Abstract
Prostate cancer is prevalent among cancers in men. A simple method for screening of reliable biomarkers is pivotal for early detection of prostate cancer. Prostate-specific antigen (PSA) has been a commonly used biomarker for prostate cancer, in spite of its false-positive limitation. On the other hand, alpha-methylacyl-CoA racemase (AMACR), a metabolic enzyme, has been proven to be a highly expressed biomarker in prostate cancer cells. Therefore, a method or tool, which can detect either PSA or AMACR or both simply, cost effectively, and with high sensitivity and selectivity is desirable. We describe a novel bioconjugated, single-use biosensor capable of detecting both PSA and AMACR antigens in undiluted human serum. The preparation of the biosensor by the bioconjugation mechanism occurred within a day, which could be completed prior to actual testing. The effectiveness of the bioconjugation mechanism and the coverage of the electrode surface of the biosensor were experimentally assessed. Measurements of PSA and AMACR antigens and the specificity of the biosensor were carried out using differential pulse voltammetry. This biosensor was single-use and cost-effective and required a small quantity of test medium and relatively short preparation time, providing a very attractive biosensor for the detection of the biomarkers of prostate cancer.
1. Introduction
Prostate cancer was the most prevalent cancer in men for new cancer diagnoses and the third leading cause of cancer death in the United States in 2017.1,2 Metabolic syndrome was considered as a possible risk factor, and there was insufficient evidence for the correlation.3,4 Prostate-specific antigen (PSA) is a widely used biomarker in clinical screening for prostate cancer.5 However, PSA also acted as a biomarker in many noncancerous conditions, such as inflammation, infection, trauma, and benign prostatic hyperplasia. Thus, the screening of PSA led to poor specificity, especially with an intermediate range of valid PSA concentration of 4–10 ng mL–1.6,7 Therefore, a more specific biomarker is desirable for early diagnosis of prostate cancer. One of the emerging biomarkers for prostate cancer is alpha-methylacyl-CoA racemase (AMACR), a metabolic enzyme, which has been proven to be a highly expressed biomarker in prostate cancer cells.8−10 AMACR, a peroxisomal enzyme, facilitates β-oxidation of branched-chain fatty acids by changing (2R,S,6R,10R)-pristanoyl-CoA to (2S,S,6R,10R)-pristanoyl-CoA.11 The mutations in the gene AMACR lead to reduced enzyme activity caused by the raised level of branched-chain fatty acids. Messenger-RNA(mRNA) of AMACR is overexpressed in prostate epithelium during the translation process. For prostate cancer patients, the mRNA level of AMACR was around 9 times higher compared to that of the control group, and the protein AMACR level increased around 2.5 times comparing to that of the normal group, making AMACR an ideal biomarker for prostate cancer.8−12 Although AMACR is a tissue-carried protein, studies proved the possibilities of detecting AMACR directly from body fluid samples of prostate cancer patients.13,14
Conventional methods for the detection of AMACR included techniques of enzyme-linked immunosorbent assay, radioimmunoassay, chemiluminescence immunoassay, and fluoro-immunoassay (FIA). The FIA method showed that the detection limit of AMACR was down to 4.6 pg mL–1.15 The accuracy of detection using these techniques was applicable for screening purposes. However, these tests were laborious, time-consuming, and expensive. Thus, the cost-effective and time-efficient advantages of biosensor technology became attractive for biomarker detection in early monitoring of disease progression. However, very limited studies were reported for the detection of AMACR using an electrochemical-based biosensor. Square wave voltammetry to directly detect AMACR using aptamers immobilized on a polymer substrate was reported with a detection limit of 1.4 fM in human plasma.16 Thus, at this juncture, a biosensor can detect the biomarkers of prostate cancer; AMACR and/or PSA will be of significance, providing a very useful tool for the diagnosis of prostate cancer, regardless of the selection of the suitable biomarker of prostate cancer: either AMACR or PSA.
Self-assembled monolayer (SAM) is a promising platform technology for biosensor applications.17−21 Typically, SAM is formed by an alkane-linked thiol molecule producing a gold–sulfur (Au–S) bond with the gold electrode surface of a biosensor. Then, activation of the terminal functional group followed, immobilizing antigen binding, such as antibody, aptamer, and specific receptor. The formation of the gold electrode elements, working and counter electrodes, of the biosensor could be accomplished by various techniques and in different dimensions. In this study, the gold electrode element was a thin gold film, 50 nm in thickness and was deposited by sputtering physical vapor deposition by a roll-to-roll cost-effective manufacturing method, producing the biosensor relatively inexpensively and effectively. This biosensor was a three-electrode configuration, and the details of the configuration and the characterization of this biosensor have been reported elsewhere.22 For a comprehensive development of this biosensor, six different SAMs were studied, compared, and assessed in this research. These general preparing procedures of a commonly used biosensor were complex and required days for preparation and consumed excess chemicals. Furthermore, the biosensors using SAMs had relatively low sensitivity and poor reproducibility, because of common monolayer defects, such as pinholes, inhomogeneity of surface coverage, and others. Therefore, a new technique for the preparation of a biosensor was used in this study, and it was the bioconjugation mechanism.
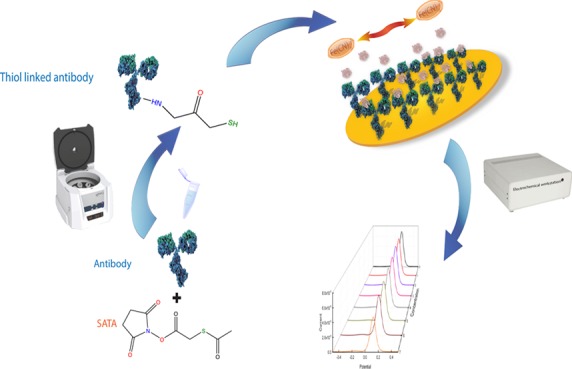
The bioconjugation mechanism conjugates two or more molecules, forming a novel complex embracing the combined properties of its individual components.16 This method makes a zero-length linkage between the protein and electrode elements of the biosensor possible. Furthermore, this bioconjugation technique will shorten the preparation process, enhancing the coverage of the biosensor surface/minimizing the pinhole effect. Consequently, this will improve the practical clinical application. The interaction between antibody and antigen remained to be the biorecognition mechanism in this research endeavor. In this study, anti-AMACR and anti-PSA were modified by the bioconjugation technique using N-succinimidyl S-acetylthioacetate (SATA) to conjugate the antibody. The final product of the conjugation reaction was a thiol group-linked AMACR antibody or PSA antibody, which directly linked with the thin gold film electrode element surfaces of the biosensor through incubation. After being modified by a thiol-linked antibody, fabrication of an AMACR or a PSA biosensor was completed. This single-step preparation took approximately one day including the incubation time for the preparation of the biosensor.
Thus, the combination of bioconjugation technique in preparation, microfabrication of a thin gold film-based biosensor prototype, and differential pulse voltammetry (DPV) measurement technique results in a single-use, cost-effective, and highly sensitive and selective biosensor for the detection of the biomarkers of prostate cancer, AMACR and PSA, a very attractive and practical diagnostic tool for the screening application of prostate cancer.
2. Results and Discussion
2.1. Evaluation of the Effectiveness of Coverage and Antibody Bonding Based on Different SAM Systems and Bioconjugated Systems
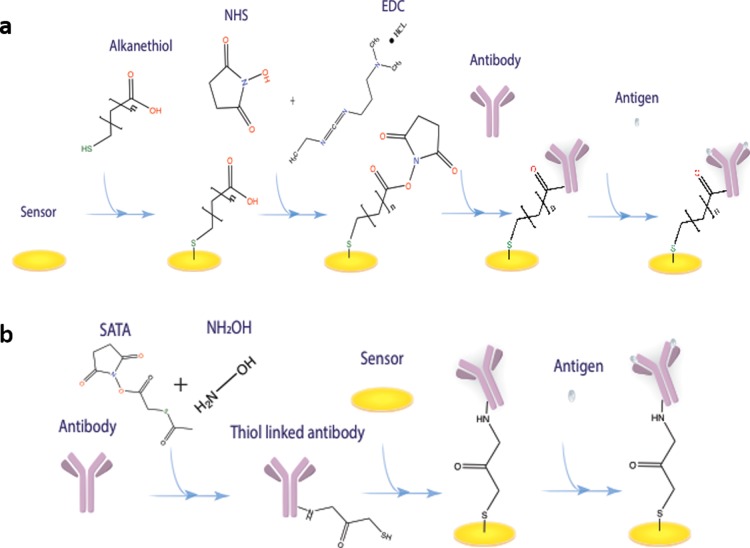
SAM is pivotal for binding the antibody. Different configurations of the SAM affect the orientation of the antibody and the electrode surface coverage of the biosensor, resulting in various binding effects and current signal outputs for electrochemical detection. Six different SAM systems were prepared examining their effectiveness in surface preparation of the biosensor in this study. The configurations of the six SAM systems are shown in Table 1. Figure 1a shows the formation of the SAM system for biosensor development. The bioconjugation mechanism aims at the modification of an antibody to produce an external thiol-linker on the antibody, which is able to directly react with the gold surface to form an Au–S bond without complex surface treatment. Figure 1b shows the process of bioconjugation-based biosensor fabrication. The detailed fabrication procedure for both processes is demonstrated in the Experiments section.
Table 1. Compositions of Six Different SAM Systemsa.
| SAM1 | SAM2 | SAM3 | SAM4 | SAM5 | SAM6 |
|---|---|---|---|---|---|
| 1.00 mM 3-MPA | 1.00 mM 3-MPA | 5.00 mM MUA | 1.00 mM MUA | 1.00 mM MUA | 3.00 mM MUA |
| 0.13 mM DTT | 10.00 mM MCH | 10.00 mM 3-MPA | 9.00 mM MCH |
3-MPA: 3-mercaptopropionic acid, MCH: 6-mercapto-1-hexanol, DTT: DL-dithiothreitol, and MUA: 11-mercaptoundecanoic acid.
Figure 1.
(a) Formation of the SAM for biosensor fabrication. (b) Formation of the bioconjugated antibody for biosensor fabrication.
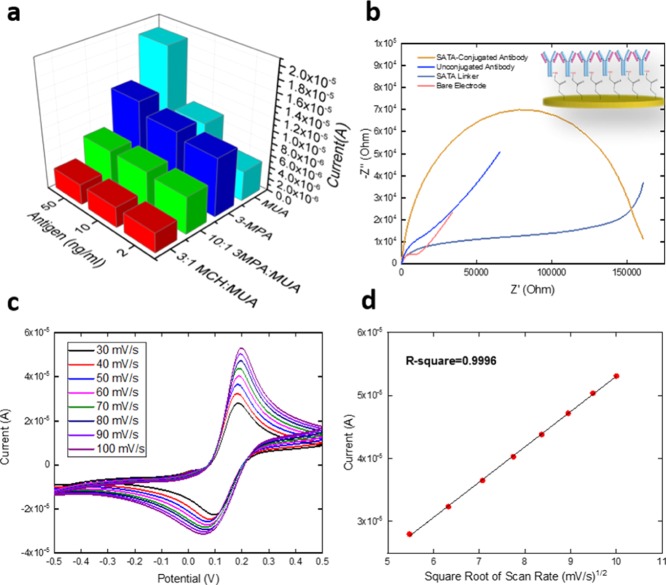
The sensitivity and the reproducibility of the biosensors prepared using different SAM systems and bioconjugation mechanism were evaluated. Three different AMACR antigen concentrations (50, 10, and 2 ng/mL) were prepared in 0.1 M phosphate-buffer saline (PBS) and drop-casted onto the biosensors with different SAM systems incubating for 2 h at room temperature. After incubation, the biosensor was rinsed by 0.1 M PBS solution and dried by nitrogen. DPV was used to measure the conductivity on the biosensor. A redox coupling solution (20 μL) of potassium ferrocyanide (K4Fe(CN)6) and potassium ferricyanide (K3Fe(CN)6) of 5 mM each was applied onto the biosensor surface. Of these six different SAM systems, SAM2- and SAM4-prepared biosensors showed unpromising reproducibility, and they were not investigated any further. Figure 3a shows the sensitivity comparison for the other four SAM systems studied as described in Table 1. SAM3 demonstrated the best sensitivity; the difference among these four SAM modifications was minute and the sensitivity was at the level of 104 to 105 μA·μM–1·cm–2 which was only fair. The SAM1 system (3-MPA) retained the highest R-square value of these four SAM systems which was 0.698 (n = 3). This R-square value was significantly lower than that of the bioconjugation-prepared biosensor [R-square = 0.967 (n = 5)], indicating a lower reproducibility of the SAM system for the detection of AMACR. The results of this study convincingly led us to apply the bioconjugation mechanism to prepare the biosensor for better achievement of an antibody–antigen recognition mechanism.
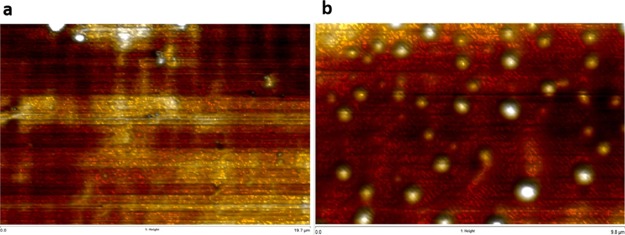
Figure 3.
(a) Topography graph of a bare gold electrode. (b) Topography graph of an antibody film-covered gold electrode.
Electrochemical impedance spectroscopy (EIS) was used to assess the effectiveness of antibody binding by different SAM techniques as well as the bioconjugation method. Using the anti-AMACR protein as the example, four selected SAM systems and the bioconjugated modified biosensors were investigated. The impedance difference between the SAM only biosensor and antibody-bonded SAM biosensor as well as that between the bare biosensor and the antibody-bonded bioconjugated biosensor was examined. A concentration of 1.25 μg/mL anti-AMACR protein was used for all the SAM and bioconjugated systems. The biosensors were incubated for 15 h at 4 °C. The biosensor was then rinsed by using 0.1 M PBS solution and dried by using nitrogen gas. The redox coupling solution (20 μL) of K4Fe(CN)6 and K3Fe(CN)6 of 5 mM of each was applied on the surface for the EIS measurement. The AC frequency range for the EIS measurement was 0.01–10 000 Hz. The Nyquist plots of this study are shown in Figure S1 for four different SAMs and Figure 2b for the bioconjugation-prepared antibody monolayer. The impedance difference before and after incubation of the antibody was calculated by EC-Lab software fitting the Randle circuit as shown in Figure S1a, in which R1 represented the solution resistance, R2 characterized the charge transfer resistance, W2 indicated the diffusion limited process, and Q2 represented the electron transfer process.22,23 The difference of R2 value of each SAM system before/after incubation of the antibody was used to display the impedance difference as shown in Table 2. The biggest impedance difference was shown from the bioconjugation method-prepared biosensor in Figure 2b with a calculated resistance value at 6635 Ω, which was significantly larger than that of any SAM system, indicating the maximum efficiency of antibody coverage by using bioconjugation for antibody monolayer formation. Figure 2b shows the EIS characterization of the bioconjugation-prepared biosensor. The unconjugated antibody (blue line) and SATA linker without antibody (gray line) were also incubated for 2 h on the bare electrode. The changes of resistance on the sensor surface because of gold–protein affinity and only SATA linking with gold were not comparable with the resistance provided by the SATA-conjugated antibody (yellow line), indicating that the best coverage of the electrode surface was produced by the SATA-conjugated antibody. High coverage of the antibody on the biosensor surface has been proved to be able to provide more sensitive quantification of analyte than lack of coverage condition.24
Figure 2.
(a) Sensitivity comparison of four different SAM systems. (b) EIS measurements of the bare electrode (pink), SATA-linker (gray), unconjugated antibody (blue), and SATA-conjugated antibody (yellow). (c) CV characterization of the stability of the bioconjugation mechanism-based biosensor based on different scan rates ranging from 30 to 100 mV/s. (d) Linear calibration curve of the CV current outputs against the square root of scan rates.
Table 2. Resistance Value Difference Modeled by the Randle Circuit.
| monolayer system | SAM1 | SAM3 | SAM5 | SAM6 | bioconjugation |
| resistance difference (Ω) | 21.3 | 116 | 51.1 | 552 | 6.64 × 103 |
To demonstrate the stability and reproducibility of the bioconjugation-prepared biosensor, after incubation with the bioconjugated antibody, cyclic voltammetry (CV) (Figure 2c) was used to examine the biosensors in the presence of [Fe(CN)6]3–/4– at different scan rates ranging from 30 to 100 mV/s. On the basis of the Randles–Sevcik equation, with a constant number of electrons transferred based on the redox event, fixed electrode surface area, diffusion coefficient, and the concentration of the redox coupling, the square root of the scan rate is linear proportional to the current outputs as shown in Figure 2d, demonstrating good stability and reproducibility of the bioconjugated antibody-covered electrode based on the R-square value of 0.9996. Detailed costs for biosensor fabrication based on the SAM method and bioconjugation method were also compared and are shown in Table S1. The bioconjugation method-fabricated sensor was around $2.10/sensor and the SAM method-fabricated sensor was around $3.03/sensor, which outlays 44% more than the cost of the bioconjugation method.
2.2. Qualification of a Bioconjugation-Based Biosensor Surface Using Atomic Force Microscopy
Atomic force microscopy (AFM) was used to confirm the surface difference between a bare gold working electrode and a thiol-linked AMACR antibody-covered gold working electrode. A scan size of 20 μm × 10 μm was applied at a scan rate of 0.513 Hz. Figure 3a,b shows the topography of a bare gold electrode image and a thiol-linked antibody-covered gold electrode image. Figure 3a demonstrates a smooth gold electrode surface with a maximum height of 148 nm. Figure 3b shows a more zigzag surface with a maximum height of 76 000 nm. The white plumped ball shapes indicate a rougher topography with the existence of the AMACR antibody. The white plumped balls also show a very similar size with radius around 200–250 nm, indicating a homogeneous distribution of the antibody on the surface. The qualification changes on the gold electrode surface observed by AFM provide solid proof of the capability of the bioconjugation mechanism on biosensor antibody film fabrication.
2.3. DPV Measurement of the AMACR Antigen in PBS and Undiluted Human Serum
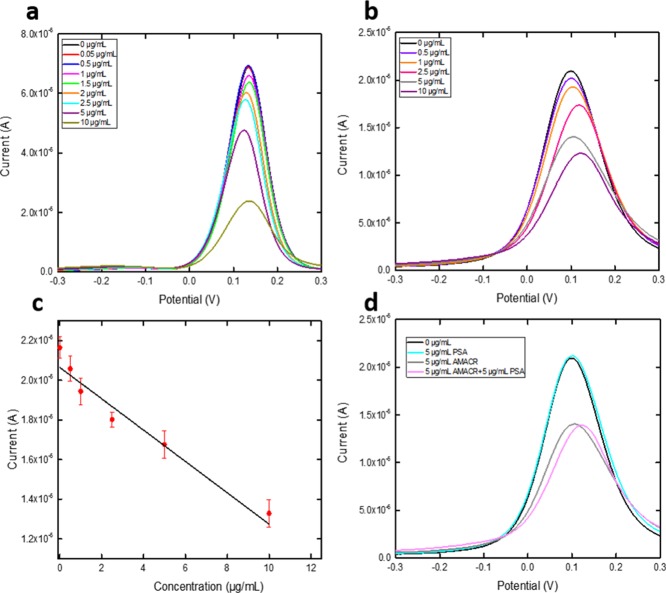
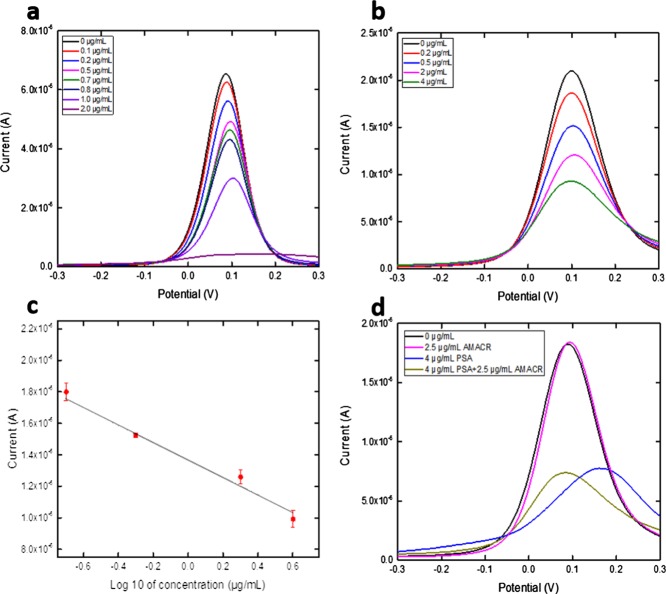
The bioconjugation method-prepared AMACR biosensor was then used for the detection of an antigen of AMACR of different concentrations. Eight different concentrations of AMACR antigen were prepared in 0.1M PBS solution ranging from 10 to 0.05 μg/mL. The antigen sample (20 μL) was placed onto the AMACR biosensor and was incubated for 1 h at room temperature. The AMACR biosensor was then rinsed by 0.1 M PBS and dried by nitrogen gas. A redox probe solution (20 μL) of K4Fe(CN)6 and K3Fe(CN)6 of 5 mM each was applied onto the biosensor surface, and DPV measurement was then made. DPV was conducted at the potential range from −0.3 to 0.3 V. Figure 4a shows the DPV measurement of eight different concentrations with a limitation of testing found at 0.05 μg/mL. The same experiment was also conducted using the AMACR antigen in undiluted human serum with a limitation of detection as shown in Figure 4b, and the calibration is shown in Figure 4c with a linear fit of Y = 2.30 × 10–5X = 6.39 × 10–6 and R-square value of 0.900 (n = 5).
Figure 4.
(a) DPV measurements of the AMACR antigen in 0.1 M PBS. (b) DPV measurements of the AMACR antigen in undiluted human serum. (c) Calibration curve based on the DPV measurement of the AMACR antigen in 0.1 M PBS. (d) Interference test on the AMACR biosensor using PSA antigen.
2.4. DPV Measurements of PSA in PBS and Undiluted Human Serum
For comprehensive detection of prostate cancer, PSA was also evaluated using the bioconjugation mechanism-prepared PSA biosensor and DPV. PSA antigen in PBS solution was firstly tested based on an antibody concentration of 0.27 μg/mL. Concentrations of PSA antigen ranging from 2 to 0.1 μg/mL were prepared in 0.1 M PBS solution. The selected PSA antigen solution (20 μL) was drop-casted on the prepared PSA biosensor and incubated for 1 h at room temperature. The PSA biosensor was then rinsed by 1 mL of 0.1 M PBS solution and dried by nitrogen gas. A redox probe solution (20 μL) of K4Fe(CN)6 and K3Fe(CN)6 of 5 mM each was applied onto the biosensor surface, and DPV measurement was then made. DPV measurement is shown in Figure 5a with a detection limit of 0.1 μg/mL. DPV measurement on PSA in undiluted human serum was also conducted with a PSA antigen concentration range of 0–4 μg/mL using an antibody concentration of 0.55 μg/mL. The same procedure as described in the PBS test was applied for the undiluted human serum test. DPV measurement is shown in Figure 5b with a detection limit of 0.2 μg/mL, and its calibration curve is shown in Figure 5c with a linear fit of Y = 2.41 × 10–5X = 4.33 × 10–7 and R-square value of 0.967 (n = 5).
Figure 5.
(a) DPV measurements of the PSA antigen in 0.1 M PBS. (b) DPV measurements of the PSA antigen in undiluted human serum. (c) Calibration curve based on the DPV measurement of the PSA antigen in undiluted human serum. (d) Interference test on the PSA biosensor using the AMACR antigen.
2.5. Interference Study of the AMACR Biosensor and PSA Biosensor
Interference studies on the AMACR biosensor and PSA biosensor were conducted to confirm the selectivity of each biosensor. For the AMACR biosensor study, undiluted human serum with a PSA antigen concentration of 5 μg/mL was mixed with 5 μg/mL of AMACR antigen. The same incubation and detection procedures were conducted as described in section 2.3. The DPV measurement result is shown in Figure 4d, in which the serum with mixed PSA/AMACR antigens showed no current output difference with only AMACR antigen in the serum, indicating that the PSA antigen did not interfere with the AMACR measurement by the AMACR biosensor. Similarly, for the PSA biosensor study, undiluted human serum with an AMACR antigen concentration of 2.5 μg/mL was mixed with 4 μg/mL PSA antigen solution. The same incubation and detection procedures were conducted as described in section 2.4. The DPV measurement result is shown in Figure 5d, in which the mixed PSA/AMACR antigens showed no current output difference with only the PSA antigen in the serum, indicating that the AMACR antigen did not interfere with the PSA measurement by the PSA biosensor.
3. Conclusion
Bioconjugation mechanism has shown promising ability in applications for biosensor development. The fabrication process using the bioconjugation mechanism demonstrated the advantages of time-efficiency, cost-effectiveness, and most importantly, a simple method that makes it possible for medical professionals to operate the biosensor fabrication process. Two different biosensors for the detection of prostate cancer were fabricated. The developed biosensors showed excellent capability in the detection of biomarkers of prostate cancer in both PBS and undiluted human serum with good selectivity proved by two different interference studies.
4. Materials
Anti-AMACR (Cat. no. HPA019527) was obtained from Sigma-Aldrich (St. Louis, MO, USA), and AMACR (Cat. no. MBS428004) was obtained from MyBioSource (San Diego, CA, USA). Anti-PSA (Cat. no. ab76113) and PSA peptide (Cat. no. ab41421) were obtained from Abcam (Cambridge, MA, USA). PBS 1.0 M (pH 7.4), human serum (Cat. no. H3667), dl-dithiolthreitol solution (Cat. no. 43816), 3-mercaptopropionic acid (3-MPA) (Cat. no. M5801), 6-mercapto-1-hexanol (Cat. no. 451088), 11-mercaptoundecanoic acid (Cat. no. 450561), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (Cat. no. E1769), N-hydroxysuccinimide (NHS) (Cat. no. 130672), and N-hydroxysulfosuccinimide sodium salt (Cat. no. 56485) were purchased from Sigma-Aldrich (St. Louis, MO, USA). SATA (Cat. #26102) and dimethyl sulfoxide (DMSO) (Cat. #BP231-1) were obtained from Thermo Fisher Scientific (Pittsburgh, PA. USA). Ethylenediaminetetraacetic acid (EDTA) (Cat. EDS) and hydroxylamine (Cat. #255580) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Concentrated H2SO4 (95.0–98.0 w/w %) and concentrated HNO3 (70% w/w %) were received from Fisher Scientific (Pittsburgh, PA, USA). Potassium hydroxide, K3Fe(CN)6, and K4Fe(CN)6 (Cat. no. P1767, P3289, and P3667) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All the chemicals were used without further purification. A CHI 660C (CH Instrument, Inc., Austin, TX, USA) electrochemical workstation was used for DPV characterization.
5. Experiments
5.1. Preparation of an SAM-Based Biosensor
All the SAM systems were prepared in ethanol solution. Thin gold film-based biosensors were first cleaned as described in previous studies22,25 and immersed in the different SAM solutions for 24 h at room temperature. After 24 h of immersion in the SAM solution, the biosensors were rinsed by using deionized water and dried by using nitrogen gas. EDC (0.2 M) and 0.05 M NHS in 0.1 M PBS solution were prepared to activate the carboxylate group on the SAM by immersing the biosensors in the prepared SAM solution for 1 h at room temperature. Using anti-AMACR as an example, 20 μL of anti-AMACR solution with a concentration of 1 μg mL–1 was drop-casted onto the biosensor after the activation process and incubated for 15 h at 4 °C. The biosensor was then ready to be assessed for the effectiveness of the SAM system. The process of fabrication of the SAM biosensor is shown in Figure 1a.
5.2. Preparation of the Thiol-Linked Anti-AMACR or Anti-PSA Protein
The bioconjugation mechanism was applied to create the thiol-linked anti-AMACR or anti-PSA protein. SATA was used to conjugate the antibody to produce a thiol-linked anti-AMACR or anti-PSA protein. Typically, for the preparation of the thiol-linked anti-AMACR, 0.5 mg of SATA was firstly dissolved in 1 mL of DMSO. The prepared SATA solution (1 μL) was mixed with 30 μL of anti-AMACR in 0.1 M PBS solution based on a molar ratio of 20:1 between SATA and the antibody.26 This mixed solution was incubated for 30 min at room temperature. The solution was then diluted to a total volume of 500 μL by using 0.1 M PBS solution and transferred into an Amicon ultra-0.5 10k filter tube, centrifuging at 12 000 rpm for 15 min at 5 °C. Twenty-five microliters of the filtered solution was obtained, and this filtered solution was reacted with 5 μL of 0.5 M hydroxylamine and 25 mM EDTA in 0.1 M PBS solution at room temperature for 2 h. An Amicon ultra-0.5 10k filter tube was used again to filter out any molecules lower than 10 kDa molecular weight. This filtered solution was diluted to 500 μL by 10 mM EDTA in 0.1 M PBS solution and centrifuged at 12 000 rpm for 15 min at 8 °C. The solution was then diluted again using 10 mM EDTA in 0.1 M PBS solution to 500 μL and centrifuged again at 12 000 rpm for 15 min at 8 °C. After this second centrifuge process, the obtained solution was thiol-linked anti-AMACR. The bioconjugation process is shown in Figure 1b. The thiol-linked anti-PSA solution was prepared in a similar manner, which is described in the Supporting Information.
5.3. AMACR Biosensor and PSA Biosensor Fabrication Based on the Thiol-Linked Antibody
Using the anti-AMACR solution as an example, it reactedwith the gold electrode surface, forming a strong Au–S bond linking the antibody onto the gold working electrode. The thiol-linked anti-AMACR was firstly diluted by 0.15 M NaCl and 10 mM EDTA in 0.1 M PBS solution to a concentration of 1.25 μg/mL. The gold biosensor was prepared in a batch of ten and cleaned as described in a previous study.22 The diluted thiol-linked anti-AMACR solution was vortexed. Twenty microliters of the solution at a concentration of 1.25 μg/mL was drop-casted onto the cleaned gold biosensor for incubation for 8 h at 4 °C. The preparation step of the PSA biosensor is similar and is described in the Supporting Information.
Acknowledgments
Support of this research by the Wallace R. Persons research fund of Case Alumni Association is gratefully appreciated and acknowledged. We acknowledge Laurie Dudik from the Electronics Design Center of Case Western Reserve University for facility usage.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00634.
Electrochemical impedance characterization of multiple SAM layers; preparation of thiol-linked anti-PSA; preparation of the PSA biosensor; cost of biosensor fabrication (PFF or comprehensive detection of prostate cancer), and PSA (PDF)
Author Contributions
§ J.Y., Y.W., and Y.D. contributed equally. C.C.L. and Y.D. designed and conceived the experiments. C.C.L. and Y.D. analyzed the data. J.Y., Y.W., and Y.D. conducted the experiments. Y.D., C.C.L., J.Y., and Y.W. contributed to the preparation of the manuscript.
The authors declare no competing financial interest.
This paper was published on June 14, 2018 with the wrong image in the Abstract. The image was corrected and the revised version was republished on June 15, 2018.
Supplementary Material
References
- Ries L. A.; Harkins D.; Krapcho M.; Mariotto A.; Miller B. A.; Feuer E. J.; Clegg L. X.; Eisner M.; Horner M.-J.; Howlader N.. SEER Cancer Statistics Review, 1975-2003, 2006.
- Siegel R. L.; Miller K. D.; Fedewa S. A.; Ahnen D. J.; Meester R. G.; Barzi A.; Jemal A. Colorectal cancer statistics. Ca-Cancer J. Clin. 2017, 67, 177–193. 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- Hsing A. W.; Sakoda L. C.; Chua S. C. Jr. Obesity, metabolic syndrome, and prostate cancer. Am. J. Clin. Nutr. 2007, 86, 843S–857S. 10.1093/ajcn/86.3.843s. [DOI] [PubMed] [Google Scholar]
- Esposito K.; Chiodini P.; Capuano A.; Bellastella G.; Maiorino M. I.; Parretta E.; Lenzi A.; Giugliano D. Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J. Endocrinol. Invest. 2013, 36, 132–139. 10.1007/bf03346748. [DOI] [PubMed] [Google Scholar]
- Yilmaz S. N.; Yildiz A. PSA, PSA derivatives, proPSA and prostate health index in the diagnosis of prostate cancer. Turk. J. Urol. 2014, 40, 82. 10.5152/tud.2014.94547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercole C. J.; Lange P. H.; Mathisen M.; Chiou R. K.; Reddy P. K.; Vessella R. L. Prostatic specific antigen and prostatic acid phosphatase in the monitoring and staging of patients with prostatic cancer. J. Urol. 1987, 138, 1181–1184. 10.1016/s0022-5347(17)43543-9. [DOI] [PubMed] [Google Scholar]
- Balk S. P.; Ko Y.-J.; Bubley G. J. Biology of prostate-specific antigen. J. Clin. Oncol. 2003, 21, 383–391. 10.1200/jco.2003.02.083. [DOI] [PubMed] [Google Scholar]
- Luo J.; Zha S.; Gage W. R.; Dunn T. A.; Hicks J. L.; Bennett C. J.; Ewing C. M.; Platz E. A.; Ferdinandusse S.; Wanders R. J. α-Methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002, 62, 2220–2226. [PubMed] [Google Scholar]
- Jiang Z.; Wu C. L.; Woda B. A.; Iczkowski K. A.; Chu P. G.; Tretiakova M. S.; Young R. H.; Weiss L. M.; Blute R. D.; Brendler C. B. Alpha-methylacyl-CoA racemase: a multi-institutional study of a new prostate cancer marker. Histopathology 2004, 45, 218–225. 10.1111/j.1365-2559.2004.01930.x. [DOI] [PubMed] [Google Scholar]
- Rubin M. A.; Zhou M.; Dhanasekaran S. M.; Varambally S.; Barrette T. R.; Sanda M. G.; Pienta K. J.; Ghosh D.; Chinnaiyan A. M. α-Methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA, J. Am. Med. Assoc. 2002, 287, 1662–1670. 10.1001/jama.287.13.1662. [DOI] [PubMed] [Google Scholar]
- Mukherji M.; Schofield C. J.; Wierzbicki A. S.; Jansen G. A.; Wanders R. J. A.; Lloyd M. D. The chemical biology of branched-chain lipid metabolism. Prog. Lipid Res. 2003, 42, 359–376. 10.1016/s0163-7827(03)00016-x. [DOI] [PubMed] [Google Scholar]
- Xu J.; Stolk J. A.; Zhang X.; Silva S. J.; Houghton R. L.; Matsumura M.; Vedvick T. S.; Leslie K. B.; Badaro R.; Reed S. G. Identification of differentially expressed genes in human prostate cancer using subtraction and microarray. Cancer Res. 2000, 60, 1677–1682. [PubMed] [Google Scholar]
- Rogers C.; Yan G.; Zha S.; Gonzalgo M.; Isaacs W.; Luo J.; DeMarzo A.; Nelson W.; Pavlovich C. Prostate cancer detection on urinalysis for α methylacyl coenzyme a racemase protein. J. Urol. 2004, 172, 1501–1503. 10.1097/01.ju.0000137659.53129.14. [DOI] [PubMed] [Google Scholar]
- Sreekumar A.; Laxman B.; Rhodes D. R.; Bhagavathula S.; Harwood J.; Giacherio D.; Ghosh D.; Sanda M. G.; Rubin M. A.; Chinnaiyan A. M. Humoral immune response to α-methylacyl-CoA racemase and prostate cancer. J. Natl. Cancer Inst. 2004, 96, 834–843. 10.1093/jnci/djh145. [DOI] [PubMed] [Google Scholar]
- Yang D.-K.; Chen L.-C.; Lee M.-Y.; Hsu C.-H.; Chen C.-S. Selection of aptamers for fluorescent detection of alpha-methylacyl-CoA racemase by single-bead SELEX. Biosens. Bioelectron. 2014, 62, 106–112. 10.1016/j.bios.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Jolly P.; Miodek A.; Yang D.-K.; Chen L.-C.; Lloyd M. D.; Estrela P. Electro-engineered polymeric films for the development of sensitive aptasensors for prostate cancer marker detection. ACS Sens. 2016, 1, 1308–1314. 10.1021/acssensors.6b00443. [DOI] [Google Scholar]
- Dai Y.; chiun Liu C. Detection of 17 β-estradiol in Environmental Estrogen Pollution and in Women’s Health Care Using a Single-Use, Cost Effective Biosensor Based on Differential Pulse Voltammetry (DPV). Biosensors 2017, 7, 15. 10.3390/bios7020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; Molazemhosseini A.; Liu C. In Vitro Quantified Determination of β-Amyloid 42 Peptides, a Biomarker of Neuro-Degenerative Disorders, in PBS and Human Serum Using a Simple, Cost-Effective Thin Gold Film Biosensor. Biosensors 2017, 7, 29. 10.3390/bios7030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W.; Sim S. J.; Cho S. M.; Lee J. Characterization of a self-assembled monolayer of thiol on a gold surface and the fabrication of a biosensor chip based on surface plasmon resonance for detecting anti-GAD antibody. Biosens. Bioelectron. 2005, 20, 1422–1427. 10.1016/j.bios.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Love J. C.; Estroff L. A.; Kriebel J. K.; Nuzzo R. G.; Whitesides G. M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- Vericat C.; Vela M. E.; Benitez G.; Carro P.; Salvarezza R. C. Self-assembled monolayers of thiols and dithiols on gold: new challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. 10.1039/b907301a. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Molazemhosseini A.; Liu C. A Single-Use, In Vitro Biosensor for the Detection of T-Tau Protein, A Biomarker of Neuro-Degenerative Disorders, in PBS and Human Serum Using Differential Pulse Voltammetry (DPV). Biosensors 2017, 7, 10. 10.3390/bios7010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lvovich V. F.Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena; John Wiley & Sons, 2012. [Google Scholar]
- Saha B.; Evers T. H.; Prins M. W. J. How antibody surface coverage on nanoparticles determines the activity and kinetics of antigen capturing for biosensing. Anal. Chem. 2014, 86, 8158–8166. 10.1021/ac501536z. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Liu C. A Simple, Cost-Effective Sensor for Detecting Lead Ions in Water Using Under-Potential Deposited Bismuth Sub-Layer with Differential Pulse Voltammetry (DPV). Sensors 2017, 17, 950. 10.3390/s17050950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen J. T. P.; Scherphof G. L. An improved method for the covalent coupling of proteins to liposomes. Biochim. Biophys. Acta 1985, 814, 151–155. 10.1016/0005-2736(85)90430-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.