Abstract
Cardiovascular disease remains the leading cause of death and disease worldwide. As demands on an already resource-constrained healthcare system intensify, disease prevention in the future will likely depend on out-of-office monitoring of cardiovascular risk factors. Mobile health tracking devices that can track blood pressure and heart rate, in addition to new cardiac ‘vital signs,’ such as physical activity level and pulse wave velocity, offer a promising solution. An initial barrier is the development of accurate and easily-scalable platforms.
In this study, we made a customized smartphone app, and used mobile health devices to track pulse wave velocity, blood pressure, heart rate, physical activity, sleep duration and multiple lifestyle risk factors in roughly 250 adults for 17 continual weeks. Eligible participants were identified by a company database, and then were consented and enrolled using only a smartphone app, without any special training given.
Study participants reported high overall satisfaction, and 73% of participants were able to measure blood pressure and pulse wave velocity, less than 1 hour apart, for at least 14 of 17 weeks. The study population’s blood pressure, pulse wave velocity, heart rate, activity levels, sleep duration, and the interrelationships among these measurements, were found to closely match either population averages, or values obtained from studies performed in a controlled setting. As a proof-of-concept, we demonstrated the accuracy and ease, as well as many challenges, of using mHealth technology to accurately track pulse wave velocity and new cardiovascular ‘vital signs’ at home.
Keywords: hypertension, pulse wave velocity, activity tracking, healthcare education, mobile health, smartphone, cardiovascular disease
Introduction
Cardiovascular (CV) disease remains the leading cause of death and disease worldwide, responsible for roughly 46% of noncommunicable disease deaths in 2012.1 As demands on an already resource-constrained healthcare system intensify, disease prevention in the future will likely depend on out-of-office monitoring of cardiovascular risk factors.2 Commerically-available mobile health tracking devices, that can continually track and wirelessly transmit blood pressure and heart rate, in addition to new ‘vital signs,’ such as physical activity level, sleep duration and pulse wave velocity, offers a promising solution.2 An initial barrier is the development of accurate, easily-scalable, home monitoring platforms.
Pulse wave velocity (PWV) is a validated measure of arterial wall stiffness, and one of the most important measures of cardiovascular risk.3–10 PWV has traditionally been measured in a controlled setting using a Sphygmometer and applanation tonometry, but can now be accurately measured out-of-the-office using “smart” weight scales.11,12 Similarly, blood pressure (BP), a powerful contributor to cardiovascular disease,13 is still measured predominantly in a clinic setting, but can now be measured accurately at home using wireless BP monitors. In fact, the American College of Cardiology (ACC) and American Heart Association (AHA) 2017 guidelines recommend taking BP measurements at home, citing strong evidence that an individual’s BP outside the clinic setting is more predictive of health outcomes.14
ACC/AHA guidelines also recommend identifying and reversing other lifestyle CV risk factors. This goal is difficult to accomplish using physician office visits because patients often lie to physicians regarding their lifestyle behaviors, and there is limited face-to-face time between doctors and patients.15 The use of new mobile health (mHealth) technologies to assess and track CV risk factors at home offers a promising solution to improve monitoring, help meet guideline recommendations, and offload some burden placed on healthcare systems. In this proof-of-concept study, we made a customized smartphone app, and then used advanced mHealth technologies to determine the feasibility, accuracy and ease of tracking an array of different CV risk factors at home.
Methods
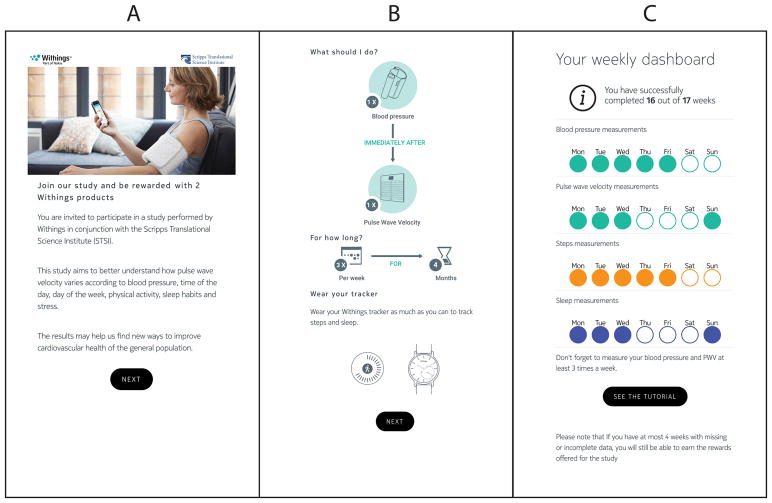
The data that support the findings of this study are available from the corresponding author upon reasonable request. Participants were enrolled and gave informed consent using a customized smartphone app with all protocols approved by an institutional review committee (IRB) at Scripps Health (La Jolla, CA). There were two inclusion criteria. First, participants had to already own three health tracking devices: a mHealth blood pressure monitor, an activity tracker, and a smart weight scale that can measure weight, heart rate, and PWV. Second, participants had to have measured their BP, on their own volition, at least once per week, for most weeks, over three months prior to enrollment. Owners of Nokia health tracking devices had agreed to share their health tracking data with the company, and so eligible study participants were identified by quering a company database. Eligible participants were contacted for enrollment through a push message on the Nokia Health Mate app (Figure 1a). Partipicants volunteered from the general public, and no exclusions were made based on sex, race, etc. Exclusion criteria included aortic artery disease, peripheral vascular disease, atrial fibrillation, weight > 396 lbs. (scale weight limit) and pregnancy.
Figure 1. Screenshots of mobile app developed for the study.
A) Screenshot of invitation sent to eligible participants for study enrollment B) Diagram used to explain the study protocol, and to obtain informed consents C) Weekly dashboard presented to app users detailing progress and successful completion of study measurements.
Participants provided demographic, medication usage, and medical history by completing a short survey, provided in Supplement S1. Stress levels were assessed using the validated Perceived Stress Scale (PSS) survey.16 Participants were asked to measure BP, heart rate (HR), PWV, and weight two days per week for 17 weeks from February to May, 2017 (Figure 1b). All measurements were transmitted wirelessly through the smartphone app to a secure database. Participants were instructed to take their BP using the Nokia wireless blood pressure monitor while sitting, but were not trained or observed or given any other special instructions. For the analysis, mean arterial pressure (MAP) was calculated based on the following formula: MAP = DBP + 0.4(SBP - DBP),17 where DBP and SBP are the diastolic and systolic BP reported by the wireless blood pressure monitor. PWV, HR, and BMI/weight were measured by standing on the smart scale. Acceptable ranges of systolic and diastolic BPs were 80–225 and 30–110 mmHg, respectively. An acceptable time between BP and PWV measurements was less than 1 hour. An acceptable range for PWV was 4–14 m/s. PWV and BP values outside the specified acceptable ranges, and BP and PWV measurements taken more than 1 hour apart, were excluded, and the individual did not receive credit for those measurements.
Participants agreed to wear activity trackers during the day and night, for at least 2 days per week, throughout the study. The accuracies of smartphone applications and wearable activity tracking devices to track physical activity have been previously reported.18,19 The smartphone app also recorded activity using the built-in activity trackers on the participants’ smartphones. In the case of activity device failure (e.g. battery exhaustion), the activity information was integrated.
Fifty-two participants used the Aura Total Sleep System to track sleep, which uses a method known as ballistocardiography, or a measurement of cardiac variability, to detect different sleep phases.20,21 The Aura system has proven accuracy by comparisons with polysomnography recordings, the current gold standard.22 Participants without an Aura device had sleep calculated by the smartphone app and wearable activity tracking devices, which has been shown to correlate strongly with research-grade devices and be of high accuracy.23
Participants received two reminder messages on their smartphone each week. A weekly “dashboard” was provided on the smartphone app providing the number of weeks where the user successfully recorded BP, PWV, activity and sleep measurements (Figure 1c). Participants were compensated with a Nokia Thermo thermometer (Estimated Retail price: $100) at eight weeks if requirements were met. Participants were compensated with a Nokia Steel HR (Estimated retail price: $180) at 17 weeks if requirements were met. Participants were allowed to miss their BP and PWV measurements 4 times (weeks) in the study, and still be rewarded.
Results
Demographics
255 individuals were enrolled, and started the study by measuring at least 1 pulse wave velocity (PWV) and blood pressure (BP). 214 (83%) participants were male with an average age of 48.7 years (see Supplement S2 for full demographic info). Females were roughly the same age at 47.4 years. A small number of participants had a history of stroke (n=2), myocardial infarction (n=9) and kidney disease (n=4). 67 (26%) study participants were taking anti-hypertensive medications. 3.5% of users had greater than 12 alcoholic drinks per week, while 47% reported no alcohol use at all. 43 (17%) participants were taking over-the-counter medications, with non-steroidal anti-inflammatory drugs (NSAIDs) comprising roughly 90% of all medications reported.
After a High Initial Drop Out, Participant Engagement and Satisfaction was High
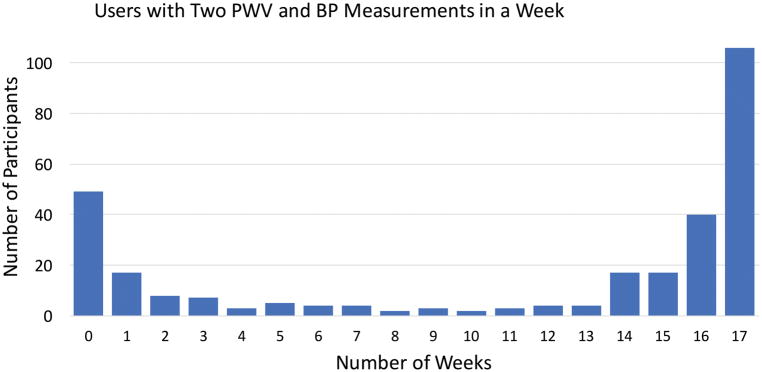
Although 295 individuals responded to the push message on the mobile app and signed the digital informed consent, 40 (14%) of these individuals never recorded a BP or PWV, and 9 (3%) participants measured a BP or PWV only once. This initial drop out was expected, as market research shows that 22–27% of smartphone users will open a downloaded app only once.24 Of the remaining 246 study participants (that started the study), 180 (73%) of participants successfully measured BP and PWV, less than 1 hour apart, for at least 14 of 17 weeks, and thereby qualified for the study rewards (Figure 2). In comparison, a study of 5115 participants that used a mHealth app to regularly measure BP, but did not give out monetary rewards, reported that 74% were using the app at 2 weeks and only 6% at 16 weeks.25 Individuals did not receive credit for BP and PWV measurements that were outside the specified acceptable ranges, or when BP and PWV measurements were taken more than 1 hour apart. These failed ‘attempts’ were not recorded, but were filtered by the app. See Supplement S3 for full details of adherence.
Figure 2. Histogram Plot of Adherence Data.
Represented is a histogram of the number of participants that successfully measured blood pressure and pulse wave velocity, less than 1 hour apart according to number of weeks in the study. For example, 49 individuals signed up for the study online, but never recorded a measurement. This is represented by the 1st column, or 0 number of weeks.
At on a scale from 1 to 10, where a 10 is considered an “extremely good” experience, the study scored an average of 9.1. The protocol design graded at 9.3, which was considered “easy to follow.” Remarkably, 99% of participants were willing to participate in another similar research study.
Study Population Device Values Match Population Means or Clinically Based Studies
The study population’s values recorded or calculated by the health tracking devices are provided in Table 1. The average body mass index (BMI) was 29.3 for men and 29.5 for women, which are above national averages at 26.6 and 26.5, respectively.26 The mean number of steps per person per day were 6,915, and values were similar between men and women. In comparison, an external study of 103,383 employees wearing wearable activity trackers reported a remarkably similar 6,886 steps per employee per day.27 As expected, the mean daily steps significantly correlated with BMI (R2=0.22), weight (R2=0.20), heart rate (HR) (R2=0.09), mean arterial pressure (MAP) (R2=0.08) and PWV (R2=0.02). In a newly identified relationship, PWV showed an inverse relationship with steps per day (pwv = 7.9 - 4.4e-5*steps/day, p=0.017, R2=0.023). See Supplements S4 and S5 for full statistical details of this analysis. We calculated that BMI decreased by roughly 1 point for every additional 1000 steps taken per day (Linear equation: BMI = 35.7 – 0.001*Steps per day; p<0.0001, R=0.48).
Table 1. Average values measured by health tracking devices.
The following values were measured by using mobile technology devices in conjunction with the customized mobile app, apart from the Perceived Stress Scores (PSS) that were obtained by surveys on the app.
| Measurement | Overall Study Values (± SE) | Study Values Men (± SE) | Study Values Women (± SE) | p-value |
|---|---|---|---|---|
| BMI (m/s) | 29.3 (± 0.4) | 29.3 (± 0.4) | 29.5 (± 1.0) | 0.9 |
| Steps per person per day | 6915 (± 217) | 6664 (± 232) | 6236 (± 514) | 0.4 |
| Sleep (hours/night) | 7.2 (± 0.1) | 7.2 (± 0.1) | 7.7 (± 0.1) | 0.0001 |
| PWV (m/s) | 7.6 (± 0.1) | 7.6 (± 0.1) | 7.3 (± 0.1) | 0.04 |
| Sys BP (mmHg) | 126.3 (± 0.7) | 127.1 (± 0.7) | 122.6 (± 1.6) | 0.01 |
| Dias BP (mmHg) | 80.0 (± 0.5) | 80.2 (± 0.6) | 78.3(± 1.4) | 0.2 |
| MAP (mmHg) | 98.4 (± 0.6) | 99.0 (± 0.6) | 96.0 (± 1.4) | 0.05 |
| Heart Rate (bpm) | 71.5 (± 0.7 | 71.3 (± 0.7) | 72.4 (± 1.6) | 0.5 |
| PSS1 | 21.2 (± 0.8) | 20.6 (± 0.9) | 24.5 (± 1.9) | 0.2 |
| PSS2 | 21.5 (± 0.8) | 21.2 (± 0.9) | 22.6 (± 1.9) | 0.5 |
Abbreviations: BMI: body mass index, BP: blood pressure, MAP: mean arterial pressure, PSS: perceived stress score
Using the activity devices to calculate sleep, we found that roughly ½ of study participants had less than 7 hours of sleep per night, the amount recommended by the American Academy of Sleep Medicine and Sleep Research. In comparison, 35% of persons surveyed by the Centers for Disease Control and Prevention (CDC) reported less than 7 hours of sleep per night,28 suggesting individuals overreport the amount of sleep they get each night. Females in the current study slept longer than males (7.7 vs. 7.2 hrs.; p<0.0001). Sleep duration had an inverse relationship with weight and BMI, but at overall low R2 values (R2 = 0.02 and 0.01, respectively).
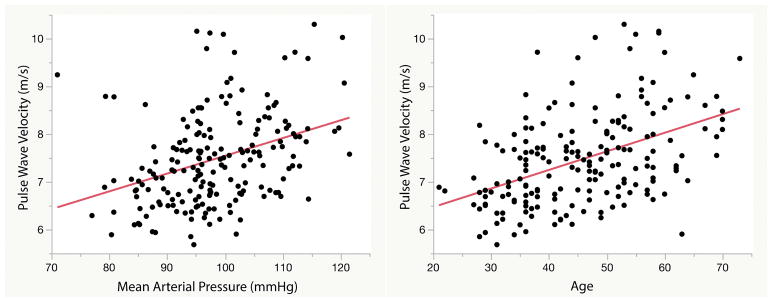
A total of 22,475 PWV measurements were recorded in the study. Men had a higher PWV than women at 7.6 m/s vs. 7.3 m/s (p=0.04), respectively. In a subanalysis of 2207 European subjects with similar age and demographics, PWV was also greater in men (7.8 m/s) vs. women (7.7 m/s; p=0.04) with comparable mean values.29 In the current study, PWV significantly correlated with age, BP, weight/BMI, and total daily steps (see Supplement S6). The strongest relationships with PWV were found with mean arterial pressure (MAP) (R2=0.13) and age (R2=0.20) (Figure 3). This finding is in agreement with a clinic-based study featuring 16,867 subjects, also finding that MAP and age best predicted PWV.30 The authors of this study calculated R2 values ranging from 0.07–0.26 for MAP, and 0.31–0.46 for age, but used full quadratic quations (a + b x age + c x age2) to improve regression equations and boister R2 values. Alternatively, another study of 174 participants that measured PWV using an invasive method demonstrated modestly lower correlations with MAP (R2=0.06) and age (R2=0.13).31 In our study, a linear regression model using MAP, age, weight, HR, and standard deviation of the PWV as dependent variables predicted PWV with an overall R2 of 0.36.
Figure 3. Correlation of pulse wave velocity with mean arterial pressure (MAP) and age.
Pulse wave velocity demonstrated the strongest relationship with A) MAP (R2=0.13) and B) age (R2=0.20) in the population not taking anti-hypertensives.
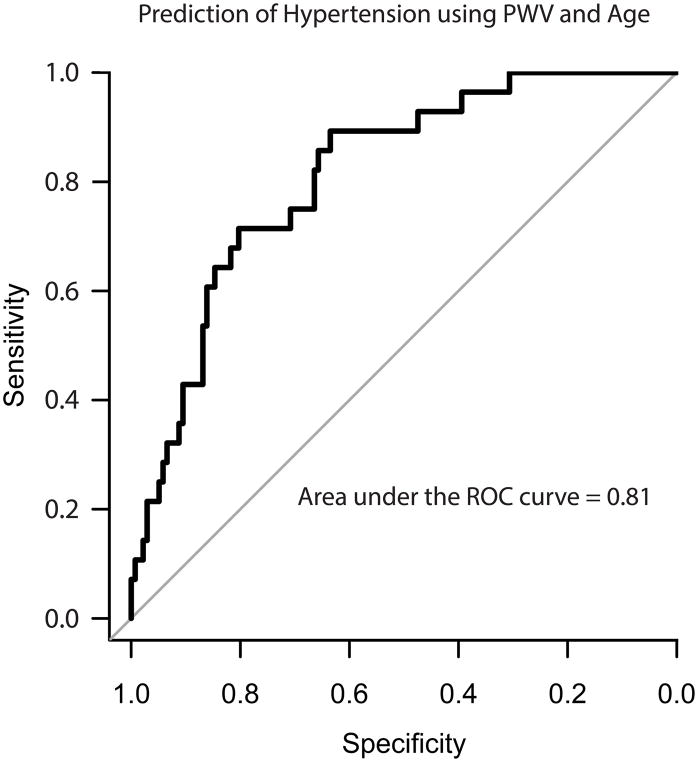
A total of 22,888 BP measurements were recorded. Roughly 1/3 of hypertensive participants had uncontrolled BPs (systolic greater > 135 mmHg or diastolic > 85 mmHg), which is actually below the national average at 47%.32 Supporting strong medication adherence as the reason for higher than average BP disease control, only 1 study participant reported missing BP meds 2–4 days/week and only 8 reported missing 1–2 days/week. As expected, participants with hypertension were found to take an average ~1200 less steps per day (6900 vs. 5700, p=0.01) and have a higher BMI (31.5 vs. 28.6, p=0.003). After excluding participants on anti-hypertensive medications, a logistic regression model using PWV, standard deviation PWV, and BMI could predict hypertension with an area under the curve (AUC) of 0.81 (sensitivity = 0.89, specificity = 0.63)(Figure 3). See Supplement S7 for statistical details.
As expected, standing and resting heartrates (HRs) taken by the weight scale, n=22,026, were significantly greater than sitting and resting HRs taken by the BP monitor (83.5 vs. 71.4 bpm, p=2.2E-16, Supplement S8). There was noted to be 449 more PWV than HR measurements recorded by the weight scale. These two measurements are calculated by independent algorithms, and this finding demonstrated a roughly 2% failure rate of the HR algorithm when the PWV is measured. HRs significantly correlated with daily steps (R2=0.10), BMI (R2=0.09), MAP (R2=0.07), weight (R2=0.06), age (R2=0.05) and Perceived Stress Scale (PSS) (R2=0.05) (see Supplement S9).
At 8 weeks, 143 participants completed the perceived stress scale (PSS) questionnaire with a median of 22 (interquartile range (IQR)=13–28), which is within the range of “moderate stress.” Roughly 1/3 of participants had “high” perceived stress. Compared to those with low or moderate stress, high stressed individuals were younger (44.7 vs. 49.6 years, p=0.02), had slightly higher heart rates (74.5 vs. 70.5, p=0.04), but demonstrated no difference in BP, BP medication use, sleep duration, alcohol use or tobacco use. PSS did not have a significant relationship with BP or PWV.
Discussion
In this proof-of-concept study, we demonstrate that advanced mHealth technology can be used to accurately track PWV and new cardiovascular ‘vital signs’ at home, while requiring minimal study personnel and no prior participant training. The study population’s mean blood pressure, pulse wave velocity, heart rate, activity levels, sleep duration, and the interrelationships among these measurements, were found to closely match reported population averages, or values obtained from studies performed in controlled, clinical settings featuring research-grade devices, strongly supporting the study’s methods and accuracy the devices. The demographics of the study population, which was comprised mostly of middle-aged and older individuals, clearly demonstrates an interest of older adults, particularly older men, to adopt new health monitoring technologies.
As mentioned, there was a significant, expected initial drop-out rate in our study, with ~17% of participants measuring BP or PWV only once or less. Yet, for those that started the study, the adherence was quite high, as 73% were able to successfully meet the study criteria and qualify for the rewards. A recent meta-analysis of 25 studies (10,487 patients) showed that BP reduction through self monitoring was most related to the intensity of the co-intervention (e.g. follow-up telephone conversations with study personnel).33 While our study maintained a relatively high adherence, we largely depended on both monitory rewards and co-interventions, i.e. a reminder system and a “dashboard” system that provided the user updated adherence numbers. Whether this level of adherence is obtainable with no, or lower, monetary awards, is an important question worth further investigation.
PWV is a validated measure of arterial wall stiffness and an important measure of cardiovascular health.34 In the study, several older individuals demonstrated high PWV values despite low or normal mean blood pressures. PWV is the cumulative effect of multiple factors on arterial wall stiffness over time, where aging, genetics and other factors are likely equally as important as chronic BP control. Thus, PWV provides an independent measure of cardiovascular risk that goes beyond BP control. Unfortunately, an inability to measure PWV outside of specialized clinic environments has limited its adoption in current clinical practice. To our knowledge, this is the first study to successfully assess PWV in the home environment. Supporting the plausibility and accuracy of our home PWV system, PWV values reported here closely matched the means reported by studies where PWV was measured in a clinical setting and/or invasively.15,29 Most importantly, PWV correlated strongest to the same covariates, age and BP, with comparable R2 values. Of interest and of obvious clinical importance, PWV in combination with BMI, i.e. measurements obtained by the smart scales, was also able to accurately predict hypertension.
As expected, BP values positively correlated with age, BMI, and PWV. Yet, as demonstrated by the heat map of Supplement S10, where yellow and blue colors represent individual values that are higher and lower than population averages, such relationships do not hold true for all individuals. For example, several individuals have normal blood pressures despite a high BMI, high PWV, and/or being of older age. While the heterogeneity that exists between BP and these other factors or clinical characteristics is well known, it underscores the need for care providers to develop medical and lifestyle recommendations that are intentionally tailored to the individual. The physician office visit often does not provide adequate time for such an assesessment using the physician-patient interview alone, but a proposed solution would be to summarize an individuals’ health tracking device data and present it as cumulative report or scorecard, such as the example provided in Table 2. An individual’s scorecard could be autopopulated by health tracking technologies, and used to quickly inform both the individual and his/her care provider of problem areas that need further assessment and intervention, providing greater efficiency and more meaning to the physician office visit.
Table 2. Proposed Individual Health Scorecard.
An individual’s mHealth measurements are compared to known population values or study’s mean values. The score is based on individual values that deviate from the population mean. Values greater than 1 standard deviation (SD) from the mean are scored +/− 0.5 point, while values greater than 2 SD are scored +/− 1 point. Red values indicates increased risk of CV disease, while green suggests decreased risk.
| Measurement | Individual Value | Study Population Mean (± SD) | Ideal Range | Points |
|---|---|---|---|---|
| Age | 49 | 47.6 (± 11.5) years | NA | 0 |
| BMI | 28 | 29 (± 6.6) m/s | 18.5–24.9 | −0.5 |
| PWV* | 8.5 | 7.6 (± 1.0) m/s | 5.9–8.6 m/s | −0.5 |
| Systolic BP† | 160 | 126 (± 11) mmHg | < 135 mmHg | −1.0 |
| Heart Rate | 99 | 71.1 (± 13.0) bpm | 60–100 bpm | −0.5 |
| Steps per day* | 13,000 | 6915 (± 3350) steps/day | >10,000 steps/day | +1.0 |
| Sleep duration | 7.5 | 7.2 (± 0.8) hours | 7–9 hours | 0 |
| Stress | 5 | 21 (± 9.7) PSS score | < 21 | +0.5 |
| Engagement | 1 | # Meas/week | 2–3 | −0.5 |
| Total Score | −3.0 |
Ideal values change based on age.
May substitute with mean arterial pressure or diastolic BP
There were several salient findings in this proof-of-concept study that can be used to improve and advance future mobile health tracking studies. Identified post hoc, HR was found to differ significantly between the sitting vs. standing position. HR is known to increase with standing because of baroreflex activation, where proportional activation of the sympathetic nervous tract maintains BP despite orthostatism,35,36 but it shows that the home environment provides endless opportunities for systematical errors to occur.
In agreement with clinical studies of BP and PWV, a significant amount of the variation seen in these measurements could not be explained with the extensive array of lifestyle factors measured. It is likely that factors not easily measured, such as current emotional state or technique differences, acted together influence BP recordings, perhaps with incalculable results. This finding underscores the need for high periodicity of measurements, something that can likely only be done using out-of-office technologies, before making medical treatment decisions.
There are several important limitations to performing mHealth studies that warrant further discussion. The difficulties in sustaining user engagement, in combination with a traditionally high initial drop-out rate, are major problems for almost all mHealth studies. A recent meta-analysis of 25 studies (10,487 patients) showed that BP reduction through self monitoring was most correlated to the intensity of the co-intervention used (e.g. follow-up telephone conversations with study personnel).33 While our study maintained a relatively high adherence rate (in comparison to similar studies), we depended on both monetary rewards and co-interventions, i.e. a reminder system and a “dashboard” system that provided the user updated adherence numbers. The monetary rewards used in this study, to maintain 4 months of adherence, totaled over $280. Although this cost per individual is arguably lower than 2 physician office visits in the same period (average physician visit cost = $200), while providing substantially more insight into cardiovascular vital signs, an ability to obtain funding for these mHealth endeavors will likely be a future challenge, and may limit access to those of lower socioeconomic status. Further, our sample size was relatively small, while scaling to a large population will be accompanied by increasing and unique expenses, such as the costs associated with large data storage.
The use of mHealth technologies are also accompanied intrinsic, perhaps underappreciated, challenges. Wearable activity tracker batteries may unexpectedly fail, limiting data captures. Activity trackers worn on the wrist may also falsely record the number steps, such as during eating or other activity, and have the potential to be less accurate when individuals walk at different paces. To limit or correct for these potential problems, we allowed users to give step data recorded by their smartphone activity trackers, integrating this data with the wearable device data, improving robustness of the physical activity data. Perhaps reassuringly, a recent study found that activity devices worn on the chest, pants pocket and wrist demonstrated good-to-excellent correlation to the gold standard, step counts during treadmill experiments when users were walking at 2.5, 5 and 8 km/h.37
Mobile health tracking devices often change, and commercial companies may tweak software algorithms, upgrade software or device versions, integrate devices, or even discontinue product lines altogether. After the study’s completion, Nokia voluntarily deactivated the PWV feature from its smartscale, perhaps temporarily, for regulatory concern that it no longer falls under the “wellness device” classification. Any of these changes have to potential to disrupt or permanently stop mHealth studies.
Perspectives
In summary, we provide new evidence that new cardiovascular ‘vital signs,’ including the newly adopted PWV, can be accurately measured and tracked outside of the physician office visit using new mobile health technologies, a system that requires minimal designated study or medical personnel, minimal participant training, and thereby is likely scalable to much larger populations. In our study, the home mHealth monitoring system was associated with high adherence, satisfaction, and participant engagement, which when taken together with strong indicators of accuracy, sets the stage to assess and potentially reduce cardiovascular risk in the general population.
Supplementary Material
Figure 4. Prediction of hypertension using PWV and BMI.
A) A logistic regression model using only PWV, standard PWV and BMI could predict hypertension with an area under the curve (AUC) of 0.81 (sensitivity = 0.89, specificity = 0.63).
Novelty and Significance.
1) What is New?
The study is the first to successfully assess and track pulse wave velocity outside of a controlled clinical setting using new smart scales.
The first study to combine multiple health tracking devices to holistically assess cardiovascular risk factors outside of the clinical setting along with demographics, medication adherence, and stress levels. Measurements taken by health monitoring devices, and the relationships among these measurements, were found to closely match national averages and/or prior studies performed in controlled, clinical settings, supporting their accuracy and reliability.
The study demonstrates the new ability to predict hypertension using BMI and PWV.
The study shows that individuals likely overreport the amount of sleep they obtain each night.
2) What is Relevant?
The study assesses hypertension in the home setting using wireless BP monitors, and assesses the influence of an array of lifestyle factors, such as stress, sleep, activity on BP.
The study correlates BP to pulse wave velocity at home, and shows that hypertension can be predicted using BMI and PWV recorded from a smart scale.
3) Summary
With high adherence, satisfaction, and participant engagement, we provide new evidence that cardiovascular risk factors, including the newly adopted PWV, can be reliably measured and tracked outside of the physician office visit using new mobile health technologies, a system that requires minimal designated study or medical personnel, and thereby is likely scalable to much larger populations.
Acknowledgments
We could not have completed this work without the help of a talented team of administrators and researchers at Scripps Research Institute and Nokia Research. We would like to especially acknowledge Dr. David Campo for his expertise and critical assessments, along with all other members of the Nokia research team.
Sources of Funding
This research was partially supported by funding provided by Nokia, along with Scripps Health and the linked NIH/NCATS Clinical Translational Science Award 5KL2TR001112 and 5UL1TR001114 to Scripps Translational Science Institute. BDM is supported by the NIH award 1K23HL144418-01. It was also supported and linked to the All of Us Research Program 1U24OD023176.
Abbreviations
- BMI
Body mass index
- BP
Blood pressure
- CV
Cardiovascular
- HR
Heart rate
- MAP
Mean arterial pressure
- PSS
Perceived Stress Scale
- PWV
Pulse wave velocity
- SE
Standard Error
Footnotes
Conflict of Interest/Disclosures
Co-authors OB, AC were employees of Nokia at the time of the study, makers of the health tracking devices used in the study. Nokia provided funding towards doing this research. The manuscript was written primarly by Scripps investigators (BDM) that no report a conflict of interest, and who had the final say on the presentation of the results.
References
- 1.Global Status Report on Noncommunicable Diseases. World Health Organization; 2010. [Accessed December 11, 2017]. http://www.who.int/nmh/publications/ncd-status-report-2014/en/ [Google Scholar]
- 2.Piette JD, List J, Rana GK, Townsend W, Striplin D, Heisler M. Mobile Health Devices as Tools for Worldwide Cardiovascular Risk Reduction and Disease Management. Circulation. 2015;132(21):2012–2027. doi: 10.1161/CIRCULATIONAHA.114.008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amar J, Ruidavets JB, Chamontin B, Drouet L, Ferrieres J. Arterial stiffness and cardiovascular risk factors in a population-based study. J Hypertens. 2001;19(3):381–387. doi: 10.1097/00004872-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63(5):1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim EJ, Park CG, Park JS, et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: invasive study. J Hum Hypertens. 2006;21(2):141–148. doi: 10.1038/sj.jhh.1002120. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 8.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21(12):2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 9.van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32(2):454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 10.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 11.Campo D, Khettab H, Yu R, et al. Measurement of Aortic Pulse Wave Velocity With a Connected Bathroom Scale. American Journal of Hypertension. 2017;30(9):876–883. doi: 10.1093/ajh/hpx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khettab H, Campo D, Yu R, Buard N, Boutouyrie P. [OP.LB03.04] FIRST IN MAN MEASUREMENT OF ARTERIAL STIFFNESS USING A CONNECTED BATHROOM SCALE: CALIBRATION AGAINST SPHYGMOCOR. Journal of Hypertension. 2016;34:e112. [Google Scholar]
- 13.Kannel WB. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA. 1996;275(20):1571–1576. [PubMed] [Google Scholar]
- 14.Cifu AS, Davis AM. Prevention, detection, evaluation, and management of high blood pressure in adults. JAMA. 2017;318(21):2132–2134. doi: 10.1001/jama.2017.18706. [DOI] [PubMed] [Google Scholar]
- 15.Palmieri JJ, Stern TA. Lies in the Doctor-Patient Relationship. Primary Care Companion to The Journal of Clinical Psychiatry. 2009;11(4):163–168. doi: 10.4088/PCC.09r00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S, Kamarck T, Mermelstein R. Perceived stress scale. Measuring stress: A guide for health and social scientists. 1994 [Google Scholar]
- 17.Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA. How to assess mean blood pressure properly at the brachial artery level. J Hypertens. 2007;25(4):751–755. doi: 10.1097/HJH.0b013e32803fb621. [DOI] [PubMed] [Google Scholar]
- 18.Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313(6):625–626. doi: 10.1001/jama.2014.17841. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell S, ÓLaighin G, Quinlan LR. When a Step Is Not a Step! Specificity Analysis of Five Physical Activity Monitors. PLOS ONE. 2017;12(1):e0169616. doi: 10.1371/journal.pone.0169616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redmond SJ, de Chazal P, O’Brien C, Ryan S, McNicholas WT, Heneghan C. Sleep staging using cardiorespiratory signals. Somnologie - Schlafforschung und Schlafmedizin. 2007;11(4):245–256. [Google Scholar]
- 21.Karlen W, Mattiussi C, Floreano D. Improving actigraph sleep/wake classification with cardio-respiratory signals. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2008;2008:5262–5265. doi: 10.1109/IEMBS.2008.4650401. [DOI] [PubMed] [Google Scholar]
- 22.The Science Behind Aura Total Sleep System. Withings Nokia; Online: https://sleeptrackers.io/wp-content/uploads/2016/06/withings-Aura-science-behind.pdf. [Google Scholar]
- 23.Ferguson T, Rowlands AV, Olds T, Maher C. The validity of consumer-level, activity monitors in healthy adults worn in free-living conditions: a cross-sectional study. The international journal of behavioral nutrition and physical activity. 2015;12:42. doi: 10.1186/s12966-015-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leger B. App User Retention Up by 10%, Up Nearly 50% on Android. [Accessed April 17, 2018, 2018];2013 [Google Scholar]
- 25.Kaplan AL, Cohen ER, Zimlichman E. Improving patient engagement in self-measured blood pressure monitoring using a mobile health technology. Health Information Science and Systems. 2017;5(1):4. doi: 10.1007/s13755-017-0026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville MUSDoH. Healthy weight, overweight, and obesity among U.S. adults. 2003 https://www.cdc.gov/nchs/data/nhanes/databriefs/adultweight.pdf.
- 27.Berko J, Goetzel RZ, Roemer EC, Kent K, Marchibroda J. Results From the Bipartisan Policy Center’s CEO Council Physical Activity Challenge to American Business. Journal of Occupational and Environmental Medicine. 2016;58(12):1239–1244. doi: 10.1097/JOM.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.1 in 3 adults don’t get enough sleep [press release]. Online2016.
- 29.Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. European Heart Journal. 2010;31(19):2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. European Heart Journal. 2010;31(19):2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EJ, Park CG, Park JS, et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: invasive study. Journal Of Human Hypertension. 2006;21:141. doi: 10.1038/sj.jhh.1002120. [DOI] [PubMed] [Google Scholar]
- 32.Merai R, Siegel C, Rakotz M, et al. CDC Grand Rounds: A Public Health Approach to Detect and Control Hypertension. MMWR Morbidity and mortality weekly report. 2016;65(45):1261–1264. doi: 10.15585/mmwr.mm6545a3. [DOI] [PubMed] [Google Scholar]
- 33.Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis. PLOS Medicine. 2017;14(9):e1002389. doi: 10.1371/journal.pmed.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic Pulse Wave Velocity as a Marker of Cardiovascular Risk in Hypertensive Patients. Hypertension. 1999;33(5):1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 35.Ewing DJ, Campbell IW, Murray A, Neilson JM, Clarke BF. Immediate heart-rate response to standing: simple test for autonomic neuropathy in diabetes. British Medical Journal. 1978;1(6106):145–147. doi: 10.1136/bmj.1.6106.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF. Autonomic mechanisms in the initial heart rate response to standing. Journal of applied physiology: respiratory, environmental and exercise physiology. 1980;49(5):809–814. doi: 10.1152/jappl.1980.49.5.809. [DOI] [PubMed] [Google Scholar]
- 37.Alinia P, Cain C, Fallahzadeh R, Shahrokni A, Cook D, Ghasemzadeh H. How Accurate Is Your Activity Tracker? A Comparative Study of Step Counts in Low-Intensity Physical Activities. JMIR mHealth and uHealth. 2017;5(8):e106. doi: 10.2196/mhealth.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.