Abstract
Background
Topical application of menthol is a popular form of cold therapy and chemically triggers cold receptors and increases cutaneous blood flow. However, although cutaneous blood flow increases, it remains unknown where this increase arises from. Intramuscular temperature assessment may indirectly indicate a change in muscular blood flow.
Purpose
To establish intramuscular temperature, blood flow responses and subjective temperature sensation following application of menthol-based cooling gel to the anterior thigh.
Study design
Controlled, randomized cross over interventional study
Methods
Twenty (age: 21.4 + 1.7) healthy males were treated on three separate days in random order with ice, a menthol-based gel or placebo gel (participant single blinded) on one anterior thigh. All measurements were taken at baseline and for 80 mins following treatment: 1) Skin, core, and intramuscular temperatures (1 & 3 cm deep); 2) femoral arterial blood flow (duplex ultrasound); 3) cutaneous blood flow (laser Doppler) and 4) subjective cold sensation.
Results
Ice and both gels decreased (p<0.0001, CI (Ice): -5.2 to -6.2 and CI (gels) -1.4 to -2.5) intramuscular temperature by 5.7 and 1.9 °C respectively, but by 80 mins were similar to each other (1.5-2 °C less than pre-treatment). Skin temperature mirrored muscle temperature with 8.8 and 4.2 °C respective decline for ice and gels. Menthol gel increased (p<0.0001) cutaneous blood flow by 0.3 ml/min compared to unaltered flow associated with the placebo gel and a decline of 0.3 ml/min for the ice. Menthol gel cold sensation was subjectively reported to be cooler (p<0.0001) than the other two treatments. Core temperature and arterial flow were unaffected.
Conclusion
This is the first study to demonstrate the intramuscular cooling effect of menthol-based gel. However, the likely cause was from evaporative cooling despite menthol-derived increases in cutaneous blood flow and cooling sensation.
Level of evidence
Treatment, level 2.
Keywords: Cold therapy, intramuscular temperature, temperature sensation
INTRODUCTION
Cold therapy is widely used to reduce the amount of soft tissue damage following an acute injury1 and has been proposed to reduce inflammatory response, haematoma, oedema and pain.2-4 Cold therapy is typically administered by either ice or gel packs, ice massage, or cold water immersion. Such forms of therapy can often be impractical due to environmental factors and access issues to freezers and ice baths when travelling. Additionally, these forms of cold therapy require at least 10 minutes of application for effect1 thus, rendering their use for minor injuries during games/competition likewise impractical. Therefore, localized topical application of menthol containing cooling gels may represent a suitable and practical alternative.
Menthol is an ingredient that activates Transient Receptor Potential Melastatin 8 (TRMP8) channels which are non–selective and are known to also open in response to cold temperature (8-28 °C).5 Menthol and cold therapy provide cold sensations6 as well as an analgesic effect.7,8 In contrast to traditional ice therapy which decreases cutaneous blood flow,9,10 menthol has been shown to increase cutaneous blood flow.11,12 This increased cutaneous flow occurs through activation of TRMP8 receptors in the vascular cells,12,13 which in turn increase Nitric Oxide production from the endothelial cells resulting in localized vasodilation.12,14,15 This, in itself, could have important clinical benefit for treating patients with peripheral neuropathy by increasing cutaneous blood flow to their hands and feet to help with distal sensation.11
The resultant increased cutaneous blood flow has to occur by diverting blood from another region and there is no topical menthol research describing where this blood may be redirected from. Therefore, it can be speculated that the menthol induced vasodilation and increased cutaneous flow, as previously described,11,12 derives from a diversion of blood from muscle and in doing so may reduce intramuscular temperature. Thus, menthol may induce intramuscular cooling, reduce inflammation and edema with similar endpoints to ice but through an alternative mechanism. However, to the authors’ knowledge, no research has demonstrated if intramuscular temperature decline occurs following treatment of topical menthol. Accordingly, the author's aim was to establish intramuscular temperature, blood flow responses and subjective temperature sensation following application of menthol-based cooling gel to the anterior thigh. In order to establish this the authors compared a menthol containing gel; a placebo gel; and a traditional ice pack, within a cross over design. To account for the effects of rest over time the authors also took measurements from the untreated contralateral leg. The authors hypothesized that the menthol gel would increase cutaneous blood flow, by reducing intramuscular temperature while maintaining arterial blood flow and increase cooling sensation.
Methods
Twenty young healthy, active males were recruited for the study, their age; mass and height were 21.4 + 1.7 years, 81.4 + 10.5 and 179.1 + 7.9 cm respectively. A difference of 3.8 °C + 1.5 °C temperature decline difference between ice and menthol gel treatment for 95% power was used to calculate a minimum sample size requirement of n=5 (G*Power) was estimated (5% significance level). All participants were to be injury free and had to avoid any unaccustomed exercise during the trial that may cause delayed onset of muscle soreness (DOMS). Participants abstained from strenuous exercise and followed their usual dietary habits for 24 h prior to test sessions, which were conducted at the same time of day to account for circadian variation.16 Skinfold measurements of the anterior thigh did not exceed 20 mm. Approval for the study was granted by the local research ethics committee in accordance with the Helsinki Declaration of 2013 and all participants signed an Informed consent form prior to testing and their rights protected throughout the entire process.
EXPERIMENTAL OVERVIEW
Participants attended the laboratory on three separate occasions, in the morning, with at least 48 hours between visits. The participants were asked to refrain from any physical activity 24 hours preceding their visit and arrive following a 10 hour fast whereupon they were fed a standardized breakfast of 30 g of cornflakes and 150 ml of semi skimmed milk one hour prior to testing. They arrived with activity and diet diaries completed for the preceding seven days. Every participant received all three different treatments (1 per visit) in random order, by using Excel (Microsoft Office 2013 Professional Plus) RAND function, in a cross over design. The laboratory conditions were kept at a constant 22 °C throughout. The leg to be treated was randomly selected, by also using Excel, on the first visit but kept the same for the remaining two visits. The trial was run by an experienced physiologist (PhD) along with a postgraduate physiologist (Mphil). No adverse side effects were reported.
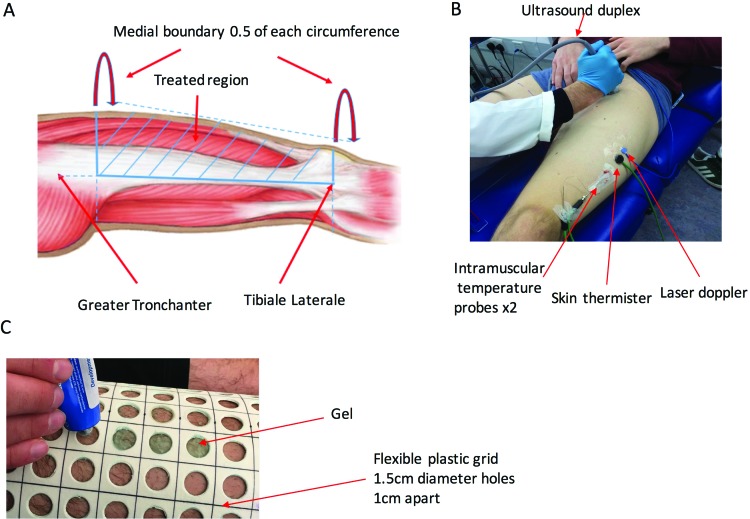
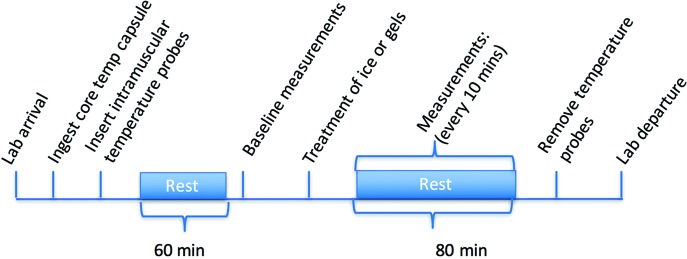
Following breakfast, the participants ingested a telemetric core temperature capsule (Philips VitalSense, Linton Instrumentation, Norfolk, UK) with the assistance of tap water. The participants then had intramuscular temperature probes (13050, 0.7 mm diameter, Ellab, Hilleroed, Denmark) inserted into both anterior thighs, along with surface skin temperature thermisters (MHC, Ellab, Hilleroed, Denmark) attached (Figure 2B) whereupon temperature was recorded prior to resting for 60 minutes. Then femoral arterial blood flow was recorded using a 10-MHz multifrequency linear array transducer attached to a high- resolution ultrasound machine (Sonosite, M Turbo, FUJIFILM SonoSite, Inc. Washington) and marked for repeat measurements along with cutaneous blood flow from the vastus lateralis. Then the treatment of either ice pack, menthol-based gel or placebo gel were applied to one thigh while the other thigh was used to control for the effects of rest. Immediately following treatment measurements of skin and intramuscular temperature, cutaneous blood flow and a visual thermal comfort scale17 were taken and repeated every 10 minutes. Femoral arterial flow was measured every 20 minutes. These measurements were continued up until 80 minutes (Figure 1).
Figure 2.
Anterior thigh - region of treatment (A), experimental measurements for femoral arterial blood flow (Ultrasound duplex), cutaneous blood flow (Laser doppler), intramuscular temperature (probes) and skin temperature (thermistor) (B) and gel application (C).
Figure 1.
Time course of the experimental procedure. During the 80 minute period the temperature measurements will be recorded every 5 minutes whereas the blood flow every 20 minutes.
INTERVENTIONS
The anterior thigh portion of the leg to be treated was marked; a line was drawn from the greater trochanter of the femur to the lateral tibial condyle, the highest thigh circumference (below the groin) was then measured from this line with half of the circumference drawn across to the other side of the leg. Then, the lowest thigh circumference was drawn from the same line just above the patella with half of this circumference drawn across to the other side of the leg. Then, the two medial half circumferences were joined up to complete the markings for the anterior region to be treated (Figure 2A). Then a flexible plastic grid containing 1.5 cm diameter holes, 1 cm apart from each other, was placed over the marked anterior thigh portion (Figure 2C). Even portions of either Deep Freeze Cold Relief Gel® (The Mentholatum Company Ltd, East Kilbride, UK) containing (3% by weight [w/w] levomenthol; 35-40% w/w ethanol) or placebo gel (The Mentholatum Company Ltd, Glasgow, Scotland) (0% w/w levomenthol; 3% w/w water; 35-40% w/w ethanol) were then placed into the holes, ensuring sufficient amounts applied by covering the skin area exposed by the holes. The quantity of gel used for each participant was measured by weighing the gel tube twice for pre and post treatment. The overall amount of menthol gel used was 5.1 + 1.2 ml/200 cm2 vs. 5.7 + 1.1 ml/200 cm2 of placebo gel with no statistical difference (paired t-test) between the two. Although this is greater than previous studies which used 1 ml/200 cm2 18,19 and 3.3 ml/200 cm2, these prior studies have used hand/wrist and forearms respectively. Whereas our the present study used the anterior thigh which is a far greater surface area therefore provides greater capacity for gel absorption during massage. The grid was then removed whereupon the gel was gently massaged using a stroking technique grade 120 into the skin for 10 minutes. The sampling area where the probes were located formed ∼14% of the overall treatment area; although this sampling area had gel applied it was carefully massaged into the skin by avoiding the probes to prevent disturbing positioning. For the ice treatment two plastic bags containing ice were secured to the marked anterior thigh portion, on top of a dry paper towel for 10 minutes. The control leg was covered with cloth to protect from the cold effects of the ice.
MEASUREMENTS
Core, skin and muscle temperature
Telemetric Tcore capsules were consumed upon arrival to the laboratory two hours prior to experimental trials to ensure passing into the gastrointestinal tract.21 These capsules then transmitted core temperature to data monitor (Equivital EQ02 LifeMonitor, Cambridge, UK) at a frequency of four samples/min which transmitted to an Android application (eqView, Equivital, Cambridge, UK) for recording. A skin thermistor was attached 5 cm proximal to the intramuscular electrodes of the vastus lateralis (Figure 2B) for the assessment of skin temperature. Thigh skinfold thickness was measured using Harpenden skinfold calipers (Baty International, West Sussex, United Kingdom) and divided by two to determine the thickness of the thigh subcutaneous fat layer over each participant's vastus lateralis, following which the surface area was cleaned with an alcohol swab. Then, intramuscular temperature was assessed using two sterilized needles (1.4 mm diameter), containing sterilized temperature probes; these contain small thermocouple probes have very low thermal inertia for a fast response and are insulated by a thin polymer layer so the surrounding environment cannot alter the measurement. The needles containing the probes were inserted into the main belly of the vastus lateralis (Figure 2B) at depths of 1 and 3 cm plus one-half the skinfold measurement, following which the needle was carefully withdrawn while ensuring that the probe remained in situ. The retracted needle was then secured to the thigh using surgical tape. Both muscle and skin temperature were then recorded using a hand-held data logger thermometer (HH378, Omega, Manchester, UK) at a frequency of one sample/second. Both intramuscular and skin probes were checked for accuracy by regularly performing two-point calibrations using known water temperatures. The validity of this technique been confirmed previously work where there was observed expected intramuscular temperature rises following exercise induced hyperthermia.22
Heart Rate and Arterial Pressure
Heart rate was continuously monitored during the experimental protocol (Accurex Plus, Polar, Kempele, Finland). Arterial blood pressure was measured noninvasively via automated brachial auscultation (Dinamap, GE Pro 300V2, Tampa, Florida), and mean arterial pressure was calculated as follows: diastolic / (0.3333 [systolic – diastolic]).
Femoral Artery Blood Flow
Femoral artery diameter and velocity were measured using a 10-MHz multifrequency linear array transducer attached to a high- resolution ultrasound machine (Sonosite, M Turbo, FUJIFILM SonoSite, Inc. Washington). The images were taken at the superficial femoral artery ∼3 cm distal to the bifurcation (Figure 2B). Ultrasound parameters were set to optimize longitudinal B-mode images of the lumen- arterial wall interface. Continuous and synchronized pulsed-wave Doppler velocities were also obtained using the ultrasound machine. Data were collected using an insonation angle of 60 °, and each measurement was recorded for 30 seconds. This position was marked on the skin for ultrasound head repositioning during the remaining measures. Post-test analysis of femoral artery diameter was performed using custom-designed edge detection which provides simultaneous and continuous measurements of arterial diameter and blood flow velocity. The assessment of blood flow velocity uses the edge detection algorithm to assess the peak velocity envelope from the Doppler gate, which is placed in the middle of the artery. From these data, the software calculates blood flow (the product of cross-sectional area and blood flow velocity) at 30 Hz. In this experiment, femoral artery blood was shown to have a coefficient of variation of 20% on the control leg repeat measurement. All data were written to a file and retrieved for analysis in the custom-designed analysis package. The diameter, velocity, and flow were then calculated as the mean of the data acquired across each 30-second period for statistical analysis.
Cutaneous blood flow
A laser Doppler probe (BL 52, Transonic, Ithaca, NY, USA) was attached to the mid-anterior thigh, midline, halfway between the inguinal line and the patella (Figure 2B) for the measurement of cutaneous blood flow. Measurements were determined by using the average of samples recorded over 30s time periods for each 10-minute interval.
Subjective measurements
The visual 7 point (1 = much too cool; 7=much too warm) Thermal Comfort Scale17 was used to establish thermal comfort every 10 minutes. The authors’ have previously used the scale during exercise induced hyperthermia22 and found a strong relationship with core temperature increases.
Statistical Analysis
All data are presented as mean ±SD. Differences between conditions for muscle temperature (1 and 3 cm depth), skin temperature, core temperature, arterial and cutaneous blood flow, subjective thermal rating index were examined using a two factor (condition [menthol gel, placebo gel, ice] 3 × time, 10) repeated measures ANOVA. Effects of rest and contralateral influences between treated leg and control leg were examined by using a two factor (condition [menthol gel; contralateral control leg, Placebo Gel; contralateral control leg], 4 × time, 10) repeated measures ANOVA. Where necessary, effects were followed by Tukey's post-hoc tests. All data analysis was performed on statistical software (Graphpad Prism v.6, USA); significance was accepted at α = 0.05.
RESULTS
Temperature
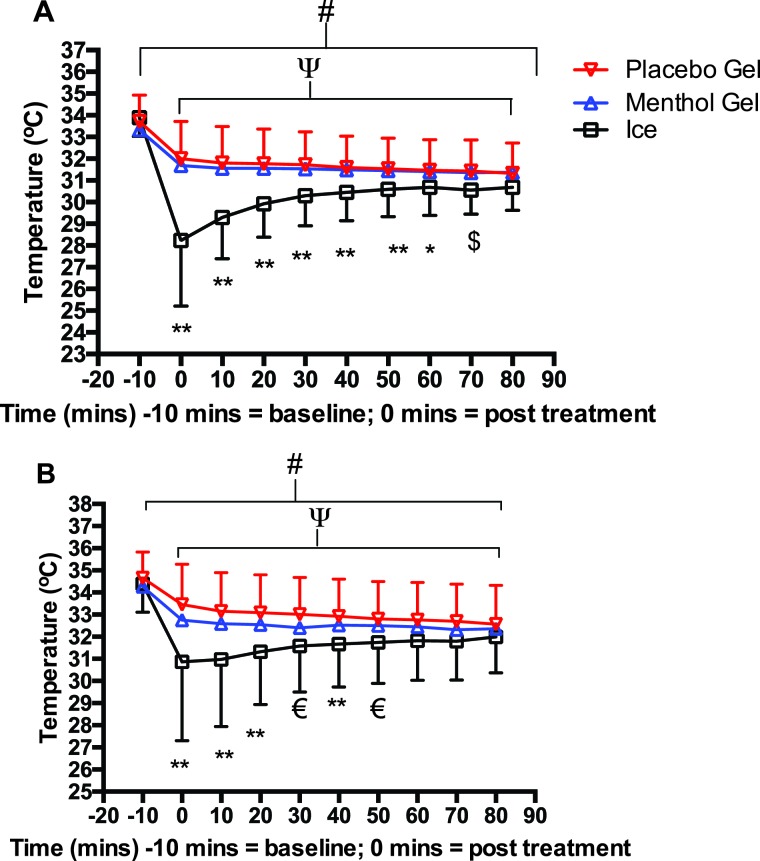
All three treatments significantly reduced intramuscular temperature at the 1 cm (F9,171, = 110.5, p<0.0001) and 3 cm depth (F9,171 = 64.9, p<0.0001) by ∼6% for both gels and ∼4% for the untreated legs across the full-time course (Table 1; Figure 3A). A significant treatment effect also existed at 1 cm depth (F2,38=9, p<0.001) and a tendency for a difference at 3 cm depth (F2,38=3, p=0.057) (Table 1; Figure 3B); whereas both depths showed significant interaction effects between the treatments and across the time course (1 cm: F18,342=30, p<0.0001, 3 cm: F18,342=7.6, p<0.0001) (Table 1; Figure 3). This interaction effect occurred from significantly (p<0.05) lower intramuscular temperatures of the ice vs. menthol and placebo gels immediately following treatment, which declined by ∼15% compared to ∼6%; the ice treatment became progressively warmer across the remaining time period with no differences by 60 and 80 min for 1 and 3 cm depths respectively. By 80 minutes all of the thighs treated by the three conditions were still significantly (p<0.0001) cooler than before treatment by ∼7% for 1 cm and ∼6% for 3 cm (Table 1; Figure 3).
Table 1.
Intramuscular temperatures in °C for Menthol and Placebo Gel treated legs alongside untreated contralateral legs at respective corresponding times from pre-treatment through to 80 minutes’ post-treatment at 1 and 3 cm depths.
| Time | Menthol Gel Leg 1cm | Untreated contralateral leg 1cm | Placebo Gel Leg 1cm | Untreated contralateral leg 1cm | Menthol Gel Leg 3cm | Untreated contralateral leg 3cm | Placebo Gel Leg 3cm | Untreated contralateral leg 3cm |
|---|---|---|---|---|---|---|---|---|
| Baseline | 33.3 ± 1.1 | 32.9 | 33.7 ± 1.2 | 33.4 ± 1.1 | 34.3 ± 1.5 | 34.1 ± 1.1 | 34.6 ± 1.2 | 34.8 ± 0.9 |
| 0 min post | 31.6 ± 1.6** | 32.5 ± 1.3 | 31.9 ± 1.7** | 33.2 ± 1.2 | 32.7 ± 1.9** | 33.6 ± 1 | 33.5 ± 1.8** | 34.6 ± 1.2 |
| 10 min post | 31.6 ± 1.5** | 32.4 ± 1.2 | 31.8 ± 1.7** | 33 ± 1.3 | 32.6 ± 1.9** | 33.5 ± 1 | 33.1 ± 1.7** | 34.5 ± 1.2 |
| 20 min post | 31.5 ± 1.5** | 32.2 ± 1.2 | 31.7 ± 1.6** | 32.9 ± 1.3 | 32.5 ± 1.7** | 33.3 ± 1 | 33 ± 1.7** | 34.3 ± 1.2 |
| 30 min post | 31.5 ± 1.4** | 32 ± 1.3 | 31.7 ± 1.5** | 32.8 ± 1.3 | 32.4 ± 1.7** | 33.3 ± 1 | 33 ± 1.6** | 34.2 ± 1.3 |
| 40 min post | 31.4 ± 1.5** | 31.9 ± 1.2 | 31.6 ± 1.4** | 32.6 ± 1.3 | 32.5 ± 1.6** | 33.2 ± 1 | 32.9 ± 1.7** | 34.1 ± 1.3 |
| 50 min post | 31.4 ± 1.4* | 31.8 ± 1.2 | 31.5 ± 1.5** | 32.5 ± 1.3 | 32.5 ± 1.6** | 33 ± 1 | 32.8 ± 1.7** | 34 ± 1.3 |
| 60 min post | 31.4 ± 1.4** | 32 ± 1.8 | 31.4 ± 1.4** | 32.3 ± 1.3 | 32.4 ± 1.6** | 32.9 ± 1 | 32.7 ± 1.7** | 33.9 ± 1.4 |
| 70 min post | 31.4 ± 1.5 | 31.6 ± 1.2 | 31.4 ± 1.4** | 32.2 ± 1.3 | 32.3 ± 1.7** | 32.9 ± 1 | 32.7 ± 1.7** | 33.8 ± 1.4 |
| 80 min post | 31.4 ± 1.5 | 31.5 ± 1.2 | 31.3 ± 1.4** | 32.1 ± 1.3 | 32.4 ± 1.7** | 32.7 ± 1.2 | 32.6 ± 1.7** | 33.7 ± 1.4 |
**p<0.0001 *p<0.01 treated vs. untreated contralateral leg
Figure 3.
Intramuscular temperatures for both legs (vastus lateralis) at depths of A: 1 cm and B: 3 cm for: both gels vs. ice. All treatments significantly declined over 80 minutes #p<0.01; time points 0-80 minutes vs. -10 minutes p<0.01 Ice significantly less than the gels **p<0.01; Deep Freeze Gel and Placebo Gel significantly greater than ice € p<0.05 p<0.01 respectively; placebo gel significantly greater than ice $ p<0.05.
When controlling for the effects of rest and massage of gel into the leg, the authors compared the treated leg with the contralateral untreated leg: both intramuscular depths displayed significant interactions for both menthol (1 cm: F9,71=9.2, p<0.0001, 3 cm: F27,513=6.8) and placebo gels (1 cm: F9,171=31.2, p<0.0001, 3 cm: F9,171=8.3, p<0.0001) between treated and contralateral untreated legs across all the time points (Table 1). This demonstrated significantly (p<0.0001) cooler values for both gels vs. contralateral untreated legs by 2-4% at all time points for 3 cm depth but at just 0-60 minutes at 1 cm depth (Table 1).
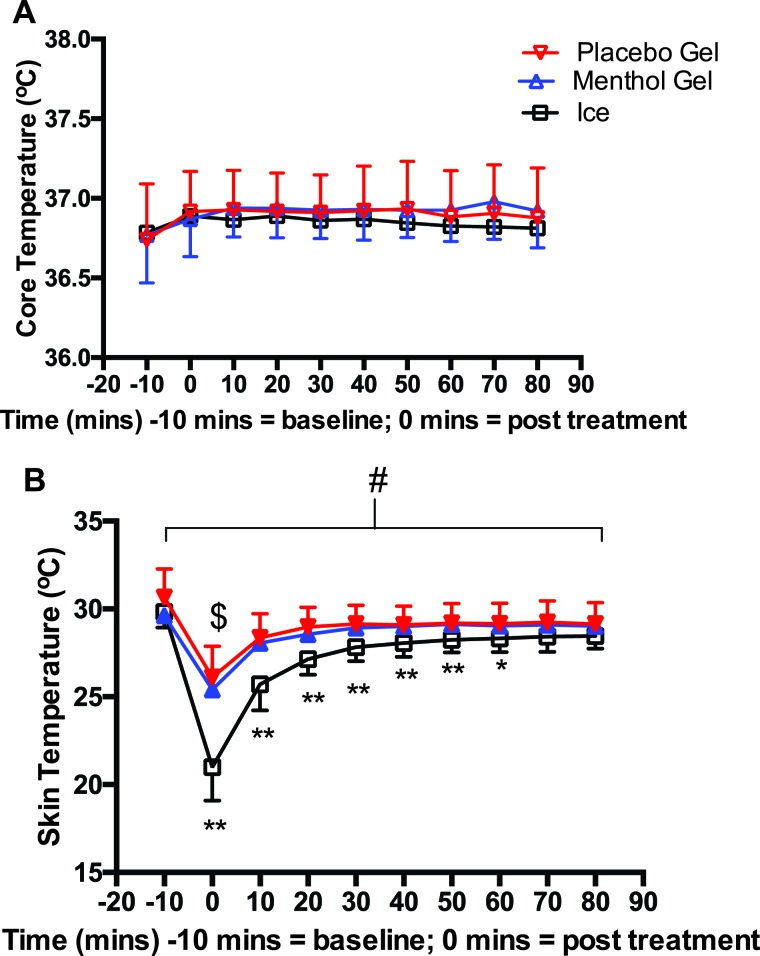
Core temperature remained unchanged across all time points with no difference between conditions (Figure 4A). Skin temperature showed a similar response to muscle temperature by significantly (F9,71=384, p<0.0001) reducing temperature in all three treatments with significantly (p<0.0001) greater reductions in the ice (∼32%) vs. both gels (∼14%) at 0-60 minutes with no differences at 70 and 80 minutes (Figure 4B). All skin temperatures still remained significantly (p<0.0001) cooler at 80 minutes by ∼5% for the ice and ∼2% for the gels when compared to baseline but this was not different than the contralateral untreated leg.
Figure 4.
Core temperature (A) and skin temperature (B) for menthol and placebo gel and ice. *p<0.001 *p<0.05 ice vs. both gels # p<0.001 all treatments change over time.
Blood Flow
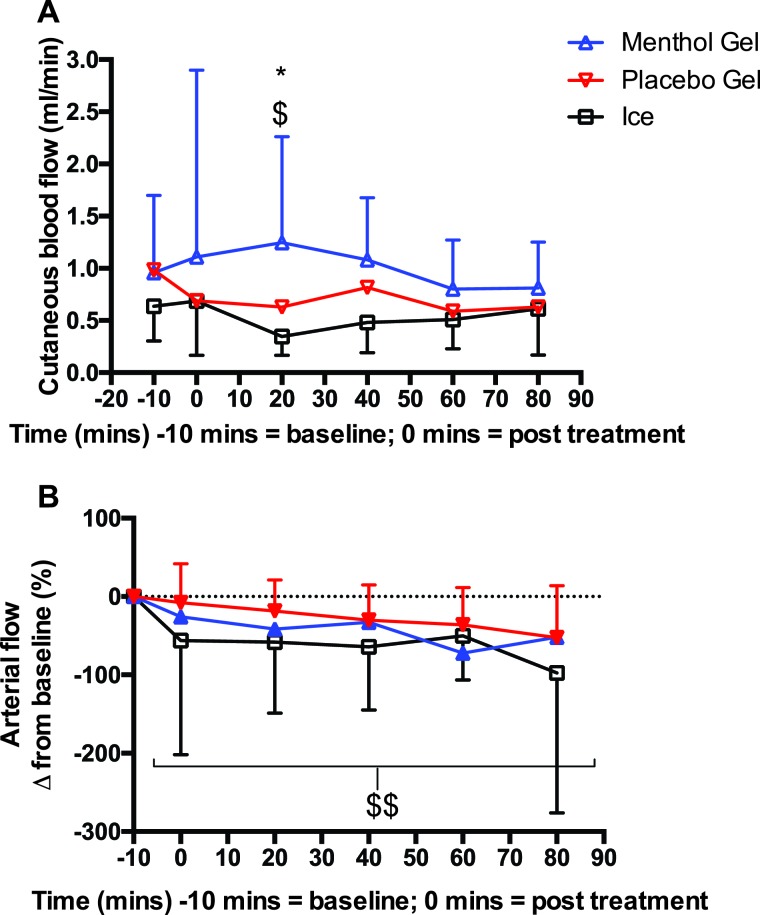
Skin perfusion showed a significant (F2,38=6.6, p<0.01) difference between treatments with menthol gel significantly higher than placebo gel (∼50%) and ice (∼72%) at 20 minutes (p<0.0001) (Figure 5A). Whereas femoral artery blood flow significantly (F8,82=325, p<0.0001) decreased by 67% for all three treatments and contralateral legs across the time course with no differences between them (Figure 5B).
Figure 5.
Cutaneous bloodflow (A) and arterial flow from baseline (pre-treatment) (B) menthol and placebo gel and ice. *p<0.05 menthol vs. placebo gel $p<0.001 menthol gel vs. ice $$ p<0.0001 all treatments declined over 80 minutes.
Heart Rate and Mean Arterial Pressure
Heart rate was not different between treatments but did significantly (F5,95=4.3, p<0.01) decline, for all treatments, over the full time period from ∼65 to ∼62 beats/minute. Mean arterial pressure was also not different between treatments but did significantly (F9,95=3.6, p<0.01) increase, for all treatments, over the full time period from ∼93 to ∼96 mmHg.
Subjective measures
Thermal comfort demonstrated significant effects across time (F9, 171 = 4.8, p<0.0001) between treatments (F2, 38 = 3.7, p<0.05) and for interaction (time 3 treatment) (F 18, 342 = 2, p<0.01). These differences occurred from: 1) ∼7% significantly (p<0.0001) cooler sensation of the menthol gel vs. ice and placebo gel from 10-80 minutes; 2) ice sensation significantly (p<0.0001) declined by ∼14% immediately following treatment and recovered by 10 minutes, whereas there was no decline in the placebo gel, but the menthol gel significantly (p<0.01) declined immediately ∼by 9% after but remained lower than baseline at 10, 40, 50, 60 and 70-minute time points.
DISCUSSION
This is the first well controlled study to demonstrate that menthol gel increased cutaneous blood flow and reduced intramuscular and skin temperature without any alterations to femoral arterial blood flow and core temperature. However, placebo gel also reduced intramuscular temperature to a similar extent but without cutaneous blood flow changes. Ice packs reduced intramuscular temperature, alongside cutaneous blood flow, to a greater extent than both gels immediately following treatment but was similar by 80 minutes. Core temperature remained unaltered, for all three treatments, while skin temperature followed a similar response to intramuscular temperature. Menthol gel provided a cooler sensation longer compared to ice and placebo gel. Thermoregulation remained unaltered for all treatments as evidenced by a decline in heart rate alongside increased MAP due to likely increase in total peripheral resistance to maintain circulation during the resting conditions.23
It is well documented that conductive heat transfer from skeletal muscle to blood occurs in an attempt to reduce muscle temperature.24 The authors hypothesized that menthol gel would increase cutaneous blood flow due to likely activation of vascular cells TRMP8 to increase endothelial function and subsequent vasodilation.12,14,15 Cutaneous blood flow did increase but arterial blood flow remained unchanged, in comparison to ice, placebo gel and control leg, therefore the authors are unsure regarding the source of the increased blood flow. Interestingly, previous studies19,25,26 examining topical menthol effects observed a decline in arterial flow due to presence of TRMP8 receptors in arterial smooth muscle.12 However, these studies applied menthol to the upper arm19,26 and the upper leg (anterior and posterior)25 and took blood flow measurements from brachial and popliteal arteries respectively; whereas the authors took their measurements from the femoral artery which is larger and deeper located than brachial and popliteal arteries,27 therefore the relative effects of menthol gel application are likely to be less. This is despite the relatively higher dosage used in the present study of 5.1 ml/200 cm2 compared to 1ml/200 cm2 used when applying to the upper arm18,19 or 3.3 ml/200 cm when applied to the forearm.19 However, when applied to the both anterior and posterior of the upper leg 7 ml/200 cm2 has been used25. Therefore, it appears that the larger the surface area treated, the more gel can be applied, most likely due to exponentially greater absorbing capacity; however, it is worth noting that the menthol gel used in the present study consisted of 3% levomenthol compared to 3.5% used in all the other reported studies, therefore relative response to the gel may have been less. Nevertheless, the menthol effect could not have caused the observed intramuscular temperature decline as this was similar to the placebo gel both superficially (1 cm depth) and deep (3 cm) into the muscle. This effect was not due to the cooling effect of slowing metabolism from rest28 as these temperatures were still lower than the untreated control leg which also reduced, over the same time period. Given the menthol content of the menthol gel did not cause the observed temperature decline, it suggests that the ethanol content of the gels, similar to the action of sweat,29 may have increased evaporation30 to reduce intramuscular and skin temperatures. Interestingly, although ice produced far lower temperature immediately after treatment, by 80 minutes the temperature was similar to that of the gels as they were still present in the skin whereas ice was removed after 10 minutes; any longer would have caused severe discomfort. Also, following ice application and removal, intramuscular temperature was becoming progressively warmer, whereas both gels maintained temperature decline for the full 80 minutes, suggesting that beyond this time point the gels could display a greater sustained cooling effect than the ice; which is an important practical consideration for practioners seeking long term cooling effects during sporting activities where ice pack application may be impractical.
Application of menthol gel most likely activated TRMP8 receptors, and have subsequently activated cold temperature sensory nerves;6 which may have caused greater cooler sensation than the other treatments and is associated with analgesic effects,7,8 which is an important consideration when seeking to treat soft tissue injury.
The exact mechanisms by which menthol provides local analgesia remain unclear with some suggested theories; these include increased pain receptor thresholds31,32 which may be as a result of “The Gate Control Theory”,31 which blocks pain transmission when alternative peripheral stimulus is received, such as hot or cold.32 Recently, a systematic review33 examined the topical analgesic clinical effectiveness of menthol Biofreeze? gel on musculoskeletal pain; the review covered neck, back, knee and hand pain and delayed onset of muscle soreness of elbow flexors and knee extensors. Most of the studies demonstrated significant reductions in pain following menthol gel application, although the knee pain studies failed to demonstrate clinically important differences. However, some of the included studies were underpowered and the authors33 recommended follow up research using large randomized clinical trials to fully establish analgesic effects. In the present study, the cold sensation of the menthol gel still existed by 70 minutes post treatment, whereas cutaneous blood flow returned to normal by 40 minutes. This is a similar occurrence to that described by Craighead et al34 who showed menthol induced cutaneous blood flow to return by 45 minutes whereas cooling sensation persisted for 60 minutes. Although the authors did not explain why there were different time effects, it is likely due to different location of TRPM8 receptors in sensory nerves, providing cool sensation35 and the vasculature,12,13 causing vasodilation. Therefore, it is possible that the threshold effects of menthol are different depending on activation of sensory nerves TRPM8 or vasculature TRPM8. Nevertheless, to fully understand menthol analgesic effects further study using menthol gel to treat exercise induced muscle damage and soft tissue injury is needed.
Limitations of this study include: 1) the population used were young and healthy males free of any acute injury or chronic illness. Therefore, the findings may not apply to both sexes of different ages that express decrements in cutaneous blood flow; 2) the measurements used were unable to determine where the increased cutaneous blood flow was redirected from; 3) although the participants where blinded to the treatments it was impossible to disguise ice vs. gel treatments and even with the placebo gel used it was difficult to mask the menthol aroma. However, the influence of bias on either the participant or investigator will have been minimal due to the objective measurements used while the participant was in the passive rested state and; 4) due to the electrode placement on the vastus lateralis it was difficult to directly massage this region which may have created a bias in measurements, although this was consistent between the 2 gels.
CONCLUSIONS
In conclusion, the results of this study has demonstrate that at rest, menthol cooling gel increases cutaneous blood flow and cooling sensation alongside reduced intramuscular and skin temperatures, which was also shown in the placebo and ice conditions. Although menthol gel intramuscular and skin temperature was reduced over an extended period of time, this was likely from the evaporative effects of alcohol content in the gel rather than from any pharmacological action of the menthol. Nevertheless, this cooling effect along with the possible analgesic effects of menthol gel, indicates that it could provide an effective practical alternative to ice treatment for practitioners seeking to treat soft tissue injury. Further study is needed to establish whether this is possible.
References
- 1.Bleakley CM Hopkins JT. Is it possible to achieve optimal levels of tissue cooling in cryotherapy? Phys Ther Rev. 2010;15(4):344-350. [Google Scholar]
- 2.Meeusen R Lievens P. The use of cryotherapy in sports injuries. Sports Med. 3(6):398-414. [DOI] [PubMed] [Google Scholar]
- 3.Swenson C Swärd L Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports. 1996;6(4):193-200. [DOI] [PubMed] [Google Scholar]
- 4.Merrick MA Rankin JM Andres FA Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31(11):1516-1521. [DOI] [PubMed] [Google Scholar]
- 5.McKemy DD Neuhausser WM Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52-58. [DOI] [PubMed] [Google Scholar]
- 6.Babes A Ciobanu AC Neacsu C Babes R-M. TRPM8, a sensor for mild cooling in mammalian sensory nerve endings. Curr Pharm Biotechnol. 2011;12(1):78-88. [DOI] [PubMed] [Google Scholar]
- 7.Liu B Fan L Balakrishna S Sui A Morris JB Jordt S-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154(10):2169-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premkumar LS Abooj M. TRP channels and analgesia. Life Sci. 2013;92(8-9):415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho SS Coel MN Kagawa R Richardson AB. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22(4):537-540. [DOI] [PubMed] [Google Scholar]
- 10.Thorsson O Lilja B Ahlgren L Hemdal B Westlin N. The effect of local cold application on intramuscular blood flow at rest and after running. Med Sci Sports Exerc. 1985;17(6):710-713. [DOI] [PubMed] [Google Scholar]
- 11.Craighead DH Alexander LM. Topical menthol increases cutaneous blood flow. Microvasc Res. 2016;107:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson CD Melanaphy D Purse A Stokesberry SA Dickson P Zholos A V. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. AJP Hear Circ Physiol. 2009;296(6):H1868-H1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X-R Lin M-J McIntosh LS Sham JSK. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. AJP Lung Cell Mol Physiol. 2006;290(6):L1267-L1276. [DOI] [PubMed] [Google Scholar]
- 14.Cheang WS Lam MY Wong WT, et al. Menthol relaxes rat aortae, mesenteric and coronary arteries by inhibiting calcium influx. Eur J Pharmacol. 2013;702(1-3):79-84. [DOI] [PubMed] [Google Scholar]
- 15.Sun J Yang T Wang P, et al. Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway. Hypertens (Dallas, Tex 1979). 2014;63(6):1354-1363. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson G Reilly T. Circadian variation in sports performance. Sports Med. 1996;21(4):292-312. [DOI] [PubMed] [Google Scholar]
- 17.Bedford T. Basic Principles of Ventilation and Heating. London, pp 101-154: HK Lewis; 1964. [Google Scholar]
- 18.Sundstrup E Jakobsen MD Brandt M, et al. Acute Effect of Topical Menthol on Chronic Pain in Slaughterhouse Workers with Carpal Tunnel Syndrome: Triple-Blind, Randomized Placebo-Controlled Trial. Rehabil Res Pract. 2014;2014:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive JL Hollis B Mattson E Topp R. Vascular conductance is reduced after menthol or cold application. Clin J Sport Med. 2010;20(5):372-376. [DOI] [PubMed] [Google Scholar]
- 20.Watt J. Massage for Sport. Malborough: Crowood Press; 1999. [Google Scholar]
- 21.Byrne C Lim CL. The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br J Sports Med. 2007;41(3):126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter A Albertus-Kajee Y St Clair Gibson A. The effect of exercise induced hyperthermia on muscle fibre conduction velocity during sustained isometric contraction. J Electromyogr Kinesiol. 2011;21(5):834-840. [DOI] [PubMed] [Google Scholar]
- 23.Widmaier EP Raff H Strang KT. Human Physiology. Ninth. Boston: Mcgraw Hill; 2004. [Google Scholar]
- 24.Raccuglia M Lloyd A Filingeri D Faulkner SH Hodder S Havenith G. Post-warm-up muscle temperature maintenance: blood flow contribution and external heating optimisation. Eur J Appl Physiol. 2016;116(2):395-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topp R Winchester LJ Schilero J Jacks D. Effect of topical menthol on ipsilateral and contralateral superficial blood flow following a bout of maximum voluntary muscle contraction. Int J Sports Phys Ther. 2011;6(2):83-91. [PMC free article] [PubMed] [Google Scholar]
- 26.Topp R Winchester L Mink AM Kaufman JS Jacks DE. Comparison of the effects of ice and 3.5% menthol gel on blood flow and muscle strength of the lower arm. J Sport Rehabil. 2011;20(3):355-366. [DOI] [PubMed] [Google Scholar]
- 27.Marieb EN. Human Anatomy and Physiology. 5th ed. San Fransico: Benjamin Cummings; 2001. [Google Scholar]
- 28.Speakman JR Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc. 2003;62(3):621-634. [DOI] [PubMed] [Google Scholar]
- 29.McArdle WD Katch FI Katch VL. Exercise Physiology: Nutrition, Energy and Human Performance. Eighth. Baltimore; 2015. [Google Scholar]
- 30.Frederick Frasch H Bunge AL. The Transient Dermal Exposure II: Post-Exposure Absorption and Evaporation of Volatile Compounds. J Pharm Sci. 2015;104(4):1499-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melzack R Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971-979. [DOI] [PubMed] [Google Scholar]
- 32.Kwon YS Robergs RA Schneider SM. Effect of local cooling on short-term, intense exercise. J Strength Cond Res. 2013;27(7):2046-2054. [DOI] [PubMed] [Google Scholar]
- 33.Page P Alexander L. The Clinical Effectiveness of Biofreeze ® Topical Analgesic on Musculoskeletal Pain: A Systematic Review. JPHR J Perform Heal Res. 2017;1(1). [Google Scholar]
- 34.Craighead DH McCartney NB Tumlinson JH Alexander LM. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc Res. 2017;110:43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhaka A Earley TJ Watson J Patapoutian A. Visualizing Cold Spots: TRPM8-Expressing Sensory Neurons and Their Projections. J Neurosci. 2008;28(3):566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]