Abstract
Introduction
Alterations in tendon structure and muscle performance have been suggested as mechanisms driving improvement in pain and function with mid-portion Achilles tendinopathy (AT). However, few trials have used consistent outcome measures to track differences in muscle structure and function, tendon structure and neural and pain associated mechanisms.
Objectives
1) Identify the outcomes measures used in trials utilising loading protocols for mid-portion AT that assess muscle structure and function, tendon structure and neural and pain associated mechanisms in order to report on the reliability of the identified measures, and 2) Propose a summary of measures for assessment of muscle structure and function, tendon structure and neural and pain associated mechanisms in patients with AT.
Design
Literature Review
Data Sources
Three electronic databases were searched from inception until May 2016 for studies using loading protocols for mid-portion AT.
Eligibility Criteria
Randomized and non-randomized trials of loading protocols for mid-portion AT.
Results
Twenty-eight studies were included; seven assessed muscle, 21 assessed tendon and two assessed neural and pain associated mechanisms. Evidence suggests that isokinetic dynamometry, eccentric-concentric heel raise tests, single leg drop counter-movement jumps or hopping are the most reliable ways to assess muscular adaptation. Assessment of tendon structure is unlikely to have any benefit given it does not appear to correlate to clinical outcomes. The neural and pain associated mechanisms have not been thoroughly investigated.
Conclusion
Further research needs to be done to determine the role of muscle, tendon and neural adaptations using reliable outcome measures during the management of mid-portion AT.
Level Of Evidence
Level Five.
Keywords: Achilles, outcome measures, reliability, tendinopathy, tendon structure, tendon function
INTRODUCTION
Achilles tendinopathy (AT) is a common running-related injury, with a prevalence of 6.2-9.5% in recreational runners.1 AT can present as either mid-portion or insertional tendinopathy. Mid-portion tendinopathy affects the mid-portion of the tendon approximately 2-4 cm proximal to the insertion whereas insertional tendinopathy affects the tendon insertion onto the calcaneus.2 Clinically, AT is characterised by pain and stiffness with these symptoms affecting athletic function.3,4 Mid-portion and insertional AT are considered different clinical entities,5 and accordingly, the scope of this review focusses purely on mid-portion AT.
Both researchers and clinicians are challenged by mid-portion AT.6 Exercise rehabilitation, specifically either eccentric only or isotonic resistance loading protocols, appear to be effective interventions over time.2,7 While these programs appear effective, a proportion of patients who complete loading protocols continue to experience symptoms at five year follow up.8,9 One potential explanation for this relates to the incomplete understanding of the mechanisms underpinning this therapy.10 Several theories have been alluded to in the literature including 1) alterations in tendon structure11 2) alterations in muscle performance12 and 3) alterations in pain mechanisms.13 Evidence supporting these theories however, is either sparse or conflicting and without clear guidance on what outcome measures should be used to assess muscle (structure and function), tendon and neural contributions.
The most commonly cited mechanism underpinning improvements in AT following a loading program is improvements in the material and mechanical properties of the tendon.11,14 This is based on the theory that disorganized fibrillar structure observed within tendinopathic tissue may be responsible for the pain associated with tendinopathy.3,4 For instance in the pathological tendon, a decrease in tendon size and signal on Magnetic Resonance Imaging (MRI) has been proposed to reflect positive improvement in the tendon.11,14 This theory has recently been challenged as the majority of work in this area indicates that changes in tendon structure and volume do not explain improvements in pain and function when assessed with greyscale ultrasound (US), ultrasound tissue characterisation (UTC) and MRI.15,16 Given the lack of correlation between tendon structure on imaging and improvement in pain and function, recent attention has been directed towards possible adaptations that may occur in either the muscular12 and/ or neural and pain associated mechanisms.13
The muscular system, specifically plantar flexor performance, may act as a ‘stress shield’ for the Achilles tendon,17 which is believed to optimize the stretch shortening cycle.18 A growing body of biomechanical literature supports the premise that muscle performance and musculotendinous behaviour are intimately linked, and can be modulated by training. In response to loading, muscle performance improves in timeframes of seconds to weeks, mediated initially by neural mechanisms,19 then subsequently by functional and structural changes within the muscle itself.20 For example, maximal voluntary isometric contraction has been shown to immediately increase following a single bout of isometric loading in patients with patellar tendinopathy and this was associated with a decrease in excess cortical motor inhibition.21 However, these findings have yet to be replicated in mid-portion AT. Medial gastrocnemius fascicles have been shown to have an intimate relationship with the stiffness of the muscle-tendon unit and can be considered in series with the Achilles tendon.22 Longer gastrocnemius fascicle length has also been shown to correlate with improved physical performance in running athletes.23 In healthy individuals, muscle strength improves within days following resistance training.19 These improvements are due to a plethora of suggested neural mechanisms such as increases in motor unit firing rates, double discharges of individual motor units, increased excitability of the motor cortex and cross transfer/ cross education.19 Changes in sensory receptors may also lead to a disinhibition or increased expression of muscle force.19 Despite these early changes in motor output, true muscular hypertrophy appears only after approximately three weeks of a resistance training protocol.20 Given that clinical improvements in response to loading interventions are typically observed prior to this time point, it is likely that other mechanisms driving this improvement are involved here also. The early improvement in pain and function without frank adaptation in muscular tissue implicates neural mechanisms as a likely mechanism underpinning this clinical observation.
The drivers of pain in both upper limb and lower limb tendinopathy remain poorly understood, although evidence exists supporting peripheral tendon-based nociceptive contributors as well as input from the central nervous system (CNS).10,24,25 Furthermore, a case control study specific to patellar and AT demonstrated a predominantly peripheral pain state.26 Importantly, immediate improvements in self-reported pain have been demonstrated following exercise rehabilitation for other conditions such as knee osteoarthritis.27 When it comes to measuring pain and function, it has also been highlighted that the reliability and validity of many outcome measures used within intervention studies for mid-portion AT have not been established.28 This means that the findings of research and clinical assessment may potentially be inaccurate or misleading.28
Thus, it is vital that outcome measures across all assessment domains are valid and reliable in order to produce high quality research which usefully informs our understanding of changes in muscle structure and function, tendon structure, and neural and pain associated mechanisms in mid-portion AT rehabilitation.
One challenge in understanding these mechanisms is that few trials have used consistent outcome measures to quantify the changes in muscle structure, muscle function, and tendon structure. The chosen outcome measures also lack sufficient reliability allow confidence in the results. It is important that clinicians and researchers are familiar with the outcome measures that have been used in clinical trials including their reliability, as sub-optimal outcome measures can produce results with questionable utility. However, while reliable measures are important for researchers it is also important for clinicians to know which measures are accessible for everyday practice. This may in turn reduce barriers to implementation of these measures.29
AIMS
The objectives of this literature review are: 1) Identify the outcomes measures used in trials utilising loading protocols for mid-portion AT that assess muscle structure and function, tendon structure and neural and pain associated mechanisms in order to report on the reliability of the identified measures, and 2) Propose a summary of measures for assessment of muscle structure and function, tendon structure and neural and pain associated mechanisms in patients with AT.
METHODS
Criteria for considering studies for this review
Types of studies
Both non-randomized cohort studies and randomized controlled trials were included if a loading protocol was used to treat mid-portion AT. Case reports, clinical observations, and systematic reviews were excluded.
Types of participants
Physically active and sedentary participants aged 18 years and over identified as having mid-portion AT for greater than three months were included. Studies including participants with insertional AT or other causes of pain (differential diagnoses) anywhere in the Achilles region were excluded from the review.
Types of interventions
Intervention studies using either isometric, eccentric, concentric or isotonic (eccentric and concentric) loading protocols were included. Studies that employed an isometric, eccentric, concentric or isotonic loading program in conjunction with a placebo therapy (for example sham laser treatment) were included.
Types of outcomes measures
Only studies that used an outcome measure of muscle structure and function, tendon structure, and neural and pain associated mechanisms in mid-portion AT were included.
Search methods for identification of studies
Electronic Searches
Searches using free text terms (Table 1) were used to identify published articles on the following electronic databases; PUBMED, CINAHL (Ovid) and CINAHL (EBSCO). Only peer reviewed, human, clinical trials and cohort studies were included.
TABLE 1.
Systematic Review Search Strategy
| Number | Combiners | Terms |
|---|---|---|
| 1 | Problem of Interest | Achilles tend* |
| 2 | Intervention | exercise OR eccentric OR isotonic OR resistance OR strength* |
| 3 | #1 AND #2 | |
| Limitations | Peer reviewed, human, clinical trials written in English were included. |
Searching other Resources
Reference lists from reviews and retrieved articles were checked and citation searches on key articles performed. The list of included studies was evaluated by content experts to help identify any additional relevant studies.
Data collection and analysis
Selection of Studies
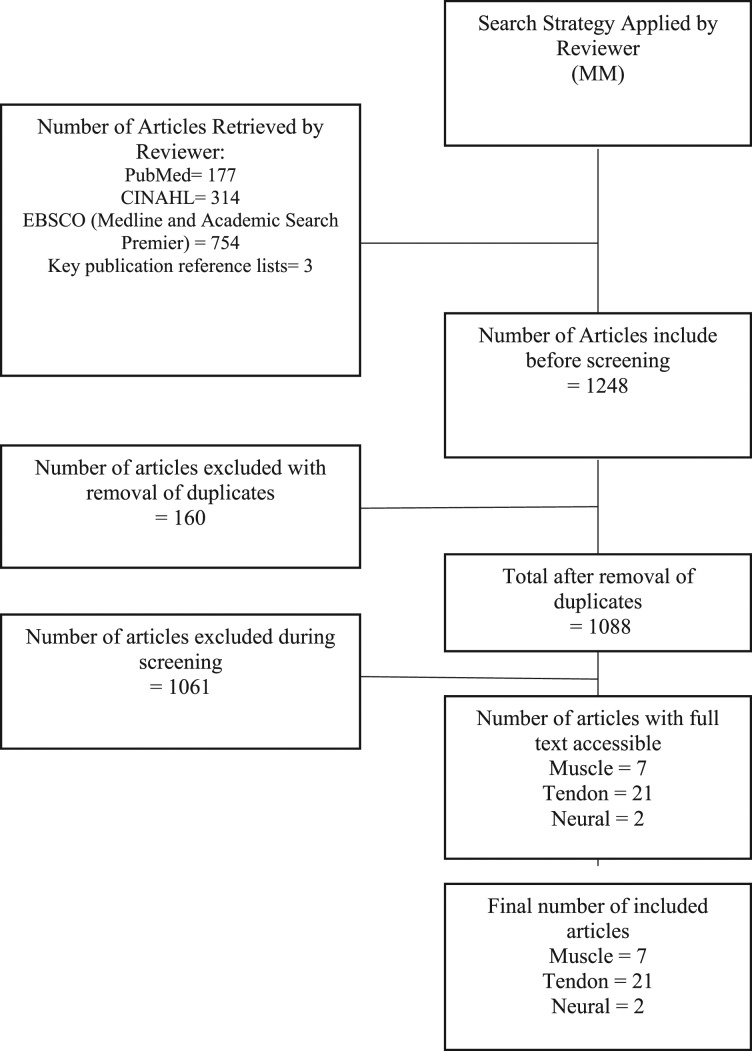
One review author (MM) searched and assessed the titles and abstracts of potential studies identified by the search strategy for their eligibility. Studies were exported to reference management software EndNote X8.0.2 (Clarivate Analytics, 2017) and duplicates were removed. If the eligibility of a study was unclear from the title and abstract the full paper was assessed. Studies that did not match the inclusion criteria for this review were excluded. Studies were not anonymised prior to assessment. A PRISMA study flow diagram was used to document the screening process.30
Data abstraction and management
One review author (MM) extracted data from all included studies using a standardized and piloted data extraction form on Microsoft Excel (Microsoft, 2016). The following information was recorded; primary author, year of publication, study design, study population (diagnosis), sample size, loading intervention (e.g. heavy eccentric calf training), outcome measures used, number of follow up points and time (weeks) at each follow up point.
Data synthesis
Reliability was reported if provided by the study using the outcome measure. If the study provided a reference to psychometric properties, the referenced study was used to extract the data.
RESULTS
Selection of Studies
A total of seven studies were included that contained a measure of muscle structure or function.31-37 A total of 21 studies were included that contained a measure of tendon structure.9,11,14,15,33,38-53 A total of two studies were included that contained a measure of neural and pain associated mechanisms.8,51 Given overlap in some studies a total of 28 studies were included for analysis. The study selection process is demonstrated in the Prisma Flow Diagram (Figure 1).
Figure 1.
PRISMA Study Flow Diagram.
Data Extraction
Data were extracted from 28 studies, of which some studies assessed a combination of muscle strength and function, tendon structure and neural and pain associated mechanisms. The characteristics of the included studies are presented in Appendix A.
MUSCLE STRUCTURE AND FUNCTION
Data Synthesis
The outcome measures used to assess muscle structure and function are presented in Table 2. Three separate measures are shown to represent muscle function; categorized into those looking at muscle strength, endurance, or power production
TABLE 2.
Outcome measures assessing muscle structure and function
| Outcome Measure | Frequency in Clinical Trials (n) | Follow up (weeks) | Reliability |
|---|---|---|---|
| STRUCTURE | |||
| Greyscale US – Gastrocnemius Fascicle Length | 132 | 4, 8 | Intra-Session Reliability in healthy adults without lower limb pain or injury: ICC=0.9154 |
| Greyscale US –Gastrocnemius Pennation Angle | 132 | 4, 8 | Intra-Session Reliability in post-stroke patients: ICC=0.69-0.8255 Inter-Session Reliability in post-stroke patients: ICC=0.70-0.9655 |
| Greyscale US –Gastrocnemius Muscle Thickness | 132 | 4, 8 | Intra-Session Reliability in post stroke patients: ICC=0.96-0.9955 Inter-Session Reliability in post stroke patients: ICC=0.97-0.9955 |
| STRENGTH | |||
| Isokinetic Dynamometry – 30 degrees / second (Peak Torque) | 136 | 8 | Test Re-Test Reliability in healthy, older women without lower limb pain or injury: ICC=0.89, SEM=8.7 units56 Inter-Session Reliability in healthy adults without lower limb pain or injury: ICC=0.9057 |
| Isokinetic Dynamometry – 60 degrees / second (Peak Torque) | 133 | 12 | Intra-Session Reliability in patients following Achilles tendon rupture: ICC=0.80-0.9058 Inter-Session Reliability in patients following Achilles tendon rupture: ICC=0.76-0.9258 |
| Isokinetic Dynamometry – 90 degrees / second (Peak Torque) | 131 | 12 | Test Re-Test Reliability in healthy, older women without lower limb pain or injury: ICC=0.85, SEM=8.9 units56 Inter-Session Reliability in healthy adults without lower limb pain or injury: ICC=0.9357 |
| Isokinetic Dynamometry – 90 degrees / second (Total Work) | 131 | 12 | Not Reported |
| Isokinetic Dynamometry – 120 degrees / second (Mean Peak Torque) | 136 | 8 | Intra-Session Reliability in healthy adults without lower limb pain or injury: r=0.9459 Inter-Session Reliability in healthy adults without lower limb pain or injury: r=0.9459 |
| Isokinetic Dynamometry – 225 degrees / second (Peak Torque) | 131 | 12 | Intra-Session Reliability in patients with chronic mid-portion Achilles tendinopathy: r=0.5531 |
| Isokinetic Dynamometry – 225 degrees / second (Total Work) | 131 | 12 | Not Reported |
| Toe Raise Test (Concentric) | 134 | 6, 12, 26, 52 | Test Re-Test Reliability in patients with mid-portion Achilles tendinopathy: ICC = 0.73-0.82, SEM=17% (0.1)60 |
| Toe Raise Test (Concentric/ Eccentric) | 134 | 6, 12, 26, 52 | Test Re-Test Reliability in patients with mid-portion Achilles tendinopathy: ICC = 0.76-0.86, SEM=15-17% (72W)60 |
| ENDURANCE | |||
| Isokinetic Dynamometry – 20 degrees / second (Mean Peak Torque for Endurance) | 133 | 12 | Not Reported |
| Toe Raise Test | 334,35,37 | 6, 12, 26, 52 | Test Re-Test Reliability in patients with mid-portion Achilles tendinopathy: r=0.5635 Test Re-Test Reliability in healthy adults without lower limb pain or injury: ICC=0.78-0.8461 |
| POWER PRODUCTION | |||
| Single Leg CMJ – Height | 135 | 6, 12, 26 | Test Re-Test Reliability in patients with mid-portion Achilles tendinopathy: r=0.9335 |
| Single Leg CMJ – Time in Air | 135 | 6, 12, 26 | Test Re-Test Reliability in patients with mid-portion Achilles tendinopathy: r=0.9335 |
| Single Leg CMJ | 134 | 6, 12, 26, 52 | Test Re-Test Reliability in patients with mid-portion Achilles tendinopathy: ICC = 0.91, SEM=8% (1.2cm)60 |
| Single Leg Drop CMJ | 134 | 6, 12, 26, 52 | Test Re-Test Reliability in patients with mid-portion Achilles tendinopathy: ICC = 0.88-0.92, SEM=11-13% (1.3-1.7cm)60 |
| Hopping (Plyometric Quotient) | 234,37 | 6, 12, 26, 52 | Test Re-Test Reliability in patients with mid-portion Achilles tendinopathy: ICC = 0.83-0.94, SEM=9-11% (0.1)60 |
| Sargant Jump Test | 136 | 8 | Test Re-Test Reliability in healthy adults without lower limb pain or injury: ICC=0.96, CV=3%62 |
US = Ultrasound; CMJ = coumter movement jump;
TABLE 3.
Outcome measures assessing tendon structure
| Outcome Measure | Frequency in Clinical Trials (n) | Follow up (weeks) | Reliability |
|---|---|---|---|
| STRUCTURE | |||
| Greyscale US – AP Tendon Thickness | 109,39,41,42,46,48-52 | 4, 6, 12, 16, 52, 3.8 years | Test Retest Reliability in healthy adults without lower limb pain or injury: Variation = 0.06(22%)63 Intra-rater Reliability in healthy adults without lower limb pain or injury: Variation = 0.05(19%)63 Inter-rater Reliability in healthy adults without lower limb pain or injury: Variation=0.16(60%)63 |
| Greyscale US – Pathology (Yes/ No) | 333,47,48 | 12, 28 months, 3.8 years | Test Retest Reliability in patients with chronic mid-portion Achilles tendinopathy: CV=1.147 |
| Greyscale US - 4 Point Pathology Grading Scale | 146 | 12, 52 | Inter-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: κ = <0.446 |
| Doppler US - Colour Fraction Measurement (%) | 139 | 12, 52 | Not reported |
| Doppler US - Ohberg Score (0-2) | 147 | 28 months | Test Retest Reliability in patients with chronic mid-portion Achilles tendinopathy: CV=1.147 |
| Doppler US - Modified Ohberg Score (0-4) | 59,40,41,43,53 | 0, 6, 12, 24, 52, 5 years | Inter-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: ICC=0.8564 |
| UTC - % of Type 1-4 Echoes | 215,42 | 2, 6, 8, 16, 24, 52 | Intra-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: ICC=0.95-0.9965 |
| MRI - 4 Point Scale of Intratendinous Signal | 114 | 12, 14 months, 4.2 years | Intra-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: κ=0.5814 Inter-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: κ=0.7114 |
| MRI - Mean Signal (Units) | 111 | 12 | Intra-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: r=0.84-0.9711 Inter-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: r=0.78-0.9511 |
| MRI - Tendon Volume | 211,14 | 12, 14 months, 4.2 years | Intra-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: r=0.98-0.9911,14 Inter-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: r=0.96-0.9911,14 |
| PARA-TENDINOUS SUBSTANCES | |||
| Micro-dialysis - Intratendinous Glutamate | 138 | 12 | Not Reported |
| Micro-dialysis - Interstitial PICP from paratendinous space just ventral to the Achilles tendon (Collagen synthesis) | 145 | 12 | Intra-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: CV=2.7% at 214 μg/L45 |
| Micro-dialysis - Interstitial ICTP from paratendinous space just ventral to the Achilles tendon (Collagen Degradation) | 145 | 12 | Intra-rater Reliability in patients with chronic mid-portion Achilles tendinopathy: CV=4.9% at 6.1 μg/L45 |
| TENDON/ PARATENON MICROCIRCULATION | |||
| Tendon and Paratenon Microcirculation using non-invasive combined laser-Doppler and Flowmetry systems | 144 | 12 | Intra-rater Reliability in healthy adults without lower limb pain or injury: CV=5%66 Inter-rater Reliability in healthy adults without lower limb pain or injury: CV=32-37%66 |
AP = anterior-posterior; US = ultrasound; UTC = ultrasound tissue characterisation; MRI = Magnetic resonance imaging; PICP = interstitial carboxyterminal propeptide of type I collagen; ICTP = interstitial carboxyterminal telopeptide region of type I collagen; CV = coefficient of variance
Structure
Ultrasound (US) has been used to examine muscle structure in a single clinical trial.32 US assessment of the calf muscle complex has shown excellent reliability when assessing gastrocnemius thickness (Intra-rater ICC=0.96-0.99, Inter-rater ICC=0.97-0.99),55 gastrocnemius fascicle length (Intra-rater ICC=0.91)54 and gastrocnemius pennation angle (Intra-rater ICC=0.69-0.82, Inter-rater ICC=0.70-0.96).55
Strength
Isokinetic dynamometry, albeit at different speeds, has been used to measure strength in three clinical trials31,33,36 while the eccentric-concentric toe/heel raise strength tests have been used in one clinical trial.34 Excluding isokinetic dynamometry at 225 degrees/second, all other speeds tested showed superior (good to excellent) reliability when compared to the eccentric-concentric heel-raise tests. Specifically, isokinetic dynamometry at 120deg/s shows excellent reliability (r=0.94).59 The eccentric-concentric heel-raise test also shows good, yet inferior, reliability (ICC = 0.76-0.86)60 when compared to isokinetic dynamometry.
Endurance
To measure muscle endurance, isokinetic dynamometry has been used in one clinical trial33 and heel-raise test for endurance have been used in two clinical trials.34,35 The reliability of slow, endurance based isokinetic dynamometry has not been assessed in the ankle, however the heel-raise test for endurance shows good reliability (ICC=0.78-0.84).60
Power Production
Power production has been assessed using a variety of different tests measuring multiple variables (e.g. height, air time etc) with the Sargent jump test being used in one clinical trial,36 the one legged counter movement jump (CMJ) being used in two clinical trials,34,35 the one legged drop CMJ being used in one clinical trial34 and hopping used in two clinical trials.34,37 The Sargent jump test (Test-retest ICC=0.96)62 reported marginally better reliability than one legged CMJ (Test-retest ICC=0.91-0.93),35,60 one legged drop CMJ's (r=0.0.88-92)60 and hopping (Test-retest ICC=0.83-0.94).60
TENDON STRUCTURE
Data Synthesis
The outcome measures used to assess tendon structure are presented in Table Three. Three separate measures are shown; categorised into those looking at structure, para-tendinous substances, and tendon/ paratenon microcirculation.
Structure
Tendon structure has been measured using Greyscale US in 10 clinical trials,9,39,41,42,46,48-52 Doppler US has been used in seven clinical trials,9,39-41,43,47,53 UTC has been reported in two clinical trials15,42 and MRI has been used in two clinical trials.11,14 Excellent intra-rater reliability was seen when classifying tendon structure into echo-types using UTC (ICC=0.95-0.99).65 MRI assessment of tendon volume showed excellent intra-rater (r=0.98-0.99)11 and inter-rater(r=0.96-0.99)11 reliability. MRI assessment of Mean Achilles Tendon Signal has shown excellent intra-rater (r=0.84-0.97)11 and inter-rater reliability (r=0.78-0.95).11 Grading tendon structure on Doppler US using the Modified Ohberg Score (0 = no vessels visible, 1 + =1 vessel, mostly anterior to the tendon, 2 + =1 or 2 vessels throughout the tendon, 3 + =3 vessels throughout the tendon, or 4 + =more than 3 vessels throughout the tendon) showed good inter-rater reliability (ICC=0.85).64 Tendon thickness measured by greyscale US has shown good intra-rater (ICC=0.78-0.90)67 and inter-rater (ICC=0.72-0.73)67 reliability.
Para-tendinous substances
One clinical trial has measured paratendinous substances using micro-dialysis.45 The variability of assessing interstitial carboxyterminal propeptide of type I collagen (PIPC) was shown to be good (CV=2.7% at 214 μg/L)45 and the variability of assessing interstitial carboxyterminal telopeptide region of type I collagen (ICTP) was also shown to be good (CV=4.9% at 6.1 μg/L).45
Tendon/ paratenon microcirculation
Tendon and paratenon micro-circulation has been measured by assessing non-invasive combined laser Doppler and flowmetry in one clinical trial. The intra-tester (CV=5%)66 and inter-tester (CV=32-37%)66 variability has shown to be good.
NEURAL AND PAIN ASSOCIATED MECHANISMS
Data synthesis
The outcome measures used to assess domains within the nervous system are listed in Table 4.
TABLE 4.
Outcome measures assessing the neural and pain associated mechanisms
| Outcome Measure | Frequency in Clinical Trials (n) | Follow up (weeks) | Reliability |
|---|---|---|---|
| Pressure Pain Threshold (Pressure on an Algometer at which pain is first experienced) | 151 | 16 | Inter-rater Reliability in chronic mid-portion Achillea tendinopathy : ICC = 0.9668 |
| Tampa Scale of Kinesiophobia | 18 | 5 Years | Test-Retest Reliability in patients with acute lower back pain: r=0.64-0.8069 |
Peripheral sensitivity
There is a dearth of information assessing the neural and pain associated mechanisms in clinical trials in AT. Mechanosensitivity has been assessed using Pressure Pain Thresholds (PPT) in one clinical trial.51 PPT's using manual algometry have been shown to have excellent intra-rater reliability (ICC=0.96)68 in the Achilles tendon.
DISCUSSION
Muscle structure and function
Structure
In patients with AT, medial gastrocnemius fascicle length significantly increases within six weeks of beginning an eccentric calf program and correlates with improvements in pain and function.32 However, while US measures of fascicle length are reliable54 these investigators did not establish the reliability of the measure themselves32 leading to potential bias. The role of soleus fascicle length has not been investigated yet in mid-portion AT. Whilst US is reliable for assessing calf muscle architecture, changes in calf muscle thickness have not been shown to correlate to improvement in pain and function in mid-portion AT leaving questions regarding its utility.32 MRI has not been used in clinical trials to assess muscle structure but it is highly reliable in assessing the muscle volume of the gastrocnemius (Intra-rater ICC=0.99-1.00, Inter-rater ICC=0.99)70 and soleus (Intra-rater ICC=0.99-1.00, Inter-rater ICC=0.99).70 US and MRI are appropriate for use in assessing muscle structure in research. However, access to these measures and the training required to report on them is likely a significant barrier to use. Quantification of muscular hypertrophy or architectural changes will not likely occur for at least three weeks following the inception of a loading protocol.20,32 Therefore, any future work investigating these changes require appropriate timeframes for follow-up.
Strength
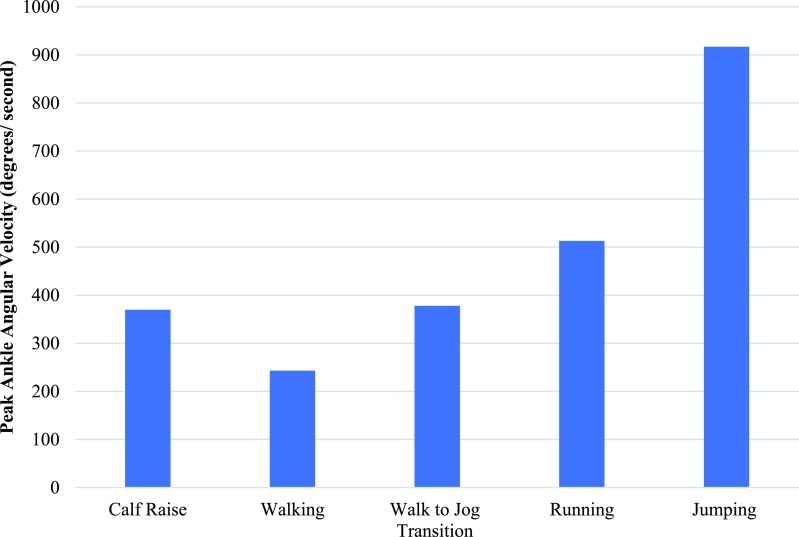
A variety of different assessments have been used to quantify muscle strength in mid-portion AT. Varied speeds for isokinetic dynamometry have been reported and speed of isokinetic dynamometry should therefore be selected based on the role of the muscle to tie into function. The required speed for performing a heel raise is 6.3-6.5 rad/s,71 walking is 4.2 rad/s,72 transitioning from walking to running is 6.6 rad/s,73 running is 8.4-9.0 rad/s74 and jumping is 16 rad/s75 (Figure 2). Given the large angular velocities required in the ankle for function, isokinetic dynamometry at 120 deg/s appears to be the fastest, yet most reliable form of measuring strength. However, even at this velocity it does not mimic functional speeds. More functional strength measures such as the eccentric-concentric heel-raise test may better mirror the required angular velocities60 and can be used clinically by recording a three-repetition maximum using weights equipment such as a Smith machine. The eccentric-concentric heel-raise test has also been validated in mid-portion AT and is able to differentiate between participants more painful and least/ non painful side (p = 0.001-0.006).60 Isokinetic dynamometry has also only assessed plantar flexor strength in a straight knee position which biases the gastrocnemius.76 To bias the soleus when assessing plantar flexion with isokinetic dynamometry, the knee must be flexed to greater than 45 degrees.77 However, it should be stated that neither of these positions eliminate the other plantar flexor muscles and purely act to selectively bias towards one muscle.77 Further research is needed to determine relevant contributions of the gastrocnemius or soleus in explaining the mechanisms in improvement in pain and function with mid-portion AT. An additional barrier is that isokinetic dynamometry is expensive and often not available in clinical settings. While isokinetic dynamometry remains the gold standard, the eccentric-concentric heel-raise test with its high test-retest reliability and good validity represents a more feasible clinical test.
Figure 2.
Peak Ankle Angular Velocity during Functional Tasks.71-75,78
Given the immediate improvements in pain and function observed in several interventional studies,15,37 performing strength testing at multiple time points may provide information around different contributors to clinical improvement. Though pain is more complex than this, if we consider strength specifically, testing at four weeks following the commencement of the loading program may highlight the neural contribution to strength19 whilst testing after this time likely includes the contribution of muscular hypertrophy to strength.20
Endurance
The heel-raise test for endurance has good reliability60 and given the simplicity of the test it represents the best outcome measure for assessing plantar flexor endurance in both research and clinical practice. However, significant variability exists in how this test can be performed and this may impact its utility both clinically and in research.76 It is also important to note that no differences were seen when using the heel-raise test for endurance in participants with AT between painful and non-painful/ least painful sides (P=0.077).60 Further research into the reliability of endurance based Isokinetic Dynamometry needs to be performed before it can be recommended for use in research or clinical practice.
Power production
A systematic review demonstrated sensory and motor deficits are present bilaterally in patients with unilateral tendon pain and disability however, this review only included one paper specifically on AT.24 Functional tasks including jumping and hopping are often the most aggravating tasks in mid-portion AT60 therefore choosing the appropriate measure to assess this functional deficit is important for both clinicians and researchers. In participants with AT, significant unilateral differences during hopping were observed when comparing the painful to the non-painful/ less symptomatic limb; height (p=0.006), plyometric quotient (p=0.001) and visual analogue scale (VAS) pain score (p=0.000).60 Significant differences were also seen during a one-legged drop CMJ task; height (P=0.049), contact time (p=0.008) and VAS pain score (p=0.001).60 However no significant differences were seen with a normal CMJ; height (p=0.665) and VAS pain score (p=0.012).60 Given the significant differences between limbs, unilateral functional tests are likely more sensitive to detecting deficits in function than bilateral tests.
The single leg drop CMJ or hopping appear to be the most appropriate measures of power production given the high test- retest reliability they demonstrate and that they correlate to deficits seen in people with AT.60
For the one-legged drop CMJ and Hopping tests reported by Silbernagel et al.60 a jump mat is required; however, this is expensive. Recently smartphone applications have been validated against jump mats (p<0.001) and been shown to have excellent test-retest reliability (ICC=0.997)79 and are more easily accessible for both clinicians and researchers.
Tests of power production represent functional performance and improve due to a combination of muscular and neural factors. These could be assessed as early as four weeks as improvements in muscle strength are potentially correlated to improvements in power production which may be reflected by an increase in the Victorian Institute of Sports Assessment-Achilles (VISA-A). However, due to a test of power likely being correlated to muscle strength and pain it is unlikely to provide specific insight into mechanisms underpinning the improvement patients obtain with loading programs. This distinction may or may not be important for the patient but improvement in functional capacity may encourage rehabilitation adherence. It would also be of great interest to the clinician and the researcher who wish to assess which components or deficits need to be addressed to optimise outcomes in the clinic and in trials.
Tendon structure
Structure
Both UTC and MRI are not easily accessible for researchers and clinicians. However, these modalities are the most reliable investigations to assess tendon structure and, unlike conventional US, allow for quantification of tendon structure. These measures have been correlated to symptoms in some studies,11,14,65 however they have also been shown to have a poor relationship to improvement in others.15,42 Doppler US has also been shown to have no correlation to patient symptoms or improvement.16 Given improvements are seen on the VISA-A as early as two weeks following the inception of a loading protocol,15,37 determining whether or not tendon structural adaptations correlate to these improvements would require assessment to be completed when the improvement in pain and function is first seen. Radiological imaging for tendon pathology is known to be highly assessor dependant with poor inter-rater reliability.65 The reliability of grading radiological imaging in tendon pathology has also been demonstrated to be better with more experienced radiologists.80 Given the extent of variation due to different assessors the use of previous reliability studies to justify a new trials reliability should be discouraged and should always be re-examined in an effort to reduce potential bias. Follow up imaging is not indicated for clinicians treating patients with loading protocols given the poor access, reporting difficulties, patient compliance, and cost. Specifically, differences in tendon structure on imaging do not correlate to improvements in clinical status and therefore are not recommended in the management of mid-portion AT.16
Para-tendinous substances and Tendon/paratenon microcirculation
Measures of para-tendinous substance and micro-circulation are expensive and can be invasive, but they may provide some insight into the mechanisms playing a role in tendinopathy. However, the authors suggest this would be a research application only due to cost and accessibility.
Neural and pain associated mechanisms
Central nervous system mechanisms modulating pain have been shown to be important in chronic musculoskeletal pain conditions such as rheumatoid arthritis, osteoarthritis and fibromyalgia,81 yet little research using these outcomes has been performed in AT. A recent systematic review demonstrated nervous system sensitization in persistent tendinopathy, however identified no Achilles tendon studies and only one patellar tendon study out of 16 studies.25
Pressure pain thresholds have been used to assess peripheral and central sensitization,82 however PPTs have also been reported as a potentially poor marker of central and peripheral sensitivity.83 Given the conflicting evidence it is unclear as to how much useful insight PPTs offer into the mechanisms underlying the improvements patients report with loading programs. However, since PPTs have been assessed using manual algometers in one clinical trial and have been shown to have excellent reliability they represent the best (currently available) measure to assess mechanosensitivity of the Achilles tendon.
No clinical trials of loading programs in mid-portion AT have investigated the contribution of different CNS driven mechanisms involved in modulating pain. Three cross sectional studies of patients with AT have identified alterations in 1) endogenous analgesia,68 2) fear of movement8 and 3) altered tactile acuity (as a suggested reflection of somatosensory cortical reorganisation).84
Endogenous analgesia is typically assessed via the conditioned pain modulation paradigm (CPM). Endogenous analgesia measured via the CPM effect is a centrally driven mechanism that modulates pain.85 Briefly, this requires measurement of the PPT of the Achilles tendon before and during/ after a painful stimulus (e.g. immersion of the participant's hand in cold water) is applied in a remote area from the painful site.68 The change in the PPT is known as the CPM effect and a reduction in mechanosensitivity during/ after the painful conditioning stimulus is regarded as normal.68 One study has demonstrated people with mid-portion AT have deficient CPM compared with controls68 suggesting that further investigation into the role this may play in people with mid-substance AT is needed. However, the pain map provided in this study included people with heel and posterior ankle pain beyond what is currently considered diagnostic of mid-portion AT5 leading to considerable heterogeneity of the group and likely involving other differential diagnoses.
‘Fear of movement’ or kinesiophobia is a centrally driven mechanism which may result in restriction of activity and has been shown to be predictive of long term disability in persistent pain conditions, such as chronic low back pain.86 The Tampa scale of Kinesiophobia is one way of measuring fear of movement and has been shown to be have moderate to good test-retest reliability (r=0.64-0.80).69 However, it has only been used in long term follow up of patients with AT and not at baseline8 so it is not possible to make inferences on its prognostic value and therefore future prospective studies quantifying its role in mid-portion AT are needed.
Impaired tactile acuity is theorised to reflect many mechanisms, including altered cortical representation of the painful region.87 A systematic review of 16 studies found that tactile acuity is impaired in persistent pain conditions such as arthritis, complex regional pain syndrome and chronic lower back pain.87 Recently, tactile acuity has been shown to be impaired in mid-portion AT when compared to the unaffected limb and healthy controls.84 Further research is needed to investigate whether alterations in tactile acuity contribute to initiation of the pain experience or are a consequence of it. Given that no investigations of CNS-driven mechanisms of pain modulation, such as the CPM effect or fear of movement, have been completed with loading protocols in mid-portion AT, future investigations should determine if differences occur with loading protocols and what the most optimal timeframes are to assess these differences.
Pressure pain thresholds and the CPM effect can be difficult to assess clinically and therefore may only be plausible for researchers. For clinicians, clinical tools to examine of fear of movement may provide insight into the role of CNS driven mechanisms that modulate pain.
LIMITATIONS
The reliability of the tests used to assess muscle structure and function, tendon structure and neural and pain associated mechanisms have been reported for the selected outcome measures in most studies. However, a small proportion of assessments used did not have the reliability assessed in a mid-portion AT population. It is unclear whether the reliability of these tests needs to be assessed in this population to remain a valid measure. It is also possible that as a measure of structure and function the tests remain valid across heterogenous populations but as this cannot be certain it may introduce bias.
CONCLUSION
Many different outcome measures have been used to assess muscular adaptations, tendon adaptations and neural and pain associated mechanisms in clinical trials treating mid-portion AT with loading protocols. Isokinetic dynamometry at 120 deg/s and eccentric-concentric heel-raise tests are the suggested best measures of plantar flexor muscle strength, US is capable of measuring muscle architectural properties and the single leg drop CMJ or hopping are the best measures of power production. UTC or MRI are acknowledged as the best ways with which to classify tendon structure and tendon signal. However, given the lack of relationship between tendon structural change and symptoms they are not recommended for clinical use. Endogenous analgesia assessed via the CPM paradigm may be completed simply using a tonic stimulus (such as cold-water immersion) with PPT assessed via manual algometry pre and post application of this tonic stimulus. It is important for clinicians and researchers to be aware of the outcome measures that have been used as well as the reliability of these measures. By identifying consistent measures that are available to both clinicians and researchers we may be able to gain further insight into the mechanisms responsible for improving pain and function in mid-portion AT.
APPENDIX A.
Individual Study Characteristics
| Study Name | Study Design | Sample Size (n) | Loading Protocol Used |
|---|---|---|---|
| Alfredson et al. (1998)31 | Cohort | 15 | Heavy Eccentric Calf Training |
| Alfredson & Lorentzon. (2003)38 | Cohort | 6 | Heavy Eccentric Calf Training |
| Beyer et al. (2015)39 | RCT | 25 | Heavy Eccentric Calf Training |
| 22 | Heavy Slow Resistance Training | ||
| Brown et al. (2006)37 | RCT | 18 | Heavy Eccentric Calf Training |
| Crill et al. (2014)32 | Cohort | 25 | Heavy Eccentric Calf Training |
| De Jonge et al. (2010)40 * | Cohort | 32 | Heavy Eccentric Calf Training |
| De Jonge et al. (2011)41 * | RCT | 27 | Heavy Eccentric Calf Training |
| De Jonge et al. (2015)42 * | RCT | 54 | Heavy Eccentric Calf Training |
| De Vos et al. (2007)53 * | Cohort | 58 | Heavy Eccentric Calf Training |
| De Vos et al. (2010)43 * | RCT | 27 | Heavy Eccentric Calf Training |
| De Vos et al. (2012)15 * | Cohort | 24 | Heavy Eccentric Calf Training |
| Gardin et al. (2010)14 * | Cohort | 24 | Heavy Eccentric Calf Training |
| Horstmann et al. (2013)33 | RCT | 19 | Heavy Eccentric Calf Training |
| Knobloch et al. (2008)44 | RCT | 59 | Heavy Eccentric Calf Training |
| Langberg et al. (2007)45 | Cohort | 6 | Heavy Eccentric Calf Training |
| Norregaard et al. (2007)46 | RCT | 21 | Heavy Eccentric Calf Training |
| Ohberg & Alfedson (2004)47 | Cohort | 30 | Heavy Eccentric Calf Training |
| Ohberg et al. (2004)48 | Cohort | 25 | Heavy Eccentric Calf Training |
| Peterson et al. (2007)49 | RCT | 37 | Modified Heavy Eccentric Calf Training |
| Rompe et al. (2007)51 | RCT | 23 | Heavy Eccentric Calf Training |
| Rompe et al. (2009)50 | RCT | 30 | Heavy Eccentric Calf Training |
| Silbernagel et al. (2001)35 | RCT | 22 | Eccentric Overload |
| Silbernagel et al. (2007)34 | RCT | 26 | Eccentric Overload with Active Rest |
| 24 | Eccentric Overload | ||
| Silbernagel et al. (2011)8 | Cohort | 34 | Eccentric Overload |
| Tumilty et al. (2016)52 | RCT | 13 | Heavy Eccentric Calf Training |
| 19 | Modified Heavy Eccentric Calf Training | ||
| Van der Plas et al. (2012)9 * | Cohort | 46 | Heavy Eccentric Calf Training |
| Yu et al. (2013)36 | RCT | 16 | Heavy Eccentric Calf Training |
| 16 | Concentric Calf Training |
The results of this study are a follow up of an included study or present different components of data from another included study.
REFERENCES
- 1.Lopes AD Hespanhol LC Yeung SS, et al. What are the main running-related musculoskeletal injuries? Sports Med. 2012;42(10):891-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habets B van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25(1):3-15. [DOI] [PubMed] [Google Scholar]
- 3.Cook JL Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409-16. [DOI] [PubMed] [Google Scholar]
- 4.Cook JL Rio E Purdam CR, et al. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research? Br J Sports Med. 2016;50(19):1187-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk CN van Sterkenburg MN Wiegerinck JI, et al. Terminology for Achilles tendon related disorders. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):835-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott A Docking S Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47(9):536-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy M Travers M Gibson W, et al. The rate of improvement of pain and function in mid-portion Achilles tendinopathy with loading protocols: A Systematic Review and Longitudinal Meta-Analysis. Sports Med. 2018; 1-17. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Silbernagel KG Brorsson A Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: a 5-year follow-up. Am J Sports Med. 2011;39(3):607-13. [DOI] [PubMed] [Google Scholar]
- 9.van der Plas A de Jonge S de Vos RJ, et al. A 5-year follow-up study of Alfredson's heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46(3):214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rio E Moseley L Purdam C, et al. The pain of tendinopathy: physiological or pathophysiological? Sports Med. 2014;44(1):9-23. [DOI] [PubMed] [Google Scholar]
- 11.Shalabi A Kristoffersen-Wilberg M Svensson L, et al. Eccentric training of the gastrocnemius-soleus complex in chronic Achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am J Sports Med. 2004;32(5):1286-96. [DOI] [PubMed] [Google Scholar]
- 12.Allison GT Purdam C. Eccentric loading for Achilles tendinopathy — strengthening or stretching? Br J Sports Med. 2009;43(4):276-79. [DOI] [PubMed] [Google Scholar]
- 13.Rio E Kidgell D Moseley GL, et al. Tendon neuroplastic training: changing the way we think about tendon rehabilitation: a narrative review. Br J Sports Med. 2016;50(4):209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardin A Movin T Svensson L, et al. The long-term clinical and MRI results following eccentric calf muscle training in chronic Achilles tendinosis. Skeletal Radiol. 2010;39(5):435-42. [DOI] [PubMed] [Google Scholar]
- 15.de Vos RJ Heijboer MP Weinans H, et al. Tendon structure's lack of relation to clinical outcome after eccentric exercises in chronic midportion Achilles tendinopathy. J Sports Rehab. 2012;21(1):34-43. [DOI] [PubMed] [Google Scholar]
- 16.Drew BT Smith TO Littlewood C, et al. Do structural changes (eg, collagen/matrix) explain the response to therapeutic exercises in tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):966-72. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill S Watson PJ Barry S. Why are eccentric exercises effective for achilles tendinopathy? Int J Sport Phys Ther. 2015;10(4):552-62. [PMC free article] [PubMed] [Google Scholar]
- 18.Debenham JR Gibson WI Travers MJ, et al. Eccentric loading of triceps surae modulates stretch shortening cycle behaviour- a possible therapeutic mechanism. J Sports Rehab. 2016:1-22. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel DA Kamen G Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36(2):133-49. [DOI] [PubMed] [Google Scholar]
- 20.DeFreitas JM Beck TW Stock MS, et al. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol. 2011;111(11):2785-90. [DOI] [PubMed] [Google Scholar]
- 21.Rio E Kidgell D Purdam C, et al. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br J Sports Med. 2015;49(19):1277-83. [DOI] [PubMed] [Google Scholar]
- 22.Farcy S Nordez A Dorel S, et al. Interaction between gastrocnemius medialis fascicle and Achilles tendon compliance: a new insight on the quick-release method. J Appl Phys. 2014;116(3):259-66. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai K Abe T Brechue WF, et al. Sprint performance is related to muscle fascicle length in male 100-m sprinters. J Appl Phys. 2000;88(3):811-6. [DOI] [PubMed] [Google Scholar]
- 24.Heales LJ Lim EC Hodges PW, et al. Sensory and motor deficits exist on the non-injured side of patients with unilateral tendon pain and disability--implications for central nervous system involvement: a systematic review with meta-analysis. Br J Sports Med. 2014;48(19):1400-6. [DOI] [PubMed] [Google Scholar]
- 25.Plinsinga ML Brink MS Vicenzino B, et al. Evidence of nervous system sensitization in commonly presenting and persistent painful tendinopathies: a systematic review. J Orthop Sports Phys Ther. 2015;45(11):864-75. [DOI] [PubMed] [Google Scholar]
- 26.Plinsinga ML van Wilgen CP Brink MS, et al. Patellar and Achilles tendinopathies are predominantly peripheral pain states: a blinded case control study of somatosensory and psychological profiles. Br J Sports Med. 2018;52(5):284-91. [DOI] [PubMed] [Google Scholar]
- 27.Vincent KR Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(5 0):S45-S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy M Rio E Debenham J, et al. Evaluating the progress of mid-portion Achilles tendinopathy during rehabilitation. A review of outcome measures for self- reported pain and function. Int J Sport Phys Ther. 2018;13(2)283-292 [PMC free article] [PubMed] [Google Scholar]
- 29.Jette DU Halbert J Iverson C, et al. Use of standardized outcome measures in physical therapist practice: perceptions and applications. Phys Ther. 2009;89(2):125-35. [DOI] [PubMed] [Google Scholar]
- 30.Liberati A Altman DG Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alfredson H Pietila T Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26(3):360-6. [DOI] [PubMed] [Google Scholar]
- 32.Crill MT Berlet G Hyer C. Plantar flexor muscle architecture changes as a result of eccentric exercise in patients with Achilles tendinosis. Foot Ankle Spec. 2014;7(6):460-5. [DOI] [PubMed] [Google Scholar]
- 33.Horstmann T Jud HM Frohlich V, et al. Whole-body vibration versus eccentric training or a wait-and-see approach for chronic Achilles tendinopathy: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43(11):794-803. [DOI] [PubMed] [Google Scholar]
- 34.Silbernagel KG Thomee R Eriksson BI, et al. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: a randomized controlled study. Am J Sports Med. 2007;35(6):897-906. [DOI] [PubMed] [Google Scholar]
- 35.Silbernagel KG Thomee R Thomee P, et al. Eccentric overload training for patients with chronic Achilles tendon pain--a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports. 2001;11(4):197-206. [DOI] [PubMed] [Google Scholar]
- 36.Yu J Park D Lee G. Effect of eccentric strengthening on pain, muscle strength, endurance, and functional fitness factors in male patients with achilles tendinopathy. Am J Phys Med Rehabil. 2013;92(1):68-76. [DOI] [PubMed] [Google Scholar]
- 37.Brown R Orchard J Kinchington M, et al. Aprotinin in the management of Achilles tendinopathy: a randomised controlled trial. Br J Sports Med. 2006;40(3):275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alfredson H Lorentzon R. Intratendinous glutamate levels and eccentric training in chronic Achilles tendinosis: a prospective study using microdialysis technique. Knee Surg Sports Traumatol Arthrosc. 2003;11(3):196-9. [DOI] [PubMed] [Google Scholar]
- 39.Beyer R Kongsgaard M Hougs Kjaer B, et al. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43(7):1704-11. [DOI] [PubMed] [Google Scholar]
- 40.de Jonge S de Vos RJ Van Schie HT, et al. One-year follow-up of a randomised controlled trial on added splinting to eccentric exercises in chronic midportion Achilles tendinopathy. Br J Sports Med. 2010;44(9):673-7. [DOI] [PubMed] [Google Scholar]
- 41.de Jonge S de Vos RJ Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39(8):1623-9. [DOI] [PubMed] [Google Scholar]
- 42.de Jonge S Tol JL Weir A, et al. The tendon structure returns to asymptomatic values in nonoperatively treated Achilles tendinopathy but is not associated with symptoms: a prospective study. Am J Sports Med. 2015;43(12):2950-8. [DOI] [PubMed] [Google Scholar]
- 43.de Vos RJ Weir A van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-9. [DOI] [PubMed] [Google Scholar]
- 44.Knobloch K Schreibmueller L Longo UG, et al. Eccentric exercises for the management of tendinopathy of the main body of the Achilles tendon with or without the AirHeel Brace. A randomized controlled trial. A: effects on pain and microcirculation. Disabil Rehabil. 2008;30(20-22):1685-91. [DOI] [PubMed] [Google Scholar]
- 45.Langberg H Ellingsgaard H Madsen T, et al. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand J Med Sci Sports. 2007;17(1):61-6. [DOI] [PubMed] [Google Scholar]
- 46.Norregaard J Larsen CC Bieler T, et al. Eccentric exercise in treatment of Achilles tendinopathy. Scand J Med Sci Sports. 2007;17(2):133-8. [DOI] [PubMed] [Google Scholar]
- 47.Ohberg L Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg Sports Traumatol Arthrosc. 2004;12(5):465-70. [DOI] [PubMed] [Google Scholar]
- 48.Ohberg L Lorentzon R Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med. 2004;38(1):8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen W Welp R Rosenbaum D. Chronic Achilles tendinopathy: a prospective randomized study comparing the therapeutic effect of eccentric training, the AirHeel brace, and a combination of both. Am J Sports Med. 2007;35(10):1659-67. [DOI] [PubMed] [Google Scholar]
- 50.Rompe JD Furia J Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2009;37(3):463-70. [DOI] [PubMed] [Google Scholar]
- 51.Rompe JD Nafe B Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35(3):374-83. [DOI] [PubMed] [Google Scholar]
- 52.Tumilty S Mani R Baxter GD. Photobiomodulation and eccentric exercise for Achilles tendinopathy: a randomized controlled trial. Lasers Med Sci. 2016;31(1):127-35. [DOI] [PubMed] [Google Scholar]
- 53.de Vos RJ Weir A Cobben LP, et al. The value of power Doppler ultrasonography in Achilles tendinopathy: a prospective study. Am J Sports Med. 2007;35(10):1696-701. [DOI] [PubMed] [Google Scholar]
- 54.Kudo S Hisada T Sato T. Determination of the fascicle length of the gastrocnemius muscle during calf raise exercise using ultrasonography. J Phys Ther Sci. 2015;27(12):3763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho KH Lee HJ Lee WH. Reliability of rehabilitative ultrasound imaging for the medial gastrocnemius muscle in poststroke patients. Clin Physiol Funct Imaging. 2014;34(1):26-31. [DOI] [PubMed] [Google Scholar]
- 56.Webber SC Porter MM. Reliability of ankle isometric, isotonic, and isokinetic strength and power testing in older women. Phys Ther. 2010;90(8):1165-75. [DOI] [PubMed] [Google Scholar]
- 57.Taskiran OO Gzdugan V Sepici V, et al. Test-retest and inter-rater reliability of isokinetic ankle dorsiflexor and plantar flexor strength measurement in healthy adults/Saglikli eriskinlerde ayak bilegi dorsifleksor ve plantar fleksor izokinetik kas kuvvet olcumlerinin gozlemci ici ve gozlemciler arasi guvenilirligi. Turk J Phys Med Rehab. 2013;59(1):32. [Google Scholar]
- 58.Chester R Costa ML Shepstone L, et al. Reliability of isokinetic dynamometry in assessing plantarflexion torque following Achilles tendon rupture. Foot Ankle Int. 2003;24(12):909-15. [DOI] [PubMed] [Google Scholar]
- 59.Karnofel H Wilkinson K Lentell G. Reliability of isokinetic muscle testing at the ankle. J Orthop Sports Phys Ther. 1989;11(4):150-4. [DOI] [PubMed] [Google Scholar]
- 60.Silbernagel KG Gustavsson A Thomee R, et al. Evaluation of lower leg function in patients with Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1207-17. [DOI] [PubMed] [Google Scholar]
- 61.Moller M Lind K Styf J, et al. The reliability of isokinetic testing of the ankle joint and a heel-raise test for endurance. Knee Surg Sports Traumatol Arthrosc. 2005;13(1):60-71. [DOI] [PubMed] [Google Scholar]
- 62.Markovic G Dizdar D Jukic I, et al. Reliability and factorial validity of squat and countermovement jump tests. J Strength Cond Res. 2004;18(3):551-5. [DOI] [PubMed] [Google Scholar]
- 63.O'Connor PJ Grainger AJ Morgan SR, et al. Ultrasound assessment of tendons in asymptomatic volunteers: a study of reproducibility. Eur Radiol. 2004;14(11):1968-73. [DOI] [PubMed] [Google Scholar]
- 64.Sengkerij PM de Vos RJ Weir A, et al. Interobserver reliability of neovascularization score using power Doppler ultrasonography in midportion achilles tendinopathy. Am J Sports Med. 2009;37(8):1627-31. [DOI] [PubMed] [Google Scholar]
- 65.van Schie HT de Vos RJ de Jonge S, et al. Ultrasonographic tissue characterisation of human Achilles tendons: quantification of tendon structure through a novel non-invasive approach. Br J Sports Med. 2010;44(16):1153-9. [DOI] [PubMed] [Google Scholar]
- 66.Ghazanfari M Vogt L Banzer W, et al. Reproduzierbarkeit nicht-invasiver Durchblutungsmessung mit der Laser-Doppler-Spektroskopie. Phys Med Rehab Kuror. 2002;12(06):330-36. [Google Scholar]
- 67.Johannsen F Jensen S Stallknecht SE, et al. Sonographic measurements of the achilles tendon, plantar fascia, and heel fat pad are reliable: A test-retest intra- and intertester study. J Clin Ultrasound. 2016;44(8):480-6. [DOI] [PubMed] [Google Scholar]
- 68.Tompra N van Dieen JH Coppieters MW. Central pain processing is altered in people with Achilles tendinopathy. Br J Sports Med. 2016;50(16):1004-7. [DOI] [PubMed] [Google Scholar]
- 69.Swinkels-Meewisse EJ Swinkels RA Verbeek AL, et al. Psychometric properties of the Tampa scale for kinesiophobia and the fear-avoidance beliefs questionnaire in acute low back pain. Man Ther. 2003;8(1):29-36. [DOI] [PubMed] [Google Scholar]
- 70.Commean PK Tuttle LJ Hastings MK, et al. Magnetic resonance imaging measurement reproducibility for calf muscle and adipose tissue volume. J Magn Reson Imaging. 2011;34(6):1285-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arai T Obuchi S Shiba Y, et al. The feasibility of measuring joint angular velocity with a gyro-sensor. Arch Phys Med Rehabil. 2008;89(1):95-9. [DOI] [PubMed] [Google Scholar]
- 72.Crowther RG Spinks WL Leicht AS, et al. Relationship between temporal-spatial gait parameters, gait kinematics, walking performance, exercise capacity, and physical activity level in peripheral arterial disease. J Vasc Surg. 2007;45(6):1172-8. [DOI] [PubMed] [Google Scholar]
- 73.Hreljac A. Determinants of the gait transition speed during human locomotion: kinematic factors. J Biomech. 1995;28(6):669-77. [DOI] [PubMed] [Google Scholar]
- 74.Heidenfelder J Sterzing T Bullmann M, et al. Heel strike angle and foot angular velocity in the sagittal plane during running in different shoe conditions. J Foot Ankle Res. 2008;1(1):O16. [Google Scholar]
- 75.Bobbert MF Huijing PA van Ingen Schenau GJ. A model of the human triceps surae muscle-tendon complex applied to jumping. J Biomech. 1986;19(11):887-98. [DOI] [PubMed] [Google Scholar]
- 76.Hebert-Losier K Newsham-West RJ Schneiders AG, et al. Raising the standards of the calf-raise test: a systematic review. J Sci Med Sport. 2009;12(6):594-602. [DOI] [PubMed] [Google Scholar]
- 77.Hebert-Losier K Schneiders AG Garcia JA, et al. Influence of knee flexion angle and age on triceps surae muscle activity during heel raises. Journal Strength Cond Res. 2012;26(11):3124-33. [DOI] [PubMed] [Google Scholar]
- 78.Neptune RR Sasaki K. Ankle plantar flexor force production is an important determinant of the preferred walk-to-run transition speed. J Exp Biol. 2005;208(Pt 5):799-808. [DOI] [PubMed] [Google Scholar]
- 79.Balsalobre-Fernandez C Glaister M Lockey RA. The validity and reliability of an iPhone app for measuring vertical jump performance. J Sports Sci. 2015;33(15):1574-9. [DOI] [PubMed] [Google Scholar]
- 80.Sein ML Walton J Linklater J, et al. Reliability of MRI assessment of supraspinatus tendinopathy. Br J Sports Med. 2007;41(8):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee YC Nassikas NJ Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13(2):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jespersen A Amris K Graven-Nielsen T, et al. Assessment of pressure-pain thresholds and central sensitization of pain in lateral epicondylalgia. Pain Med. 2013;14(2):297-304. [DOI] [PubMed] [Google Scholar]
- 83.Kamper SJ Maher CG Hush JM, et al. Relationship between pressure pain thresholds and pain ratings in patients with whiplash-associated disorders. Clin J Pain. 2011;27(6):495-501. [DOI] [PubMed] [Google Scholar]
- 84.Debenham J Butler P Mallows A, et al. Disrupted tactile acuity in people with Achilles tendinopathy: a preliminary case-control investigation. J Orthop Sports Phys Ther. 2016;46(12):1061-64. [DOI] [PubMed] [Google Scholar]
- 85.Locke D Gibson W Moss P, et al. Analysis of meaningful conditioned pain modulation effect in a pain-free adult population. J Pain. 2014;15(11):1190-8. [DOI] [PubMed] [Google Scholar]
- 86.Swinkels-Meewisse IE Roelofs J Schouten EG, et al. Fear of movement/(re)injury predicting chronic disabling low back pain: a prospective inception cohort study. Spine 2006;31(6):658-64. [DOI] [PubMed] [Google Scholar]
- 87.Catley MJ O'Connell NE Berryman C, et al. Is tactile acuity altered in people with chronic pain? a systematic review and meta-analysis. J Pain. 2014;15(10):985-1000. [DOI] [PubMed] [Google Scholar]