Synopsis
Left ventricular dysfunction remains one of the best prognostic determinants of survival in patients with coronary artery disease. Revascularization has been shown to improve survival compared to medical therapy alone. Viability testing can help direct patients whom will benefit the most from revascularization. Single-Photon Emission CT (SPECT), dobutamine stress echo, Cardiac Magnetic Resonance Imaging (CMR), and Positron Emission Tomography (PET) imagining with F18-fluorodeoxyglocse (FDG) are the most common modalities for assessing myocardial viability. Viability testing can help differentiate which patients benefit most from chronic total occlusion interventions.
Keywords: Viability, hibernation, revascularization, chronic total occlusion
1. Introduction
Left ventricular (LV) dysfunction remains one of the best prognostic determinants of survival in patients with coronary artery disease (CAD).1,2 It was originally thought that dysfunctional myocardium after an infarction was irreversibly damaged.3 However, it was later recognized that some of the involved tissue remained viable and contractility may be restored with revascularization.4,5 Given that worsening LV systolic function secondary to ischemia has been shown to be associated with worse outcomes, but not all myocardium improves with revascularization, viability testing has since been well studied and utilized. We will review the pathophysiology and mechanism of myocardial viability, the most commonly used non-invasive modalities to assess myocardial viability and their strengths and weaknesses, the utility of viability testing for chronic total occlusion (CTO) interventions, and the STICH trial.6
2. Pathophysiology and mechanism of myocardial viability
After a myocardial infarction, the myocardium will usually demonstrate one of 5 pathophysiologies: 1) normal myocardial perfusion and function, 2) myocardial ischemia, 3) stunned myocardium, 4) myocardial hibernation, and 5) non-viable infarction.3 Prompt reperfusion or the presence of collateral vessels and intact coronary microvasculature function may preserve myocardial perfusion. Ischemia occurs as a result of decreased blood flow resulting in low ATP production and subsequent LV dysfunction.3
2.1. Myocardial Stunning
Myocardial stunning is a reversible state of regional contractile dysfunction that occurs after transient ischemia without ensuing necrosis.7 Myocardial stunning is believed to play an important role in persistent contractile dysfunction seen in acute myocardial infarction patients after successful reperfusion.8 In general, myocardial perfusion is normal and function recovers relatively quickly.
2.2. Myocardial Hibernation
More than 40 years ago physicians noticed that chronic myocardial dysfunction before coronary bypass often improved after revascularization.4,5 Myocardial hibernation is a state of persistent left ventricular dysfunction that results from chronically reduced blood flow or repetitive stunning without infarction and necrosis. A downregulation in contractile function at rest is thought to represent a protective mechanism to reduce myocardial oxygen requirements and ensure myocyte survival. When severe cellular hypoperfusion and damage occurs, only cellular function that is essential for survival, such as membrane integrity, is preserved. Preserved or increased myocardial glucose metabolism also occurs during this state.
2.3. Nonviable myocardium
If myocardial perfusion is not restored, irreversible myocardial necrosis can occur. The goal of viability testing, detailed in the next section, is to determine if a large portion of dysfunctional myocardium is nonviable in which case the risks would likely outweigh benefit of revascularization.
3. Viability and Noninvasive Imaging Methods of Assessment
Viability testing can predict improvement of heart failure symptoms and exercise capacity after revascularization.9,10 The ability to distinguish viable from non-viable myocardium that is able to recover contractile function following revascularization presents a clinical challenge in current practice.11 Furthermore, viability testing can have a lower specificity related to the fact that not all patients with viable myocardium improve function after revascularization.12
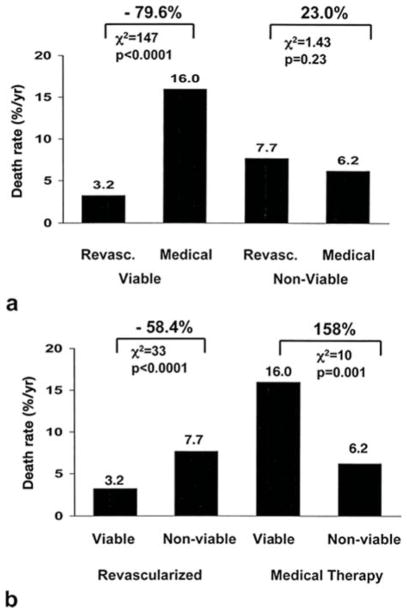
Medical therapy with revascularization in patients with ischemic cardiomyopathy has been shown to decrease mortality compared to medical therapy alone.13 The probability of reversing LV remodeling and improving LV systolic function with medical therapy and/or revascularization has been shown to be greater with increased proportions of viable myocardium on noninvasive imaging.14,15 As shown in Figure 1, Allman et al. demonstrated in a meta-analysis of mostly observational studies that patients with viability treated by revascularization had a near 80% reduction in mortality. Those without viability had no difference in mortality between medical therapy or revascularization.16 We will review each of the currently most commonly used modalities for viability testing below.
Figure 1.
(a) Death rates for patients with and without myocardial viability treated by revascularization or medical therapy. (b) same data as (a) with comparisons based on treatment strategy in patients with and without viability. (From Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002 Apr 3:39(7):1151–8, with permission.)
3.1 Single-Photon Emission CT (SPECT)
SPECT utilizes radionuclide-labeled tracer to measure regional tracer concentration in the myocardium and can measure viability by determining percentage of peak uptake of the tracer. This can be interpreted with rest images only or with a stress/rest testing protocol. The most common tracers used are 99mTc-sestamibi or 201Tl. The two tracers have been shown to have comparable results in predicting recovery of resting defects.17 Radiotracers sequester within myocytes with intact cell membrane. Thus myocardial viability is interpreted as an all-or-none phenomenon as SPECT cannot assess the transmural extent of variability within the left ventricular wall. The advantage of 99mTc-sestamibi is its much shorter protocol duration with rest imaging occurring approximately 1 hour after tracer administration. 201T viability imaging is based on its redistributive property of 201Tl in the myocardium and thus requires 4-hour and 24-hour delayed imaged in order to assess viability.18
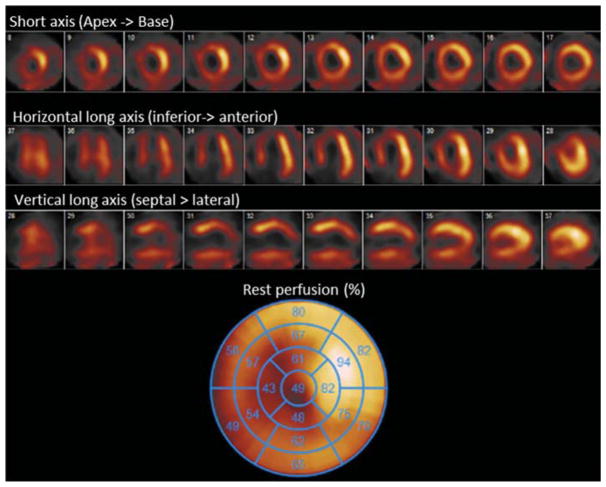
A cutoff of >50% tracer activity is the most commonly used criteria to identify viable myocardium (Figure 2). When viability is clearly present on rest images, generally no further imaging is necessary to determine viability. It has been shown that the presence of inducible ischemia is of additive value and more predictive of functional recovery than comparable images with similar peak uptake of tracer on rest images but no ischemia.19 SPECT has been shown to have a mean sensitivity of 84% and mean specificity of 77% in predicting recovery of global LV function after revascularization.7
Figure 2.
SPECT rest only myocardial perfusion imaging with 99mTc-sestamibi for viability assessment of a patient with severe three vessel disease and LV EF 35%. Visual and quantitative analysis reveals a large region of infarct in the apical to mid anterior, anteroseptal and inferoseptal segments. The quantitative polar plot shown reveals viability in all coronary territories except in segments of the apex with perfusion under 50%.
3.2 Dobutamine Stress Echocardiography (DSE)
Assessment for augmentation of contractility, or contractile reserve, in response to dobutamine stress is the basis for the use of DSE as a measurement of viability.20 An initial infusion of dobutamine at 2.5 μg/kg/min, with gradual increase to 5, 7.5, 10, and 20 μg/kg/min, is commonly used.21 Wall thickness should be assessed on resting images as segments that are thinned (≤0.5 or 0.6 cm) and bright (suggesting advanced fibrosis) rarely recover.21–23 DSE has higher specificity (mean 79% vs 59%) but lower sensitivity (mean 82% vs 86%) in detection of viable myocardium compared to 201Tl rest-redistribution imaging.21,24 Less scar and greater percentage of viable myocytes are needed to detect contractile reserve by DSE.25
Multicenter studies have shown worse outcomes when viable myocardium is identified by DSE and no revascularization is pursued.26,27 Four distinct responses to dobutamine echo have been described.11,21,28 A “biphasic response” can occur in which contractility improves in dysfunctional segments with low-dose dobutamine and then becomes dysfunctional again at higher doses due to ischemia. The biphasic response is 60% sensitive and 88% specific in assessing recovery of contractile function 6 weeks after coronary angioplasty.28 Another study in patients with ischemic cardiomyopathy undergoing CABG showed a 75% improvement in regional ventricular function after 14 months.29 Hibernating myocardium is thought to occur with “worsening contractile function” as dobutamine doses increase. Hibernating myocardial tissue has no contractile reserve and increases in demand result in ischemia and further worsens contractility. This response to dobutamine has been shown to be the second most predictive of functional recovery.28,30 Combining biphasic response with worsening response improves sensitivity to 74% but decreases specificity to 73% in assessing recovery of contractile function.28 Myocardial stunning is believed to be present when there is “sustained improvement” with increasing dobutamine dose. Finally, lack of viability is believed to be present when there is “no response” to dobutamine, with only 4% of segments recovering after revascularization.11,29
3.3 Cardiac Magnetic Resonance Imaging (CMR)
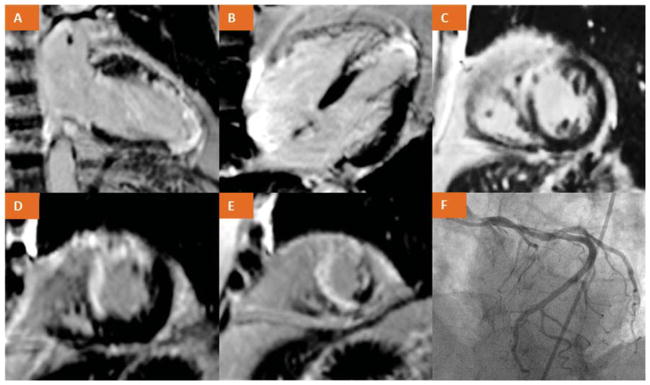
CMR provides information in regards to global left ventricular function and regional wall motion. Viability can be assessed using LV end-diastolic wall thickness (EDWT) or response to dobutamine stress similar to DSE as previously described. The most commonly used technique is late gadolinium enhancement (LGE) imaging (Figure 3). Gadolinium should be avoided in those with advanced renal disease due to the risk of nephrogenic systemic fibrosis although its incidence has virtually disappeared after adopting guidelines restricting its use.31,32 Other contraindications to MRI include claustrophobia, certain metallic hardware, and inability to breath hold. Pacemakers and defibrillators are no longer absolute contraindications.33 Benefits of CMR over DSE and SPECT include excellent spatial imaging and ability to determine transmural variations in viability.
Figure 3.
(A–E) show >75% transmural late gadolinium enhancement in the mid to distal LAD territories suggesting no viability. (F) Coronary angiography demonstrating occluded LAD after late presentation from a myocardial infarct.
Nearly two decades ago, Kim et al. demonstrated that reversibly myocardial dysfunction could be identified by contrast-enhanced CMR before coronary revascularization. Fifty patients with planned revascularization (CABG or PCI) and regional wall motion abnormalities without unstable angina or NYHA class IV heart failure were included in this study. Of the patients with dysfunctional segments, 78% of the segments without enhancement (deemed to be completely viable) had an improvement in contractility 79±36 days after revascularization.34 Reasons for incomplete recovery could be premature reevaluation of ventricular function, tethering to infarcted segments, other nonischemic reasons for LV dysfunction, or incomplete revascularization. Complete recovery of hibernating myocardium may take more than 12 months because prolonged ischemia may result in sarcomere loss, glycogen accumulation, disarray of mitochondria, and fibrosis.7 Sixty-five percent of segments with 1–25% hyperenhancement and wall segment dysfunction severity of at least severe hypokinesia had recovery in ventricular function after revascularization. A cut-off value of 50% transmural hyperenhancement resulted in a negative predictive value of 92%. An impressive negative predictive accuracy of 100% was seen in segments with at least 75% transmural hyperenhancement and at least severe hypokinesia. LGE CMR has been studied vs PET, the prior gold standard for viability assessment, and found to closely agree in identifying myocardial scar.35
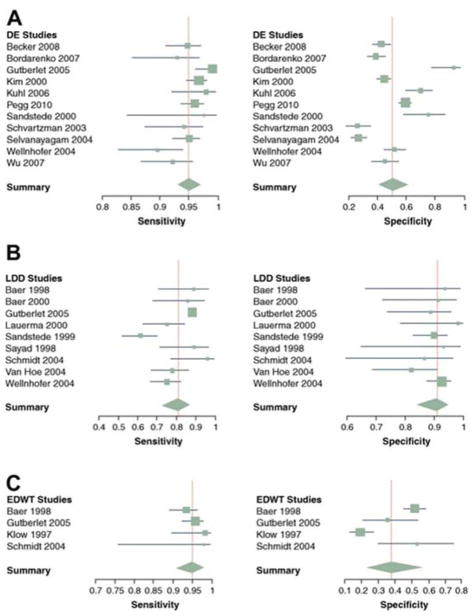
A recent meta-analysis by Romero et al. studied the three aforementioned CMR methods to assess viability.36 A cut-off for viability of < 50% transmural LGE allowed for a high sensitivity (95%) and negative predictive value (90%) but low specificity (51%). Low dose dobutamine (LDD) response had the highest specificity (91%) and positive predictive value (93%) with a lower sensitivity (81%) and negative predictive value (75%). LDD was more accurate than LGE (84% vs 70%). An LV EDWT of 5.5 to 6.0 mm has a high sensitivity (96%) arguing that thin and dysfunctional segments can accurately be classified as nonviable without further assessment with LGE. Specificity however is only 38% with this method (Figure 4).36 A recent multicenter prospective study using CMR in patients with CAD and regional myocardial thinning found that even in myocardial regions with LV EDWT ≤ 5.5 mm an inverse relationship between scar burden (LGE) and viability exist.37 This suggests that even myocardial segments with regional wall thinning warrant further viability assessment.
Figure 4.
Forest plots of sensitivity and specificity for delayed enhancement (DE) CMR, low-dose dobutamine (LDD) CMR, and resting LV end-diastolic wall thickness (EDWT). (From Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012 May;5(5):494–508, with permission.)
3.4 Positron Emission Tomography (PET) imagining with F18-fluorodeoxyglocse (FDG)
FDG PET is a very effective imaging technique for differentiating among normal, infarcted, stunned, and hibernating myocardium. PET has better spatial resolution than SPECT. Rest perfusion can be assessed with multiple tracers, including 13N-ammonia or 82Rb.38 Hibernating myocardium can be determined by assessment of glucose uptake in the myocardium. At rest the myocardium will generally oxidize free fatty acids to produce ATP. However, in the setting of myocardial ischemia, there is a shift to glucose metabolism with up-regulation of glucose transporters. When fasting, FDG is taken up mainly by ischemic myocardium. Scar tissue and normal myocardium do not take up FDG. However, oral glucose loading can stimulate FDG uptake in viable and normal myocardium. Insulin can be given to correct for hyperglycemia as needed.39 In patients with insulin resistance, FDG uptake in normal regions may remain less than that of ischemic or hibernating regions. One limitation to PET is the variability of FDG uptake which can be impacted by cardiac output, heart failure, degree of ischemia, and sympathetic activity.11
The typical appearance of hibernation on PET with FDG is a perfusion-metabolism mismatch, which involves decreased 13N-ammonia uptake, indicating decreased perfusion, and increased or preserved FDG uptake due to up-regulation of glucose transporters.38 A reduction in blood flow and metabolism is indicative of myocardial scar (Figure 5). FDG PET can identify stunned myocardium by demonstrating normal perfusion in an area of regional contractile dysfunction.
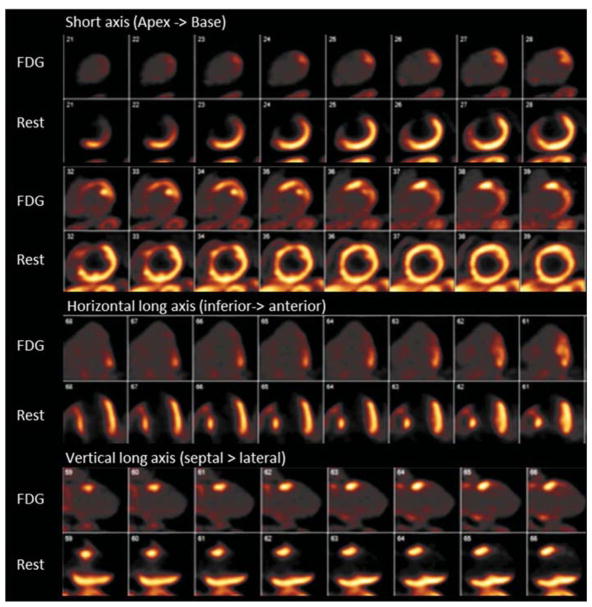
Figure 5.
PET myocardial perfusion imaging using N-13 ammonia rest perfusion images (bottom row images) and F-18 fluorodeoxyglucose (FDG) myocardial metabolic images (top row images). A large fixed perfusion defect with akinesis on gated images is seen in the mid to basal anterior and septal segments of the LAD territory. No FDG uptake is seen in this territory suggesting no viability.
Tillisch et al. demonstrated that FDG PET could predict reversible segments (85 percent predictive accuracy) and irreversible (92 percent predictive accuracy) abnormal contraction in patients with LV systolic dysfunction undergoing coronary-artery bypass.40 Eitzman et al. used perfusion and FDG PET to assess viability prior to revascularization (CABG or PCI) in 82 patients with advanced CAD and LV dysfunction.10 Those who had evidence of viability who did not undergo revascularization were more likely to experience a myocardial infarction, death, cardiac arrest or later revascularization (p<0.01). Those with viability that underwent revascularization had improvement in symptoms. Those without viability had no difference in outcome comparing revascularization or no revascularization.
A meta-analysis of 20 studies with 598 patients undergoing viability with FDG before revascularization showed a high sensitivity (93%) but low specificity (58%) for identifying LV recovery.24 Sensitivity was higher than other nuclear imaging techniques and dobutamine echocardiography. The lower specificity was thought to be in part due to variation of follow-up duration with studies varying from 7 days to 14 months.24
To date one randomized control trial, the PET and Recovery Following Revascularization-2 (PARR-2) trial, evaluated the efficacy of FDG PET viability imaging in identification of patients with ischemic cardiomyopathy who would benefit most from revascularization.41 In the FDG PET assisted management guided arm, when significant viable myocardium was identified revascularization work-up was recommended. When predominantly scar tissue was identified no revascularization work-up was recommended. There was a non-statistically significant trend (p=0.16) towards fewer cardiovascular events within one year in the FDG-PET assisted management group. A post hoc analysis showed a significant reduction in adverse outcomes (p=0.019) when there was adherence to PET recommendations. This was a major limitation of the study as only 75% of the patient’s clinicians adhered to PET recommendation.41
4. Surgical Treatment for Ischemic Heart Failure (STICH) trial
STICH, was a multicenter, unblinded, randomized control trial evaluating the role of surgical coronary artery revascularization in ischemic cardiomyopathy with EF ≤ 35%. The main finding was that after a median follow-up of 10 years, surgical revascularization improved all-cause mortality (58.9% vs 66.1%, p=0.02) and cardiovascular mortality (40.5% vs 49.3%, p=0.006).13 A substudy of this trial assessed the 601 patients who underwent myocardial viability evaluation with SPECT (n=321), DSE (n=130), or both (n=150) and viability was determined in binary fashion. Mortality was lower in those with viable myocardium (37%) vs without viable myocardium (51%), however after adjustment for other prognostic variable (age, EF, heart failure class, etc.) this association was no longer significant (p=0.21). There was also no significant interaction between viability status and treatment assignment with respect to mortality (p=0.53).6
Some have interpreted these results as viability testing having little value in determining who should undergo revascularization in ischemic cardiomyopathy and as discordance between observational studies with the findings in this trial.42 However, viability testing in the substudy was not randomized and thus the data was prone to the same biases as an observational study.43 When this study was initially planned, PET and CMR methods were not available in sufficient numbers of centers to include in the study protocol. Multiple studies have demonstrated superior accuracy of PET and CMR for assessment of viability compared to T1-201 SPECT and dobutamine echo.15 One aspect of the trial which could account for its failure to identify benefit from viability testing is that there was a lack of standardized protocols for SPECT viability evaluation. Each center adopted their own SPECT protocol and ischemia assessment was not included. There was also difficulty with patient enrollment which may be due to perception of a lack of equipoise among many clinicians. The STICH population also was skewed in that the patients most likely to benefit from CABG may have been selected out either by design or clinician preference.
5. Chronic total occlusion (CTO) and viability
The benefits of CTO interventions are controversial given the increased complexity of the intervention and the results of the Occluded Artery Trial (OAT) which showed a lack of benefit of PCI versus medical therapy in patients with an occluded infarct-related artery.44 However, these results can only apply to patients with an occluded infarct related artery 3–28 days after an acute myocardial infarction and PCI was not guided by ischemia nor myocardial viability testing.45
Baks et al. studied 27 patients who underwent successful CTO recanalization with DES and whom had CMR before and after intervention. They found that segmental wall thickening (SWT) improved most significantly in segments with <25% transmural extent of infarction by LGE CMR.46 A recent single-center prospective study used stress CMR to guide CTO intervention on candidate patients with stable angina and estimated occlusion duration of ≥ 3 months felt to be suitable for recanalization based on review of their coronary angiogram.45 Those felt to have viability based on a criteria of <75% transmural late gadolinium enhancement (LGE) in the majority of the CTO segments and an inducible perfusion defect, proceeded with intervention and underwent repeat CMR 3 months after successful CTO recanalization. Myocardial perfusion reserve (MPR) improved significantly in the CTO region (2.3 ±0.9 vs 1.8 ±0.72; p= 0.02) with complete or near-complete resolution of CTO related perfusion defect in 90% of patients, LV ejection fraction increased from 63±13% to 67±17% (p<0.0001), end-systolic volume decreased from 65±38 to 56±38 ml (p<0.001) and the patients showed improvement in symptoms based on the Seattle Angina Questionnaire score 3 months after CTO PCI.
Kirschbaum et al. evaluated LV function recovery with CMR pre-procedure, 5 months, and 3 years after CTO percutaneous recanalization. SWT significantly improved at 5-months follow-up (p<0.001) in those with < 25% transmural infarct but not in those with 25% to 75% transmural infarct (p = 0.89).47 However at 3 years there was an improvement in SWT in those with 25% to 75% transmural infarct suggesting that the recovery time of dysfunction myocardium was related to the extent of damage on a cellular level.
6. Summary
In summary, patients with obstructive CAD and severe LV dysfunction are known to have a poor prognosis. Those who can undergo successful revascularization have a decrease in mortality. The goal of viability testing is to identify whether there is significant viable myocardium that would likely result in an improved outcome with coronary revascularization. If no significant viability is identified, the risk for perioperative morbidity is likely higher than the gain from revascularization. Table 1 summarizes the advantages and disadvantages of the most common modalities currently available for assessment of viability. It is imperative that interventional cardiologists understand the advantages and limitations of each method when trying to make decisions on revascularization. Viability testing is not needed in all patients with ischemic cardiomyopathy such as those with angina or documented ischemia which by definition is associated with viable myocardium. However, more studies have recently been published in viability assessment prior to CTO interventions, a growing field with some controversy as how to manage these lesions. There remains room for clinical trials using viability testing to guide patient management.
Table 1.
Pros and cons of each viability modality
| Modality | Advantages | Disadvantages |
|---|---|---|
| SPECT |
|
|
| DSE |
|
|
| CMR |
|
|
| PET |
|
|
CMR = Cardiac Magnetic Resonance; DSE = Dobutamine Stress echo; EDWT = End diastolic wall thickness; PET = Positron Emission Tomography; SPECT = Single-Photon Emission CT.
Key Points.
Left ventricular dysfunction remains one of the best prognostic determinants of survival in patients with coronary artery disease and revascularization improves survival.
Patients with myocardial viability have increased mortality if treated medically and do not undergo revascularization.
Out of the most commonly used modalities to assess viability, CMR and PET offer the highest sensitivity and specificity.
Patients undergoing CMR guided CTO intervention have been shown to have improvement in LV ejection fraction, myocardial perfusion reserve, and symptoms.
Footnotes
Disclosures: Supported in part by 5T32EB003841
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burns RJ, Gibbons RJ, Yi Q, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. Journal of the American College of Cardiology. 2002;39:30–36. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 2.Møller JE, Egstrup K, Køber L, Poulsen SH, Nyvad O, Torp-Pedersen C. Prognostic importance of systolic and diastolic function after acute myocardial infarction. American Heart Journal. 2003;145:147–153. doi: 10.1067/mhj.2003.46. [DOI] [PubMed] [Google Scholar]
- 3.Marwick TH. The viable myocardium: Epidemiology, detection, and clinical implications. Lancet. 1998;351:815–819. doi: 10.1016/S0140-6736(97)08080-X. [DOI] [PubMed] [Google Scholar]
- 4.Helfant RH, Pine R, Meister SG, Feldman MS, Trout RG, Banka VS. Nitroglycerin to unmask reversible asynergy. correlation with post coronary bypass ventriculography. Circulation. 1974;50:108–113. doi: 10.1161/01.cir.50.1.108. [DOI] [PubMed] [Google Scholar]
- 5.Dyke SH, Cohn PF, Gorlin R, Sonnenblick EH. Detection of residual myocardial function in coronary artery disease using post-extra systolic potentiation. Circulation. 1974;50:694–699. doi: 10.1161/01.cir.50.4.694. [DOI] [PubMed] [Google Scholar]
- 6.Bonow RO, Maurer G, Lee KL, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103–114. doi: 10.1161/CIRCULATIONAHA.107.702993. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E, Kloner RA. The stunned myocardium: Prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- 9.Di Carli MF, Asgarzadie F, Schelbert HR, et al. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation. 1995;92:3436–3444. doi: 10.1161/01.cir.92.12.3436. [DOI] [PubMed] [Google Scholar]
- 10.Eitzman D, Al-Aouar Z, Kanter HL, et al. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. Journal of the American College of Cardiology. 1992;20:559–565. doi: 10.1016/0735-1097(92)90008-b. [DOI] [PubMed] [Google Scholar]
- 11.Bhat A, Gan GC, Tan TC, Hsu C, Denniss AR. Myocardial viability: From proof of concept to clinical practice. Cardiol Res Pract. 2016;2016:1020818. doi: 10.1155/2016/1020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bax JJ, Schinkel AF, Boersma E, et al. Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse long-term prognosis. Circulation. 2004;110:II18–22. doi: 10.1161/01.CIR.0000138195.33452.b0. [DOI] [PubMed] [Google Scholar]
- 13.Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desideri A, Cortigiani L, Christen AI, et al. The extent of perfusion-F18-fluorodeoxyglucose positron emission tomography mismatch determines mortality in medically treated patients with chronic ischemic left ventricular dysfunction. J Am Coll Cardiol. 2005;46:1264–1269. doi: 10.1016/j.jacc.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 15.Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: Diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32:375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: A meta-analysis. Journal of the American College of Cardiology. 2002;39:1151–1158. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 17.Udelson JE, Coleman PS, Metherall J, et al. Predicting recovery of severe regional ventricular dysfunction. comparison of resting scintigraphy with 201Tl and 99mTc-sestamibi. Circulation. 1994;89:2552–2561. doi: 10.1161/01.cir.89.6.2552. [DOI] [PubMed] [Google Scholar]
- 18.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol. 2016;23:606–639. doi: 10.1007/s12350-015-0387-x. [DOI] [PubMed] [Google Scholar]
- 19.Kitsiou AN, Srinivasan G, Quyyumi AA, Summers RM, Bacharach SL, Dilsizian V. Stress-induced reversible and mild-to-moderate irreversible thallium defects: Are they equally accurate for predicting recovery of regional left ventricular function after revascularization? Circulation. 1998;98:501–508. doi: 10.1161/01.cir.98.6.501. [DOI] [PubMed] [Google Scholar]
- 20.Buckley O, Di Carli M. Predicting benefit from revascularization in patients with ischemic heart failure: Imaging of myocardial ischemia and viability. Circulation. 2011;123:444–450. doi: 10.1161/CIRCULATIONAHA.109.903369. [DOI] [PubMed] [Google Scholar]
- 21.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG American Society of Echocardiography. American society of echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Cwajg JM, Cwajg E, Nagueh SF, et al. End-diastolic wall thickness as a predictor of recovery of function in myocardial hibernation: Relation to rest-redistribution T1-201 tomography and dobutamine stress echocardiography. J Am Coll Cardiol. 2000;35:1152–1161. doi: 10.1016/s0735-1097(00)00525-8. [DOI] [PubMed] [Google Scholar]
- 23.Biagini E, Galema TW, Schinkel AF, Vletter WB, Roelandt JR, Ten Cate FJ. Myocardial wall thickness predicts recovery of contractile function after primary coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2004;43:1489–1493. doi: 10.1016/j.jacc.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Bax JJ, Poldermans D, Elhendy A, Boersma E, Rahimtoola SH. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Current Problems in Cardiology. 2001;26:147–181. doi: 10.1067/mcd.2001.109973. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Mikati I, Weilbaecher D, et al. Relation of the contractile reserve of hibernating myocardium to myocardial structure in humans. Circulation. 1999;100:490–496. doi: 10.1161/01.cir.100.5.490. [DOI] [PubMed] [Google Scholar]
- 26.Afridi I, Grayburn PA, Panza JA, Oh JK, Zoghbi WA, Marwick TH. Myocardial viability during dobutamine echocardiography predicts survival in patients with coronary artery disease and severe left ventricular systolic dysfunction. J Am Coll Cardiol. 1998;32:921–926. doi: 10.1016/s0735-1097(98)00321-0. [DOI] [PubMed] [Google Scholar]
- 27.Meluzin J, Cerny J, Frelich M, et al. Prognostic value of the amount of dysfunctional but viable myocardium in revascularized patients with coronary artery disease and left ventricular dysfunction. investigators of this multicenter study. J Am Coll Cardiol. 1998;32:912–920. doi: 10.1016/s0735-1097(98)00324-6. [DOI] [PubMed] [Google Scholar]
- 28.Afridi I, Kleiman NS, Raizner AE, Zoghbi WA. Dobutamine echocardiography in myocardial hibernation. optimal dose and accuracy in predicting recovery of ventricular function after coronary angioplasty. Circulation. 1995;91:663–670. doi: 10.1161/01.cir.91.3.663. [DOI] [PubMed] [Google Scholar]
- 29.Cornel JH, Bax JJ, Elhendy A, et al. Biphasic response to dobutamine predicts improvement of global left ventricular function after surgical revascularization in patients with stable coronary artery disease: Implications of time course of recovery on diagnostic accuracy. J Am Coll Cardiol. 1998;31:1002–1010. doi: 10.1016/s0735-1097(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 30.Perrone-Filardi P, Pace L, Prastaro M, et al. Dobutamine echocardiography predicts improvement of hypoperfused dysfunctional myocardium after revascularization in patients with coronary artery disease. Circulation. 1995;91:2556–2565. doi: 10.1161/01.cir.91.10.2556. [DOI] [PubMed] [Google Scholar]
- 31.Collidge TA, Thomson PC, Mark PB, et al. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: Retrospective study of a renal replacement therapy cohort. Radiology. 2007;245:168–175. doi: 10.1148/radiol.2451070353. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines. Radiology. 2011;260:105–111. doi: 10.1148/radiol.11102340. [DOI] [PubMed] [Google Scholar]
- 33.Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;14:e97–e153. doi: 10.1016/j.hrthm.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 35.Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: Comparison with positron emission tomography. Circulation. 2002;105:162–167. doi: 10.1161/hc0202.102123. [DOI] [PubMed] [Google Scholar]
- 36.Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: A meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012;5:494–508. doi: 10.1016/j.jcmg.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Shah DJ, Kim HW, James O, et al. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA. 2013;309:909–918. doi: 10.1001/jama.2013.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 39.Schelbert HR, Beanlands R, Bengel F, et al. PET myocardial perfusion and glucose metabolism imaging: Part 2-guidelines for interpretation and reporting. J Nucl Cardiol. 2003;10:557–571. doi: 10.1016/j.nuclcard.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Tillisch J, Brunken R, Marshall R, et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884–888. doi: 10.1056/NEJM198604033141405. [DOI] [PubMed] [Google Scholar]
- 41.Beanlands RSB, Nichol G, Huszti E, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: A randomized, controlled trial (PARR-2) Journal of the American College of Cardiology. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Panza JA, Bonow RO. Ischemia and viability testing in ischemic heart disease: The available evidence and how we interpret it. JACC Cardiovasc Imaging. 2017;10:365–367. doi: 10.1016/j.jcmg.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Chareonthaitawee P, Gersh BJ, Panza JA. Is viability imaging still relevant in 2012? JACC: Cardiovascular Imaging. 2012;5:550–558. doi: 10.1016/j.jcmg.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Hochman JS, Lamas GA, Buller CE, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395–2407. doi: 10.1056/NEJMoa066139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bucciarelli-Ducci C, Auger D, Di Mario C, et al. CMR Guidance for Recanalization of Coronary Chronic Total occlusion. JACC: Cardiovascular Imaging. 2016;9:547–556. doi: 10.1016/j.jcmg.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 46.Baks T, van Geuns R, Duncker DJ, et al. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. Journal of the American College of Cardiology. 2006;47:721–725. doi: 10.1016/j.jacc.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 47.Kirschbaum SW, Baks T, van den Ent M, et al. Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. The American Journal of Cardiology. 2008;101:179–185. doi: 10.1016/j.amjcard.2007.07.060. [DOI] [PubMed] [Google Scholar]