Case Summary
A 63-year-old man presents to his primary care doctor with a one-month history of blood in his stools. He is referred for a colonoscopy and found to have a friable mass along the anterior wall just proximal to the second rectal fold. A biopsy confirms moderately-differentiated adenocarcinoma. The patient then obtains pelvic magnetic resonance imaging and chest, abdomen, and pelvis computed tomography demonstrating local invasion of the primary tumor into the mesorectal fat (T3), no suspicious regional lymph nodes (N0), and no evidence of distant metastatic disease (M0).
Background
Local recurrence from rectal cancer surgery historically exceeded 20% at 2 years with locally invasive lesions such as this patient’s harboring the highest risk.1 The near-simultaneous introduction of total mesorectal excision and neoadjuvant radiotherapy in late 1980s radically reduced the risk of 2-year local recurrence to 3-5%.2 The basis for both of these additions is that local recurrence occurs due to tumor cells remaining in the mesorectal fat and that sterilization with radiation therapy and complete removal with surgery offers the best oncologic outcomes. Since these early advances, approaches to neoadjuvant therapy have evolved in many disparate ways. The practicing colorectal surgeon is faced with a number of options and subtle differences in patient-specific factors often determine the best course of action.
Presentation and Diagnosis
Current guidelines for rectal cancer recommend neoadjuvant therapy for all T3 (Stage IIA) lesions extending through the muscularis propria into pericolorectal tissues, any locoregional nodal metastases (Stage III), or distant metastatic disease.3,4 Therefore, the critical criteria for neoadjuvant therapy are primary tumor stage and extrarectal tumor burden.
Recommendations for staging of local invasion and locoregional pathologic lymph nodes include both endorectal ultrasound and pelvic MRI.3,4 These modalities are typically viewed as complementary with case-specific factors often dictating preference for one over the other. In the invasive lesions most suited to neoadjuvant therapy, endorectal ultrasound has been criticized as an inadequate evaluation due to the technical proficiency required to identify pathologic mesorectal lymph nodes and its limited radiologic view.3 European practice and United States-based Commission on Cancer rectal cancer accreditation standards reflect this view, and pelvic MRI will likely become the preferred modality in the future.5–7 Importantly, standard pelvic MRI is not adequate for rectal cancer staging due to volume-quality relationships and specific rectal protocols required.7,8
To assess distant disease, all patients should obtain a chest, abdomen, and pelvis computed tomography scan.4 Small pulmonary metastases will be missed with plain-film chest radiography. No current evidence supports positron emission tomography as a superior study due to the false negative non-avid lesions and false positive inflammatory, non-malignant findings.3
Management
Once a patient is deemed eligible for neoadjuvant therapy, a number of different approaches are available that vary by institutional practice patterns. The three evidence-based approaches currently accepted include: 1) long-course chemoradiotherapy, 2) induction chemotherapy followed by long-course chemoradiotherapy, and 3) short-course radiotherapy.
Long-course chemoradiotherapy
Patients receiving long-course chemoradiotherapy regimens obtain a total of 45 to 50Gy of external beam, intensity-modulated radiation therapy over 25 to 28 daily fractions while also receiving concomitant radiation-sensitizing fluoropyrimidine-based chemotherapy. Radio-sensitizing chemotherapy prevents DNA repair of radiation-damaged tumor cells with the use of oral capecitabine, continuous infusion 5-fluorouracil, or bolus 5-fluorouracil with leucovorin. The choice of sensitizing chemotherapy will likely be driven by patient fitness and the experience of the partnering medical oncologist.
Timing of surgery following long-course chemoradiotherapy continues to be widely debated. Consensus exists that surgery after neoadjuvant chemoradiotherapy should be delayed no longer than 12 weeks.3,4 Traditionally, the shortest acceptable interval between chemoradiotherapy and surgery has been 8 weeks to allow time for downstaging and tumor cell death.3 However, anecdotal evidence suggested that radiation-associated fibrosis led to technically more difficult operations in a time-dependent fashion. Recent observational studies and the GRECCAR6 multi-institutional randomized control trial support these beliefs and have demonstrated that waiting more than 8 weeks contributes to lower rates of sphincter preservation, higher postoperative morbidity, and lower success achieving complete total mesorectal excision.4,9 Although current practice guidelines remain unreconciled, this evidence currently supports surgery 5 to 12 weeks following completion of long-course chemoradiotherapy.
Short-course radiotherapy
Much of the recently observed controversy surrounding technical dissection difficulties post-long-course chemoradiotherapy harkens back to the earliest radiation modalities of the neoadjuvant era. Although much less commonly used in the United States, short-course radiotherapy is widely practiced in Northern Europe. This approach aims to sterilize the mesorectal fat prior to surgery in manner that is better tolerated, more convenient for patients, and lower cost. Patients undergoing short-course radiotherapy receive a higher dose per fraction and complete a biologically equivalent radiation therapy course with 5 Gy per day for 5 days total. This regimen does not include chemotherapy, and patients typically proceed to surgery 1 to 2 weeks following radiotherapy.
A landmark Australian/New Zealand head-to-head trial comparing traditional long-course chemoradiotherapy and short-course radiotherapy demonstrated non-inferiority in oncologic outcomes between the two approaches.10 Two important disease presentations that are ineligible for short-course chemotherapy include T4 tumors and those threatening the anal sphincter due to the lack of downsizing observed with immediate surgery.3,4 Although most patients in the United States receive long-course chemoradiotherapy, contraindications for the short-course radiotherapy regimen remain limited. The short-course radiotherapy’s role in current American rectal cancer care practice remains controversial, but the approach may offer clinically relevant advantages for a well-selected subset of rectal cancer patients.
Induction chemotherapy with long-course chemoradiotherapy
While radiation-based neoadjuvant therapies have been important for reducing local recurrence rates, these approaches offered no protection against systemic spread leading up to surgery. Many studies have combined long-course chemoradiotherapy with a course of induction chemotherapy that precedes radiation therapy in order to obtain early systemic control and potentially avoid postoperative chemotherapy-related toxicity.4 Induction chemotherapy regimens vary widely but typically include combination chemotherapy such as 5-fluorouracil (or capecitabine), oxaliplatin, and leucovorin given for 3 to 6 cycles prior to initiation of traditional long-course radiotherapy. The GCR-3 Spanish randomized clinical trial supports that induction chemotherapy provides at least similar oncologic outcomes as postoperative chemotherapy and receiving definitive chemotherapy before surgery – increasingly referred to as “total neoadjuvant therapy” – reduced toxicity and improved completion rates.11 Since 2015, the National Comprehensive Cancer Network (NCCN) includes induction chemotherapy as an equivalent approach to the prior two discussed above.
Expected new directions and future alternatives
The last decade has been further characterized by many modifications to these accepted approaches. Three important trends that need to be watched closely are: 1) induction chemotherapy with selective chemoradiotherapy; 2) “watch and wait” protocols for clinical complete responders; and 3) hybrid-approaches that merge elements of both short- and long-course neoadjuvant therapies.
Unresolved toxicity and treatment completion rates have challenged the supremacy of the multimodal neoadjuvant approach. Some have argued that the risk-benefit of neoadjuvant radiotherapy may be less attractive due to the success of total mesorectal excision alone for improving local recurrence, the long-term toxicity associated with radiation, and the fear of distant metastases occurring during the neoadjuvant treatment interval. The PROSPECT trial (NCT01515787) is currently recruiting locally invasive rectal cancer patients at over 1,000 sites in the United States and Canada with randomization to traditional long-course chemoradiotherapy versus induction chemotherapy followed by selective chemoradiotherapy only for nonresponders. The trial with its planned eight-year follow-up is expected to finish in 2021.
“Watch and wait” approaches are becoming increasingly popular due to the potential for avoiding surgery entirely in carefully selected clinical complete responders after traditional long-course chemoradiotherapy. No results from randomized trials currently exist to support this approach but the early data from select centers is promising.4 There is not yet adequate evidence to recommend a “watch and wait” approach over traditional options described here, but it is likely that a non-surgical option may be available to patients in the future with a United States-based trial (NCT02008656) results currently pending. When we do offer patients a “watch and wait” paradigm, we only consider patients with demonstrated, reliable long-term follow-up capabilities and emphasize the stringent mandatory surveillance schedule.
There are also numerous hybrid approaches between traditional short- versus long-course neoadjuvant therapy. In one closely watched example, the Stockholm III trial has combined short-course radiotherapy with a prolonged 4 to 8 week post-radiation delay to allow for the downsizing typically not provided by short-course radiotherapy.12 Oncologic outcomes from the trial are expected soon.
Conclusions
Neoadjuvant therapy remains a critical component of locally invasive and metastatic rectal cancer care. The approach one chooses balances a number of influential factors including baseline patient condition, tumor conformation and stage, and psychosocial risks with extended compliance needs. Assessment of one’s institutional environment and a multidisciplinary, shared-decision making approach are necessary to determine the best option for each patient.
Evaluation and Treatment Algorithm (Figure 2)
Figure 2.
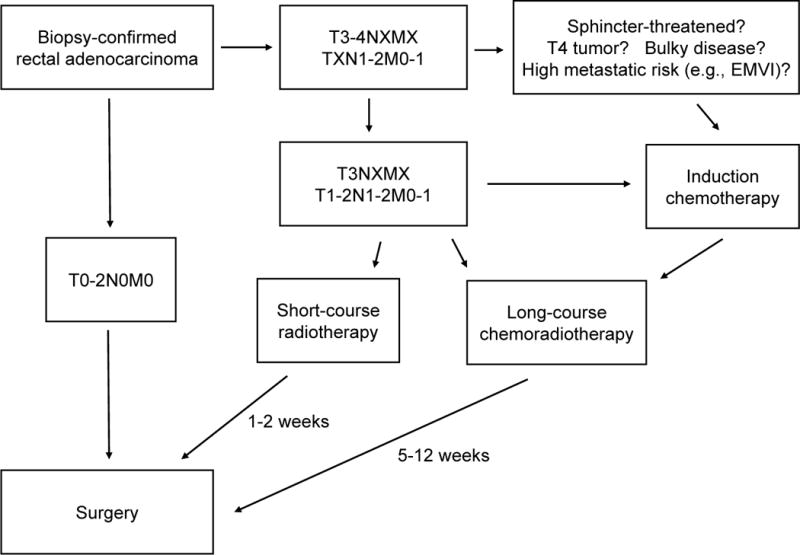
Evaluation and treatment algorithm for neoadjuvant-eligible rectal cancer.
Figure 1.
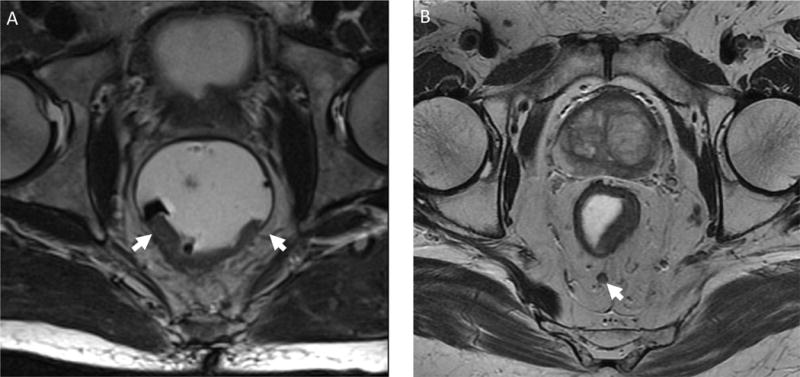
Neoadjuvant-eligible staging findings using pelvic magnetic resonance imaging. A) T3 midrectal lesion with posterior invasion through the muscularis propria (low intensity signal encircling rectum) into the mesorectal fat with arrows marking lateral extent. B) T2 mid-rectal, posterior lesion with thinning of the muscularis propria and a 5mm pathologic lymph node (arrow) located in the posterior mesorectal fat.
Clinical Questions.
Which rectal cancer lesions are best suited to receive neoadjuvant therapy?
What practices are current standards of care for neoadjuvant therapy for rectal cancer?
What controversies remain for neoadjuvant therapy for rectal cancer?
Acknowledgments
Funding Support: I.L. received salary support for the preparation of this manuscript from a National Cancer Institute T32 Institutional Training Grant (5T32CA126607) and a Research Foundation of the American Society of Colon and Rectal Surgeons Resident Research Initiation Grant (GSRRIG-031)
Footnotes
Conflicts of Interest: None
Earn Continuing Education (CME) credit online at cme.lww.com. This activity has bee approved for AMA PRA Category 1 credit.™
References
- 1.Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Dutch Colorectal Cancer Group Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 3.Monson JRT, Weiser MR, Buie WD, et al. Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised) Dis Colon Rectum. 2013;56:535–550. doi: 10.1097/DCR.0b013e31828cb66c. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Rectal Cancer (Ver 3.2017) 2017 Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal_blocks.pdf. Accessed December 21, 2017.
- 5.Nougaret S, Rouanet P, Molinari N, et al. MR volumetric measurement of low rectal cancer helps predict tumor response and outcome after combined chemotherapy and radiation therapy. Radiology. 2012;263:409–418. doi: 10.1148/radiol.12111263. [DOI] [PubMed] [Google Scholar]
- 6.Taylor FGM, Quirke P, Heald RJ, et al. MERCURY study group Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 7.Commission on Cancer - American College of Surgeons. The National Accreditation Program for Rectal Cancer Standards Manual - 2017 Edition. (Revised October 2017) Available from: https://www.facs.org/~/media/files/qualityprograms/cancer/naprc/naprcstandardsmanual.ashx. Accessed December 21, 2017.
- 8.Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol. 2005;78:245–251. doi: 10.1259/bjr/33540239. [DOI] [PubMed] [Google Scholar]
- 9.Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6) J Clin Oncol. 2016;34:3773–3780. doi: 10.1200/JCO.2016.67.6049. [DOI] [PubMed] [Google Scholar]
- 10.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Martos C, Pericay C, Salud A, et al. Three-year outcomes of GCR-3: A phase II randomized trial comparing conventional preoperative chemoradiation (CRT) followed by surgery and postoperative adjuvant chemotherapy (CT) with induction CT followed by CRT and surgery in locally advanced rectal ca. J Clin Oncol. 2011;29(suppl):3552–3552. [Google Scholar]
- 12.Pettersson D, Cedermark B, Holm T, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg. 2010;97:580–587. doi: 10.1002/bjs.6914. [DOI] [PubMed] [Google Scholar]


