Abstract
Parkinson’s disease (PD), an age-related neurodegenerative disorder that results from a progressive loss of dopaminergic neurons has an enormous economical and human cost. Unfortunately, only symptomatic treatment such as dopamine replacement therapy is available. Therefore, drugs with new mechanisms of action able to protect against neuronal cell death are an urgent need. We here report the in vivo efficacy on dopaminergic neuronal protection in a PD mouse model and the lack of toxicity in zebrafish and Ames test of benzothiazole-based casein kinase-1δ (CK-1δ) nanomolar inhibitors. On the basis of these results, we propose protein kinase CK-1δ inhibitors as the possible disease-modifying drugs for PD, benzothiazole 4 being a promising drug candidate for further development as a new therapy of this neurodegenerative disease.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease. It is characterized by diverse motor symptoms (tremor, bradykinesia or slowness of movement, and rigidity or stiffness) and cognitive decline (hallucinations and dementia). These symptoms appear as direct consequence of a dopamine deficit in the nigrostriatal brain region due to the loss of dopamine-producing neurons in the substantia nigra pars compacta (SNpc). Intracellular α-synuclein inclusions called Lewy bodies and dystrophic neurites are other prominent neuropathological hallmarks.1,2 The incidence of PD in the general population increases with age, and around 1–2% of those over 65 years of age suffer from this disorder, with more than 3 million patients currently diagnosed.3 As the global life expectancy grows, a twofold increase in PD is expected by 2030.4 The discovery in the 1960s that the selective loss of dopaminergic neurons was the main cause of PD directed the pharmacological therapies toward neurotransmitters replacement drugs, such as the dopamine precursor levodopa, which is currently the standard clinical treatment. However, levodopa treatment is only effective during a limited period. Eventually, other motor symptoms, including dyskinesias, are experienced by PD patients as the disease progresses and the number of the remaining dopaminergic neurons decrease.5 Currently, there is no cure for PD and novel effective drug treatments for this devastating disease are urgently needed. Mainly drugs that control the motor and nonmotor symptoms of the pathology, as well as enable the protection of the dopaminergic neurons from progressive death, are highly desirable. Although the important role of casein kinase-1 (CK-1) in different neurodegenerative diseases6 and the association of this protein kinase in the phosphorylation of α-synuclein7 has been previously described,7 in this work, we report for the first time the discovery of δ isoform of CK-1 (CK-1δ) as a potential neuroprotective target for the treatment of PD and the value of benzothiazole-based CK-1δ inhibitors as the new drug candidates for a future disease-modifying treatment of this pathology, as they show dopaminergic neuroprotection in vivo.
Results and Discussion
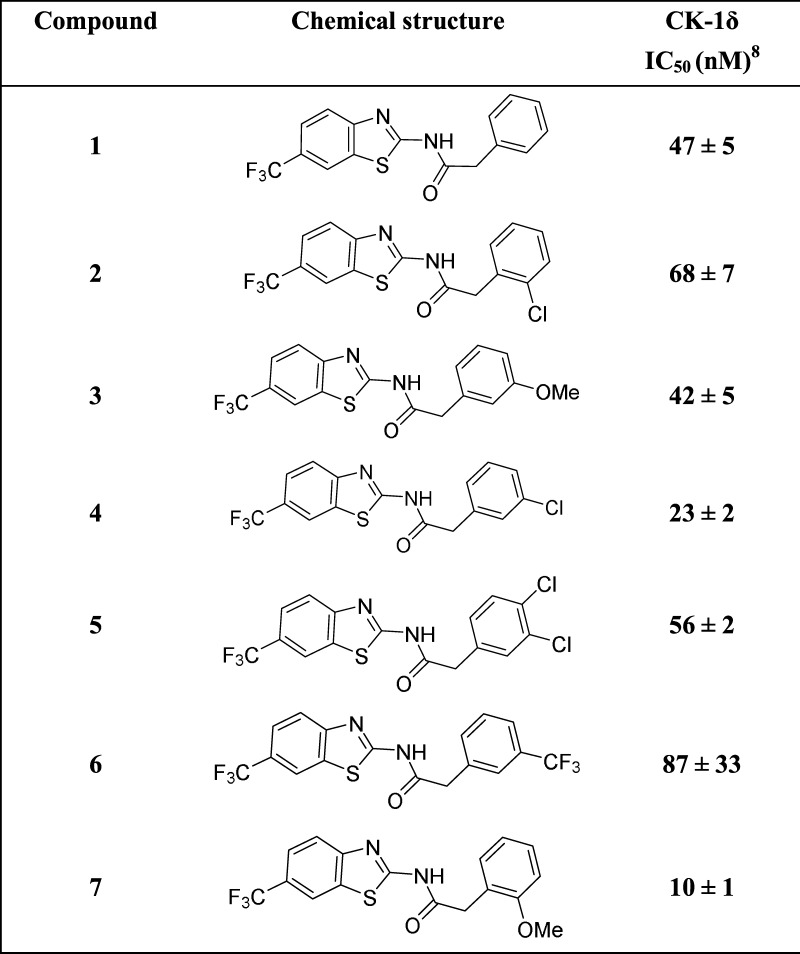
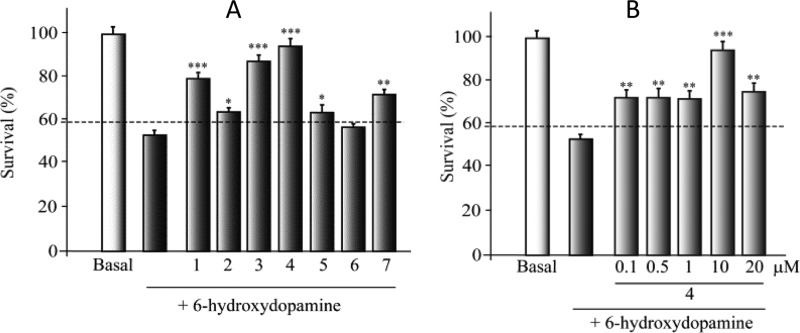
First, to check if SH-SY5Y cell line expressed CK-1δ, we performed the Western blot and immunocytochemistry analyses using a specific anti-CK-1δ antibody (Figure 1S). Results showed that CK-1δ protein is not only present in this cell line, but more interestingly, CK-1δ levels are increased after the treatment with 6-hydroxydopamine (6-OHDA). These results suggest the involvement of CK-1δ in PD. For this reason we selected some of the N-(benzothiazolyl)-2-phenylacetamides derivatives (1–7) previously described by our group as the nanomolar CK-1δ inhibitors8 (Table 1). To further characterize the pharmacological activity of these selective inhibitors, we evaluated them in different cellular models. The biological relevance of CK-1δ as novel neuroprotective drug candidates for PD was assessed using the SH-SY5Y dopaminergic cell line using the neurotoxin (6-OHDA) to induce the cell death. Thus, before 6-OHDA exposure, cells were pretreated with different doses (0.1–20 μM) of the CK-1δ inhibitors (1–7) for 1 h (Figure 2S). Cell viability was analyzed 24 h later. Although treatment of this cell line with 6-OHDA induced a substantial cell death (more than 40%, dotted line in the figure), the addition of these compounds triggered a neuroprotection against 6-OHDA in a dose-dependent manner, with the best effects at 10 μM (Figure 1). It is worth mentioning that the cell activity of a new compound is not only based on its evidence in vitro target activity or potency but also on the different drug-like properties such as solubility or absorption that finally determine the rate of compound penetration inside the cell such as compound 6, for which no neuroprotection was observed at different doses (Figure 2S). Thus, although IC50 values against CK-1δ of the tested compounds are in the same range, the cell neuroprotection values show some differences among the evaluated derivatives, with compound 4 being the most potent. Altogether, the present data confirm that N-(benzothiazolyl)-2-phenylacetamides protect dopaminergic neuronal cell death from 6-OHDA toxicity, where CK-1δ may be the pharmacological target involved.
Table 1. Some N-(Benzothiazolyl)-2-phenylacetamides (1–7) Previously Described8 and Their Potency as Inhibitors of CK-1δ Inhibitors.
Figure 1.
Effect of protein kinase CK-1δ inhibitors on 6-hydroxydopamine (6-OHDA)-induced SH-SY5Y cell death. SH-SY5Y cells were exposed for 24 h to 6-OHDA (35 μM) in the presence or absence of the CK-1δ inhibitors (10 μM) (A) and compound 4 (0.1–20 μM) (B). The number of viable cells was measured by (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Each data point represents the mean ± SD of six replications in three different experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 statistically significant differences between CK-1δ inhibitors and 6-OHDA-treated cultures.
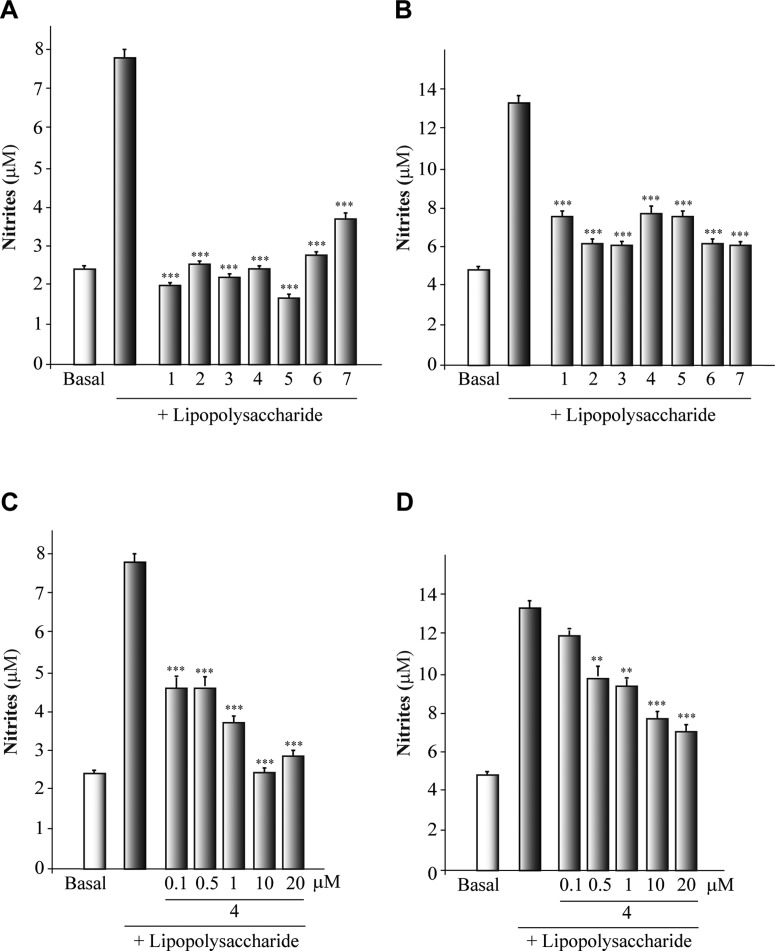
Compounds (1–7) were then evaluated as anti-inflammatory agents, as it is well known that neuroinflammation underlies many neurodegenerative diseases, including PD.9 To produce the inflammatory activation in astrocytes and microglia (primary cultures), lipopolysaccharide (LPS) was employed. The inflammatory reaction was measured by nitrite production using the well-established Griess methodology. Cultures were incubated with different concentrations (0.1–20 μM) of the different CK-1δ inhibitors for 1 h and then cells were cultured for another 24 h in the presence of LPS (Figure 3S). A significant induction of nitrite production in the culture medium of astrocytes and microglial cells (4- and 2.5-fold, respectively) was observed when primary cells were stimulated with LPS. This effect was significantly diminished by CK-1δ inhibitors in a dose-dependent manner. The 10 μM dose is the most effective form of treatment (Figure 2). Given the in vitro results showing a neuroprotective and anti-inflammatory action of CK-1δ inhibitors, we next assessed the efficacy of one of the most potent, selective, and neuroprotective CK-1δ inhibitor in the 6-OHDA model, derivative 4, in a rodent model of PD. To this end, we analyzed whether compound 4 administered in vivo affected the dopaminergic cell loss in the SNpc as well as glial activation. Adult rats were injected unilaterally with LPS into the SNpc. This injection into the SNpc of the rodents produces the activation of microglial cells with a release of neurotoxic factors leading to dopaminergic cell death.10
Figure 2.
Anti-inflammatory effect of protein kinase CK-1δ inhibitors on the glial primary cultures. Astrocytes (A, C) and microglial cells (B, D) cultures were isolated, plated, and later treated with lipopolysaccharide (LPS, 10 μg/mL) in the presence of the different CK-1δ inhibitors (10 μM) and compound 4 (0.1–20 μM). The production of nitrite was measured by the Griess reaction. Each data point represents the mean ± SD of six replications in three different experiments. ***p < 0.001, statistically significant differences between CK-1δ inhibitors and LPS-treated cultures.
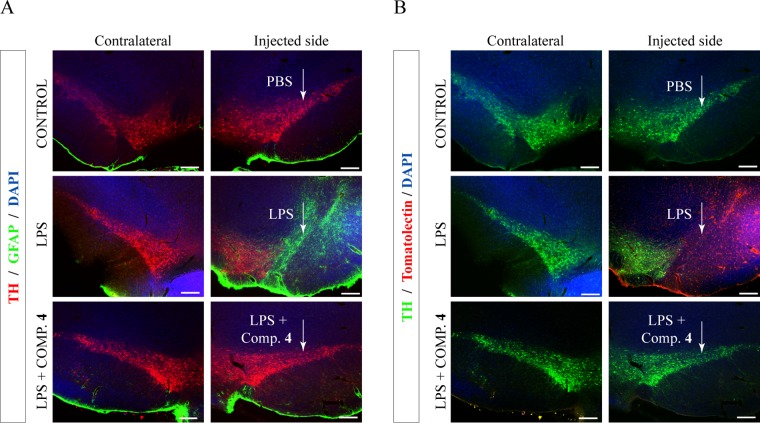
The results obtained from the fluorescence immunohistochemical analysis of LPS-lesioned animal brains indicated a substantial neuronal death in the SNpc of these animals (Figure 3). The administration of compound 4 directly into the brain together with LPS resulted in a significant protection of tyrosine hydroxylase (TH)-positive cells against the LPS-induced damage, compared with the abundant dopaminergic neuronal loss observed in the lesioned animals.
Figure 3.
In vivo neuroprotective and anti-inflammatory effect of derivative 4. Lipopolysaccharide (LPS, 10 μg) was injected unilaterally into the adult substantia nigra pars compacta (SNpc) of adult rats together with the CK-1δ inhibitor 4 (15 nmol). Control animals were injected with phosphate-buffered saline (PBS). After 72 h, the brains were removed and sections processed for immunodetection of tyrosine hydroxylase (TH) and inflammatory markers. (A) Double immunostaining showing the expression of an astrogial marker (glial fibrillary acidic protein (GFAP), green) together with tyrosine hydroxylase (TH, red) in SNpc (injected and contralateral hemispheres, as control, are shown). When LPS is administrated, a decrease in the number of dopaminergic neurons and an increase in astrocytosis are observed. These facts are abolished when the CK-1 inhibitor (compound 4) is administrated. (B) Immunostaining showing the expression of tomato lectin (red) as a marker of activated microglia in SNpc (injected and contralateral hemispheres). Dopaminergic neurons are shown in green (TH immunoreactivity). Compound 4 avoids the loss of dopaminergic neurons produced by LPS and the microglia activation. Scale bar, 200 μm.
N-(Benzothiazolyl)-2-phenylacetamide 4 is an important lead compound that targets CK-1δ with an IC50 value of 23 nM and shows neuroprotective and anti-inflammatory properties both in cell cultures and in animal models. To confirm this lead compound as a drug candidate for further in vivo studies after systemic administration, we look for several drug-like properties of this CK-1δ inhibitor. To become a drug for the treatment of a neurological disorder, it is absolutely necessary that the compound crosses the blood–brain barrier, being safe and nonmutagenic. Moreover, the compound should be orally bioavailable to be further developed as an oral medication. Previous in vivo pharmacokinetics results showed that this compound was able to penetrate into the brain by oral absorption and intraperitoneal injection.11 To assess the safety of the CK-1δ inhibitor 4, two experiments were done. First, and taking into account that more than 70% of human genes have their corresponding genes in the zebrafish (Danio rerio),12 we employed them as a useful pharmacological tool for biosafety studies. In fact, it has been used extensively for testing toxicity in the whole organism.13 Different readouts are considered, the zebrafish tail being one of them. The tail is crooked when the tested compound has toxic properties for the zebrafish embryo development. Thus, to assess the safety of the CK-1δ inhibitor 4, which has shown good results in the protection of dopaminergic neurons in mice brains, we tested this compound at different concentrations (0.5, 1, 2.5, 5, 7.5, 10, and 20 μM) in the zebrafish embryos. We performed the assay by triplicate and used 10 embryos per compound concentration. The results are depicted in Figure 4S, showing a safe therapeutic window for compound 4 in a range of concentration between 0.5 and 10 μM.
Additionally, we evaluated the in vitro mutagenic and genotoxic potential using the well-known Ames test.14 A positive result indicates that the chemical is mutagenic and may be carcinogen, ruling out any possibilities for further development. Compound 4 was examined for its ability to produce and/or revert mutations in the bacterial assay conducted without the metabolic activation using two Salmonella typhimurium strains, such as TA100 and TA98. We used two positive controls, sodium azide and 2-nitrofluorene (2-NF), which are suspected to be carcinogenic agents (Table 2).
Table 2. Mutagenic Activity of CK-1δ Inhibitor 4 Using S. typhimurium Strains, without S9 Activation, Scored at Day 5.
| TA100
strain |
TA98
strain |
||||
|---|---|---|---|---|---|
| compound | number positive wells/total number of wells | resultsa | compound | number positive wells/total number of wells | resultsa |
| background (TA100) | 3/96 | background (TA98) | 21/96 | ||
| background (TA100) | 7/96 | background (TA98) | 26/96 | ||
| blank | 0/96 | blank | 0/96 | ||
| control (NaN3) | 74/96 | + | control (2-NF) | 95/96 | + |
| 4 (20 μM) | 1/96 | – | |||
| 4 (1 μM) | 8/96 | – | 4 (10 μM) | 9/96 | – |
| 4 (0.5 μM) | 5/96 | – | |||
+, Significant increase in the number of positive wells compared with the related control; −, no significant effect observed.
All of the tested concentrations of derivative 4 (20–0.5 μM) were found to be not mutagenic in the absence of S9 metabolic activation, as the numbers of positive wells in the treated plates were not statistically different from those obtained in the background plates for both S. typhimurium strains TA98 and TA100.
Consequently, the results obtained under the experimental conditions used in the present study provide the evidence that compound 4 is not mutagenic, giving the green light for further pharmaceutical development.
Conclusions
We have discovered that targeting the protein kinase CK-1δ may be a new mechanism of action to modulate the death of dopaminergic neurons. Thus, benzothiazole-based CK-1δ inhibitors may be promising compounds for the PD treatment with very potent neuroprotective and anti-inflammatory activity both in in vitro and in vivo models of PD. Moreover, we have shown for the first time that CK-1δ is involved in the activation of inflammatory cell response to LPS, its inhibitors being good modulators of this pathological process. The selection of benzothiazole 4 as the drug candidate was done based on the in vivo data of efficacy and safety properties. Thus, we can summarize that CK-1δ inhibitor 4 is a small molecule with an IC50 of 23 nM and is able to protect the death of dopaminergic in vivo neurons induced by 6-OHDA and to decrease the inflammatory activation by LPS in the primary cell cultures of astrocytes and microglia. Moreover, this compound is not toxic in a zebrafish model, having a negative Ames test. Further studies are in progress to show the efficacy of this new compound in the well-established models of PD (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) after chronic oral administration. Altogether, the present data led us to propose N-(benzothiazolyl)-2-phenylacetamide inhibitor 4, a selective CK-1δ inhibitor, as a lead compound for further pharmacological development in the search of future disease-modifying drugs for Parkinson’s disease.
Experimental Section
Protein Kinase CK-1δ Expression in Human Dopaminergic SH-SY5Y Cell Line
Neuronal Cell Culture
The human dopaminergic SH-SY5Y cell line was obtained from Sigma-Aldrich and propagated in F12 medium/Eagle’s Minimum Essential Medium (EMEM) containing glutamine (2 mM), 1% of nonessential amino acids, and 15% of fetal bovine serum under humidified 5% CO2 and 95% air. The human recombinant casein kinase-1δ was purchased from Millipore (Millipore Iberica S.A.U.).
Immunocytochemistry
The cultures were processed immunocytochemically as previously described.15 In brief, after 1 h incubation with a rabbit anti-CK-1δ primary antibody (Thermo Scientific Pierce), the cells were washed with PBS and incubated with an AlexaFluor-488 goat anti-rabbit secondary antibody (Molecular Probes) for 45 min at 37 °C. Later, the images were obtained using a LSM710 laser scanning spectral confocal microscope (Zeiss). Confocal microscope settings were adjusted to produce the optimum signal-to-noise ratio. The staining of the nuclei was performed using 4′,6-diamidino-2-phenylindole.
Immunoblot Analysis
Proteins were isolated from basal and 6-OHDA-treated SH-SY5Y cell cultures by standard methods. The total protein extraction and Western blot analysis were performed as previously described.16
Effect of Protein Kinase CK-1δ Inhibitors on 6-OHDA
Neuronal Cell Culture
On attaining semiconfluence, the SH-SY5Y cells were treated with 6-OHDA (35 μM, Sigma) for 24 h. Some cultures were pretreated for 1 h with the different compounds at 0.1, 0.5, 1, 10, and 20 μM. After the treatment, cultures were processed for cell viability as previously described.17
Anti-Inflammatory Effect of Protein Kinase CK-1δ Inhibitors on Glial Primary Cultures
Primary Glial Cells Cultures
Primary glial cultures were prepared from the cerebral cortex of 2 day old rats as previously described.16,17 The cultures were pretreated with the different CK-1δ inhibitors (0.1, 0.5, 1, 10, and 20 μM) 1 h before exposure to LPS (10 μg ml–1), and nitrites production was measured after 24 h in the culture.
In Vivo Neuroprotective and Anti-Inflammatory Effect
Animal Experiments
Adult male Wistar rats (8–12 weeks old) were used in this study. All of the procedures with animals were in accordance with the protocols issued, which followed the National (normative 53/2013) and International recommendations (normative 2010/63 from the European Communities Council). Adequate measures were taken to minimize pain or discomfort of the animals.
LPS was injected in vivo. The animals were divided into three groups, with at least four rats in each group, properly anesthetized, and placed in a stereotaxic apparatus (Kopf Instruments, CA). LPS (10 μg in 2.5 μL PBS) alone or in combination with compound 4 (15 nmol) was injected into the right side of the SNpc (coordinates from Bregma: posterior −4.8 mm; lateral +2.0 mm; ventral: +8.2 mm, according to the atlas of Paxinos and Watson). The dose of LPS was chosen on the basis of previous published data.16
Histology and Immunohistochemistry
The animals previously anesthetized were perfused transcardially with 4% paraformaldehyde and the brains obtained as previously described.16,18
Acknowledgments
We thank Prof. Dr. Boris Schmidt for helpful advice in the zebra fish evaluation.
Glossary
ABBREVIATIONS
- PD
Parkinson’s disease
- SNPc
substantia nigra pars compacta
- CK-1δ
casein kinase-1δ
- 6-OHDA
6-hydroxydopamine
- LPS
lipopolysaccharide
- TH
tyrosine hydroxylase
- 2-NF
2-nitrofluorene
- NaN3
sodium azide
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00869.
Experimental procedures and assay protocols; protein kinase CK-1δ expression in human dopaminergic SH-SY5Y cell line (Figure 1S); effect of protein kinase CK-1δ inhibitors on 6-hydroxydopamine (6-OHDA)-induced SH-SY5Y cell death by 6-OHDA (35 μM) in the presence or absence of the CK-1δ inhibitors (0.1–20 μM) (Figure 2S); anti-inflammatory effect of protein kinase CK-1δ inhibitors on glial primary cultures; astrocytes (A) and microglial (B) cells cultures treated with lipopolysaccharide (LPS, 10 μg/mL) in the presence of the different CK-1δ inhibitors (0.1–20 μM) (Figure 3S); safety of the CK-1δ inhibitor 4, in zebrafish embryos (Figure 4S) (PDF)
This work was supported by grants from MINECO (SAF2012-37979-C03-01 to A.M. and SAF2014-52940-R to A.P.-C.) and from MICINN (grant SAF2010-16365 to A.P.-C.). I.G.S. and D.I.P. acknowledge a pre- and postdoctoral fellowship from MICINN (FPI program) and CSIC (JAE program), respectively. CIBERNED is funded by the Instituto de Salud Carlos III. J.A.M.-G. is a fellow from CIBERNED.
The authors declare no competing financial interest.
Supplementary Material
References
- Campbell A. W. Parkinson’s disease: a brief review. Adv. Mind Body Med. 2014, 28, 4–5. [PubMed] [Google Scholar]
- Weiner W. J. Parkinson’s disease and movement disorders. Rev. Neurol. Dis. 2008, 5, 86–89. [PubMed] [Google Scholar]
- de Lau L. M.; Giesbergen P. C.; de Rijk M. C.; Hofman A.; Koudstaal P. J.; Breteler M. M. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology 2004, 63, 1240–1244. [DOI] [PubMed] [Google Scholar]
- Dorsey E. R.; Constantinescu R.; Thompson J. P.; Biglan K. M.; Holloway R. G.; Kieburtz K.; Marshall F. J.; Ravina B. M.; Schifitto G.; Siderowf A.; Tanner C. M. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Whitfield A. C.; Moore B. T.; Daniels R. N. Classics in chemical neuroscience: levodopa. ACS Chem. Neurosci. 2014, 5, 1192–1197. 10.1021/cn5001759. [DOI] [PubMed] [Google Scholar]
- Perez D. I.; Gil C.; Martinez A. Protein kinases CK1 and CK2 as new targets for neurodegenerative diseases. Med. Res. Rev. 2011, 31, 924–954. 10.1002/med.20207. [DOI] [PubMed] [Google Scholar]
- Okochi M.; Walter J.; Koyama A.; Nakajo S.; Baba M.; Iwatsubo T.; Meijer L.; Kahle P. J.; Haass C. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J. Biol. Chem. 2000, 275, 390–397. 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- Salado I. G.; Redondo M.; Bello M. L.; Perez C.; Liachko N. F.; Kraemer B. C.; Miguel L.; Lecourtois M.; Gil C.; Martinez A.; Perez D. I. Protein kinase CK-1 inhibitors as new potential drugs for amyotrophic lateral sclerosis. J. Med. Chem. 2014, 57, 2755–2772. 10.1021/jm500065f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R.; Tiwari P. C.; Nath R.; Pant K. K. Role of neuroinflammation and latent transcription factors in pathogenesis of Parkinson’s disease. Neurol. Res. 2016, 38, 1111–1122. 10.1080/01616412.2016.1249997. [DOI] [PubMed] [Google Scholar]
- Dutta G.; Zhang P.; Liu B. The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam. Clin. Pharmacol. 2008, 22, 453–464. 10.1111/j.1472-8206.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquezar C.; Salado I. G.; de la Encarnacion A.; Perez D. I.; Moreno F.; Gil C.; de Munain A. L.; Martinez A.; Martin-Requero A. Targeting TDP-43 phosphorylation by Casein Kinase-1delta inhibitors: a novel strategy for the treatment of frontotemporal dementia. Mol. Neurodegener. 2016, 11, 36. 10.1186/s13024-016-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K.; Clark M. D.; Torroja C. F.; Torrance J.; Berthelot C.; Muffato M.; Collins J. E.; Humphray S.; McLaren K.; Matthews L.; McLaren S.; Sealy I.; Caccamo M.; Churcher C.; Scott C.; Barrett J. C.; Koch R.; Rauch G. J.; White S.; Chow W.; Kilian B.; Quintais L. T.; Guerra-Assuncao J. A.; Zhou Y.; Gu Y.; Yen J.; Vogel J. H.; Eyre T.; Redmond S.; Banerjee R.; Chi J.; Fu B.; Langley E.; Maguire S. F.; Laird G. K.; Lloyd D.; Kenyon E.; Donaldson S.; Sehra H.; Almeida-King J.; Loveland J.; Trevanion S.; Jones M.; Quail M.; Willey D.; Hunt A.; Burton J.; Sims S.; McLay K.; Plumb B.; Davis J.; Clee C.; Oliver K.; Clark R.; Riddle C.; Elliot D.; Threadgold G.; Harden G.; Ware D.; Begum S.; Mortimore B.; Kerry G.; Heath P.; Phillimore B.; Tracey A.; Corby N.; Dunn M.; Johnson C.; Wood J.; Clark S.; Pelan S.; Griffiths G.; Smith M.; Glithero R.; Howden P.; Barker N.; Lloyd C.; Stevens C.; Harley J.; Holt K.; Panagiotidis G.; Lovell J.; Beasley H.; Henderson C.; Gordon D.; Auger K.; Wright D.; Collins J.; Raisen C.; Dyer L.; Leung K.; Robertson L.; Ambridge K.; Leongamornlert D.; McGuire S.; Gilderthorp R.; Griffiths C.; Manthravadi D.; Nichol S.; Barker G.; Whitehead S.; Kay M.; Brown J.; Murnane C.; Gray E.; Humphries M.; Sycamore N.; Barker D.; Saunders D.; Wallis J.; Babbage A.; Hammond S.; Mashreghi-Mohammadi M.; Barr L.; Martin S.; Wray P.; Ellington A.; Matthews N.; Ellwood M.; Woodmansey R.; Clark G.; Cooper J.; Tromans A.; Grafham D.; Skuce C.; Pandian R.; Andrews R.; Harrison E.; Kimberley A.; Garnett J.; Fosker N.; Hall R.; Garner P.; Kelly D.; Bird C.; Palmer S.; Gehring I.; Berger A.; Dooley C. M.; Ersan-Urun Z.; Eser C.; Geiger H.; Geisler M.; Karotki L.; Kirn A.; Konantz J.; Konantz M.; Oberlander M.; Rudolph-Geiger S.; Teucke M.; Lanz C.; Raddatz G.; Osoegawa K.; Zhu B.; Rapp A.; Widaa S.; Langford C.; Yang F.; Schuster S. C.; Carter N. P.; HHarrow J.; Ning Z.; Herrero J.; Searle S. M.; Enright A.; Geisler R.; Plasterk R. H.; Lee C.; Westerfield M.; de Jong P. J.; Zon L. I.; Postlethwait J. H.; Nusslein-Volhard C.; Hubbard T. J.; Roest Crollius H.; Rogers J.; Stemple D. L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy S.; Sadagopan S.; Nair A.; Sukumaran S. K. Zebrafish as an in vivo high-throughput model for genotoxicity. Zebrafish 2014, 11, 154–166. 10.1089/zeb.2013.0924. [DOI] [PubMed] [Google Scholar]
- Ames B. N.; Gurney E. G.; Miller J. A.; Bartsch H. Carcinogens as frameshift mutagens: metabolites and derivatives of 2-acetylaminofluorene and other aromatic amine carcinogens. Proc. Natl. Acad. Sci. U.S.A. 1972, 69, 3128–3132. 10.1073/pnas.69.11.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Garcia J. A.; Luna-Medina R.; Alonso-Gil S.; Sanz-Sancristobal M.; Palomo V.; Gil C.; Santos A.; Martinez A.; Perez-Castillo A. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem. Neurosci. 2012, 3, 963–971. 10.1021/cn300110c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Garcia J. A.; Redondo M.; Alonso-Gil S.; Gil C.; Perez C.; Martinez A.; Santos A.; Perez-Castillo A. Phosphodiesterase 7 inhibition preserves dopaminergic neurons in cellular and rodent models of Parkinson disease. PLoS One 2011, 6, e17240 10.1371/journal.pone.0017240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-García J. A.; Susin C.; Alonso-Gil S.; Perez D. I.; Palomo V.; Perez C.; Conde S.; Santos A.; Gil C.; Martinez A.; Perez-Castillo A. Glycogen synthase kinase-3 inhibitors as potent therapeutic agents for the treatment of Parkinson disease. ACS Chem. Neurosci. 2013, 4, 350–360. 10.1021/cn300182g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Garcia J. A.; Aguilar-Morante D.; Hernandez-Encinas E.; Alonso-Gil S.; Gil C.; Martinez A.; Santos A.; Perez-Castillo A. Silencing phosphodiesterase 7B gene by lentiviral-shRNA interference attenuates neurodegeneration and motor deficits in hemiparkinsonian mice. Neurobiol. Aging 2015, 36, 1160–1173. 10.1016/j.neurobiolaging.2014.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.