Abstract
Homocysteine, a redox-active metabolite of the methionine cycle, is of particular clinical interest due to its association with various neurodegenerative diseases including Amyotrophic Lateral Sclerosis. It has been previously established that homocysteine exacerbates damage to motor neurons from reactive oxygen species (ROS) such as hydrogen peroxide. To assess the role of homocysteine at the mammalian neuromuscular junction, neurotransmission was monitored via electrophysiology at the mouse Epitrochleoanconeus (ETA) muscle. Preparations were pre-incubated in homocysteine prior to inducing ROS and recordings were taken before and after ROS treatment. In this study, homocysteine was observed to sensitize the neuromuscular junction to ROS-induced depression of spontaneous transmission frequency, an effect we found to be mediated via an NMDA receptor and nitric oxide. The NMDA receptor antagonist DL-2- Amino-5-phosphonopentanoic acid prevented the depression from homocysteine-induced sensitization to oxidative stress. Disrupting nitric oxide activity with either the NOS I antagonist Nω-Nitro-L-Arginine methyl ester hydrochloride or the NO scavenger 2-(4-Carboxyphenyl)-4,4,5,5-tetramethyl- imidazoline-1-oxyl-3-oxide potassium salt also prevented the depression. Moreover, replacing homocysteine with the exogenous NO donor Diethylamine NONOate diethylammonium was sufficient to reconstitute the effects of homocysteine-induced sensitization to ROS. Interestingly, a novel secondary effect was observed where homocysteine itself depresses quantal content, an effect found to be mediated by NMDARs independently of nitric oxide and ROS. Collectively, these data present a novel model of two distinct pathways through which homocysteine alters neurotransmission at the neuromuscular junction. Characterizing homocysteine’s mechanism of action is of particular clinical relevance as many treatments for ALS are centered on mitigating homocysteine-induced pathologies.
Keywords: Homocysteine, Nitric Oxide, Neuromuscular Junction, Amyotrophic Lateral Sclerosis
Introduction
Homocysteine (HCY), a metabolic intermediate of the methionine cycle, is also a redox-active neurotoxin that markedly reduces cell viability.[1, 2] It is of particular biological interest as elevated levels of HCY have been observed in the blood plasma of patients diagnosed with Amyotrophic Lateral Sclerosis (ALS),[3] a neurodegenerative disease characterized by the progressive deterioration of neuromuscular junctions (NMJ) contributing to muscle weakness and paralysis.[4] Its specific mechanism of action remains yet to be fully characterized, but an association has been found linking HCY to the promotion of free radicals and accumulation of cytosolic calcium.[3, 5] The balance of free radicals at the NMJ is of particular importance as they can cause motor deficits via synaptic pruning of existing synapses.[6] Recently, HCY has been shown to impair motor neurons by sensitizing the NMJ to oxidative stress, a general term that refers to the damage and disruption of cellular machinery due to an imbalance of oxidant and antioxidant levels.[7] This only further confirms it as a risk factor for ALS[8] and has prompted interest in a more in-depth examination of HCY’s mechanism of action at the NMJ.
Historically, the NMJ has been characterized as an exclusively cholinergic synapse,[9] but more recent findings suggest that the neurotransmitter glutamate functions at the NMJ.[10–12] Several types of glutamate receptors, including the N-methyl-D-aspartate receptor (NMDAR), have been identified at the NMJ.[11, 13] Since HCY is known to activate the GluN2A subunit of NMDARs[5] this would provide a possible path through which HCY might damage motor neurons. Hyperactivity of NMDARs is concerning as it has been linked to heightened calcium influx resulting in ROS generation and elevated levels of oxidative stress.[14] Subsequent studies have demonstrated a close association between NMDARs and Nitric Oxide Synthase I,[15,16] suggesting a potential involvement of NO in the effects of activating NMDARs at the neuromuscular junction, providing another line of investigation into HCY’s potential mechanism of action. Characterization of this pathway may provide further insight into treatment for ALS as it will illuminate the pathological implications of the elevated levels of HCY found in the blood plasma of ALS patients.[3] In this report, we describe a bifurcated mechanism of HCY at the NMJ wherein it (1) aggravates ROS-induced depression of neurotransmitter release frequency via a NMDAR-NOS I complex and (2) depresses quantal content independently of NOS I or ROS.
Materials and Methods
Experimental Preparation and Solutions
C57BL/6 Mice (aged 6–8 weeks, Jackson Laboratories, Bar Harbor, ME, USA) were anesthetized using a CO2 induction chamber. The mouse Epitrochleoanconeus (ETA) muscle and its associated nerve were extracted as described in Bradley et al. (1989)[17] and pinned into a Sylgard coated chamber containing fresh mouse ringer solution: 114 mM NaCl, 3.45 mM KCl, 0.7 mM MgCl2-6H2O, 1.7 mM NaH2PO4-H2O, 1.7 mM NaH2PO4-7H2O, 26.2 mM NaHCO3, 1.8 mM CaCl2-2H2O, and 11.2 mM D-Glucose; pH was adjusted to 7.4 adjusted using 1M NaOH. The bath was continuously perfused with a gas mixture of 95% O2 and 5% CO2. The procedure outlined above was approved by the Institutional Animal Use and Care Committee at Grinnell College.
In all of our experiments, drugs were administered through a perfusion bath of mouse ringer solution. All drugs were stored at −20°C at a stock concentration until the day of the experiment when the stock was diluted to the appropriate concentration for each respective experiment. In all experiments, μ-Conotoxin GIIIB (Alomone Labs, Jerusalem, Israel) was applied to the preparation thirty minutes prior to electrophysiology to inhibit action potentials in the muscle. For all experiments that required homocysteine incubation, 500 μM D-L Homocysteine (Sigma-Aldrich, St. Louis, MO, USA) was administered for three hours prior to electrophysiological recordings and sustained throughout the entire experiment. To induce acute oxidative stress from reactive oxygen species, 300 μM H2O2 was administered for thirty minutes prior to recording as per previously documented methodology wherein a 300 μM H2O2 dosage was sufficient to induce acute depression of evoked end-plate potential amplitudes.[8, 18] All other drugs were administered for the duration of their respective experiments. In Figure 2, a concentration of 50 μM DL-2-Amino-5-phosphonopentanoic acid (AP-5; Sigma-Aldrich, St. Louis, MO, USA), 300 μM Nω-Nitro-L-Arginine methyl ester hydrochloride (L-NAME; Sigma Aldrich, St. Louis, MO, USA), 40 μM 2-(4-Carboxyphenyl)-4,4,5,5-tetramethyl- imidazoline-1-oxyl-3-oxide potassium salt (CPTIO; Sigma-Aldrich, St. Louis, MO, USA), and 100 μM Diethylamine NONOate diethylammonium (DEANO; Sigma-Aldrich, St. Louis, MO, USA) with 100 μM L-NAME were administered.
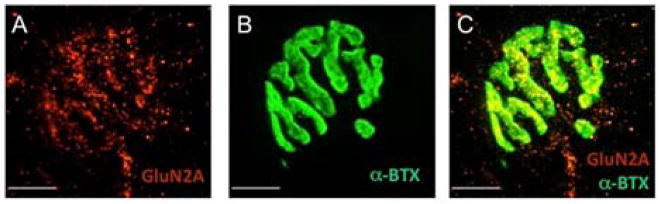
Figure 2. NMDA receptors are present at the mouse ETA neuromuscular junction.
(A) Immunofluorescence image of NMDAR2A (red). (B) The same NMJ as in A, stained with α-Bungarotoxin (green) (C) Images A and B are superimposed. All images shown are maximum projections of 14 images collected at 0.5 μm intervals. Calibration bars, 10 μm.
Electrophysiology
End-plate potentials were evoked by stimulating the ETA muscle motor nerve axon with single depolarizing square pulses using a Grass SD-9 Stimulator set at a frequency of 0.2 Hz, duration of 0.2 ms, and a suprathreshold voltage to induce an action potential in the motor nerve via a suction electrode. End-plate potentials were recorded using glass microelectrodes (3M KCl; resistance: 5–20 MΩ) prepared from 1.2 mm borosilicate glass capillaries with filament (World Precision Instruments, Sarasota, FL, USA). Membrane potentials were amplified using an A-M Systems Model 1600 Amplifier (A-M Systems, Sequim, WA, USA) and recorded with AD-Instruments PowerLab 4/25 paired with its associated LabChart software (AD Instruments, Colorado Springs, CO, USA). Recordings were taken in randomly selected ETA muscle fibers and the microelectrode was inserted into each cell for roughly 100 seconds, collecting a minimum of at least 20 end-plate potentials and 50 miniature end-plate potentials.
Immunofluorescence
ETA muscles were fixed in 4% paraformaldehyde for 15 min at room temperature. After rinsing, they were permeabilized with 0.3% TritonX-100 for 30 min at 37°C, and then rinsed for 1 hr (3×20 min) in Blocking Solution (BS; 0.01% TritonX-100, 1% bovine serum albumin) to reduce non-specific staining. Polyclonal rabbit anti-NMDAR2A IgG (A-6473; Thermo Fisher Scientific, Waltham, MA) was applied at 1:200 dilution in BS overnight at 4°C. Goat anti-rabbit IgG conjugated to Alexa Fluor 555 (Thermo Fisher Scientific) was then applied at 1:500 dilution for 3 hrs. at 37°C and washed in BS. α-Bungarotoxin conjugated to Alexa Fluor 488 (Thermo Fisher Scientific, Waltham, MA) was applied at 1:200 for 15 minutes to label the nicotinic acetylcholine receptors. After further rinsing, the muscles were mounted on microscope slides in Slow Fade Diamond Antifade Mountant (Thermo Fisher Scientific, Waltham, MA).
Muscles were imaged using an Olympus IX81 inverted microscope with a spinning-disk confocal attachment and Hamamatsu Orca EM camera. A standard FITC filter set (Ex 470/90 nm; DM 495 nm; Em 525/50 nm) and TRITC filter set (Ex 545/30 nm; DM 570 nm; Em620/60 nm) were used to image the fluorophores. All images were acquired, adjusted for optimal brightness, and analyzed with SlideBook 6 software (3i, Intelligent Imaging Innovations, Denver, CO, USA).
Data Analysis
All data is presented as a mean ± SE. Statistical significance was assessed using Student’s paired t-test for measurements taken before and after H2O2 treatment on the same preparation. For measurements across different preparations, Student’s two sample t-test was used.
Results
HCY Sensitizes the NMJ to ROS-induced Depression of Transmitter Release
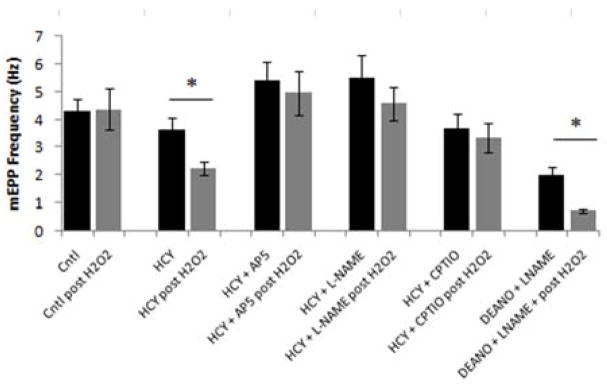
Our first endeavor was to confirm the previous findings of Bukharaeva et al.,[8] which showed that HCY sensitizes the neuromuscular junction to oxidative stress. mEPP frequencies were used to quantify spontaneous release of ACh from the nerve terminal and serve as a measure of neurotransmitter release. In our control experiments, mEPP frequencies were initially measured at 4.29 ± 0.44 Hz (mean ± SE). Application of H2O2 to induce mild oxidative stress caused an insignificant change to 4.37 ± 0.74 Hz (Figure 2). It was only when the ETA muscle was incubated in D-L Homocysteine (HCY) for three hours prior to exposure to H2O2 that a significant 31% decrease in MEPP frequency from 3.65 ± 0.39 to 2.23 ± 0.24 Hz was observed (Figure 2), thus supporting Bukharaeva et al..[8] For ease of reference in future comparison, we will refer to back to this significant 31% decrease in mEPP frequency from HCY-induced sensitization of the NMJ to oxidative stress as the standard HCY- H2O2 effect.
HCY- H2O2 induced depression of transmitter release is mediated by NMDARs and Nitric Oxide
There is pharmcological evidence that HCY activates the GluN2A subunit of NMDARs.[5] To explore whether this mediates HCY’s effects at the mouse NMJ as proposed by Bukharaeva et al.,[8] we first confirmed the presence of NMDARs using immunofluorescence. Application of an NMDAR2A antibody shows specific staining at the NMJ (Figure 1A). Co-staining with α-bungarotoxin (Figure 1B), which labels nicotinic acetylcholine receptors, confirmed the presence of NMDARs at the NMJ (Figure 1C); however, it was not possible to localize these receptors to any specific component of the NMJ (e.g. the nerve terminal, muscle, or Schwann cell).
Figure 1. HCY-induced sensitization to ROS is mediated by NMDA receptors and nitric oxide.
mEPP frequencies were recorded before and after application of H2O2 in each condition in mouse ETA muscles. Each data point represents the mean mEPP frequency calculated from each condition (1) Control Pre/Post H2O2: 50 synapses across 10 mice (2) Homocysteine Pre/Post H2O2: 75 synapses across 15 mice (3) Homocysteine + AP-5 Pre/Post H2O2: 35 synapses across 7 mice (4) Homocysteine + L-NAME Pre/Post H2O2: 30 synapses across 6 mice (5) Homocysteine + CPTIO: 25 synapses across 5 mice (6) DEANO + L-NAME: 20 synapses across 4 mice. Data were analyzed using Student’s paired t-test (where p < 0.05 denoted by *)
After ascertaining that NMDARs are present, we applied the NMDAR antagonist DL-2-Amino-5-phosphonopentanoic acid (AP-5). As seen in Figure 2, applying AP-5 blocked the effect of HCY as subsequent addition of H2O2 produced an insignificant 11% decrease in MEPP frequency from 5.41 ± 0.67 to 4.96 ± 0.80 Hz as compared to the characteristic 31% decrease observed from the standard HCY- H2O2 effect. Therefore, the standard HCY- H2O2 effect is indeed mediated through NMDARs as the antagonist successfully blocks mEPP frequency depression.
After confirming the role of NMDARs in HCY-induced sensitization to oxidative stress, we sought to characterize the next target in the pathway. Since previous work has shown that nitric oxide synthase I (NOS l) co-localizes with NMDARs at the neuromuscular junction,[15, 16] we posited that the HCY pathway utilizes NO. Therefore, we applied the nitric oxide synthase antagonist Nω-Nitro-L-Arginine methyl ester hydrochloride (L-NAME) to disrupt the pathway and see if this blocked the effect of HCY. As seen in Figure 2, application of L-NAME successfully blocked the HCY-induced depression of mEPP frequency. With L-NAME, an insignificant 14% decrease from 5.51 ± 0.81 to 4.58 ± 0.58 Hz was observed upon application of H2O2 as compared to the significant 31% decrease observed from the standard HCY- H2O2 effect. To determine whether NO must pass through the extracellular space to reach its target, we applied the membrane-impermeable NO scavenger 2-(4-Carboxyphenyl)-4,4,5,5-tetramethyl- imidazoline-1-oxyl-3-oxide potassium salt (CPTIO). Application of CPTIO blocked the standard HCY- H2O2 effect as only an insignificant 7% decrease from 3.66 ± 0.55 to 3.35 ± 0.52 Hz in mEPP frequency was observed (Figure 2). Having shown that disrupting either NO production or its diffusion through the extracellular space blocks the depression of mEPP frequencies from HCY-induced sensitization of the NMJ to oxidative stress, the next step was to determine whether it was possible to re-constitute the standard HCY- H2O2 effect by replacing HCY with an exogenous NO donor.
HCY treatment was replaced with exposure to the exogenous NO donor Diethylamine NONOate diethylammonium (DEANO). DEANO successfully reconstituted the standard HCY- H2O2 effect in the absence of HCY. A significant 62% decrease in mEPP frequency from 2.02 ± 0.26 to 0.744 ± .07 Hz was observed upon treatment with DEANO which was even more potent than the standard HCY- H2O2 effect of a 31% decrease (Figure 2).
HCY can independently depresses quantal content at the NMJ
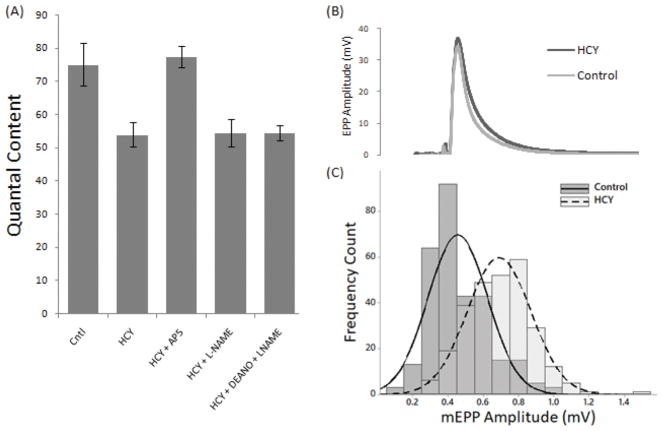
In addition to sensitizing the NMJ to oxidative stress, HCY also independently decreased evoked neurotransmitter release. As seen in Figure 3A, HCY treatment alone causes a significant decrease in quantal content from 75.14 ± 6.39 to 54.02 ± 3.69. Moreover, treatment with the NMDAR antagonist AP-5 blocks this effect and restores quantal content levels to 77.53 ± 3.31. Surprisingly, NO does not seem to be involved in this effect of HCY. Inhibiting NOS with L-NAME did not block the HCY effect, as a decrease in quantal content to 54.60 ± 4.11 was still observed. Applying an exogenous donor of NO also had no effect as a decrease in quantal content to 54.61 ± 2.31 was observed once again (Figure 3). Therefore, HCY can independently induce a depression of quantal content, an effect that is mediated through NMDARs with a mechanism unrelated to NO. A closer examination of the electrophysiological traces revealed that HCY increases mEPP amplitudes whilst having no significant effect on EPP amplitudes thus resulting in the overall decrease of quantal content (Figures 3B and 3C).
Figure 3. Homocysteine induces quantal content depression through NMDARs independent of nitric oxide.
(A) Quantal contents were calculated from the ratio of EPP amplitude to mEPP amplitude. Each data point represents the mean quantal content calculated from each condition (1) Control: 50 synapses across 10 mice (2) Homocysteine: 75 synapses across 15 mice (3) Homocysteine + AP-5: 35 synapses across 7 mice (4) Homocysteine + L-NAME: 30 synapses across 6 mice (5) Homocysteine + DEANO + L-NAME: 25 synapses across 5 mice. Data were analyzed using Student’s t-test (significant differences from control of p < 0.05 are denoted by *) (B) Representative electrophysiological traces of an EPP measured under control conditions versus HCY treatment (C) Distribution of mEPP frequencies comparing control conditions (n=272 traces) versus HCY treatment (n=296 traces).
Discussion
HCY sensitizes the NMJ to the effects of oxidative stress via nitric oxide
We conducted this investigation because recent research had shown that HCY aggravates the negative effects of ROS on neurotransmitter release at the mouse NMJ.[8] As demonstrated in Figure 1, pre-incubation of the mouse ETA muscle in HCY aggravated decreases in mEPP frequency upon exposure to oxidative stress, thus confirming the results of Bukharaeva et al..[8] Since previous work has shown HCY activates the GluN2A subunit of NMDA receptors,[5] we expected that HCY’s mechanism of action at the mouse NMJ would be mediated through NMDARs. After demonstrating the presence of NMDARs at the NMJ (Figure 2), we showed that application of a NMDAR antagonist successfully blocked the HCY- H2O2-induced depression of mEPP frequency (Figure 1) thus providing anatomical evidence and further confirming Bukharaeva et al’s[8] pharmacological evidence for the involvement of NMDARs.
To our knowledge, this paper provides the first pharmacological evidence for the next target in this pathway. Although it has been previously shown that NOS I co-localizes with the NMDAR Subunit 1 at the mouse neuromuscular junction,[15] this is the first description of a NMDAR-NOS I complex mediating HCY-induced sensitization of the neuromuscular junction to oxidative stress. We have clear pharmacological evidence that inhibiting either NO synthesis or its diffusion through the extracellular space blocks the characteristic depression of mEPP frequency from the HCY- H2O2 effect (see Figure 1). Since the target of NO is typically the presynaptic nerve terminal, we speculate that the source of NO is either the muscle or the Schwann cell.
The involvement of NO is particularly relevant because there is strong evidence that nitric oxide is involved in modulating the release of ACh at the rat NMJ[16, 19] and multiple studies have specifically linked NO activity to inhibiting release of ACh in amphibian and mammalian synapses.[20, 21] We propose that the HCY-induced nitric oxide diffuses from the postsynaptic cell to the presynaptic cell where nitric oxide then undergoes a favorable reaction with a reactive oxygen species to generate peroxynitrite.[22] Interestingly, peroxynitrite has been directly linked to the impairment of SNAP-25, a synaptic release protein whose activity facilitates synaptic transmission.[22, 23] This model we are proposing explains why synaptic transmission is reduced only during the coincidence of homocysteine and reactive oxygen species, as the formation of peroxynitrite is dependent on the presence of both.
HCY reduces quantal content at the mouse NMJ
We also observed a novel secondary effect of HCY where incubation in HCY alone was sufficient to significantly decrease quantal content at the mouse NMJ even in the absence of ROS. This effect is mediated by NMDARs as application of an antagonist effectively abolishes the depression in quantal content. Yet it is clearly a distinct pathway from the standard HCY- H2O2 effect as interference with nitric oxide production and activity did not block the effect. This complicates the mechanism of HCY action at the NMJ as it suggests that HCY acts through a bifurcated pathway.
Since HCY-induced depression of quantal content is mediated by NMDARs, but not NOS, we propose that the NMDA-induced calcium influx is targeting something entirely different than NOS to inhibit presynaptic release of ACh. Instead, it likely triggers a calcium-dependent cascade that may involve CaMKII, a kinase that increases cholinergic receptor density on the postsynaptic cell[24], a possibility which is consistent with our electrophysiological results detailing HCY-induced increases in mEPP amplitudes. It is known that there are various synaptic homeostatic mechanisms that involve retrograde signaling that can modulate presynaptic activity based on the state of the postsynaptic cell.[25] Therefore, we propose that the synapse adapts to this increase in postsynaptic receptor density in the postsynaptic cell by releasing fewer quanta of ACh from the presynaptic cell.
Accounting for HCY’s bifurcated mechanism of action at the NMJ, we propose a two-factor model where HCY lowers neurotransmission at the NMJ through (1) an indirect effect wherein HCY aggravates the effects of oxidative stress via a NMDAR-NOS I complex and (2) a direct effect where HCY induces a calcium-dependent cascade modifying synaptic machinery. A better understanding of HCY’s role at the NMJ is critical to developing therapies for ALS as many potential treatments focus on degrading or inhibiting HCY activity at the NMJ,[3] a task that requires recognition of HCY’s multifaceted effects.
Conclusions
Our data show that HCY acts synergistically with ROS to induce significant decreases in neurotransmission at the NMJ through a mechanism mediated by nitric oxide. Prior to this study, there were only hypothetical models for the mediator between NMDAR activation from HCY and its induced pathologies. To our knowledge, this study provides the first pharmacological data implicating nitric oxide’s involvement in HCY sensitizing the NMJ to ROS-induced decrease of neurotransmission. Interestingly, HCY was observed to also decrease quantal content in a separate mechanism mediated by NMDARs independent of both nitric oxide and ROS. These results demonstrate a more complicated mechanism of action than previously hypothesized where HCY acts in a bifurcated pathway at the NMJ to reduce neurotransmission in two independent mechanisms.
Acknowledgments
Funding was provided by NIH grant R15NS072735-02.
The authors thank Dr. Steve Ryan for his invaluable technical assistance. The work was supported by NIH grant R15NS072735-02.
Footnotes
Author contributions: JSW and CAL wrote the manuscript. All of the authors contributed to the experiments described in the manuscript.
There are no conflicts of interest.
References
- 1.Levin J, Bötzel K, Giese A, Vogeser M, Lorenzl S. Elevated Levels of Methylmalonate and Homocysteine in Parkinson’s Disease, Progressive Supranuclear Palsy and Amyotrophic Lateral Sclerosis. Dementia and Geriatric Cognitive Disorders. 2010;29:553–559. doi: 10.1159/000314841. [DOI] [PubMed] [Google Scholar]
- 2.Kolling J, Scherer EBS, Siebert C, Hansen F, Torres FV, Scaini G, et al. Homocysteine induces energy imbalance in rat skeletal muscle: Is creatine a protector? Cell Biochemistry and Function. 2013;31:575–584. doi: 10.1002/cbf.2938. [DOI] [PubMed] [Google Scholar]
- 3.Zoccolella S, Bendotti C, Beghi E, Logroscino G. Homocysteine levels and amyotrophic lateral sclerosis: A possible link. Amyotrophic Lateral Sclerosis. 2010;11:140–147. doi: 10.3109/17482960902919360. [DOI] [PubMed] [Google Scholar]
- 4.Arbour D, Vande Velde C, Robitaille R. New perspectives on amyotrophic lateral sclerosis: the role of glial cells at the neuromuscular junction. J Physiol (Lond ) 2017;595:647–661. doi: 10.1113/JP270213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abushik PA, Giniatullin R, Antonov SM, Sibarov DA. GluN2A Subunit-Containing NMDA Receptors Are the Preferential Neuronal Targets of Homocysteine. Frontiers in Cellular Neuroscience. 2016;10:246. doi: 10.3389/fncel.2016.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidlauskaitea E, Gibsonalan JW, Megson IL, Whitfield PD, Tovmasyan A, Batanic-Haberle I, et al. Mitochondrial ROS cause motor deficits induced by synaptic inactivity: Implications for synapse pruning. Redox Biology. 2018;16:344–351. doi: 10.1016/j.redox.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sies H. Oxidative Stress: a concept in redox biology and medicine. Redox Biology. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukharaeva E, Shakirzyanova A, Khuzakhmetova V, Sitdikova G, Giniatullin R. Homocysteine aggravates ROS-induced depression of transmitter release from motor nerve terminals: potential mechanism of peripheral impairment in motor neuron diseases associated with hyperhomocysteinemia. Frontiers in cellular neuroscience. 2015;9:391. doi: 10.3389/fncel.2015.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 10.Berger UV, Carter RE, Coyle JT. The immunocytochemical localization of N-acetylaspartyl glutamate, its hydrolysing enzyme NAALADase, and the NMDAR-1 receptor at a vertebrate neuromuscular junction. Neuroscience. 1995;64:847–850. doi: 10.1016/0306-4522(95)92578-8. [DOI] [PubMed] [Google Scholar]
- 11.Mays TA, Sanford JL, Hanada T, Chishti AH, Rafael-Fortney JA. Glutamate receptors localize postsynaptically at neuromuscular junctions in mice. Muscle & Nerve. 2009;39:343–349. doi: 10.1002/mus.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walder K, Ryan S, Bzdega T, Olszewski R, Neale J, Lindgren C. Immunohistological and electrophysiological evidence that N-acetylaspartylglutamate is a co-transmitter at the vertebrate neuromuscular junction. The European Journal of Neuroscience. 2013;37:118–129. doi: 10.1111/ejn.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todd Keith J, Slatter Carrie AB, Ali Declan W. Activation of Ionotropic Glutamate Receptors on Peripheral Axons of Primary Motoneurons Mediates Transmitter Release at the Zebrafish NMJ. Journal of Neurophysiology. 2004;91:828–840. doi: 10.1152/jn.00599.2003. [DOI] [PubMed] [Google Scholar]
- 14.Lee YS, Lee SJ, Seo KW, Bae JU, Park SY, Kim CD. Homocysteine induces COX-2 expression in macrophages through ROS generated by NMDA receptor-calcium signaling pathways. Free Radical Research. 2013;47:422–431. doi: 10.3109/10715762.2013.784965. [DOI] [PubMed] [Google Scholar]
- 15.Grozdanovic Z, Gossrau R. Co-localization of nitric oxide synthase I (NOS I) and NMDA receptor subunit 1 (NMDAR-1) at the neuromuscular junction in rat and mouse skeletal muscle. Cell Tissue Res. 1998;291:57–63. doi: 10.1007/s004410050979. [DOI] [PubMed] [Google Scholar]
- 16.Petrov KA, Malomouzh AI, Kovyazina IV, Krejci E, Nikitashina AD, Proskurina SE, et al. Regulation of acetylcholinesterase activity by nitric oxide in rat neuromuscular junction via N-methyl-d-aspartate receptor activation. European Journal of Neuroscience. 2013;37:181–189. doi: 10.1111/ejn.12029. [DOI] [PubMed] [Google Scholar]
- 17.Bradley SA, Lyons PR, Slater CR. The epitrochleoanconeus muscles (ETA) of the mouse: a useful muscle for the study of motor innervation. Journal of Physiology. 1989;415:3P. [Google Scholar]
- 18.Giniatullin AR, Giniatullin RA. Dual Action of Hydrogen Peroxide on Synaptic Transmission at the Frog Neuromuscular Junction. The Journal of Physiology. 2003;552:283–293. doi: 10.1113/jphysiol.2003.050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribera J, Marsal J, Casanovas A, Hukkanen M, Tarabal O, Esquerda JE. Nitric oxide synthase in rat neuromuscular junctions and in nerve terminals of Torpedo electric organ: its role as regulator of acetylcholine release. Journal of neuroscience research. 1998;51:90–102. doi: 10.1002/(SICI)1097-4547(19980101)51:1<90::AID-JNR10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Hebeiss K, Kilbinger H. Nitric oxide-sensitive guanylyl cyclase inhibits acetylcholine release and excitatory motor transmission in the guinea-pig ileum. Neuroscience. 1997;82:623–629. doi: 10.1016/s0306-4522(97)00308-4. [DOI] [PubMed] [Google Scholar]
- 21.Lindgren CA, Laird MV. Nitroprusside inhibits neurotransmitter release at the frog neuromuscular junction. NeuroReport. 1994;5:2205–8. doi: 10.1097/00001756-199410270-00054. [DOI] [PubMed] [Google Scholar]
- 22.Shimohama MU, Shun The role of nitric oxide in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 2001;2:71–81. doi: 10.1080/146608201316949415. [DOI] [PubMed] [Google Scholar]
- 23.Giniatullin AR, Darios F, Shakirzyanova A, Davletov B, Giniatullin R. SNAP25 is a pre-synaptic target for the depressant action of reactive oxygen species on transmitter release. Journal of Neurochemistry. 2006;98:1789–1797. doi: 10.1111/j.1471-4159.2006.03997.x. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Pena y Valenzuela I, Mouslim C, Akaaboune M. Calcium/calmodulin kinase II-dependent acetylcholine receptor cycling at the mammalian neuromuscular junction in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12455–12465. doi: 10.1523/JNEUROSCI.3309-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeates CJ, Zwiefelhofer DJ, Frank CA. The Maintenance of Synaptic Homeostasis at the Drosophila Neuromuscular Junction Is Reversible and Sensitive to High Temperature. eNeuro. 2017;4(6):1–18. doi: 10.1523/ENEURO.0220-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]