalyst. The method operates under mild conditions (−10 °C), displays good functional-group compatibility, and employs commercially available catalyst components. A trapping experiment with TEMPO is consistent with C–N bond formation via an alkyl radical in an out-of-cage process.
Because amines are a privileged functional group in bioactive molecules,1 the development of more versatile methods for their synthesis is an important objective.2 Whereas the alkylation of an amine by an alkyl halide via an SN2 pathway is a classic transformation, at the same time the process represents an ongoing challenge in synthesis.3 Thus, rather than the desired C–N bond formation, undesired pathways such as E2 reactions and over-alkylation often intervene. Furthermore, because SN2 reactions are sensitive to steric effects, unactivated secondary and tertiary alkyl halides oftentimes do not serve as useful electrophilic partners. Due in part to these limitations, an array of methods other than the substitution reaction of an amine with an alkyl halide have been developed in order to selectively and efficiently introduce an alkyl group to an amine.2
Whereas transition-metal catalysis has been pursued very extensively to address the challenge of effecting substitution reactions of aryl halides by nitrogen nucleophiles,4 until recently there were essentially no systematic investigations of corresponding metal-catalyzed substitution reactions of alkyl halides.5 During the past few years, this deficiency has begun to be addressed, including through our work on photoinduced, copper-catalyzed processes (carbazoles, carboxamides, and indoles as nucleophiles)6,7 and a study by Hartwig on palladium-catalyzed reactions (benzophenone imines as nucleophiles).8
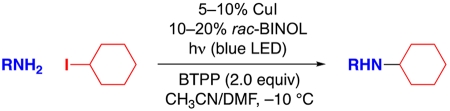
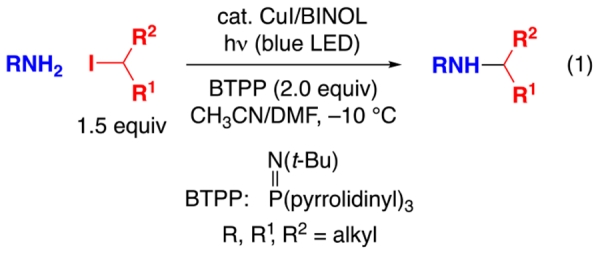
Nevertheless, to date a general method for transition-metal-catalyzed substitution of an alkyl halide by an aliphatic amine, which can be regarded as the prototypical nitrogen nucleophile, has not been described. In this study, we report a photoinduced, copper-catalyzed process that achieves the selective mono-alkylation of an array of aliphatic amines with unactivated secondary alkyl halides under mild conditions (−10 °C; eq 1).
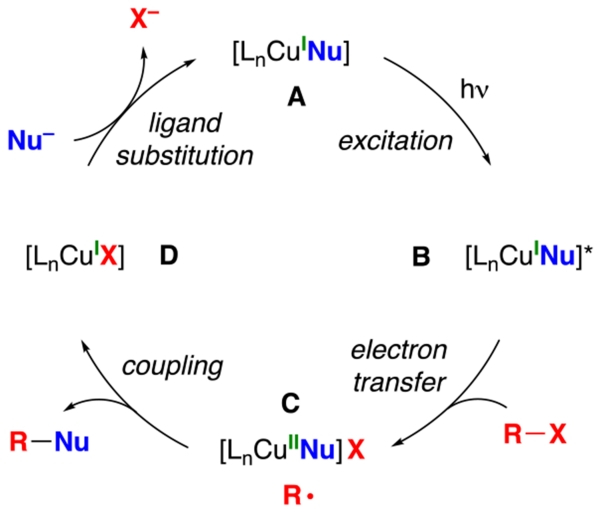
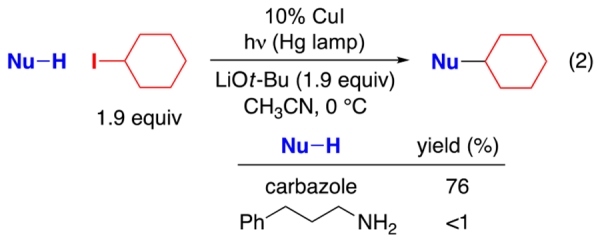
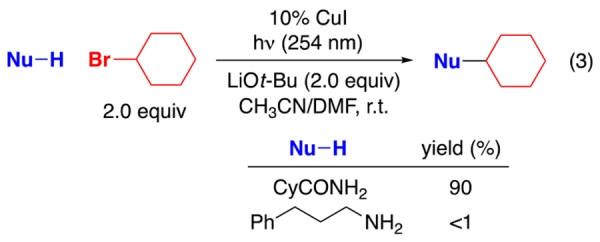
In earlier work, we have described a variety of coupling reactions of nucleophiles with organic (aryl, alkenyl, alkynyl, and alkyl) electrophiles that are induced by light and catalyzed by copper;6,9 an outline of one of the possible pathways for such processes is provided in Figure 1.10,11 To date, all of our reported couplings have employed nucleophiles wherein the nucleophilic site is part of a π system (N: carbazole, indole, and imidazole; S: aryl thiol; O: phenol; C: cyanide). On the other hand, our initial efforts to utilize nucleophiles that lack this feature were unsuccessful. For example, under conditions in which carbazole6a and cyclohexanecarboxamide6b undergo alkylation by an unactivated secondary halide in good yield, the corresponding alkylation of a primary aliphatic amine does not proceed (eq 2 and eq 3). Having the nucleophilic site incorporated within a π system might be important for any of a variety of reasons, including determining the viability of the initial photoexcitation (A → B in Figure 1)12 and/or of electron transfer from that excited state to the electrophile to generate a copper(II) complex (B → C).13
Figure 1.
Outline of one of the possible pathways for photoinduced, copper-catalyzed coupling reactions.
 |
 |
 |
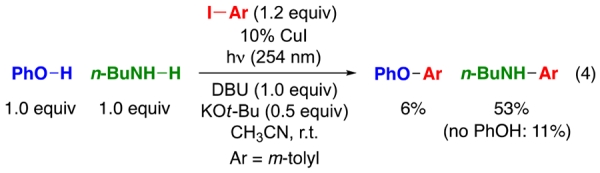
While examining the functional-group compatibility of a method that we had developed for photoinduced, copper-catalyzed arylations of phenols,9d we discovered that the presence of 1.0 equiv of an aliphatic amine additive unexpectedly leads to predominant N-arylation of the aliphatic amine, rather than O-arylation of the phenol (eq 4; in the absence of n-BuNH2: 80% yield of PhO–Ar).
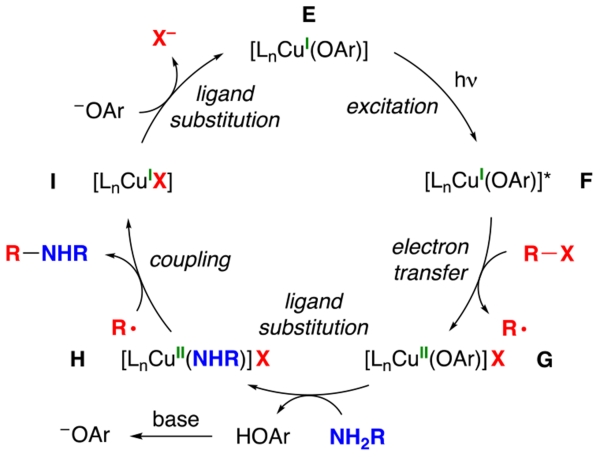
One of the possible pathways by which phenol might enable the photoinduced, copper-catalyzed cross-coupling of an aliphatic amine is depicted in Figure 2. Thus, photoexcitation of a copper(I)–phenoxide complex (E → F) and then electron transfer to an electrophile (R–X) affords a copper(II)–phenoxide (G) and an organic radical (R•). Ligand exchange of the copper(II)–phenoxide with an amine (NH2R) leads to a copper(II)–amido (H)14 that engages in C–N bond formation with the organic radical to furnish the cross-coupling product (R–NHR) and a copper(I) complex (I).15 Ligand substitution then regenerates a copper(I)–phenoxide complex (E).
Figure 2.
Simplified outline of one of the possible pathways for the photoinduced, copper-catalyzed coupling of an aliphatic amine in the presence of a phenol.
 |
Given the paucity of systematic studies of metal-catalyzed substitution reactions of unactivated alkyl halides by aliphatic amines, we attempted to exploit our initial observation (eq 4) to devise a photoinduced, copper-catalyzed process that would address this deficiency. Indeed, building on this lead result, we have been able to develop a method that achieves the selective mono-alkylation of a primary aliphatic amine by an unactivated secondary alkyl halide under mild conditions (−10 °C) in good yield (92%).
Control reactions establish that essentially none of the coupling product is generated in the absence of CuI, rac-BINOL, light, or BTPP (Table 1, entries 2–6). A variety of copper(I) and copper(II) sources furnish a good yield of the desired secondary amine, whereas copper nanopowder does not (entries 7–11). N-Alkylation proceeds less efficiently in the presence of less BINOL (entries 12 and 13) and when BINOL is replaced with related ligands (entries 14 and 15). The use of other Brønsted bases (entries 16 and 17), a smaller excess of electrophile or BTPP (entries 18 and 19), or a lower catalyst loading (entry 20; no further reaction after 24 h) also leads to significantly lower yields. Under our standard conditions, other cyclohexyl electrophiles (bromide, chloride, and tosylate) do not serve as suitable coupling partners (entries 21–23). Cross-coupling does occur in the presence of a small amount of air or water, although less effectively (entries 24 and 25).
Table 1.
Photoinduced, copper-catalyzed coupling of an aliphatic amine with an unactivated secondary alkyl iodide: Effect of reaction parameters.
| entry | variation from the “standard” conditions | yield (%)a |
|---|---|---|
| 1 | none | 92 |
| 2 | no Cul | <1 |
| 3 | no rac-BINOL | <1 |
| 4 | no hv | <1 |
| 5 | no BTPP | <1 |
| 6 | no Cul, no rac-BINOL, no light | <1 |
| 7 | CuBr, instead of Cul | 84 |
| 8 | CuCI, instead of Cul | 86 |
| 9 | CuBr2, instead of Cul | 81 |
| 10 | Cu(OTf)2, instead of Cul | 82 |
| 11 | copper nanopowder, instead of Cul | <1 |
| 12 | 6% rac-BINOL | 70 |
| 13 | 4% rac-BINOL | 48 |
| 14 | 2-naphthol, instead of rac-BINOL | 14 |
| 15 | rac-BINOL dimethyl ether, instead of rac-BINOL | <1 |
| 16 | 1,1,3,3-tetramethylguanidine, instead of BTPP | 50 |
| 17 | LiOt-Bu, instead of BTPP | 14 |
| 18 | room temperature | 56 |
| 19 | 1.2 equiv Cyl | 62 |
| 20 | 1.0 equiv BTPP | 62 |
| 21 | 2.5% Cul, 5% rac-BINOL | 54 |
| 22 | CyBr, instead of Cyl | <1 |
| 23 | CyCI, instead of Cyl | <1 |
| 24 | CyOTs, instead of Cyl | <1 |
| 25 | under air (capped vial) | 39 |
| 26 | 0.1 equiv H2O added | 78 |
Yields were determined via 1H NMR analysis versus an internal standard (average of two experiments).
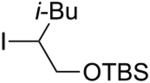
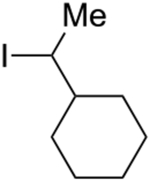
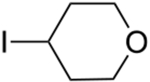
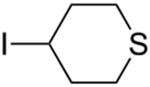
An array of unactivated secondary alkyl iodides, both cyclic and acyclic, serve as suitable electrophiles in this photoinduced, copper-catalyzed mono-alkylation of aliphatic amines (Table 2).16 The efficiency of the coupling is sensitive to steric effects, with more hindered electrophiles furnishing more modest yields (entries 6 and 7). Saturated oxygen and sulfur heterocycles are compatible with the reaction conditions (entries 8 and 9), and C–N bond formation can be achieved with excellent diastereoselectivity (entries 11 and 12; >20:1). In a gram-scale reaction, the alkylation illustrated in entry 1 proceeds in good yield with 10% CuI/20% BINOL (1.32 g, 81%).
Table 2.
Yields of purified product (average of two experiments).
Catalyst loading: 10% Cul, 20% rac-BINOL.
Starting material: cis/trans = 5/1; product: trans/cis >20/1.
Starting material: β/α >20/1; product: α/β >20/1.
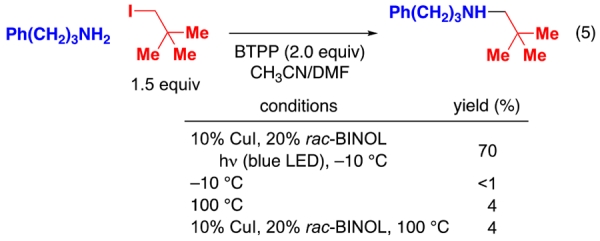
Although many unactivated primary alkyl halides can serve as useful electrophiles in SN2 reactions, neopentyl halides typically are rather poor substrates.17 Nevertheless, the combination of a CuI/BINOL catalyst and blue-LED irradiation enables the alkylation of an aliphatic amine by neopentyl iodide in good yield at −10 °C (eq 5). In contrast, a simple SN2 reaction proceeds very slowly even at 100 °C, and the addition of CuI/BINOL is not beneficial (eq 5).
 |
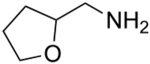
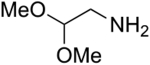
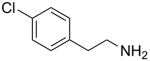
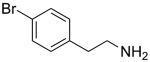
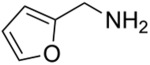
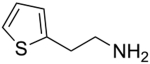
We have also examined the scope of this photoinduced, copper-catalyzed N-alkylation with respect to the nucleophile (Table 3). Thus, the efficiency of C–N bond formation does not appear to be highly sensitive to the steric demand of the aliphatic amine (entries 1 and 2). The method is compatible with a variety of functional groups, including an ether, an acetal, an aryl chloride, an aryl bromide, a furan, and a thiophene (entries 3–10).
Table 3.
Yields of purified product (average of two experiments).
Isolated as the trifluoroacetamide derivative.
Through an additive study, we have further assessed the functional-group compatibility of this method. For the coupling illustrated in entry 1 of Table 2, the addition of 1.0 equiv of an alcohol (5-nonanol), an alkyne (5-decyne), an ester (methyl octanoate), a ketone (2-nonanone), a cis olefin (cis-5-decene), and a trans olefin (trans-5-decene) has little impact on N-alkylation (>75% yield), and the additive is virtually unaffected (>90% recovery). On the other hand, the addition of a nitroalkane (nitrocyclopentane) or an aldehyde (cyclohexanecarboxaldehyde) impede coupling (<5% and 51% yield, respectively).
If desired, N-protection of the secondary amine can be effected in situ in good yield. For example, upon completion of the alkylation illustrated in entry 1 of Table 2, direct trifluoroacetylation followed by purification provides the TFA-protected amine in 86% yield. Similarly, a 73% yield of the purified carbamate can be obtained after in situ protection with Boc2O.
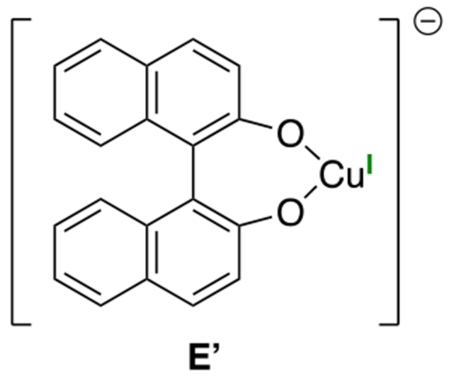
Although reaction development is the primary focus of this investigation, we have also carried out preliminary mechanistic studies; as mentioned earlier, one of the possible pathways for this process is outlined in Figure 2. With regard to the identity of the primary photoreductant, ESI–MS of a reaction mixture after partial conversion reveals the presence of copper(I)–binaphtholate complex E’; alternatively, deprotonated BINOL itself could also fill this role.18,19 The illustrated mechanism includes d9 copper(II) complexes as intermediates, and we have indeed detected such species via EPR spectroscopy by sampling a catalyzed coupling at partial conversion; at least two copper(II) species are evident (hyperfine coupling to copper), which together account for ~60% of the total copper that is present in the reaction mixture.
According to the pathway depicted in Figure 2, C–N bond formation occurs through out-of-cage coupling of an organic radical (R•) with a copper(II)–amido complex.15 Consistent with this hypothesis, the addition of TEMPO (1.5 equiv) to a reaction mixture leads to the formation of a TEMPO adduct (eq 6).
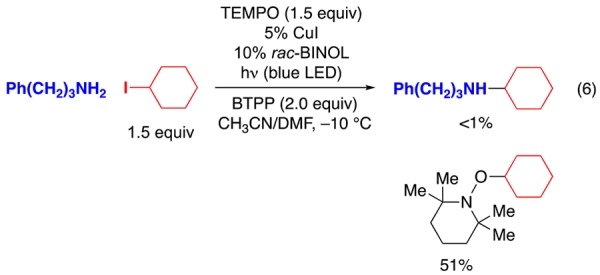 |
In summary, we have determined that the combination of visible light and a copper catalyst provides the first general method for the transition-metal-catalyzed alkylation of aliphatic amines by unactivated secondary alkyl halides. This process addresses some of the deficiencies of the classic SN2 approach, including its need for reactive electrophiles and its propensity for over-alkylation. With respect to our efforts to expand photoinduced, copper-catalyzed coupling reactions, this represents our first success with nucleophiles wherein the nucleophilic site is not part of a π system. With our optimized method, C–N bond formation proceeds without significant over-alkylation (<1%) under mild conditions (−10 °C) in the presence of a variety of functional groups, upon irradiation by blue-LED lamps of a catalyst derived from commercially available components. A preliminary mechanistic study is consistent with the formation of an alkyl radical that engages in out-of-cage C–N bond formation. Our future work will focus on expanding photoinduced, copper-catalyzed couplings to other classes of non-conjugated nucleophiles, as well as on elucidating the mechanisms of these processes.
ACKNOWLEDGMENT
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, grant R01–GM109194) and the Alexander von Humboldt Foundation (fellowship for J.S.). We thank Jun Myun Ahn, Bradley J. Gorsline, Dr. Paul H. Oyala (Caltech EPR Facility, supported by National Science Foundation grant NSF-1531940), Dr. Mona Shahgholi (Caltech Mass Spectrometry Facility), Dr. Yichen Tan, Dr. David G. VanderVelde (Caltech NMR Facility), and Dr. Scott C. Virgil (Caltech Center for Catalysis and Chemical Synthesis) for assistance and helpful discussions.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b09582.
Procedures and characterization data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).For example, see: Alkaloids: A Treasury of Poisons and Medicines; Funayama S, Cordell GA, Eds.; Academic Press: Waltham, MA, 2014.
- (2).For leading references, see: Lawrence SA Amines: Synthesis, Properties, and Applications; Cambridge University Press: Cambridge, U.K., 2004. Yang Q; Wang Q; Yu Z Chem. Soc. Rev 2015, 44, 2305–2329. Schafer LL; Yim JC-H; Yonson N In Metal-Catalyzed Cross-Coupling Reactions and More; De Meijere A, Br\l=a"\se S, Oestreich M, Eds.; Wiley\p=n-\VCH: Weinheim, Germany, 2014. Margaretha P In Science of Synthesis, Knowledge Updates; Georg Thieme Verlag: Hamburg, Germany, 2010; pp 405–442.
- (3).For leading references, see: Marvin CC In Comprehensive Organic Synthesis; Knochel P, Molander GA, Eds.; Elsevier: Amsterdam, 2015; Vol. 6, pp 34–99.
- (4).For reviews and leading references, see: Jiang Y; Ma D In Copper-Mediated Cross-Coupling Reactions; Evano G, Blanchard N, Eds.; John Wiley & Sons: Hoboken, NJ, 2014; pp 3–40. Ruiz-Castillo P; Buchwald SL Chem. Rev 2016, 116, 12564–12649. Hartwig JF; Shekhar S; Shen Q; Barrios-Landeros F In Chemistry of Anilines; Rapaport Z, Ed.; John Wiley & Sons: New York, 2007; Vol. 1, pp 455–536.
- (5).For early examples of reactions of unactivated alkyl electrophiles (all are primary alkyl bromides) that proceed in the presence of a substoichiometric quantity of a transition metal, see: Aydin A; Kaya I Electrochimica Acta 2012, 65, 104–114 (165 \s=deg\C). Tu X; Fu X; Jiang Q; Liu Z; Chen G Dyes and Pigments 2011, 88, 39–43 (80 \s=deg\C). Kozuka M; Tsuchida T; Mitani M Tetrahedron Lett 2005, 46, 4527–4530 (83 \s=deg\C).
- (6). Bissember AC; Lundgren RJ; Creutz SE; Peters JC; Fu GC Angew. Chem. Int. Ed 2013, 52, 5129–5133. Do H-Q; Bachman S; Bissember AC; Peters JC; Fu GC J. Am. Chem. Soc 2014, 136, 2162–2167. (c) For activated tertiary alkyl halides, see: Kainz QM; Matier CM; Bartoszewicz A; Zultanski SL; Peters JC; Fu GC Science 2016, 351, 681–684.
- (7).For examples of work by others on photoinduced, copper-catalyzed couplings (not with alkyl electrophiles), see: Paria S; Reiser O ChemCatChem 2014, 6, 2477–2483. Ye Y; Sanford MS J. Am. Chem. Soc 2012, 134, 9034–9037. Sagadevan A; Hwang KC Adv. Synth. Catal 2012, 354, 3421–3427.
- (8). Peacock DM; Roos CB; Hartwig JF ACS Cent. Sci 2016, 2, 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Creutz SE; Lotito KJ; Fu GC; Peters JC Science 2012, 338, 647–651. [DOI] [PubMed] [Google Scholar]; (b) Uyeda C; Tan Y; Fu GC; Peters JC J. Am. Chem. Soc 2013, 135, 9548–9552. [DOI] [PubMed] [Google Scholar]; (c) Ziegler DT; Choi J; Munoz-Molina JM; Bissember AC; Peters JC; Fu GC J. Am. Chem. Soc 2013, 135, 13107–13112. [DOI] [PubMed] [Google Scholar]; (d) Tan Y; Munoz-Molina JM; Fu GC; Peters JC Chem. Sci 2014, 5, 2831–2835. [Google Scholar]; (e) Ratani TS; Bachman S; Fu GC; Peters JC J. Am. Chem. Soc 2015, 137, 13902–13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).For mechanistic studies, see: Johnson MW; Hannoun KI; Tan Y; Fu GC; Peters JC Chem. Sci 2016, 7, 4091–4100. Ahn JM; Ratani TS; Hannoun KI; Fu GC; Peters JC J. Am. Chem. Soc 2017, 139, 12716–12723.
- (11).For leading references on photoredox catalysis, see: Prier CK; Rankic DA; MacMillan DWC Chem. Rev 2013, 113, 5322–5363. Skubi KL; Blum TR; Yoon TP Chem. Rev 2016, 116, 10035–10074.
- (12).For example, see: Lotito KJ; Peters JC Chem. Commun 2010, 46, 3690–3692. Miller AJM; Dempsey JL; Peters JC Inorg. Chem 2007, 46, 7244–7246.
- (13).For examples of studies that show a role for the \g=p\ system of the nucleophile in the electronic structure of copper(I) and copper(II) intermediates in photoinduced cross-couplings, see ref 10.
- (14).For ease of discussion, the transformation of G → H in Figure 2 is drawn as a simple ligand exchange, whereas our data (e.g., out-of-cage C\p=n-\N bond formation (vide infra)) point to a more complex pathway.
- (15).In the case of a photoinduced, copper-catalyzed decarboxylative coupling, we have recently described evidence for out-of-cage C\p=n-\N coupling after ligand exchange: Zhao W; Wurz RP; Peters JC; Fu GC J. Am. Chem. Soc 2017, 139, 12153–12156. See also ref 10b.
- (16).Notes: (a) For all N-alkylations that are illustrated in Tables 2 and 3, essentially no over-alkylation is observed (<1%). (b) Preliminary observations: this process can be conducted under flow conditions; an unactivated tertiary alkyl halide is not a suitable electrophile; in a light-on/light-off experiment, alkylation stops when irradiation stops, and alkylation resumes when irradiation resumes.
- (17).Relative to Me\p=n-\X, neopentyl\p=n-\X is typically 3.3 \m=x\ 10\m=-\7 less reactive in SN2 reactions. For example, see Table 11.5 in: Anslyn EV; Dougherty DA Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, 2006.
- (18).(a) Upon irradiation at 350 nm, 2-naphtholate has been reported to serve as a photoreductant of aryl and alkyl halides. For example, see: Arg\l=u"\ello JE; Pe\l=n~\e\l=n~\ory AB J. Org. Chem 2003, 68, 2362–2368.12636403 Legros B; Vandereecken P; Soumillion JP J. Phys. Chem 1991, 95, 4752–4761. (b) We have observed that deprotonated BINOL can serve as a photoreductant of iodocyclohexane upon irradiation with a blue LED lamp.
- (19).For an example of a photoinduced, copper-catalyzed coupling wherein both a copper-bound and a copper-free nucleophile (carbazolide) appear to serve as photoreductants, see ref 10b.