ABSTRACT
Chronic ankle instability (CAI) is a common dysfunctional state in the basketball population accompanied by pain, weakness and proprioceptive deficits which greatly affect performance. Research evidence has supported the use of blood flow restriction (BFR) training as an effective treatment strategy for improving muscle strength, hypertrophy and function following injury in a variety of patient populations. In managing CAI, it is important to address proximal and distal muscle weakness, pain, and altered proprioception to reduce the likelihood of re-occurring ankle injury. The ability to mitigate acute and cumulative strength and muscle volume losses through the integration of BFR after injury has been supported in research literature. In addition, applications of BFR training for modulating pain, improving muscle activation and proximal muscle strength have recently been suggested and may provide potential benefit for athletes with CAI. The purpose of this clinical commentary is to discuss background evidence supporting the implementation of blood flow restriction training and use a theoretical model for managing CAI as well as to suggest novel treatment strategies using this method.
Level of Evidence
5
Keywords: Ankle instability, blood flow restriction, strength training
BACKGROUND AND PURPOSE
Chronic ankle instability (CAI) consists of characteristic sequelae including recurrent sprain, pain, instability and avoidance of activities1 and is typically defined as mechanical or functional instability.2 Mechanical instability is anatomical laxity in the stabilizing structures around the ankle mortise where functional instability is a subjective feeling of giving away despite the lack of displacement beyond the normal physiological range of talar motion.3 It can be a particularly debilitating condition in the athletic population, with an associated high risk for re-injury.4,5,6 Time loss from sport due to this injury is typically associated with increased pain and crepitus as well as decreased strength, proprioception, range of motion and balance.7,8 Multiple proprioceptive impairments have been linked to persistent functional instability including altered muscle spindle activation of the peroneal musculature, abnormal reflex responses to inversion or supination and proximal kinetic chain deficits as exhibited by presence of neural inhibition and associated weakness in the hip abductors.9,10,11 Furthermore, deficits in postural control and ankle arthro-kinematic motion quality were identified in athletes with CAI using accelerometry.12 Repeat episodes of ankle sprains commonly occur in CAI and appear to further exacerbate instability, associated functional deficits and the ability to maintain a competitive status.5
The multidirectional and repetitive movement aspects associated with the sport of basketball can predispose these athletes to ankle injuries. While jump landing has been identified as the most common mechanism of injury in this population, change of direction, cutting and pivoting movements also contribute to injury occurrence.5 Over half of total time missed due to injury in this population has been attributed to ankle injury.13 Both elite and recreational basketball players of both genders with a previous ankle injury have also been identified as being at 4.9 times greater risk for subsequent ankle injury.5 Therefore, it appears that CAI leads to significant time lost in sport and previous injury can result in greater re-injury risk.
Increased pain associated with CAI greatly affects a basketball athlete's ability to return to sport and perform at an elite level. CAI typically presents with articular changes and chondral lesions within the ankle joint which can manifest as chronic pain.14 The combination of altered somatosensory afferent signaling and efferent motor control deficits resulting from ankle ligament sprains negatively affects the ability to produce desired protective motor responses in the event of an inversion mechanism.15 The resulting injury pathology then can further exacerbate faulty mechanical patterns and subsequent pain presentation associated with repetitive injury and articular changes.15
Muscle inhibition which occurs following an ankle sprain has been documented.8 Perron et al. have found that significant strength deficits exist up to six months following injury and may contribute to the high recurrence of lateral ankle sprain.16 Furthermore, increased resting motor thresholds have been demonstrated in the peroneus longus muscle, bilaterally, in subjects with CAI.17 This would indicate deficits in corticomotor excitability of the peroneus longus to control subsequent inversion mechanisms which may result in re-injury.17 Decreased corticomotor excitability was also moderately correlated to self-reported function which would indicate that subjects’ perceptions of functional limitations likely manifests in resulting neuromuscular deficits and poor motor control.17
Altered neuromuscular recruitment and motor control patterns are not strictly isolated to the ankle joint in presentations of CAI. Proximal muscle weakness can contribute to functional deficits which persist following an ankle sprain.9 In particular, weakness of the gluteal musculature can contribute to altered landing mechanics as a result of poor shock absorption and decreased force attenuation throughout the lower quarter.18 In the case of basketball athletes, landing has been identified as a common mechanism of injury for ankle sprains so failing to address these limitations is problematic.5 Therefore, it is important to focus on the entire kinetic chain during the rehabilitation process with strategies which effectively address these underlying neuromechanical deficiencies and altered movement patterns.
The purpose of this clinical commentary is to discuss background evidence supporting the implementation of blood flow restriction training and use a theoretical model for managing chronic ankle instability in the basketball athlete to suggest novel treatment strategies using this method.
DESCRIPTION OF TOPIC: THEORETICAL APPLICATIONS FOR CAI
The inability to utilize loads sufficient to induce strength and hypertrophy responses after ankle injury due to pain or healing processes may limit rehabilitation progression. Recently, blood flow restriction (BFR) training has emerged as a novel treatment technique due to its ability to create robust muscle anabolic responses similar to high load training while utilizing very low loads.19 BFR has shown to be an effective treatment strategy for diminishing disuse atrophy and weakness during periods of immobilization as well as increasing strength and hypertrophy in post-op patient populations.20,21,22 Additionally, BFR has been shown to enhance function in blast trauma patients and injured military personnel23 as well as improve performance outcomes as part of a comprehensive strength and conditioning program in the high-performance athlete.24,25 American College of Sports Medicine guidelines recommend utilization of 60-80% of a one rep max (1 RM) load targeting major muscle groups 2-3 days per week in order to achieve strength and hypertrophy gains from resistance training.26 However, similar gains utilizing BFR have been shown within a two-week training period at loads of much lower intensity (20-30% 1 RM).27,28 Although the exact mechanism is not fully understood, increased muscular fatigue under hypoxia, cellular swelling and upregulation of muscle protein synthesis via mammalian target of rapamycin complex 1 and mitogen-activated protein kinases (MTORC1/MAPK) which are responsible for protein synthesis and cell signaling, have been suggested to play a role.29,30,31 While most of these aforementioned studies focused on the lower extremity in general and post-operative conditions, currently no studies examining the utilization of BFR with CAI have been published.
Chronic Pain Management
Chronic pain which results from CAI can be debilitating if not properly managed. Recently published clinical trials that have assessed pain have reported significant reductions in pain if BFR is incorporated into the intervention. Giles at al found that, when compared to standard quadriceps strengthening, low load exercise with BFR greatly reduced pain in daily living in subjects with patellofemoral pain (PFP) following an eight-week program.32 The conceptual understanding for these changes is that subjects were able to improve quadriceps muscle strength with BFR while tolerating loads lower than those required to make similar gains utilizing traditional quadriceps strengthening activities.32 Given the association of quadriceps muscle weakness and PFP and the increases of knee extensor torque with significantly decreased reported pain in this BFR study group, it has been hypothesized that BFR training can potentially modulate pain through central and neural adaptations which influence strength gains.32 Additionally, subjects with anterior knee pain demonstrated an acute reduction in pain immediately after BFR resisted quad exercises up to 45 minutes post treatment.33 Although, mechanisms behind a potential reduction in pain through the application of BFR have not been elucidated, intensity of exercise may play a role in the endogenous opioid response.34 Despite BFR being performed under low loads, when performed under continuous occlusion (ie..no deflation cycles during rest periods), the metabolic stress produced in the working muscle is similar to exercise at much higher loads.35 This may allow patients in the early stages of rehabilitation or with chronic painful injuries to promote cortical release of opioids to allow for tolerance of rehabilitation programs. Although not directly related to pain inhibition, increases in corticomotor excitability have been demonstrated for up to 60-minutes post continuous BFR exercise possibly due to altered sensory feedback via group III and IV afferents.36 Future BFR studies are warranted that assess pain in clinical populations and the peripheral and central mechanisms of pain modulation that may be involved.
Muscle Inhibition
The work by Brandner et al. provides promise as to the applications of BFR as a potential neuromodulator.36 Following an acute bout of upper extremity exercise utilizing BFR, corticomotor excitability of the biceps brachii rapidly increased and remained elevated for up to 60 minutes post exercise.36 It is theorized that these adaptations result from increased excitability of corticospinal circuits which results in long-lasting adaptations similar to those which occur following heavy-load resistance training.36 Further research evidence is needed to suggest whether utilization of BFR can potentially improve motor recruitment and neural excitability of inhibited or weak musculature following injury, such as CAI as addressed conceptually in this commentary.
Kinetic Chain Considerations and Proximal Strengthening
BFR training would appear to be an effective treatment strategy that can be implemented to improve proximal muscle strength. Abe et al. found significant increases in gluteal muscle strength and hypertrophy following a two-week BFR program compared to a control group, implementing BFR with exercises that included the low load squat and leg curl.37 Despite distal occlusion, proximal gains may result from fatigue of musculature below the cuff requiring more recruitment of synergistic proximal muscles, a backflow effect into musculature above the area of restriction or a potential systemic effect secondary to the anabolic cascade created by BFR.38,39,40,41 Potential benefits of proximal effects from this training strategy may allow safe implementation during both early rehabilitation and return to sport specific exercises which challenge proprioception and balance to incorporate proximal and distal effects.
DISCUSSION: CLINICAL INTEGRATION
With a comprehensive understanding of the research evidence behind BFR training, one can then attempt to translate this information into applied clinical practice. Loenneke et al. proposed a clinical integration model which advocates for utilization of BFR throughout various phases of injury rehabilitation.42 The four proposed phases of BFR implementation include: 1) BFR alone during periods of bed rest, 2) BFR combined with low-workload walking exercise, 3) BFR combined with low-load resistance exercise, and 4) low-load BFR training combined with traditional high-load resistance exercise.42 In cases of prolonged immobilization or restricted weightbearing where BFR walking may not be appropriate, low-load, resisted, open kinetic chain exercises, such as ankle ROM, leg lift (Figure 1) and bridge variations may be used safely and effectively with implementation of BFR (Table 1) to attenuate muscle atrophy and weakness commonly associated with disuse.20,21 For optimal motor control in these early stages of rehab, it is important integrate external focus of attention strategies which have been shown to increase muscle excitability.43,44 These strategies may include use of a metronome for repetition pacing or biofeedback, especially during exercises which actively recruit the peroneal and gluteal muscles which have been identified as commonly inhibited and weakened muscle groups in CAI.9,17,43,44
Figure 1.
Open kinetic chain leg lift variation for hip muscle strengthening with BFR (Delfi Medical Innovations Inc. Vancouver, BC Canada).
Table 1.
Integration of BFR with Rehabilitation for Ankle Instability
| Phase | Description | Goals | Therapeutic Exercises |
|---|---|---|---|
| Phase 1 | Initial strengthening and range of motion progressions | Reduce pain | •4-way band exercises (ankle) •Straight leg raise variations •Ankle pumps •Seated heel raises •Intrinsic foot muscle activation, toe scrunches •Resisted manual ROM •Double and single leg bridge variations |
| Establish functional movements | |||
| Initiate resisted activities | |||
| Ensure adequate motion is available in all planes | |||
| Phase 2 | Static stability and closed-kinetic chain strengthening | Enhance proprioceptive ability | •Standing heel raises (double to single leg) •Four-way hip with elastic resistance •Drop step squats •Lateral slide steps •Dynamic stabilization exercises (unstable surface) •Multi-directional lunge variations (e.g. furniture slider) |
| Enhance neuromuscular control | |||
| Improve lower extremity strength under dynamic conditions | |||
| Phase 3 | Multi-planar balance and reactive series | Increase degrees of freedom during functional activities | •Plyo-toss on unstable surface •Visual light reactive drills in double and single-leg stance •Manual perturbations to surface with upper extremity activities (catch and throw, wall taps, ball dribble, jab steps) |
| Elicit adaptive reactive strategies with external attention cueing | |||
* Phase progressions should include consideration of: tissue healing parameters, quality of movement, and demonstration of adequate strength
•BFR applied to the involved extremity at 60-80% arterial occlusion for all exercises
•Standard repetition scheme of 30/15/15/15 with 30 second rest periods in between sets should be used for isotonic exercises
•Balance/stability exercises can utilize 30:30 second repetition:rest ratio x4 sets
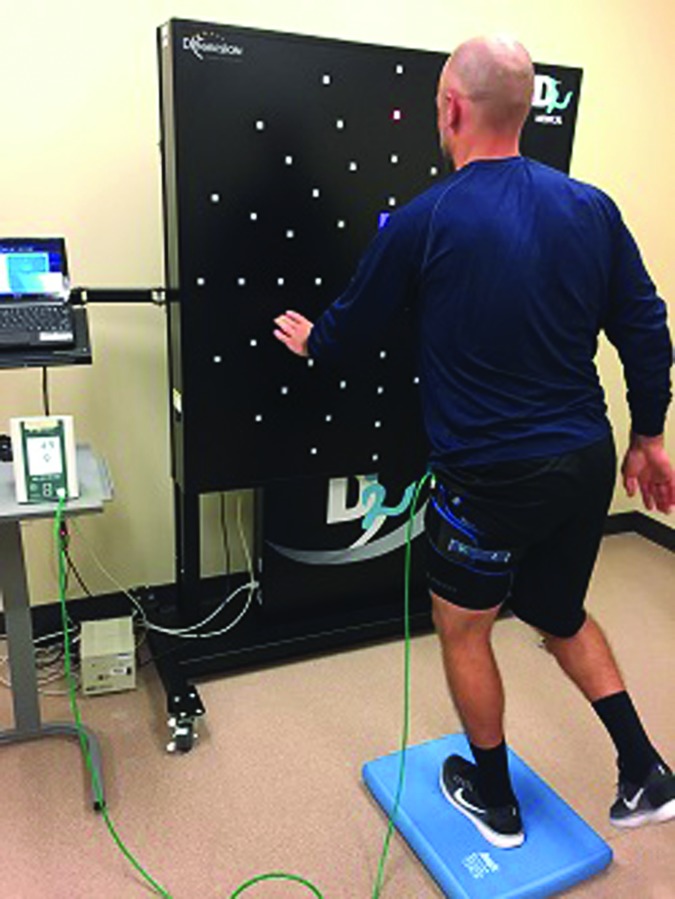
The proprioceptive, balance and motor control deficits associated with CAI have been well documented.8,15,17 Exercises which challenge the proprioceptive system on both stable and unstable surfaces with altered visual and somatosensory feedback have been effective for reducing the recurrence of ankle sprains.45 Examples would include single leg static balance on an unstable surface, multidirectional weight shifting activities and reactive drills (Figures 2 and 3). These exercises can target basketball specific demands by integrating passing (Figure 3), ballhandling and reactive movement drills utilizing verbal or visual cues, with resources such as a light reactive system (Figure 2) or a laser pointer, in order to emphasize an external focus of attention. Exercise progressions would include band-resisted movements replicating basketball specific patterns such as jab steps, close out steps, drop step squats and lateral slides (Figure 4). These exercises can be safely integrated with BFR training to further enhance proximal strengthening and neuromuscular control (Table 1).
Figure 2.
BFR static single leg balance on unstable surface with integration of light system (Dynavision D2™ -Dynavision International, LLC West Chester Township, OH; Delfi Medical Innovations Inc. Vancouver, BC Canada)
Figure 3.
BFR single leg stability with reactive passing component (BOSU® Ashland, OH; Delfi Medical Innovations Inc. Vancouver, BC Canada).
Figure 4.
BFR band resisted drop step squat (Delfi Medical Innovations Inc. Vancouver, BC Canada).
BFR training can both complement and enhance the conditioning aspects of a return to play rehab program. A BFR treadmill walking protocol, performed six days per week for two weeks, resulted in significant improvements in maximal aerobic capacity, maximal ventilation and anaerobic capacity in male collegiate basketball athletes.46 The protocol consisted of five periods of three-minute working sets at 4-6 km/h and 5% grade with 60 second inter-set rest.46 Similar findings occurred, in addition to increased size and strength of the leg muscles, following both BFR cycling and low-intensity walking programs.47,48 This indicates that, when utilized as part of a non-impact conditioning program, BFR training can improve overall cardiovascular fitness when combined with aerobic activity. This is especially important during rehab phases where limitations in mobility and dynamic loading do not permit the athlete to run or perform on-court conditioning activities. Athletes with CAI who sustain recurrent ankle injuries would likely benefit from such a program during periods when dynamic loading activities are limited in order to promote tissue healing and recovery from acute injury.
As the athlete progresses back into sport specific activities, BFR training can still play an integral role in developing strength and performance attributes when combined with a traditional strength and conditioning program. Implementation of BFR would follow a high load-low load model whereas BFR exercises would be implemented intra-session in a low-load strategy following traditional high load resistance exercises to enhance hormonal responses and strengthening benefits following the session.49 An example as it applies to the lower extremity would be completing modified bodyweight squats or leg press exercise at 20% maximal load utilizing BFR at the end of a lifting session which consisted of near maximal (80% max) squat and deadlift exercises. This strategy would be especially beneficial in transferring BFR training adaptations which occurred during the rehab process over to individualized strength and conditioning programs that are implemented upon return to play in athletes with CAI. Lastly, nutritional considerations must be addressed in the rehabilitating an athlete using BFR as part of a comprehensive performance enhancement program. Due to the increased protein synthesis response following BFR training, it is important the athlete consume 20-35 grams of protein post-activity for muscle tissue healing and recovery.50 Whey protein is preferred due to both its rapid digestion and absorption as well as greater leucine content which further enhances protein synthesis.50
SAFETY CONSIDERATIONS
Safety is of paramount concern when considering rehabilitation programs for injured individuals. BFR utilizes non-traditional methods of building strength and inducing muscle hypertrophy, so reasonable vigilance is required of rehabilitation professionals to maintain safety.
In the instance of tourniquet systems, research on safety and efficacy has consistently been monitored with technological advancements.51,52,53 In the surgical environment, tourniquets are applied for upwards of several hours at a time to provide a bloodless operating field.51 Generally, it is suggested that tourniquet time is minimized as much as possible during surgeries such as total knee arthroplasty (TKA) to mitigate risks such as deep vein thrombosis (DVT), wound infection, hematoma, and ecchymosis.53 Similar risks can be expected during BFR training as with the use of tourniquet devices including pneumatic cuffs and straps. However, at lower levels of constriction and reduced tourniquet time, these risks are likely minimal.
Research on safety during BFR and exercise is currently limited, however several measures have been examined with regard to outcomes of exercise with BFR training versus regular exercise. A recent review by Loenneke and colleagues examined peripheral blood flow hemodynamics with BFR and determined that changes in blood flow appear to occur in a “similar fashion as regular exercise”.54 Furthermore, Lida et al observed certain central cardiovascular responses to BFR and found that subjects exhibit elevated cardiac markers (blood pressures, heart rate) in occlusive protocols compared to control groups.55 However, these values are still far less than those performing high intensity exercise, and low-intensity exercise (20% 1RM) with occlusion may be a safe alternative.55
The potential for thrombosis and clotting can be a serious risk when considering patients in a rehabilitative state. Complete vascular occlusion can be experienced with strenuous, high-intensity exercise with tourniquets and has been shown to increase the formation of thrombus.56 However, reported issues of thrombosis with BFR are as little as 0.06% in some studies.57 Others have found that neither prothrombin time (PT) nor D-dimer levels increased following BFR training.58 This may be due to the fact that occlusion levels during most researched BFR protocols do not promote maximal occlusion, given that the device has a means of pressure regulation. suggesting cuff pressures be relative to cuff width and limb circumference to protect the neurovasculature of the extremity.54,59,60 Nerve conduction velocity (NCV) can be affected significantly by tourniquet pressure, giving rise to further research into electrophysiological changes during BFR.61 The use of wide tourniquets significantly reduces pressures needed for vascular occlusion and has been recommended in clinical settings.62 Additionally, personalized BFR utilizes Doppler technologies to individualize tourniquet pressures for each patient, providing further safety benefit.63
Ultimately, further investigation into BFR protocols and their safety is warranted. However, most protocols have demonstrated positive efficacy and good safety with very few ill effects. Under skilled supervision and with appropriate equipment, BFR can be used as a safe alternative to traditional high-intensity exercise to develop strength and muscular hypertrophy.
SUMMARY
BFR training could theoretically be safely and effectively implemented as part of a rehabilitation program addressing deficits associated with CAI. Benefits of BFR training include minimizing muscle weakness and atrophy associated with the acute phase of injury, potentially modulating pain related to injury conditions, facilitating tissue healing and enhancing muscle hypertrophy and strength gains when combined with low load exercise. Evidence also suggests that BFR training can enhance both aerobic and anaerobic properties when integrated as part of a cycling or walking protocol. These strategies and suggestions provided in this commentary could be especially helpful in cases of CAI where recurrent injury due to both local and proximal weakness, pain, and both decreased proprioception and function contribute to periods of low loading or limited sports specific activities during rehab where tissue healing and recovery is prioritized. Safety of the athlete should be prioritized through utilization of appropriate medical devices by trained medical personnel.
REFERENCES
- 1.Guillo S Bauer T Lee J et al. Consensus in chronic ankle instability: aetiology, assessment, surgical indications and place for arthroscopy. Orthop Traumatol Surg Res. 2013:99:S411-S419. [DOI] [PubMed] [Google Scholar]
- 2.Hong C Tan K. Concepts of ankle instability: A review. OA Sports Med. 2014;2:3. [Google Scholar]
- 3.Freeman M Dean M Hanham I. The etiology and prevention of functional instability of the foot. J Bone Jt Surg. 1965;47:678–85. [PubMed] [Google Scholar]
- 4.Ekstrand J Tropp H. The incidence of ankle sprains in soccer. Foot Ankle. 1990;11:41-44. [DOI] [PubMed] [Google Scholar]
- 5.McKay G Goldie P Payne W Oakes B. Ankle injuries in basketball: injury rate and risk factors. Br J Sports Med. 2001;35:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milgrom C Shlamkovitch N Finestone A et al. Risk factors for lateral ankle sprain: a prospective study among military recruits. Foot Ankle. 1991;12:26-30. [DOI] [PubMed] [Google Scholar]
- 7.Yeung M Chan K So C, et al. An epidemiological survey on ankle sprain. Br J Sports Med. 1994;28:112–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willems T Witvrouw E Verstuyft J Vaes P De Clercq D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J Athl Train. 2002;37:487-493. [PMC free article] [PubMed] [Google Scholar]
- 9.Friel K McLean N Myers C Caceres M. Ipsilateral hip abductor weakness after inversion ankle sprain. J Athl Train. 2006;41:74-78. [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnel F Toullec E Mabit C, et al. Chronic ankle instability: biomechanics and pathomechanics of ligaments injury and associated lesions. Orthop Traumatol Surg Res. 2010, 96:424-32. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez G Kaminski T Douex A. Neuromuscular control and ankle instability. PM&R. 2009;1:359–65. [DOI] [PubMed] [Google Scholar]
- 12.Baczkowicz D Falkowski K Majorczyk E. Assessment of relationships between joint motion quality and postural control in patients with chronic ankle joint instability. J Orthop Sports Phys Ther. 2017;47:570-577. [DOI] [PubMed] [Google Scholar]
- 13.McKay G Payne W Goldie P, et al. A comparison of the injuries sustained by female basketball and netball players. Aust J Sci Med Sport. 1996;28:12-17. [PubMed] [Google Scholar]
- 14.Taga I Shino K Inoue M et al. Articular cartilage lesions in ankles with lateral ligament injury: an arthroscopic study. Am J Sports Med. 1993;21:120-127. [DOI] [PubMed] [Google Scholar]
- 15.Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med. 2008;28:353-370. [DOI] [PubMed] [Google Scholar]
- 16.Perron M Moffet H Nadeau S Hebert LJ Belzile S. Persistence of long term isokinetic strength deficits in subjects with lateral ankle sprain as measured with a protocol including maximal preloading. Clin. Biomech. 2014;29:1151-1157. [DOI] [PubMed] [Google Scholar]
- 17.Pietrosimone B Gribble P. Chronic ankle instability and corticomotor excitability of the fibularis longus muscle. J Athl Train. 2012;47:621-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard C Sigward S Powers C. Limited hip and knee flexion during landing is associated with increased frontal plane motion and moments. Clin Biomech. 2010:25:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes L Paton B Rosenblatt B et al. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51:1003-1011. [DOI] [PubMed] [Google Scholar]
- 20.Takarada Y Takazawa H Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc. 2000;32:2035-2039. [DOI] [PubMed] [Google Scholar]
- 21.Kubota A Sakuraba K Sawaki K Sumide T Tamura Y. Prevention of disuse muscular weakness by restriction of blood flow. Med Sci Sports Exerc. 2008;40:529-534. [DOI] [PubMed] [Google Scholar]
- 22.Ohta H Kurosawa H Ikeda H Iwase Y Satou N Nakamura S. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand. 2003;74:62-68. [DOI] [PubMed] [Google Scholar]
- 23.Hylden C Burns T Stinner D Owens J. Blood flow restriction rehabilitation for extremity weakness: a case series. J Spec Oper Med. 2015;15:50-6. [PubMed] [Google Scholar]
- 24.Abe T Kawamoto K Yasuda T et al. Eight days KAATSU-resistance training improved sprint but not jump performance in collegiate male track and field athletes. Int J KAATSU Train Res. 2005;1:19-23. [Google Scholar]
- 25.Cook C Kilduff L Beaven C. Improving strength and power in trained athletes with 3 weeks of occlusion training. Int J Sports Physiol Perform. 2014;9:166-172. [DOI] [PubMed] [Google Scholar]
- 26.ACSM Position Stand. Progression Models in Resistance Training for Healthy Adults. Med Sci Sports Exerc. 2009;41:687-708. [DOI] [PubMed] [Google Scholar]
- 27.Abe T Yasuda T Midorikawa T Sato Y Kearns C Inoue K Koizumi K Ishii N. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int J Kaatsu Training Res. 2005;1:6-12. [Google Scholar]
- 28.Yasuda T Fujita S Ogasawara R Sato Y Abe T. Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: a pilot study. Clin Physiol Funct Imaging. 2010;30:338-343. [DOI] [PubMed] [Google Scholar]
- 29.Fry C Glynn E Drummond M Timmerman K Fujita S Abe T Rasmussen B. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108:1199-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita S Abe T Drummond M Cadenas J Dreyer H Sato Y Volpi E Rasmussen B. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Apply Physiol. 2007;103:903-910. [DOI] [PubMed] [Google Scholar]
- 31.Loenneke J Fahs C Thiebaud R, et al. The acute muscle swelling effects of blood flow restriction. Acta Physiologica Hungarica. 2012;99:400-410. [DOI] [PubMed] [Google Scholar]
- 32.Giles L Webster K McClelland J Cook J. Quadriceps strengthening with and without blood-flow restriction in the treatment of patellofemoral pain-a double blind randomized trial. Br J Sports Med. Published online: 12 May 2017. 10.1136. [DOI] [PubMed] [Google Scholar]
- 33.Korakakis V Whiteley R Epameinontidis K. Blood flow restriction-induced analgesia in patients with anterior knee pain. J Sci Med Sport. 20 (2017):e100. [DOI] [PubMed] [Google Scholar]
- 34.Saanijoki T Tuominen L Tuulari J et al. Opioid release after high-intensity interval training in healthy human subjects. Neuropsychopharmacology. 2017, 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suga T Okita K Takada S. Effect of multiple set on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. 2012;Eur J Appl Physiol. 112:3915-3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandner C Warmington S Kidgell D. Corticomotor excitability is increased following an acute bout of blood flow restriction resistance exercise. Front Hum Neurosci. 2015;9:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe T. Effects of short-term low intensity KAATSU training on strength and skeletal muscle size in young men. J Train Sci Exerc Sport. 2004;16:199-207. [Google Scholar]
- 38.Yasuda T Fujita T Miyagi Y et al. Electromyographic responses of arm and chest muscle during bench press exercise with and without KAATSU. Int J KAATSU Train Res. 2006;2:15-18. [Google Scholar]
- 39.Madarame H Neya M Ochi E, et al. Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc. 2008;40:258-263. [DOI] [PubMed] [Google Scholar]
- 40.Dankel SJ Jessee MB Abe T Loenneke JP. The Effects of Blood Flow Restriction on Upper-Body Musculature Located Distal and Proximal to Applied Pressure. Sport Med. 2016;46:23-33. [DOI] [PubMed] [Google Scholar]
- 41.Umbel JD Hoffman RL Dearth DJ, et al. , Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur J Appl Physiol. 2009;107:687-95. [DOI] [PubMed] [Google Scholar]
- 42.Loenneke J Abe T Wilson J et al. Blood flow restriction: an evidence based progressive model (review). Acta Physiol Hung. 2012;99:235-250. [DOI] [PubMed] [Google Scholar]
- 43.Leung M Rantalainen T Teo W et al. Motor cortex excitability is not differentially modulated following skill and strength training. Neuroscience. 2015;305:99-108. [DOI] [PubMed] [Google Scholar]
- 44.Pietrosimone B McLeod M Florea D Gribble P Tevald M. Immediate increases in quadriceps corticomotor excitability during an electromyography biofeedback intervention. J Electromyogr Kinesiol. 2015;25:316-322. [DOI] [PubMed] [Google Scholar]
- 45.Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35:922-926. [DOI] [PubMed] [Google Scholar]
- 46.Park S Kim J Choi H et al. Increase in maximal oxygen uptake following 2-week walk training with blood flow occlusion in athletes. Eur J Appl Physiol. 2010;109:591-600. [DOI] [PubMed] [Google Scholar]
- 47.Abe T Fujita S Nakajima T et al. Effects of low-intensity cycle training restricted leg blood flow on thigh muscle volume and VO2 max in young men. J Sport Sci Med. 2010;9:452-458. [PMC free article] [PubMed] [Google Scholar]
- 48.Ozaki H Sakamaki M Yasuda T et al. Increases in thigh muscle volume and strength by walk training with leg blood flow reduction in older participants. J Gerontol A Biol Sci Med Sci. 2011; 66:257-263. [DOI] [PubMed] [Google Scholar]
- 49.Scott B Loenneke J Slattery K Dascombe B. Exercise with blood flow restriction; an updated evidence-based approach for enhanced muscular development. Sports Med. 2015;45:313-325. [DOI] [PubMed] [Google Scholar]
- 50.Tang J Moore D Kujbida G Tarnopolsky M Phillips S. Ingestion of whey hydrolysate , casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107:987-992. [DOI] [PubMed] [Google Scholar]
- 51.Sato J Ishii Y Noguchi H Takeda M. Safety and efficacy of a new tourniquet system. BMC Surg. 2012;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Estebe JP Malledant Y. Pneumatic tourniquets in orthopedics. Ann Fr Anesth Reanim. 1996;15:162-178. [DOI] [PubMed] [Google Scholar]
- 53.He T Cao L Yang D, et al. A meta-analysis for the efficacy and safety of tourniquet in total knee arthroplasty. Zhonghua Wai Ke Za Zhi. 2011;49:551-557. [PubMed] [Google Scholar]
- 54.Loenneke JP Fahs CA Rossow LM, et al. Blood flow restriction pressure recommendations: a tale of two cuffs. Frontiers in Physiology. 2013;4:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lida H Takano H Meguro K. Hemodynamic and autonomic nervous responses to the restriction of femoral blood flow by KAATSU. Int J KAATSU Training Res. 2005;2:57-64. [Google Scholar]
- 56.Strock P Majno G. Vascular responses to experimental tourniquet ischemia. Surg Gynecol Obstet. 1969;129:309-318. [PubMed] [Google Scholar]
- 57.Nakajima T Kurano M Lida H et al. Use and safety of KAATSU training: results of a national survey. Int J KAATSU Training Res. 2006;2:5-13. [Google Scholar]
- 58.Clark B Manini T Hoffman R et al. Relative safety of 4 weeks of blood-flow restricted resistance exercise in young, healthy adults. Scand J Med Sci Sports. 2011;21:653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loenneke JP Fahs CA Rossow LM, et al. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol. 2012;112:2903-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laurentino GC Loenneke JP Teixeira EL Nakajima E Iared W Tricoli V. The effect of cuff width on muscle adaptations after blood flow restriction training. Med Sci Sports Exerc. 2016;48:920-925. [DOI] [PubMed] [Google Scholar]
- 61.Mittal P Shenoy S Sandhu JS. Effect of different cuff widths on the motor nerve conduction velocity of the median nerve: an experimental study. J Orthop Surg Res. 2008;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noordin S McEwen J Kragh J et al. Surgical tourniquets in orthopedics. J Bone Joint Surg Am. 2009;91:2958-2967. [DOI] [PubMed] [Google Scholar]
- 63.Younger A McEwen J Inkpen K. Wide contoured thigh cuffs and automated limb occlusion measurement allow lower tourniquet pressures. Clin Orthop Relat Res. 2004;428:286-293. [DOI] [PubMed] [Google Scholar]