Abstract
Introduction
A combination of platelet and lymphocyte to monocyte ratio (LMR) (abbreviated as COP-LMR) has been recently evaluated as systemic inflammatory marker for prognostication in lung cancer. While previous study on COP-LMR has evaluated its prognostic value in NSCLC patients who underwent curative resections, the combination of these two markers has not been evaluated in advanced NSCLC yet.
Objectives
In this study, we evaluated the prognostic value of COP-LMR in stage IV NSCLC with malignant pleural effusion under active anticancer treatment.
Methods
Between January 2012 and July 2016, 217 patients with stage IV NSCLC and MPE undergoing active anticancer treatment were selected for evaluation. If patients had both low LMR (< 2.47) and increased platelet (> 30.0 ×104 mm-3), they were assigned to COP-LMR group 2. Patients with one parameter were assigned to COP-LMR group 1. If none, patients were assigned to COP-LMR group 0.
Results
Median overall survival (OS) (P < 0.001), progression free survival (PFS) (P < 0.001) and histological feature (P = 0.003) showed significant differences among COP-LMR groups. For COP-LMR groups 0, 1 and 2, median survival times were 35.9, 14.7 and 7.4 months, respectively, while median progression free times were 19.2, 13.3 and 7.4 months, respectively. Older age, male, low albumin, high CRP and high COP-LMR (0 vs 1, P = 0.021, hazard ratio (HR): 1.822, 95% confidence interval (CI): 1.096–3.027 and 0 vs 2, P = 0.003, HR: 2.464, 95% CI: 1.373–4.421) were independent predictive factors for shorter OS. Age, sex, histology, albumin, or CRP had no significant influence on PFS. High COP-LMR was the significant factor in predicting shorter PFS (0 vs 1, P = 0.116 and 0 vs 2, P = 0.007, HR: 1.902, 95% CI: 1.194–3.028).
Conclusions
A combination of pretreatment LMR and platelet levels can be used to predict short survival in stage IV NSCLC patients who underwent active anticancer treatment.
Introduction
Lung cancer is a major cause of cancer-related death worldwide [1]. Among lung cancers, non-small cell lung cancer comprises a large proportion [2]. Of all lung cancers, non-small lung cancer (NSCLC) accounts for 85%, with majority of patients initially diagnosed at advanced stage [3]. Despite improvement in treatment modalities, treatment outcomes of patients with advanced lung cancer remain poor, with median survival less than 12 months [3]. Nevertheless, prognosis can vary according to pretreatment patients’ factors. Among patient related factors, host inflammatory response to tumor can contribute significantly to cancer progression by promoting cancer cell proliferation, evasion of immune-surveillance, tumor metastasis and angiogenesis [4, 5], thus playing key roles in survival of patients with various cancers [6].
Reliable pretreatment prognostic factor is vital to treatment of cancer by allowing risk assessment and appropriate choice of treatment modalities. Inflammatory biomarkers reflect host responses to malignant cells. Recent studies have focused on their predictive ability in many cancers [7–9]. More specifically, several notable inflammatory prognostic markers in NSCLC have been evaluated for their prognostic values. Higher neutrophil to lymphocyte ratio (NLR) can predict shorter survival in advanced NSCLC [10, 11]. NLR can also predict postoperative outcome after curative resection [12–14]. Platelet to lymphocyte ratio is also a prognostic marker in advanced NSCLC [15–17]. Moreover, lymphocyte to monocyte ratio (LMR) is an independent prognostic factor in NSCLC [18]. Thrombocytosis also predicts worse outcomes in lung cancer [19]. In addition to single inflammatory biomarkers, combinations of single biomarkers with known predictability have also been evaluated for their prognostic values [12, 20]. A combination of platelet and LMR (abbreviated as COP-LMR) has been recently evaluated as systemic inflammatory marker for prognostication in lung cancer [21]. While previous study on COP-LMR has evaluated its prognostic value in NSCLC patients who underwent curative resections [21], the combination of these two markers has not been evaluated in advanced NSCLC yet. In this study, we evaluated the prognostic value of COP-LMR in stage IV NSCLC with malignant pleural effusion under active anticancer treatment by assessing its predictability for overall survival and disease progression.
Material and methods
Patients’ selection
A total of 217 stage IV NSCLC patients were consecutively selected from a cohort of NSCLC patients with malignant pleural effusion (MPE). These cohort patients were diagnosed with NSCLC between January 2012 and July 2016. These patients were enrolled from Seoul St. Mary’s Hospital, Incheon St. Mary’s Hospital, Yeouido St. Mary’s Hospital, Bucheon St. Mary’s Hospital, St. Paul’s Hospital and Uijeongbu St. Mary’s Hospital. They had MPE confirmed either cytologically or by pleural biopsy simultaneously. Inclusion criteria for the present study were as follows: 1) histologically confirmed NSCLC; 2) underwent active anticancer treatment; and 3) all clinical data were available. Exclusion criteria were: 1) small cell lung cancer (SCLC) patients; 2) patients who underwent curative lung resection; 3) patients who had significant infection at the time of diagnosis; 4) patients with underlying hematologic disease.
Clinical and laboratory data
Clinicopathological data including age, sex, histological feature, tumor stages according to tumor–node–metastasis (TNM) criteria (AJCC criteria 2009), smoking status, first line treatment modality and Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) were evaluated for all enrolled patients. From laboratory data, white blood cell (WBC) count, hemoglobin, platelet, c-reactive protein (CRP), carcinoembryonic antigen (CEA) and lactate dehydrogenase (LDH) were assessed.
Positive driver mutation
The patients with positive EGFR mutation and/or ALK translocation at diagnosis were classified into a positive driver mutation subgroup. EGFR mutations were defined as exon 19 deletion or exon 21 point mutations. Other more uncommon EGFR mutation profiles were excluded. Genotyping of EGFR was done using peptide nucleic acid (PNA)-mediated PCR clamping methods, such as the PNAClamp TM EGFR Mutation Detection Kit (PANAGENE, Inc., Daejeon, Korea), using real-time PCR.
Specimens for fluorescence in situ hybridization (FISH) acquired from the enrolled hospitals were prepared simultaneously using a molecular analysis platform and were analyzed over a 3-day period at Yeouido St. Mary s Hospital Central Molecular Laboratory. ALK rearrangement positivity was defined as an isolated red signal or split signal. A minimum two-probe diameter distance was necessary for determination of true positive signal splitting. Positive cases were defined as those with>15% of counted nuclei within tumor cells exhibiting a split signal or isolated red signal. For surgical resection specimens, 100 tumor cells were scored. An ALK FISH split signal rate less than 15% was interpreted as negative.
Chemotherapy and adverse reactions
Systemic conventional chemotherapy regimens were as follows: docetaxel, gemcitabine, pemetrexed, or paclitaxel combined with carboplatin/cisplatin. For targeted therapy, patients with positive epidermal growth factor receptor (EGFR) mutations were treated with gefitinib, erlotinib and afatinib. Patients with positive anaplastic lymphoma kinase (ALK) translocations were treated with crizotinib.
Chemotherapy related adverse reactions for patients enrolled for this study were checked either at the outpatient clinic or at the time of admissions. If patients experienced grade III or IV adverse reactions, treatment was either delayed or converted to other regimens.
Overall survival and progression free survival
Using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, response to treatment was evaluated by treating physicians and independent radiologists. After completing every two cycles of treatment, computed tomography (CT) was performed. OS was defined as the time duration from the date of diagnosis to the date of death. PFS was defined as the time duration from lung cancer diagnosis to disease progression during 1st line treatment. If patients were dead or lost during the follow up period, they were considered censored.
Categorization by COP-LMR
Blood samples were acquired at the time of diagnosis of NSCLC. LMR was defined as a ratio of peripheral lymphocyte count to peripheral monocyte count. Optimal cutoff values for LMR and platelet counts were calculated based on receiver operating characteristics (ROC) curve analysis. Survival outcomes were dichotomized by survival (alive or death) in ROC analysis. All study patients’ survival status were assessed in October 2017. For LMR, cutoff point was 2.47 with area under curve (AUC) of 0.632. The point on the ROC curve with minimum distance to the upper left corner indicated a cut-off value of 30.05 ×104 mm-3 for platelet counts. The AUC was 0.554. Hence, the optimal cut-off value of platelet counts at 30.0 ×104 mm-3 was chosen. COP-LMR was calculated from platelet count and LMR. If patients had both low LMR (< 2.47) and increased platelet (> 30.0 ×104 mm-3), they were assigned a score of 2 (COP-LMR group 2). Patients with one of these two abovementioned parameters were assigned a score of 1 (COP-LMR group 1). If none of these parameters were found, patients were assigned a sore of 0 (COP-LMR group 0).
Defining cutoff values
For other laboratory values entered into univariate analyses on OS and PFS, cutoff points were also calculated from ROC curve analysis to categorize patients into a high or a low group. Laboratory parameters were selected based on their correlations with clinical outcomes in NSCLC [14, 22–25]. Similar to LMR and platelet count, survival outcomes were dichotomized by survival (alive or death) in ROC analysis. Optimal cutoff values for continuous variables were determined, using maximally selected log-rank statistics for time to primary endpoint.
The cut-off values for protein, albumin, LDH and CRP were 6.7g/dL, 3.1 g/dL, 488 IU/L and 2.68 mg/dL, respectively. Cutoff values of hemoglobin was 12.9 mg/d. Hence, the optimal cut-off value of hemoglobin was decided as 13.0 mg/dL.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences software program version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean or median value with range of values. A receiver operating characteristics (ROC) curve was constructed to estimate optimal cut-off values for continuous variables including LMR. For comparison of categorical variables among three COP-LMR groups, Chi-squared test was performed. One-way analysis of variance (ANOVA) was used to compare continuous variables among three COP-LMR groups after normal distribution status had been confirmed.
Cox regression hazard model univariate analysis was performed to determine the significance of variables for OS and PFS. Survival curve graphs were presented after Kaplan–Meier analysis. OS and PFS are shown as median values. Log-rank test was performed to determine the significance of difference in OS and PFS among the three COP-LMR groups. Statistically significant variables from univariate analyses were entered into Cox proportional hazards regression model for multivariate analysis. For all analyses, a P value of less than 0.05 was considered statistically significant.
Ethics statement
The study was approved by the ethics committee at each center (XC17REDI0069U). The requirement for informed consent was waived due to the retrospective nature of this study.
The names of ethics committees are as follows: Seoul St. Mary’s Hospital, Incheon St. Mary’s Hospital, Yeouido St. Mary’s Hospital, Bucheon St. Mary’s Hospital, St. Paul’s Hospital and Uijeongbu St. Mary’s Hospital.
Results
Patients characteristics
A total of 217 NSCLC patients were enrolled in this study. Their median age was 68 years (range, 35–92 years). Among all patients, 120 (55.3%) were males and 187 (86.2%) patients were diagnosed with adenocarcinoma. For 1st line anticancer treatment, 148 (68.2%) patients underwent conventional systemic chemotherapy and 69 (31.8%) patients underwent targeted therapy. Median OS and PFS were 16.2 months (range, 12.8–19.6 months) and 14.2 months (range, 12.2–16.2 months), respectively.
Table 1 shows distribution of clinicopathological parameters of study patients in three categories grouped according to COP-LMR. As shown in Table 1, median OS (P < 0.001), PFS (P < 0.001) and histological feature (P = 0.003) showed significant differences among COP-LMR groups. For COP-LMR groups 0, 1 and 2, median survival times were 35.9, 14.7 and 7.4 months, respectively, while median progression free times were 19.2, 13.3 and 7.4 months, respectively. The proportion of adenocarcinoma was the highest in COP-LMR group 0 (90.6%) and the lowest in COP-LMR group 2 (71.4%). Regarding laboratory features, white blood cell (WBC) level (P = 0.002), hemoglobin (P < 0.001), platelet (P < 0.001), albumin (P = 0.003) and CRP (P<0.001) showed significant differences among three COP-LMR groups.
Table 1. Correlation of COP-LMR with the clinicopathological and laboratory parameters of NSCLC patients.
| COP-LMR = 0 (N, %) |
COP-LMR = 1 (N, %) |
COP-LMR = 2 (N, %) |
p-value* | |
|---|---|---|---|---|
| Number of patients | 64 | 104 | 49 | |
| Median age, range | 66.4 (37–92) | 68.9 (47–90) | 68.3 (35–87) | 0.552 |
| Median OS (months) | 35.9 | 14.7 | 7.4 | <0.001 |
| Median PFS (months) | 19.2 | 13.3 | 7.4 | <0.001 |
| Sex | 0.094 | |||
| Male | 30 (46.9) | 57 (54.8) | 33 (67.3) | |
| Female | 34 (53.1) | 47 (45.2) | 16 (32.7) | |
| ECOG | 0.717 | |||
| 0 and 1 | 53 (82.8) | 90 (86.5) | 43 (87.8) | |
| ≥2 | 11 (17.2) | 14 (13.5) | 6 (12.2) | |
| Histologic features | 0.003 | |||
| Adenocarcinoma | 58 (90.6) | 94 (90.4) | 35 (71.4) | |
| Squamous | 6 (9.4) | 10 (9.6) | 14 (28.6) | |
| Smoking | 0.139 | |||
| Never smoker | 39 (60.9) | 59 (56.7) | 21 (42.9) | |
| Ever smoker | 25 (39.1) | 45 (43.3) | 28 (57.1) | |
| T-factor | 0.622 | |||
| T1/T2/T3/T4 | 4 (6.2)/ 8 (12.5)/ 6 (9.4)/ 27 (42.2) |
3 (2.9)/ 12 (11.5)/ 14 (13.5)/ 37 (35.6) | 2 (4.1)/ 7 (14.3)/ 6 (12.2)/ 24 (49) |
|
| N-factor | 0.226 | |||
| N0/N1/N2/N3 | 4 (6.2)/ 1 (1.6)/ 15 (23.4)/ 27 (42.2) |
3 (2.9)/ 7 (6.7)/ 17 (16.3)/ 37 (35.6) |
4 (8.2)/ 2 (4.1)/ 9 (18.4)/ 25 (51) |
|
| M-factor | 0.294 | |||
| M1a/M1b | 26 (46.4)/ 30 (53.6) | 45 (52.3)/ 41 (47.7) | 28 (60.9)/ 18 (39.1) | |
| Treatment modality (1st line) | 0.494 | |||
| Conventional chemotherapy | 40 (62.5) | 74 (71.2) | 34 (69.4) | |
| Targeted therapy | 24 (37.5) | 30 (28.8) | 15 (30.6) | |
| Positive driver mutation | 34 (57.6) | 40 (42.6) | 16 (34.8) | 0.051 |
| WBC count (x109/L) | 7411.6±2091.6 | 9012.9±4580.8 | 9823.5±3336.2 | 0.002 |
| Hemoglobin (g/dL) | 13.6±1.6 | 13.4±1.6 | 12.3±1.6 | <0.001 |
| Platelet (per/uL) | 247,690±34,673 | 297,080±66,894 | 370,490±72,106 | <0.001 |
| Albumin (g/dL) | 3.0±0.6 | 2.9±0.7 | 2.6±0.6 | 0.003 |
| CRP (mg/L) | 6.2±11.5 | 15.4±28.5 | 27.5±38.3 | <0.001 |
| CEA (ng/mL) | 61.1±167.4 | 229.2±847.4 | 108.7±244.8 | 0.398 |
| LDH (IU/L) | 500.9±248.0 | 475.7±150.5 | 518.6±367.9 | 0.577 |
CI: confidence interval; COP-LMR: combination of platelet and lymphocyte to monocyte ratio; CEA: carcinoembryonic antigen; CRP: c-reactive protein; ECOG: Eastern Cooperative Oncology Group; HR: hazard ratio; LDH: lactate dehydrogenase; OS: overall survival; PFS: progression free survival; WBC: white blood cell.
*p-value between COP-LMR
Association of COP-LMR with overall survival and progression free survival
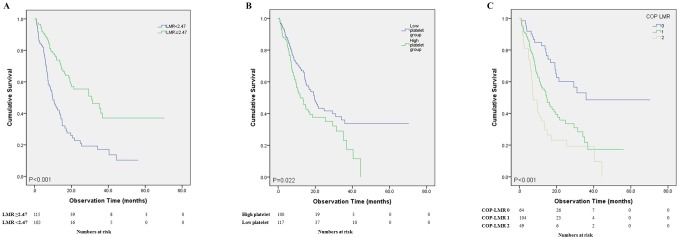
Results of univariate analyses for overall survival and progression free survival are shown in Table 2. COP-LMR was a significant factor in predicting OS (0 vs 1: P <0.001, hazard ratio (HR): 2.257, 95% confidence interval (CI): 1.393–3.658 and 0 vs 2: P<0.001, HR: 3.768, 95% CI: 2.207–6.433) and PFS (0 vs 1: P = 0.010, HR: 1.583, 95% CI: 1.118–2.241 and 0 vs 2: P<0.001, HR: 2.281, 95% CI: 1.499–3.472). Older age, male and squamous features were also significant predictors for shorter OS and PFS. Of laboratory factors, low LMR (Fig 1A), high platelet count (Fig 1B), low hemoglobin, low albumin and high CRP were significant predictors for shorter OS while low LMR, high platelet count, low albumin and high CRP were significant predictors for shorter PFS.
Table 2. Univariate analysis on OS and PFS.
| Characteristics | OS | PFS | ||||
|---|---|---|---|---|---|---|
| P | HR | 95% CI |
P | HR | 95% CI |
|
| Age (≤65/>65) | 0.004 | 1.719 | 1.184–2.497 | 0.02 | 1.420 | 1.056–1.911 |
| Male | 0.001 | 1.931 | 1.327–2.811 | 0.003 | 1.577 | 1.170–2.126 |
| Smoking status (ever/never) | 0.097 | 1.166 | 0.971–1.399 | 0.068 | 1.148 | 0.989–1.332 |
| Histology (adenocarcinoma/squamous cell) | 0.005 | 1.932 | 1.204–3.103 | 0.029 | 1.593 | 1.049–2.420 |
| Driver mutation (positive/negative) | 0.052 | 1.469 | 0.996–2.166 | 0.056 | 1.354 | 0.992–1.850 |
| ECOG (0-1/≥2) | 0.170 | 1.197 | 0.925–1.548 | 0.835 | 1.026 | 0.811–1.297 |
| First line treatment (1st line) (conventional chemotherapy/ targeted therapy) | 0.138 | 1.355 | 0.906–2.026 | 0.442 | 1.132 | 0.825–1.551 |
| M stage (M1a/M1b) | 0.266 | 1.230 | 0.843–1.849 | 0.323 | 1.177 | 0.852–1.626 |
| Hemoglobin, g/dL (<13/≥13) | 0.011 | 1.597 | 1.112–2.294 | 0.237 | 1.198 | 0.888–1.616 |
| Platelet, per uL (<300,000/≥300,000) | 0.023 | 1.523 | 1.059–2.191 | 0.010 | 1.485 | 1.098–2.008 |
| Protein, g/dL (<6.7/≥6.7) | 0.180 | 1.392 | 0.968–2.001 | 0.807 | 1.098 | 0.815–1.478 |
| LMR (<2.47/≥2.47) | <0.001 | 2.487 | 1.717–3.602 | 0.002 | 1.607 | 1.193–2.165 |
| Albumin, g/dL (<3.1/≥3.1) | <0.001 | 2.813 | 1.800–4.395 | 0.002 | 1.955 | 1.276–2.994 |
| LDH, IU/L (<488/≥488) | 0.316 | 1.332 | 0.918–1.933 | 0.587 | 1.089 | 0.801–1.481 |
| CRP, mg/dL (<2.68/≥2.68) | 0.001 | 2.204 | 1.498–3.243 | 0.005 | 1.553 | 1.145–2.106 |
| COP-LMR | <0.001 | <0.001 | ||||
| COP-LMR 0 | - | 1 | Referent | - | 1 | Referent |
| COP-LMR 1 | 0.001 | 2.257 | 1.393–3.658 | 0.010 | 1.583 | 1.118–2.241 |
| COP-LMR 2 | <0.001 | 3.768 | 2.207–6.433 | <0.001 | 2.281 | 1.499–3.472 |
CI: confidence interval; COP-LMR: combination of platelet and lymphocyte to monocyte ratio; CRP: c-reactive protein; ECOG: Eastern Cooperative Oncology Group; HR: hazard ratio; LDH: lactate dehydrogenase; LMR: lymphocyte to monocyte ratio; OS: overall survival; PFS: progression free survival.
Fig 1. Overall survival of the stage IV NSCLC patients.
(A): OS between high and low LMR patients; (B): OS between high and low platelet groups; (C): Overall survival of the stage IV NSCLC patients between the different COP LMR groups. OS showed significant difference between COP LMR groups 0 vs. 1 (P < 0.001) and 1 vs. 2 (P = 0.023). COP LMR: combination of platelet and lymphocyte to monocyte ratio; NSCLC: non-small cell lung cancer; OS: overall survival.
Significant variables from univariate analysis were assessed by multivariate analysis. Histology and hemoglobin had no significant influence on OS. Older age, male, low albumin, high CRP and high COP-LMR (0 vs 1, P = 0.021, HR: 1.822, 95% CI: 1.096–3.027 and 0 vs 2, P = 0.003, HR: 2.464, 95% CI: 1.373–4.421) were independent predictive factors for shorter OS. Age, sex, histology, albumin, or CRP had no significant influence on PFS. High COP- LMR was the significant factor in predicting shorter PFS (0 vs 1, P = 0.116, and 0 vs 2, P = 0.007, HR: 1.902, 95% CI: 1.194–3.028). Results are shown in Table 3.
Table 3. Multivariate analysis on OS and PFS.
| Characteristics | OS | PFS | ||||
|---|---|---|---|---|---|---|
| P | HR | 95% CI |
P | HR | 95% CI |
|
| Age (≤65/>65) | 0.049 | 1.497 | 1.002–2.235 | 0.120 | 1.289 | 0.922–1.803 |
| Male | 0.027 | 1.620 | 1.057–2.481 | 0.059 | 1.385 | 0.988–1.941 |
| Histology (adenocarcinoma/squamous cell) | 0.766 | 1.092 | 0.610–1.955 | 0.693 | 1.108 | 0.665–1.847 |
| Hemoglobin, g/dL (<13/≥13) | 0.317 | 1.245 | 0.810–1.914 | |||
| Albumin, g/dL (<3.1/≥3.1) | 0.035 | 1.724 | 1.039–2.861 | 0.091 | 1.478 | 0.939–2.326 |
| CRP, mg/dL (<2.68/≥2.68) | 0.011 | 1.713 | 1.130–2.599 | 0.138 | 1.289 | 0.922–1.803 |
| COP-LMR (0,1,2) | 0.009 | 0.025 | ||||
| COP-LMR 0 | - | 1 | Referent | - | 1 | Referent |
| COP-LMR 1 | 0.021 | 1.822 | 1.096–3.027 | 0.116 | 1.347 | 0.929–1.951 |
| COP-LMR 2 | 0.003 | 2.464 | 1.373–4.421 | 0.007 | 1.902 | 1.194–3.028 |
CI: confidence interval; COP-LMR: combination of platelet and lymphocyte to monocyte ratio; CRP: c-reactive protein; HR: hazard ratio; OS: overall survival; PFS: progression free survival.
Fig 1C shows graphs from Kaplan-Meier analysis assessing predictive value of COP-LMR for OS. There were significant (P < 0.001) differences in OS among the three COP-LMR groups. Statistically significant (P < 0.001) differences in PFS among the three COP-LMR were also found (Fig 2). Between COP LMR groups 0 vs. 1 and 1 vs. 2, OS showed significant differences (P < 0.001 and P = 0.023, respectively). OS and PFS decreased as COP-LMR group changed from 0 to 2.
Fig 2. Progression free survival of the stage IV NSCLC patients.
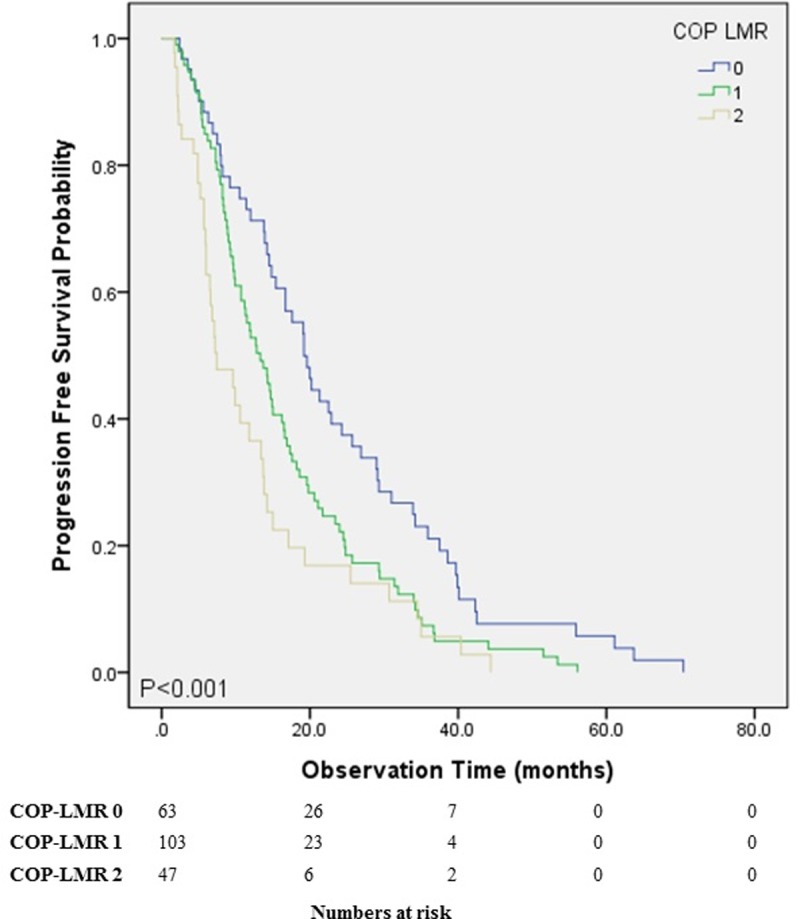
PFS between the different COP LMR groups (P<0.001). COP-LMR: combination of platelet and lymphocyte to monocyte ratio; PFS: progression free survival.
NSCLC patients without driver mutation
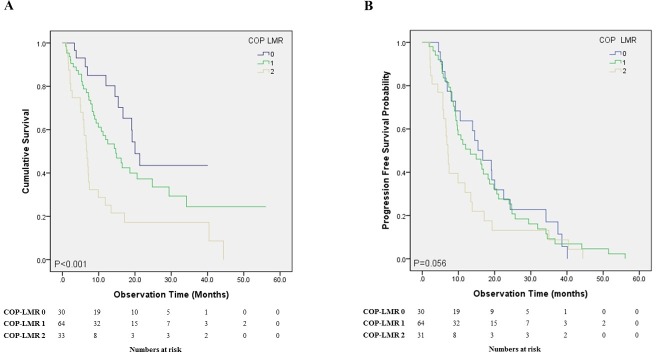
Among 127 NSCLC patients without positive driver mutations, Kaplan-Meier analysis showed significant (P < 0.001) difference in OS among three COP-LMR groups (Fig 3A), but not in PFS (P = 0.056) (Fig 3B). Median survival times for COP-LMR groups 0, 1 and 2 were 20.0, 14.7 and 6.8 months, respectively. Between COP-LMR groups 1 and 2, OS showed significant difference (P = 0.001). However, OS was not significantly different between COP-LMR groups 0 and 1 (P = 0.071).
Fig 3. Overall survival and progression free survival of the stage IV NSCLC patients without positive driver mutations between the different COP LMR groups.
A: OS showed significant difference (P = 0.001) between COP-LMR groups 1 and 2. OS showed no significant difference between COP-LMR groups 0 and 1 (P = 0.071); B: PFS showed decreasing tendency as the COP-LMR score is higher, however with no statistical significance (P = 0.056). COP LMR: combination of platelet and lymphocyte to monocyte ratio; NSCLC: non-small cell lung cancer; OS: overall survival; PFS: progression free survival.
Discussion
Our results showed that COP-LMR was an independent predictor for shorter overall survival and progression free survival in stage IV NSCLC. Liu et al. have shown that COP-LMR has prognostic value in patients with NSCLC who underwent curative resection [21]. On the other hand, our study population were comprised of stage IV NSCLC patients with MPE. Our results showed that a combination of pretreatment LMR and platelet levels also had prognostic value in more advanced NSCLC.
Thrombocytosis is associated with worse outcomes in NSCLC [19, 26, 27]. Past studies have shown that platelets have significant roles in tumor activity. Platelet is a source of various proangiogenic and anti-angiogenic proteins [28]. It secretes vascular epidermal growth factor (VEGF), transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF) that contribute to cancer metastasis, tumor cell invasion, migration and arrest within blood vessels [29–31].
PDGF promotes epithelial to mesenchymal transition by activating Smad and NF-kB pathways, which in turn promote tumor metastasis [32]. On the other hand, tumor cells induce differentiation of megakaryocytes to platelets and proliferation of platelets [33]. Furthermore, tumor cells induce aggregation of platelets to evade immune surveillance [34]. Hence, platelet counts are associated with cancer progression. The cutoff value of platelet counts has not been clearly defined yet, although platelet count of 30.0 x 104 mm-3 is often used in previous publications on prognostic value of platelet in cancer [21, 35]. Consistent with these previous publications, the cutoff value of platelet count in our study was also 30.0 x 104 mm-3.
It has been suggested that LMR has prognostic value in advanced lung cancer patients who received platinum-based chemotherapies [36] and in advanced-stage EGFR-mutant NSCLC receiving EGFR-TKIs [37]. Furthermore, LMR is an independent prognostic factor in NSCLC who underwent complete resection [18]. In lung cancer, cytotoxic T lymphocytes play a crucial role in anticancer activity [38]. Macrophages are involved in tumor growth through immunosuppression tumor angiogenesis [39] and metastasis [40]. Recruitment of macrophages to tumor site is associated with poor prognosis in various cancers [41]. While the cutoff value of LMR separating high and low groups has not been set, the cutoff value of LMR can influence its predictability. In the present study, cutoff of LMR was 2.47. LMR cutoff values used in previous studies varied from 3.29 to 4.56 [18, 21, 37], higher than the cutoff in the present study. The relatively low cutoff of LMR in our study could be due to the exclusion of patients who had concurrent infections requiring antibiotics therapy.
Both platelet and LMR play a key role in cancer progression. Derived from these two factors, COP-LMR can increase the predictive value in lung cancer patients by combining unfavorable effects and lessening the impact of bias which could happen if only one factor is used.
In the present study, multiple clinical variables were compared among three COP-LMR groups (0, 1 and 2). OS and PFS gradually decreased as COP-LMR changed from group 0 to group 2. Furthermore, OS was significantly shorter in the higher score group when two contiguous groups were compared (0 vs. 1 and 1 vs. 2). There were significant differences in WBC count, hemoglobin, platelet, albumin and CRP levels among these three groups. WBC count, hemoglobin, platelet and CRP levels are associated with poor prognosis in lung cancer [24, 25], while low hemoglobin and albumin reflect poor nutritional status associated with cachexia in cancer patients [12]. It is rational to assert that COP-LMR can categorize NSCLC patients into independent groups in terms of prognosis.
We also noted that the increase in squamous cancer portion and the increase in COP-LMR show positive relation. From the previous study on COP-LMR, the same clinical correlation is also shown [21]. We assume that squamous lung cancer could have accompanied more active systemic inflammation compared to non-squamous NSCLC. However further study with larger study population is necessary to confirm the assumption.
In comparison with a previous study [21], we tried to minimize nonmalignant factors that could influence levels of platelet and LMR. We excluded patients who had infections requiring antibiotics treatment such as bacterial pneumonia and pulmonary tuberculosis at the time of diagnosis of lung cancer. Patients with underlying hematologic diseases were also excluded.
This study has a few limitations. First, due to the retrospective nature of this study, selection bias or unidentified factors might have influenced outcomes. However, we consecutively collected data from six centers in order to minimize selection bias. Second, prevalence of driver mutations among study patients, mostly EGFR mutation, was high (41.5%) compared to that in other advanced NSCLC populations [42]. However, this proportion is not unusual in Asian NSCLC populations [43, 44]. Furthermore, Kaplan Meier curve analysis of NSCLC patients without driver mutations showed significant difference in OS among the three COP-LMR groups (Fig 3A).
Conclusions
A combination of pretreatment LMR and platelet levels can be used to predict short survival in stage IV NSCLC patients who underwent active anticancer treatment. Further research is needed to elucidate the underlying pathophysiological background for predictability of combined inflammatory biomarkers.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605–44. 10.1016/j.ccm.2011.09.001 ; PubMed Central PMCID: PMCPMC3864624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–80. 10.1056/NEJMra0802714 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moro-Sibilot D, Smit E, de Castro Carpeno J, Lesniewski-Kmak K, Aerts J, Villatoro R, et al. Outcomes and resource use of non-small cell lung cancer (NSCLC) patients treated with first-line platinum-based chemotherapy across Europe: FRAME prospective observational study. Lung Cancer. 2015;88(2):215–22. 10.1016/j.lungcan.2015.02.011 . [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. 10.1038/nature07205 . [DOI] [PubMed] [Google Scholar]
- 5.O'Callaghan DS, O'Donnell D, O'Connell F, O'Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5(12):2024–36. . [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 . [DOI] [PubMed] [Google Scholar]
- 7.Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197(4):466–72. 10.1016/j.amjsurg.2007.12.057 . [DOI] [PubMed] [Google Scholar]
- 8.Ishizuka M, Oyama Y, Abe A, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol. 2014;110(8):935–41. 10.1002/jso.23753 . [DOI] [PubMed] [Google Scholar]
- 9.Absenger G, Szkandera J, Stotz M, Postlmayr U, Pichler M, Ress AL, et al. Preoperative neutrophil-to-lymphocyte ratio predicts clinical outcome in patients with stage II and III colon cancer. Anticancer Res. 2013;33(10):4591–4. . [PubMed] [Google Scholar]
- 10.Cedres S, Torrejon D, Martinez A, Martinez P, Navarro A, Zamora E, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14(11):864–9. 10.1007/s12094-012-0872-5 . [DOI] [PubMed] [Google Scholar]
- 11.Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62(3):471–9. 10.1007/s00262-012-1347-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu J, et al. Prognostic Significance of Combination of Preoperative Platelet Count and Neutrophil-Lymphocyte Ratio (COP-NLR) in Patients with Non-Small Cell Lung Cancer: Based on a Large Cohort Study. PLoS One. 2015;10(5):e0126496 10.1371/journal.pone.0126496 ; PubMed Central PMCID: PMCPMC4423976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31(9):2995–8. . [PubMed] [Google Scholar]
- 14.Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res. 2012;32(8):3535–8. . [PubMed] [Google Scholar]
- 15.Wang YQ, Zhi QJ, Wang XY, Yue DS, Li K, Jiang RC. Prognostic value of combined platelet, fibrinogen, neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with lung adenosquamous cancer. Oncol Lett. 2017;14(4):4331–8. 10.3892/ol.2017.6660 ; PubMed Central PMCID: PMCPMC5594242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang HB, Xing M, Ma LN, Feng LX, Yu Z. Prognostic significance of neutrophil-lymphocyteratio/platelet-lymphocyteratioin lung cancers: a meta-analysis. Oncotarget. 2016;7(47):76769–78. 10.18632/oncotarget.12526 ; PubMed Central PMCID: PMCPMC5363548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toda M, Tsukioka T, Izumi N, Komatsu H, Okada S, Hara K, et al. Platelet-to-lymphocyte ratio predicts the prognosis of patients with non-small cell lung cancer treated with surgery and postoperative adjuvant chemotherapy. Thorac Cancer. 2017. 10.1111/1759-7714.12547 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9(9):e108062 10.1371/journal.pone.0108062 ; PubMed Central PMCID: PMCPMC4183469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holgersson G, Bergstrom S, Hallqvist A, Liv P, Nilsson J, Willen L, et al. The prognostic value of pre-treatment thrombocytosis in two cohorts of patients with non-small cell lung cancer treated with curatively intended chemoradiotherapy. Neoplasma. 2017;64(6):909–15. 10.4149/neo_2017_614 . [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109(2):401–7. 10.1038/bjc.2013.350 ; PubMed Central PMCID: PMCPMC3721384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Ha M, Yin N. Combination of platelet count and lymphocyte to monocyte ratio is a prognostic factor in patients undergoing surgery for non-small cell lung cancer. Oncotarget. 2017;8(42):73198–207. 10.18632/oncotarget.18336 ; PubMed Central PMCID: PMCPMC5641206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasapoglu US, Arinc S, Gungor S, Irmak I, Guney P, Aksoy F, et al. Prognostic factors affecting survival in non-small cell lung carcinoma patients with malignant pleural effusions. Clin Respir J. 2016;10(6):791–9. 10.1111/crj.12292 . [DOI] [PubMed] [Google Scholar]
- 23.Liu HB, Gu XL, Ma XQ, Lv TF, Wu Y, Xiao YY, et al. Preoperative platelet count in predicting lymph node metastasis and prognosis in patients with non-small cell lung cancer. Neoplasma. 2013;60(2):203–8. . [DOI] [PubMed] [Google Scholar]
- 24.Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer Res. 2009;29(7):2687–90. . [PubMed] [Google Scholar]
- 25.Jing X, Huang C, Zhou H, Li C, Fan L, Chen J, et al. Association between serum C-reactive protein value and prognosis of patients with non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med. 2015;8(7):10633–9. ; PubMed Central PMCID: PMCPMC4565237. [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Lee HW, Go SI, Lee SI, Lee GW. Clinical significance of the preoperative platelet count and platelet-to-lymphocyte ratio (PLT-PLR) in patients with surgically resected non-small cell lung cancer. Oncotarget. 2016;7(24):36198–206. 10.18632/oncotarget.8809 ; PubMed Central PMCID: PMCPMC5094993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KH, Park TY, Lee JY, Lee SM, Yim JJ, Yoo CG, et al. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci. 2014;29(4):507–11. 10.3346/jkms.2014.29.4.507 ; PubMed Central PMCID: PMCPMC3991793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–49. 10.1111/j.1538-7836.2010.04131.x . [DOI] [PubMed] [Google Scholar]
- 29.Sarach MA, Rovasio RA, Eynard AR. Platelet factors induce chemotactic migration of murine mammary adenocarcinoma cells with different metastatic capabilities. Int J Exp Pathol. 1993;74(5):511–7. ; PubMed Central PMCID: PMCPMC2002173. [PMC free article] [PubMed] [Google Scholar]
- 30.Belloc C, Lu H, Soria C, Fridman R, Legrand Y, Menashi S. The effect of platelets on invasiveness and protease production of human mammary tumor cells. Int J Cancer. 1995;60(3):413–7. . [DOI] [PubMed] [Google Scholar]
- 31.Felding-Habermann B, O'Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98(4):1853–8. 10.1073/pnas.98.4.1853 ; PubMed Central PMCID: PMCPMC29346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90. 10.1016/j.ccr.2011.09.009 ; PubMed Central PMCID: PMCPMC3487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229(8):1005–15. 10.1002/jcp.24539 . [DOI] [PubMed] [Google Scholar]
- 34.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet—cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143(7):819–26. 10.1038/sj.bjp.0706013 ; PubMed Central PMCID: PMCPMC1575943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012;106(7):887–91. 10.1002/jso.23163 . [DOI] [PubMed] [Google Scholar]
- 36.Lin GN, Peng JW, Xiao JJ, Liu DY, Xia ZJ. Prognostic impact of circulating monocytes and lymphocyte-to-monocyte ratio on previously untreated metastatic non-small cell lung cancer patients receiving platinum-based doublet. Med Oncol. 2014;31(7):70 10.1007/s12032-014-0070-0 . [DOI] [PubMed] [Google Scholar]
- 37.Chen YM, Lai CH, Chang HC, Chao TY, Tseng CC, Fang WF, et al. Baseline and Trend of Lymphocyte-to-Monocyte Ratio as Prognostic Factors in Epidermal Growth Factor Receptor Mutant Non-Small Cell Lung Cancer Patients Treated with First-Line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. PLoS One. 2015;10(8):e0136252 10.1371/journal.pone.0136252 ; PubMed Central PMCID: PMCPMC4552380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013;73(8):2381–8. 10.1158/0008-5472.CAN-12-3932 . [DOI] [PubMed] [Google Scholar]
- 39.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796(1):11–8. 10.1016/j.bbcan.2009.02.004 . [DOI] [PubMed] [Google Scholar]
- 40.Guruvayoorappan C. Tumor versus tumor-associated macrophages: how hot is the link? Integr Cancer Ther. 2008;7(2):90–5. 10.1177/1534735408319060 . [DOI] [PubMed] [Google Scholar]
- 41.Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123(2):97–102. 10.1016/j.imlet.2009.02.011 . [DOI] [PubMed] [Google Scholar]
- 42.D'Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29(15):2066–70. 10.1200/JCO.2010.32.6181 ; PubMed Central PMCID: PMCPMC3296671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–62. 10.1097/JTO.0000000000000033 ; PubMed Central PMCID: PMCPMC4132036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L, Zhou CC, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2(5):430–9. 10.1097/01.JTO.0000268677.87496.4c . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.