Abstract
Arsenic, a priority Superfund contaminant and carcinogen, is a legacy pollutant impacting aquatic ecosystems in urban lakes downwind of the former ASARCO copper smelter in Ruston, WA, now a Superfund site. We examined the mobility of arsenic from contaminated sediments and arsenic bioaccumulation in phytoplankton and zooplankton in lakes with varying mixing regimes. In lakes with strong seasonal thermal stratification, high aqueous arsenic concentrations were limited to anoxic bottom waters that formed during summer stratification, and arsenic concentrations were low in oxic surface waters. However, in weakly-stratified lakes, the entire water column, including the fully oxic surface waters, had elevated concentrations of arsenic (up to 30 µg L−1) during the summer. We found enhanced trophic transfer of arsenic through the base of the aquatic food web in weakly-stratified lakes; plankton in these lakes accumulated up to an order of magnitude more arsenic on multiple sampling days than plankton in stratified lakes with similar levels of contamination. We posit that greater bioaccumulation in weakly-stratified lakes was due to elevated arsenic in oxic waters. Aquatic life primarily inhabits oxic waters and in the oxic water column of weakly-stratified lakes arsenic was speciated as arsenate, which is readily taken up by phytoplankton because of its structural similarities to phosphate. Our study indicates that mobilization of arsenic from lake sediments into overlying oxic water columns in weakly-stratified lakes leads to increased arsenic exposure and uptake at the base of the aquatic food web.
Keywords: trace metal, freshwater, stratification, bioaccumulation, phytoplankton, zooplankton
Graphical abstract
1. Introduction
Arsenic, a neurotoxin and carcinogen, is a common pollutant in urban waters that poses risks to both environmental and human health (NRC, 1999; Vahidnia et al., 2007). Lake sediments that have accumulated arsenic from legacy pollution represent a long-term source of arsenic contamination to overlying waters and aquatic ecosystems. Reductive dissolution of arsenic-bearing minerals in anoxic sediments may facilitate a diffusive flux of arsenic from porewaters into the overlying water column. Previous studies have demonstrated that arsenic is readily mobilized from contaminated sediments in seasonally-stratified lakes, resulting in elevated aqueous arsenic concentrations, typically speciated as arsenite (As(III)), in anoxic hypolimnetic waters (Aggett et al., 1985; Aggett and Kriegman, 1988; Azcue and Nriagu, 1995; Barringer et al., 2001; Selyer & Martin, 1989; Senn and Hemond, 1994; Sohrin et al., 1997). In these lakes, elevated aqueous arsenic concentrations are generally restricted to anoxic bottom waters because under oxic conditions arsenic is usually speciated as arsenate (As(V)), which readily adsorbs onto iron oxyhydroxides on settling particles in the water column (Dixit and Hering, 2003; Tufano and Fendorf, 2008). Thus, particle scavenging processes commonly result in low aqueous arsenic concentrations in oxic waters.
In fully oxic water columns, diffusion of arsenic from contaminated sediments into lake waters is expected to be limited by adsorption and precipitation of arsenic by iron oxyhydroxides at the sediment-water interface (de Vitre et al., 1991). However, while relatively unusual, a few previous studies have demonstrated that arsenic may be mobilized from lake-bed sediments into a well-mixed, oxic water column in absence of a well-developed hypolimnion to maintain anoxic conditions at the sediment-water interface. Conditions that promote the flux of aqueous arsenic across an oxic sediment-water interface and allow for maintenance of elevated aqueous arsenic in an oxic water column are not well understood, but may include decreased thickness of the oxic sediment layer due to high rates of organic matter oxidation (Martin and Pedersen, 2002; Senn et al., 2007) and slow kinetics of arsenate adsorption onto iron oxyhydroxides in both sediment and settling particles due to the presence of competing ions (Couture et al., 2010). The prevalence of such systems is unknown, as are the implications for arsenic exposure and bioaccumulation in aquatic organisms in these systems.
Arsenic is bioavailable to aquatic organisms (Eisler, 1988) and may be transferred through lacustrine food webs to species consumed by humans. Uptake and bioaccumulation of arsenic in phytoplankton is dependent on the chemical speciation of aqueous arsenic (Hellweger et al., 2004; Rahman et al., 2012). In freshwater systems, inorganic arsenic species As(III) and As(V) dominate total aqueous arsenic concentrations and their distribution is related to lake redox status, primarily controlled by physical mixing and oxygenation of the water column. Phytoplankton primarily accumulate arsenic via active uptake of As(V), the thermodynamically stable arsenic species in oxygenated waters, as a byproduct of phosphate transport (Hellweger and Lall, 2004; Rahman et al., 2012). The concentration of arsenic in freshwater phytoplankton has been observed to be up to 5 orders of magnitude higher than ambient water concentrations (Caumette et al., 2011; Caumette et al., 2014; Kaise et al., 1997). Field studies have indicated that zooplankton primarily accumulate arsenic through consumption of contaminated food (Chen et al. 2000, Caumette et al., 2012), although laboratory studies have shown that in culture, zooplankton exposed to arsenic in water but not in dietary sources also bioconcentrate arsenic (Caumette et al., 2014). Trophic transfer up the food web is largely responsible for arsenic accumulation in fish, whose primary exposure is from dietary sources (Chen et al., 2000; Culioli et al., 2009; Erickson et al., 2011; Rahman et al., 2012). Hence, the exposure and bioavailability of arsenic to plankton is a critical step in the transfer of arsenic from contaminated environments into the upper trophic levels of aquatic foodwebs.
The Puget Sound lowland region in Washington State contains a large number of densely-populated urban lakes with sediments contaminated with arsenic by a century of aerosol emissions from the smokestack of the former American Smelting and Refining Company (ASARCO) copper smelter, now designated the Commencement Bay/Nearshore Tideflats Superfund Site. We hypothesized that in thermally stratified lakes, potential transfer of arsenic through the food web is likely limited as arsenic is expected to be largely sequestered in anoxic waters avoided by aquatic organisms. However, arsenic mobilization in unstratified lakes may increase the potential for biological exposure by producing high aqueous arsenic concentrations in oxic biological habitat. The objective of this study was to determine the impact of different redox states resulting from different mixing behaviors in lakes with similar levels of arsenic contamination in sediments on aqueous arsenic concentrations and on arsenic bioavailability to the base of the aquatic food web.
2. Methods
2.1. Field sites
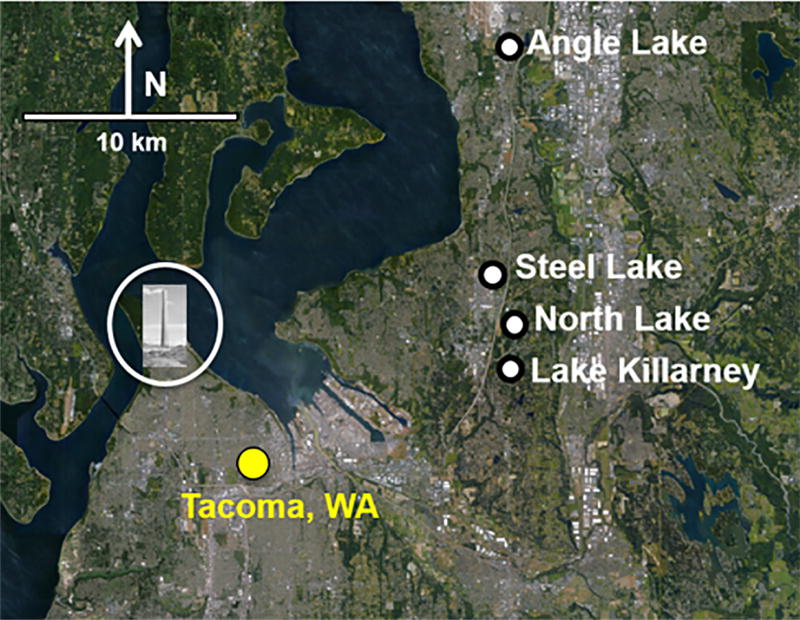
Four study lakes were chosen as representative examples of different combinations of arsenic contamination in sediments and lake mixing behaviors: strong seasonal stratification with high levels of arsenic contamination (Angle Lake); strong seasonal stratification with moderate levels of arsenic contamination (North Lake); intermittent weak stratification with high levels of arsenic contamination (Lake Killarney); and intermittent weak stratification with moderate levels of arsenic contamination (Steel Lake) (Table 1). All study lakes are within 25 km of the former ASARCO copper smelter in Ruston, WA (Fig. 1) in the direction of prevailing winds (S/SW) and within the predicted deposition field for smelter emissions (Gawel et al., 2014). Between September 2015 and August 2016, each of the study lakes was generally sampled monthly in winter and early spring (Nov-Mar) and twice monthly in late spring, summer, and fall (Apr-Oct), although sampling was occasionally missed due to weather conditions or restricted site access (Table S1).
Table 1.
Characteristics of lakes monitored in this study.
| study site | lake area (km2) |
average depth (m) |
max depth (m) |
stratification status | surface sedimenta [As] (µg g−1) |
|---|---|---|---|---|---|
| Angle Lake | 0.42 | 7.5 | 15.8 | strongly stratified | 208 |
| Lake Killarney | 0.12 | 2.6 | 4.5 | weakly stratified | 206 |
| North Lake | 0.23 | 4.2 | 9.0 | strongly stratified | 85 |
| Steel Lake | 0.19 | 3.6 | 7.6 | weakly stratified | 48 |
top 10–20 cm (Gawel et al., 2014)
Fig. 1.
Location of four study lakes in south-central Puget Sound relative to Tacoma, WA and former ASARCO copper smelter (Commencement Bay/Nearshore Tideflats Superfund Site), shown by the circled image of the smelter smokestack. Base photo credit: Google Earth.
2.2. Sample collection
Water column temperature, dissolved oxygen, specific conductivity, and pH profiles were measured using a multi-parameter water quality probe (In-Situ smarTROLL MP) deployed from a boat at set sampling stations located at approximately the deepest point in each lake. Probe sensors were calibrated daily. The Schmidt stability index (S) (Idso 1973; Schmidt, 1928) was calculated from temperature-depth profiles on each sampling day using LakeAnalyzer (Read et al., 2011).
Onboard water sampling was carried out at four predetermined depths in the water column representing surface waters, thermocline waters, hypolimnetic waters, and 0.5–2 m above the lake bed (Table S1). A peristaltic pump with acid-washed tubing was used to collect both unfiltered and filtered (0.45 µm Geotech cartridge filter) water samples. Samples for filtered and unfiltered total arsenic concentrations were collected in acid-washed 60 mL polypropylene bottles and acidified in the laboratory within 4 hours with trace metal grade nitric acid (1% v/v). Unfiltered samples were stored a minimum of 60 days prior to analysis to release refractory metal species into the dissolved phase. The difference between arsenic concentrations in filtered and unfiltered waters samples was used to estimate the relative contribution of the particulate phase to total arsenic concentrations (e.g., Nimick et al., 1998). Several times a year, unfiltered water samples were collected in acid-washed and ashed 300 mL biochemical oxygen demand (BOD) bottles for arsenic speciation analysis. These samples were filtered in the laboratory in an anoxic glove box using 0.45 µm and 0.025 µm mixed cellulose membrane filters (Millipore), then acidified with trace metal grade HCl to achieve a 1% v/v concentration and stored in amber HDPE bottles at 4°C until analysis.
Plankton biomass samples were collected in duplicate by vertical net tows from a depth of 1–2 m above bottom and stored in acid-washed polypropylene bottles on ice. Zooplankton samples were collected using a 153 µm mesh net. Phytoplankton samples were collected using 20 µm mesh net and filtered through a 153 µm sieve to remove zooplankton. In the laboratory, samples were filtered onto preweighed 5 µm polycarbonate membrane filters (Millipore) and placed in a drying oven overnight at low temperature (60°C). The average sample size for phytoplankton and zooplankton samples was 14 mg and 52 mg, respectively.
2.3. Analytical methods
Concentrations of total arsenic in water and plankton samples were determined by inductively-coupled plasma mass spectrometry (ICP-MS) on an Agilent 7900 at the University of Washington Tacoma. Calibration was performed using certified multi-element standards. Analytical accuracy of the ICP-MS method was assessed using certified reference material NIST 1640a (trace elements in natural water), which had a recovery of 107 ± 12% (n = 10) for arsenic. Total arsenic concentrations in plankton biomass were determined by ICP-MS following a microwave-assisted (CEM MARS 5) total digestion protocol (modified EPA method 3015) using trace metal grade nitric acid in pressurized digestion vessels. After digestion, sample solutions were diluted to 2% v/v nitric acid. Efficacy of the digestion procedure was verified using certified reference material BCR-414 (trace elements in plankton), which yielded a recovery of 95 ± 7% (n=8). The total method blank for arsenic, assessed by digestion of a blank polycarbonate filter, was 0.8 ng As (n = 3), equivalent to a concentration of 0.06 ug g−1 and 0.01 ug g−1 for the average phytoplankton and zooplankton sample weight, respectively.
In samples preserved for analysis of arsenic speciation, total As and As(III) were determined by hydride generation atomic-absorption spectrometry (HG–AAS) at the University of Colorado Boulder using methods detailed in McCleskey et al. (2004). Total As was measured by pre-reducing As(V) to As(III) using potassium iodide and L-ascorbic acid. The As(V) concentration was calculated as the difference between total As and As(III) concentrations. Detection limits were 0.8 µg L−1 for As(III) and 0.1 µg L−1 for total As concentrations.
3. Results and Discussion
3.1. Physical parameters and lake stratification status
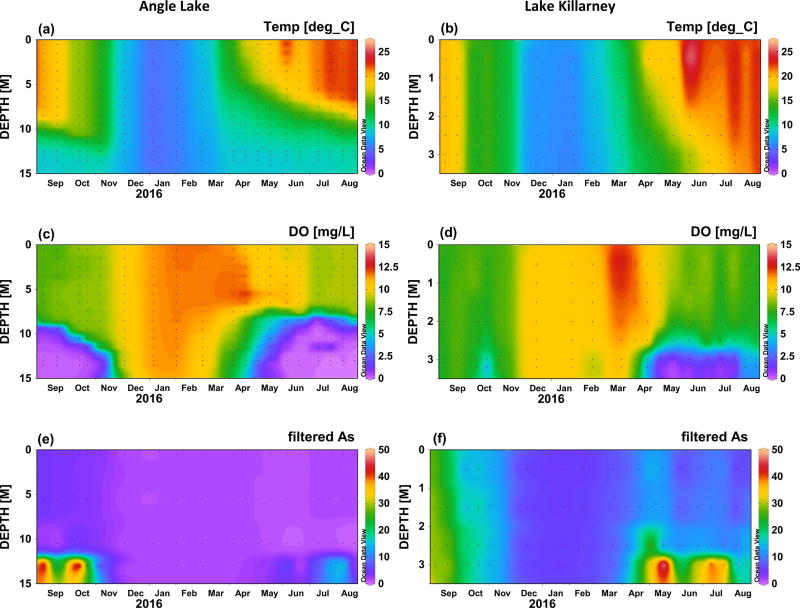
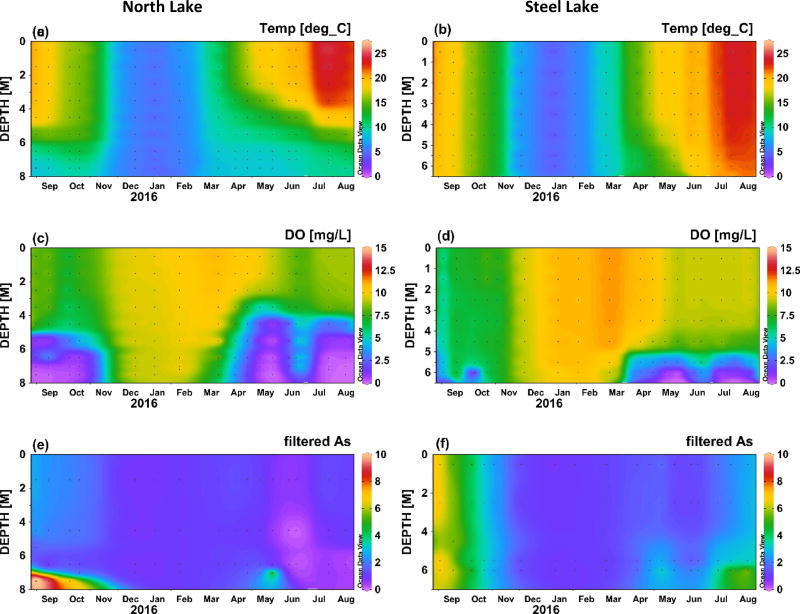
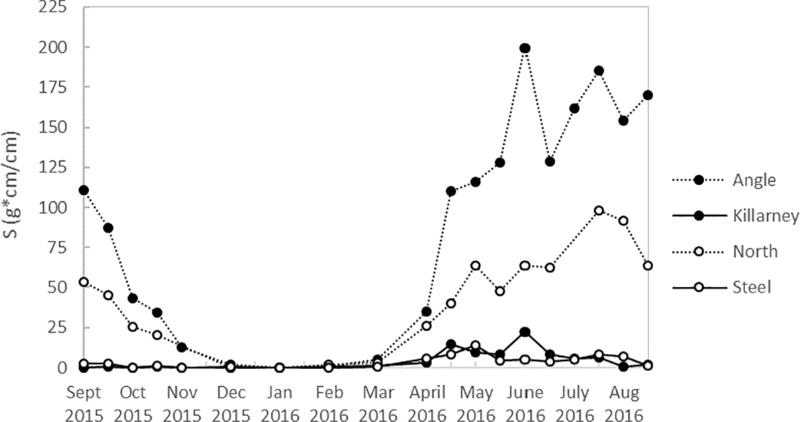
Temperature and dissolved oxygen concentrations over the course a full year (Sept 2015 to Aug 2016) are shown in Figure 2 for Angle Lake and Lake Killarney, the paired study lakes with high levels of arsenic contamination in sediments (>200 ppm) (Gawel et al., 2014), and in Figure 3 for North and Steel Lakes, the paired study lakes with lower levels of arsenic contamination in sediments (<100 ppm). The Schmidt stability index (S), was used to determine the water column stability, or the inertial resistance to complete mixing (Fig. 4). The time series show the onset of thermal stratification in late spring (April) in both Angle Lake (and North Lake, which persisted through the summer until fall overturn (November). During this period of continuous seasonal thermal stratification of the water column, dissolved oxygen in Angle Lake decreased sharply with depth to concentrations of <0.2 mg L−1 near the sediment-water interface (Fig. 2c,). In North Lake, similar anoxic conditions were observed throughout most of the summer, although there is evidence of a mixing event in North Lake in late June that temporarily re-oxygenated the hypolimnion (Fig. 3c). The thickness of the hypolimnion varied with time, reaching a maximum of ~7 m in Angle Lake and ~3 m in North Lake in July 2016. The water columns in Lake Killarney and Steel Lake remained relatively well-mixed year-round, with only weak stratification observed in early summer (Fig. 4). Despite the lack of persistent thermal stratification, bottom waters in both of these lakes (averaging 0.8 m above bottom) were occasionally hypoxic (<2 mg L−1), as observed during sampling in May and July 2016 (Figs. 2d, 3d).
Fig. 2.
Temperature (°C), dissolved oxygen (mg L−1), and total arsenic in filtered water samples (µg L−1) in Angle Lake (left) and Lake Killarney (right) between September 2015 and August 2016. Black dots indicate individual data points. Plots produced using Ocean Data View (Schlitzer, 2010).
Fig. 3.
Temperature (°C), dissolved oxygen (mg L−1), and total arsenic in filtered water samples (µg L−1) in North Lake (left) and Steel Lake (right) between September 2015 and August 2016. Plots produced using Ocean Data View (Schlitzer, 2010).
Fig. 4.
Schmidt stability index values (S) for all study lakes.
3.2 Aqueous arsenic concentrations
Similar spatial and temporal trends in total arsenic concentrations in filtered (Fig. 2e–f, Fig. 3e–f) and unfiltered water samples (Fig. S1) were observed for all lakes. Comparison of arsenic concentrations in filtered and unfiltered water samples shows that typically 70–90% of total arsenic in the water column in all of the study lakes was in the aqueous phase and apparent concentrations of particulate arsenic were small (Table 2). Additionally, a subset of samples was also filtered through a 0.025 µm filter; in the majority of samples, >90% of the 0.45 µm-filtered arsenic also passed through the 0.025 µm filter, suggesting the contribution of colloids was minimal (supporting information Table S2). Hence, the discussion below focuses on aqueous arsenic concentrations measured in filtered water samples.
Table 2.
Average concentration of arsenic (±1 standard deviation) in filtered (<0.45 µm) and unfiltered water samples and aqueous (filtered) arsenic as a percentage of total (unfiltered) in samples collected from surface waters (<2 m) in all study lakes between September 2015 and August 2016 (n = 93).
| site | filtered As (µg L−1) | unfiltered As (µg L−1) | % aqueous As |
|---|---|---|---|
| Angle Lake | 2.0 (±1.1) | 2.2 (±0.9) | 91 |
| Lake Killarney | 11.4 (±6.0) | 16.2 (±7.8) | 70 |
| North Lake | 1.3 (±0.7) | 1.4 (±0.5) | 93 |
| Steel Lake | 2.3 (±1.6) | 3.1 (±2.3) | 74 |
Elevated aqueous arsenic concentrations (up to 56 µg L−1 in Angle Lake; up to 11 µg L−1 North Lake) were observed in thermally stratified lakes in hypoxic bottom waters during summer months (May–October) (Figs. 2e, 3e). With physical mixing inhibited by strong stratification (Fig. 4), arsenic supplied from sediments was largely restricted to the hypolimnion. Relatively low arsenic concentrations (1–4 µg L−1 in Angle Lake; <2 µg L−1 North Lake) were observed in the oxic upper water columns of these two lakes throughout the year, similar to background arsenic concentrations typical of non-contaminated lakes (<1 µg L−1) (Chen et al., 2000; Gawel et al., 2014; Smedley and Kinniburgh, 2002). There was slight seasonal variability in arsenic concentrations measured in oxic surface waters of these stratified lakes. The highest concentrations were observed in late summer 2015 with slightly lower concentrations observed in winter and early summer 2016, mirroring temporal trends in arsenic concentrations in the hypolimnion (Figs. 2e, 3e). The vertical profiles of arsenic concentrations observed in Angle Lake and North Lake are typical of contaminated, thermally-stratified lakes with elevated arsenic observed in anoxic bottom waters during periods of seasonal stratification (Aggett et al., 1985; Aggett and Kriegman, 1988; Azcue and Nriagu, 1995; Barringer et al., 2011; Ford et al., 2006; Hasegawa et al., 1997; Kuhn and Sigg, 1993; Smedley and Kinniburgh, 2002). The higher maximum arsenic concentrations observed in the bottom waters of Angle Lake compared to North Lake are consistent with the more highly-contaminated surface sediments present in Angle Lake (208 mg kg−1 dry weight) compared to North Lake (85 mg kg−1 dry weight) (Gawel et al., 2014).
By contrast, elevated aqueous arsenic concentrations were observed in the oxic water columns of both Lake Killarney and Steel Lake. From September to early November 2015, arsenic concentrations of up to 30 µg L−1 in Lake Killarney and up to 7 µg L−1 in Steel Lake were observed at all depths throughout the well-mixed, oxic water column (Figs. 2f, 3f). Arsenic concentrations then decreased and were lowest (5–7 µg L−1 in Lake Killarney and ~1 µg L−1 in Steel Lake) over the winter (December 2015 to March 2016). In spring and early summer in 2016, arsenic concentrations again increased throughout the water column with the onset of intermittently hypoxic bottom waters. During this time, arsenic concentrations were highest in the hypoxic bottom waters (>50 µg L−1 in Lake Killarney and >7 µg L−1 in Steel Lake), but concentrations above background were also found in the overlying oxic waters (up to 15 µg L−1 in Lake Killarney and 4 µg L−1 in Steel Lake) (Figs. 2f, 3f). The higher arsenic concentrations observed in Lake Killarney relative to Steel Lake reflect the larger arsenic source insurface sediments in Lake Killarney (206 mg kg−1 versus 48 mg kg−1) (Gawel et al., 2014).
The speciation of aqueous arsenic in filtered water samples from Angle Lake and Lake Killarney is shown in Table 3 for samples collected in summer, when both relatively high arsenic concentrations and ecosystem productivity are expected. In Angle Lake, the elevated total arsenic found in the hypolimnion was primarily speciated as As(III) (89–97%). The relative proportion of As(III) decreased higher in the water column and was below detection (<0.8 µg L−1) in surface waters. By comparison, in Lake Killarney, As(III) concentrations were typically below detection throughout the water column, and were not significant compared to high total arsenic concentrations (8–13 µg L−1). Additional water samples from Lake Killarney collected prior to our study show similar trends in arsenic speciation in the late summer, with As(III) being a minor component (3–4 %) of total arsenic and As(V) dominating.
Table 3.
Concentrations of As(III) and total As in filtered (<0.45 µm) water samples collected at Angle Lake (August 2016) and at Lake Killarney (August 2016 and September 2006). Percentage As(III) of total arsenic concentrations given in parentheses.
| site | date | depth (m) | As(III) (µg L−1) | total As (µg L−1) |
|---|---|---|---|---|
| Lake Killarney | August 2016 | 0.5 | BDL | 8.2 |
| 1.5 | 2.2 (26%) | 8.6 | ||
| 2.5 | BDL | 11.6a | ||
| 3.0 | BDL | 13.2 | ||
| September 2006b | 0.2 | 0.9 (3%) | 25.8 | |
| 1.5 | 1.0 (4%) | 25.1 | ||
| 1.9 | 1.0 (4%) | 24.3 | ||
|
| ||||
| Angle Lake | August 2016 | 0.5 | BDL | 1.7 |
| 5.5 | 0.8 (53%) | 1.5 | ||
| 10.5 | 0.8 (89%) | 0.9 | ||
| 13.0 | 9.4 (97%) | 9.7 | ||
average value of duplicate samples
samples from September 2006 were analyzed by ion chromatography inductively coupled plasma dynamic reaction cell mass spectrometry by Applied Speciation and Consulting (Tukwila, WA)
BDL = below detection limit
Although high aqueous arsenic concentrations are expected under reducing conditions in the hypolimnion of stratified lakes, we observed high arsenic contamination in both of the relatively well-mixed lakes examined in this study. Elevated arsenic observed in the oxic water columns of Lake Killarney and Steel Lake can be attributed largely to a flux of aqueous arsenic from sediment porewaters and not simply re-suspension of arsenic-rich surface sediments during mixing events, as indicated by comparison of arsenic concentrations in filtered and unfiltered water samples (Table 2). The urban setting of our study lakes and high population densities in the immediate watersheds result in eutrophication (supporting information Table S5) and organic-rich sediments (47 wt% organic matter in Lake Killarney, loss on ignition) that likely enable strongly reducing conditions and high rates of arsenic mobilization within sediments. The eutrophic conditions and organic-rich sediments likely also provide an ample supply of ions known to compete with arsenic for sorption sites in sediments and on settling particles, such as phosphate (Manning and Goldberg, 1996) and aromatic organic matter (Redman et al., 2002; Weng et al., 2009). Competition with other ions for sorption sites on iron oxides would inhibit particle scavenging of arsenic at the oxic sediment-water interface and in the overlying water column and increase the residence time of aqueous arsenic (Couture et al., 2010; Ford et al., 2006; Martin and Pedersen, 2002; Senn et al., 2007). The weakly-stratified oxic lakes examined in this study, Lake Killarney and Steel Lake, are also relatively shallow, with average depths of 4 m and 7 m, respectively, making them potentially sensitive to wind-driven mixing events that have been shown to enhance turbulent transfer of porewaters across the sediment-water interface (Higashino and Stefan, 2012). Future work is planned to determine the specific mechanisms by which elevated arsenic is maintained in the oxic water columns of these study lakes.
3.3 Arsenic bioaccumulation in plankton
Phytoplankton populations in study lakes are generally dominated by Coscinodiscophyceae (8–96%), Pennales (0–79%), or Zygnematales (0–85%), and zooplankton communities are made up of Copepoda (29–89%) and Cladocera (11–71%) as detailed in supporting information Tables S3 and S4. Bioaccumulation of arsenic in both phytoplankton and zooplankton was enhanced in lakes with weakly-stratified, oxic water columns compared to stratified lakes with similar levels of legacy arsenic pollution in surface sediments (Table 4). During the summer growing season in Lake Killarney, average arsenic concentrations in phytoplankton (366 µg g−1 dry weight) and zooplankton (32 µg g−1 dry weight) were significantly higher than concentrations observed in these populations in Angle Lake (two-tailed t-test, p<0.01 for both populations), and up to an order of magnitude higher on any given sampling day. Likewise, both phytoplankton (61 µg g−1) and zooplankton (16 µg g−1) in Steel Lake had higher average arsenic bioaccumulation than phytoplankton (18 µg g−1) and zooplankton (5 µg g−1) populations in North Lake despite a larger sedimentary source of arsenic in North Lake. In all study lakes and on all sampling dates, arsenic accumulation was higher in phytoplankton than in zooplankton, reflecting biodiminution of arsenic in higher trophic levels in the aquatic food web (Chen et al., 2000; Culioli et al., 2009; Rahman et al., 2012).
Table 4.
Maximum arsenic concentration in the water column, average (±1 standard deviation) arsenic concentration in the oxic portion of the water column (>1 O2 mg L−1), average phytoplankton arsenic, and average zooplankton arsenic in summer months (May–Oct) on all sampling dates (n=47).
| stratification | stratification | max [As] (µg L−1) |
oxic [As] (µg L−1) |
phytoplankton [As] (µg g−1) |
zooplanktona [As] (µg g−1) |
|---|---|---|---|---|---|
| strongly stratified | Angle Lake | 56.3 | 2.3 (±1.4) | 54 (±39) | 11 (±4) |
| weakly stratified | Lake Killarney | 52.7 | 17.3 (±6.1) | 366 (±270) | 32 (±22) |
| strongly stratified | North Lake | 11.2 | 1.3 (±0.7) | 18 (±18) | 5 (±1) |
| weakly stratified | Steel Lake | 7.7 | 3.2 (±1.7) | 61 (±52) | 16 (±22) |
zooplankton data set excludes one outlying data point that had poor reproducibility between duplicate samples and an average arsenic concentration more than twice as high as any other sample in the dataset; collected from Lake Killarney on September 1, 2015.
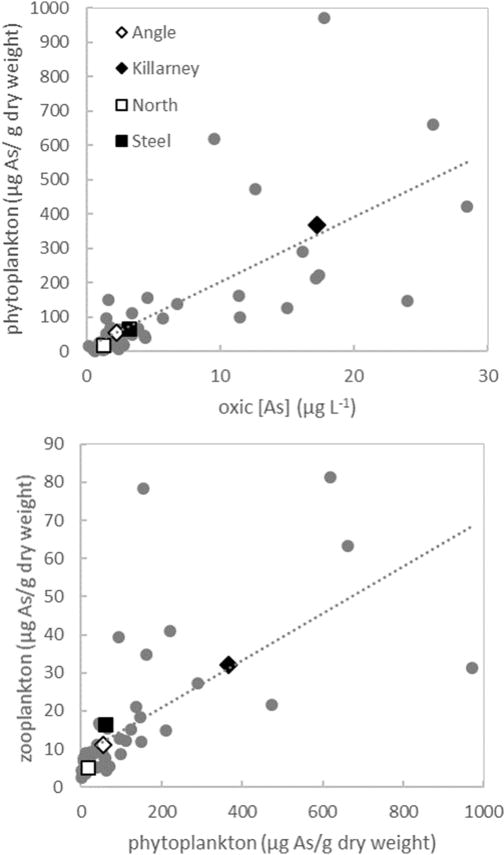
The arsenic concentration of phytoplankton was significantly correlated (R2 = 0.50, p<0.01) with average aqueous arsenic concentrations in oxic waters of study lakes (Figure 5). Hence, seasonal trends in phytoplankton arsenic content was generally similar to aqueous arsenic concentrations in lake surface waters, with lowest arsenic concentrations in phytoplankton populations in late winter and early spring and highest concentrations in late summer and early fall. Maximum aqueous arsenic concentration (found in anoxic hypolimnetic waters in the stratified lakes and at varying depths in weakly-stratified lakes) was not a good predictor of arsenic accumulation in phytoplankton, but rather, arsenic concentrations in the oxic mixed layer appeared to control phytoplankton arsenic content (Table 5).
Fig. 5.
Relationship between average total arsenic concentrations in the oxic portion of the water column (>1 mg O2 L−1) and total arsenic in phytoplankton samples (top). Relationship between total arsenic in phytoplankton samples and total arsenic in zooplankton samples, excluding outlier described in Table 3 (bottom). Colored diamond symbols indicate seasonal averages for each lake in both plots. Grey circles represent each sampling date (n=47) during summer months (May–Oct) with the dashed lines showing the least-squares linear regression in each panel for all sampling dates.
Table 5.
Coefficients of determination (R2) for the linear relationship between phytoplankton arsenic and two metrics for lake water column arsenic concentrations; between zooplankton arsenic and two metrics for lake water column arsenic concentrations; and between zooplankton arsenic and phytoplankton arsenic on all summer sampling dates (n=47).
| max [As] (µg L−1) |
oxic [As] (µg L−1) |
phytoplankton [As] (µg g−1) |
|
|---|---|---|---|
| phytoplankton [As] (µg g−1) | R2 = 0.11* | R2 = 0.50** | --- |
| zooplankton [As] (µg g−1) | R2 = 0.06 | R2 = 0.29** | R2 = 0.43** |
excludes outlier described in Table 3
correlation is significant (p<0.01)
correlation is significant (p<0.05)
Although average zooplankton arsenic was also correlated with water column arsenic concentrations, it was more strongly correlated (R2 = 0.43, p<0.01) with average phytoplankton arsenic (Figure 5). Hence, our data suggest that dietary transfer of arsenic rather than direct exposure from the environment may be a primary mechanism for bioaccumulation, as has also been indicated by previous field studies (Caumette et al. 2012; Chen et al., 2000). In our study lakes, where physical mixing processes and resulting water column redox status exert significant control over phytoplankton bioaccumulation, these factors ultimately impact the transfer of environmental contaminants into the upper trophic levels of aquatic foodwebs.
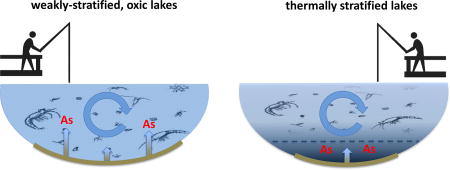
The enhanced bioaccumulation of arsenic in phytoplankton and zooplankton populations in weakly stratified lakes is hypothesized to be the result of spatial overlap between contamination and lake biota and speciation of arsenic in the water column. Anoxic conditions restrict suitable habitat for aquatic organisms (Vaquer-Sunyer and Duarte, 2008), and likely minimized exposure of lake biota to arsenic contamination in Angle Lake and North Lake where elevated dissolved arsenic concentrations were confined to anoxic bottom waters. However, in Lake Killarney and Steel Lake, mobilization of arsenic from contaminated sediments into overlying oxic water columns likely enabled spatial overlap between high aqueous arsenic concentrations and biological habitat, increasing the potential for uptake and accumulation in aquatic organisms. Additionally, in these oxic water columns, arsenic is primarily speciated as arsenate (Table 3), a phosphate analog that is readily taken up by phytoplankton (Rahman et al., 2012 and references therein). Plankton in the contaminated, stratified lakes did still accumulate higher concentrations of arsenic than plankton populations in non-contaminated systems, where plankton arsenic concentrations are generally <15 µg g−1 (Chen et al., 2000; Rahman et al., 2012 and references therein). The high concentrations of arsenic in plankton in the well-mixed lakes in this study (up to 970 µg g−1 in phytoplankton and up to 80 µg g−1 in zooplankton) are comparable to maximum plankton arsenic concentrations observed in other highly-contaminated freshwater lakes (Caumette et al., 2011; 2014), although other systems frequently have higher aqueous arsenic levels (>100 ppb) than found in this work.
4. Broader Implications
Site-specific environmental factors in freshwater systems can influence both mobilization of arsenic from sediments and bioaccumulation of arsenic in aquatic organisms (US EPA, 2003; Williams et al., 2009). In complex natural environments, levels of sediment contamination or maximum measured aqueous arsenic concentrations may not be proper benchmarks for protecting human and environmental health. Currently, the recommended EPA limit for freshwater total arsenic concentrations is 0.14 µg L−1 when aquatic organisms chronically exposed to arsenic are consumed by humans (US EPA, 2009). As of 2015, the human-health water quality criteria for arsenic was targeted for revision (US EPA, 2015) and new criteria will be set using arsenic bioaccumulation factors that relate total aqueous arsenic concentrations to the expected body burden in aquatic organisms (US EPA, 2000). This work demonstrates that the transfer of arsenic into plankton in arsenic-contaminated lakes is enhanced when arsenic is mobilized from sediments into well-mixed, oxic waters compared to systems where the water column experiences seasonal stratification. Although Lake Killarney and Angle Lake have comparable levels of arsenic contamination in surface sediments (>200 mg kg−1 dry weight) and maximum observed aqueous arsenic concentrations (Table 4), the bioaccumulation of arsenic in both phytoplankton and zooplankton was significantly higher in Lake Killarney than in Angle Lake. Well-mixed or weakly-stratified, oxic water columns likely promote arsenic bioaccumulation both by creating overlap between elevated aqueous arsenic concentration and biological habitat, and favoring speciation of arsenic as As(V). Hence, lake redox status, controlled by lake mixing processes, exerts notable control over bioaccumulation and concentrations of aqueous arsenic in oxic waters could be a better metric for predicting arsenic bioaccumulation in aquatic life. Because higher trophic level aquatic organisms consumed by humans (e.g., fish) are at risk of arsenic bioaccumulation primarily via exposure from food sources (Erickson et al., 2011), the elevated arsenic concentrations that we observed in the base of the aquatic food web could potentially represent human health risks from fish consumption. Our future work will determine the arsenic bioaccumulation in recreationally-fished species in these lakes to evaluate potential human arsenic exposure.
Anthropogenic arsenic contamination of lake sediments has been documented at sites across the United States, resulting from historical application of arsenical herbicides to lakes (Durant et al., 2004) or surrounding landscapes (Whitmore et al., 2008), hazardous waste disposal (Nikolaidais et al., 2014), leaching of mine tailings (Harrington et al., 1998), or as in this study, historical smelter emissions (Gawel et al., 2014). The characteristics of the weakly-stratified study lakes suspected to facilitate elevated levels of arsenic in the well-mixed, oxic water columns include arsenic-contaminated sediments, eutrophic conditions, and shallow depths, which are common to many small lakes in densely-populated watersheds. Hence, these lakes may be uniquely vulnerable to environmental and human health risks from legacy arsenic contamination in sediments. Small urban lakes are under-studied compared to large, thermally-stratified lakes, particularly with respect to arsenic contamination (Arnold and Oldham, 1997; Birch and McCaskie, 1999), yet these shallow lakes play an important role in supporting human recreation and food needs.
Data
The water column and plankton data presented in this manuscript have been submitted to the Pangaea data repository and are publicly accessible at https://doi.pangaea.de/10.1594/PANGAEA.884327.
Supplementary Material
Highlights.
High concentrations of arsenate were observed in waters of weakly-stratified lakes
Plankton accumulated up to 10× more arsenic than in stratified lakes
Lake mixing status impacts arsenic transfer from sediments to the base of food web
Acknowledgments
This work was sponsored by the University of Washington Superfund Research Program, funded by NIEHS grant P42ES004696 to RBN and JEG, and the University of Washington Tacoma Research and Teaching Fund. The authors would like to thank Jonathan McLean for assistance with plankton identification and Blaine McCleskey (U.S. Geological Survey) for assistance with arsenic speciation measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggett J, O’Brien GA. Detailed model for the mobility of arsenic in lacustrine sediments based on measurements in Lake Ohakuri. Environ. Sci. Technol. 1985;19:231–238. doi: 10.1021/es00133a002. [DOI] [PubMed] [Google Scholar]
- Aggett J, Kriegman MR. The extent of formation of arsenic(III) in sediment interstitial waters and its release to hypolimnetic waters in Lake Ohakuri. Wat. Res. 1988;22:407–411. [Google Scholar]
- Arnold TN, Oldham CE. Trace-element contamination of a shallow wetland in Western Australia. Marine Freshwater Res. 1997;48(6):531. [Google Scholar]
- Azcue JM, Nriagu JO. Impact of abandoned mine tailings on the arsenic concentrations in Moira Lake, Ontario. J. Geochem. Explor. 1995;52:81–89. [Google Scholar]
- Barringer JL, Szabo Z, Wilson TP, Bonin JL, Kratzer T, Cenno K, Romagna T, Alebus M, Hirst B. Distribution and seasonal dynamics of arsenic in a shallow lake in northwestern New Jersey, USA. Environ. Geochem. Health. 2011;33:1–22. doi: 10.1007/s10653-010-9289-7. [DOI] [PubMed] [Google Scholar]
- Birch S, McCaskie J. Shallow urban lakes: a challenge for lake management. Hydrobiologia. 1999;395:365–378. [Google Scholar]
- Caumette G, Koch I, Estrada E, Reimer KJ. Arsenic distribution and speciation in Daphnia pulex. Environ. Sci. Tech. 2011;45:9917–9923. [Google Scholar]
- Caumette G, Koch I, Moriarty M, Reimer KJ. Arsenic distribution and speciation in Daphnia pulex. Sci. Tot. Environ. 2012;432:243–250. doi: 10.1016/j.scitotenv.2012.05.050. [DOI] [PubMed] [Google Scholar]
- Caumette G, Koch I, MoriHouse K, Reimer KJ. Arsenic cycling in freshwater phytoplankton and zooplankton cultures. Environ. Chem. 2014;11:496–505. [Google Scholar]
- Chen CY, Stemberger RS, Klaue B, Blum JD, Pickhardt PC, Folt CL. Accumulation of heavy metals in food web components across a gradient of lakes. Limnol. Oceanogr. 2000;45:1525–1536. [Google Scholar]
- Couture R-M, Gobeil C, Tessier A. Arsenic, iron, and sulfur co-diagenesis in lake sediments. Geochim. Cosmochim. Acta. 2010;74:1238–1255. [Google Scholar]
- Culioli J-L, Fouquoire A, Calendini S, Mori C, Orsini A. Trophic transfer of arsenic and antimony in a freshwater ecosystem: A field study. Aquat. Toxicol. 2009;94:286–293. doi: 10.1016/j.aquatox.2009.07.016. [DOI] [PubMed] [Google Scholar]
- De Vitre R, Belzile N, Tessier A. Speciation and adsorption of arsenic on diagenetic iron oxyhydroxides. Limnol. Oceanogr. 1991;36:1480–1485. [Google Scholar]
- Dixit S, Hering J. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003;37:4182–4189. doi: 10.1021/es030309t. [DOI] [PubMed] [Google Scholar]
- Durant JL, Ivushkina T, MacLaughlin K, Lukacs H, Gawel J, Senn D, Hemond HF. Elevated levels of arsenic in the sediments of an urban pond: sources, distribution and water quality impacts. Wat. Res. 2004;38:2989–3000. doi: 10.1016/j.watres.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Eisler R. Arsenic hazards to fish, wildlife and invertebrates: a synoptic review. In: Eisler R, editor. Biological Reports: Contaminant Hazard Reviews. U.S. Fish and Wildlife Service; Laurel, Maryland: 1988. [Google Scholar]
- Erickson RJ, Mount DR, Highland TL, Russell Hockett J, Jenson CT. The relative importance of waterborne and dietborne arsenic exposure on survival and growth of juvenile rainbow trout. Aquat. Toxicol. 2011;104:108–115. doi: 10.1016/j.aquatox.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Ford RG, Wilkin RT, Hernandez G. Arsenic cycling within the water column of a small lake receiving contaminated ground-water discharge. Chem. Geol. 2006;228:137–155. [Google Scholar]
- Gawel JE, Asplund JA, Burdick S, Miller M, Peterson SM, Tollefson A, Ziegler K. Arsenic and lead distribution and mobility in lake sediments in the south-central Puget Sound watershed: The long-term impact of a metal smelter in Ruston, Washington, USA. Sci. Total Environ. 2014;472:530–537. doi: 10.1016/j.scitotenv.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Harrington JM, Fendorf SE, Rosenzweig RF. Biotic Generation of Arsenic(III) in Metal(loid)-Contaminated Freshwater Lake Sediments. Environ. Sci. Technol. 1998;32:2425–2430. [Google Scholar]
- Hasegawa H. The behavior of trivalent and pentavalent methylarsenicals in Lake Biwa. Appl. Organomet. Chem. 1997;11:305–311. [Google Scholar]
- Hellweger FI, Lall U. Modelling the effect of algal dynamics on arsenic speciation in Lake Biwa. Environ. Sci. Tech. 2004;38:6716–6723. doi: 10.1021/es049660k. [DOI] [PubMed] [Google Scholar]
- Higashino M, Stefan HG. Model of turbulence penetration into a suspension layer on a sediment bed and effect on vertical solute transfer. Environ. Fluid Mech. 2012;12:451–469. [Google Scholar]
- Idso SB. On the concept of lake stability. Limnol. Oceanogr. 1973;18:681–683. [Google Scholar]
- Kaise T, Ogura M, Nozaki T, Saitoh K, Sakurai T, Matsubara C, Wanatabe C, Hanaoka K. Biomethylation of arsenic in an arsenic-rich freshwater environment. Appl. Organometal. Chem. 1997;11:297–304. [Google Scholar]
- Kuhn X, Sigg X. Arsenic cycling in eutrophic Lake Greifen, Switzerland: Influence of seasonal redox processes. Limnol. Oceanogr. 1993;38:1052–1059. [Google Scholar]
- Manning BA, Goldberg S. Modeling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals. Soil Sci. Soc. Am. J. 1996;60:121–131. [Google Scholar]
- Martin AJ, Pedersen TF. Seasonal and Interannual Mobility of Arsenic in a Lake Impacted by Metal Mining. Environ. Sci. Technol. 2002;36:1516–1523. doi: 10.1021/es0108537. [DOI] [PubMed] [Google Scholar]
- McCleskey RB, Nordstrom DK, Maest AS. Preservation of water samples for arsenic(III/V) determinations: an evaluation of the literature and new analytical results. Appl. Geochem. 2004;19:995–1009. [Google Scholar]
- National Research Council. 1999. Arsenic in Drinking Water. Washington, DC: The National Academies Press; 1999. [DOI] [Google Scholar]
- Nikolaidais NP, Dobbs GM, Chen J, Lackovic JA. Arsenic mobility in contaminated lake sediments. Environ. Pollut. 2014;129:479–487. doi: 10.1016/j.envpol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Nimick DA, Moore JN, Dalby CE, Savka MW. The fate of geothermal arsenic in the Madison and Missouri Rivers, Montana and Wyoming. Water Resour. Res. 1998;34:3051–3067. [Google Scholar]
- Rahman MA, Hasegawa H, Lim RP. Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ. Res. 2012;116:118–135. doi: 10.1016/j.envres.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Read J, Hamilton D, Jones I, Muraoka K, Winslow L, Kroiss R, Wu C, Gaiser E. Derivation of lake mixing and stratification indices from high-resolution lake buoy data. Environ. Model. Softw. 2011;26:1325–1336. [Google Scholar]
- Redman AD, Macalady DL, Ahmann D. Natural Organic Matter Affects Arsenic Speciation and Sorption onto Hematite. Environ. Sci. Technol. 2002;36:2889–2896. doi: 10.1021/es0112801. [DOI] [PubMed] [Google Scholar]
- Schlitzer R. Ocean Data View. 2010 http://odv.awi.de.
- Schmidt W. Über Temperatur- und Stabilitätsverhältnisse von Seen. Geogr. Ann. 1928;10:145–177. [Google Scholar]
- Selyer P, Martin J-M. Biogeochemical processes affecting arsenic species distribution in a permanently stratified lake. Environ. Sci Technol. 1989;23:1258–1263. [Google Scholar]
- Senn DB, Hemond HF. Particulate arsenic and iron during anoxia in a eutrophic, urban lake. Environ. Toxicol. Chem. 2004;23:1610–1616. doi: 10.1897/03-243. [DOI] [PubMed] [Google Scholar]
- Senn DB, Gawel JE, Jay JA, Hemond HF, Durant JL. Long-Term Fate of a Pulse Arsenic Input to a Eutropic Lake. Environ. Sci. Technol. 2007;41:3062–3068. doi: 10.1021/es062444m. [DOI] [PubMed] [Google Scholar]
- Smedley P, Kinniburgh D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002;17:517–568. [Google Scholar]
- Sohrin Y, Matsui M, Kawashima M, Hojo A, Hasegawa H. Environ. Sci. Technol. 1997;31:2712–2720. [Google Scholar]
- Tufano K, S. Fendorf S. Confounding impacts of iron reduction on arsenic retention. Environ. Sci Technol. 2008;42:4777–4783. doi: 10.1021/es702625e. [DOI] [PubMed] [Google Scholar]
- US EPA. Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health. Office of Water; Washington, DC: 2000. http://www.nj.gov/drbc/library/documents/EPA_human-health-criteria2000.pdf. [Google Scholar]
- US EPA. Technical Summary of Information Available on the Bioaccumulation of Arsenic in Aquatic Organisms. Office of Science and Technology & Office of Water; Washington, DC: 2003. nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1002YTX.TXT. [Google Scholar]
- US EPA. National Recommended Water Quality Criteria: 2009. Office of Science and Technology & Office of Water; Washington, DC: 2009. https://hero.epa.gov/hero/index.cfm/reference/download/reference_id/644569. [Google Scholar]
- US EPA. Human Health Ambient Water Quality Criteria: 2015 Update. Office of Water; Washington, DC: 2015. https://www.epa.gov/sites/production/files/2015-10/documents/human-health-2015-update-factsheet.pdf. [Google Scholar]
- Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity: A review. Hum. Exp. Toxicol. 2007;26:823–832. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- Vaquer-Sunyer R, Duarte CM. Thresholds of hypoxia for marine biodiversity. Proc. Nat. Acad. Sci. 2008;105:15452–15457. doi: 10.1073/pnas.0803833105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L, van Riemsdijk WH, Hiemstra T. Effects of Fulvic and Humic Acids on Arsenate Adsorption to Goethite: Experiments and Modeling. Environ. Sci. Technol. 2009;43:7198–7204. doi: 10.1021/es9000196. [DOI] [PubMed] [Google Scholar]
- Whitmore TJ, Riedinger-Whitmore MA, Smoak JM, Kolasa KV, Goddard EA, Bindler R. Arsenic contamination of lake sediments in Florida: evidence of herbicide mobility from watershed soils. J. Paleolimnol. 2008;40:869–884. [Google Scholar]
- Williams L, Schoof RA, Yager JW, Goodrich-Mahoney JW. Arsenic Bioaccumulation in Freshwater Fishes. Hum. Ecol. Risk Assess. 2006;12:904–923. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.