Abstract
Background
Patellofemoral pain syndrome (PFPS) is a prevalent knee disorder. A novel yet increasingly popular treatment for PFPS is trigger point dry needling (DN).
Purpose
The purpose of this study was to determine if DN is more effective at reducing pain and disability than a sham treatment in individuals with PFPS.
Study design
Randomized trial.
Materials/Methods
Sixty military health care beneficiaries (36 males) with a clinical diagnosis of PFPS were recruited and completed the study. Subjects underwent a standardized clinical examination and were randomized into a DN or sham treatment group. DN treatment consisted of insertion of an acupuncture-like needle into six sites in the quadriceps femoris muscles of the symptomatic lower extremity based on a palpation examination. The sham grouped received a simulated treatment with a sharp object and needle guide tube without puncturing the skin. Self-reports of pain, disability, and overall status were collected before treatment, immediately after treatment and at 72 hours. Data were analyzed with separate 2x2 repeated measures analysis of variance, with independent variables being Group (DN vs. sham) and Time (pre-treatment vs. immediately post-treatment, and pre-treatment vs. 72 hours). The hypothesis of interest in each case was the Group*Time interaction. The alpha-level was set a priori to .05 using 2-tailed tests.
Results
Both groups exhibited a clinically meaningful reduction in pain based on numeric pain rating scale scores immediately post-treatment and at 72 hours, but there was no statistically significant difference between groups (p = 0.219, 0.310). There was no significant difference between groups for any other outcome measures.
Conclusion
These data suggest that DN treatment is not more effective than a sham DN treatment at reducing short-term pain and disability in individuals with PFPS when used as an isolated treatment approach.
Level of Evidence
2
Keywords: Dry needling, knee pain, patellofemoral pain syndrome, rehabilitation
INTRODUCTION
Patellofemoral pain syndrome (PFPS) is a prevalent knee disorder seen among young, active individuals and in general practice,1 orthopedic,2 sports3,4 and military clinics.5,6 Dye7 described PFPS as a clinical enigma, and one of the most challenging knee pathologies to manage. The problematic nature of PFPS is highlighted by the fact that 70% to 90% of individuals with the condition have recurrent or chronic symptoms.3,8 Despite the prevalence of the disorder, the etiology of PFPS is poorly understood. A number of abnormal biomechanical and neuromuscular factors may contribute to increased stresses on the patellofemoral joint which in turn can ultimately lead to pain and dysfunction.9-12 Because the etiology may be multifactorial in nature, and due to the variations in the clinical presentation of patients with PFPS, numerous non-operative interventions have been proposed for treatment of the disorder.
Dry needling (DN) is a therapeutic intervention that has been growing in both popularity and supportive research evidence.13-16 DN involves the insertion of small solid filament needles directly into myofascial trigger points in an attempt to reduce muscle tension, restore normal muscle function, and relieve pain.17-19 Myofascial trigger points are locally tender and palpable bands of muscle tissue that can cause pain and muscle dysfunction.20,21 Patients with PFPS often present with weakness and poor motor control of the quadriceps muscles.11,22-25 Restoration of quadriceps muscle strength and function is predictive of a successful rehabilitation outcome for patients with PFPS.26,27 Travell and Simons described trigger points in three of the four quadriceps femoris muscles which, when palpated, could generate the peripatellar and anterior knee pain that is characteristic of PFPS.21 They proposed that treatment of myofascial trigger points may be an effective way to diminish the pain associated with PFPS and to help restore quadriceps muscle function.21 To date, the effects of DN on patients with PFPS have not been investigated. Two previous studies described the use of acupuncture for the management of anterior knee pain,28,29 however the treatment techniques used in those studies and the proposed mechanisms for traditional acupuncture are substantially different than those associated with DN.
While recent investigations have shown that DN can be an effective treatment for patients with a variety of musculoskeletal dysfunctions,14,15,30,31 no study to date has investigated the use of the treatment technique in patients with PFPS. The current level of evidence regarding the use of DN for the management of PFPS is limited to that of expert opinion.21 Research is needed in the form of a randomized controlled trial to determine whether DN is an effective intervention for individuals with PFPS. Therefore, the purpose of this study was to determine if DN is more effective at reducing pain and disability than a sham treatment in individuals with PFPS. The authors hypothesized that the DN group would experience a significantly greater reduction in pain and disability than the sham group.
METHODS
Sixty participants were recruited from the military health care beneficiary population at Fort Sam Houston in San Antonio, Texas. All volunteers provided informed consent for participation in the study, which was approved by the Institutional Review Board at Brooke Army Medical Center. Participants were required to be 18-40 years of age and have a clinical diagnosis of PFPS. Participants were determined to have PFPS if they had a complaint of retropatellar or anterior knee pain that was provoked by two or more of the following activities: squatting, stair ascent, stair descent, prolonged sitting, kneeling or isometric quadriceps contraction.9 Individuals were excluded if they had a history of prior knee surgery, any competing knee pathology (meniscal tears, patellar tendinopathy, ligamentous sprains, osteoarthritis, etc.), any systemic disease and/or connective tissue disorders, or signs of lumbosacral nerve root compression. Individual were also excluded those who had received acupuncture, injection, or DN treatment for the knee or quadriceps femoris muscles within the prior six months, individuals who were currently taking anticoagulant medications or had a medical history of a bleeding disorder, and pregnant females.
Volunteers initially received a screening examination consisting of a medical history questionnaire and a standard physical examination to confirm a clinical diagnosis of PFPS and to rule out any competing knee pathologies. All screening examinations were performed by licensed physical therapists, who were board certified as a sports clinical specialist (JHM) or orthopedic clinical specialist (TGS). The screening examination consisted of palpation, special tests to rule out competing pathologies such as meniscal tears, patellar tendinopathy, and ligamentous injuries. Altman's criteria32 were used to screen for individuals with possible knee osteoarthritis. Additionally, a comprehensive neurological screening examination was performed to rule the possibility of lumbosacral nerve root or peripheral nerve involvement. Individuals who met the inclusion and exclusion criteria then underwent a standardized history and physical examination, which included selected testing of lower extremity muscle strength, muscle length, and range of motion. Isometric strength of the knee extensors and flexors, and hip abductors, flexors, internal rotators and external rotators was assessed with a hand-held dynamometer. Knee and hip range of motion (ROM) measures were performed using a standard goniometer. To assess muscle length, examiners conducted the Thomas, Ober's and hamstring 90-90 tests.33 A bubble goniometer was used to measure the angle of hip and knee flexion during the Thomas test, hip abduction during the Ober's test, and knee flexion during the hamstring 90-90 test. The Q-angle was assessed in the standing position as previously described by Iverson and colleagues.34 Evaluation of a possible leg length discrepancy was performed using a Palpation Meter.35
Participants completed two self-report questionnaires, the Kujala Anterior Knee Pain Scale (AKPS)36,37 and the Lower Extremity Functional Scale (LEFS),38,39 as well as a body chart to provide a thorough description of the location and nature of their symptoms. Demographic information was collected to include age, gender, height, and weight. Participants also rated their pain level during performance of three functional activities (stepping up a 20-cm step, stepping down from the same step, and squatting)34,40 on an 11-point numeric pain rating scale (NPRS).41-43 During the squat test, participants were instructed to maintain their trunk in an upright position and to look straight ahead, while keeping their heels on the floor. As they performed the squat, the angle of knee flexion at which the participants first experienced their pain was measured with a goniometer and recorded. Documenting the angle of knee flexion at onset of pain allowed the examiners to ask the subject to assess the pain experienced at the same angle with subsequent squats immediately following the intervention and at a 72-hour follow-up appointment. Examiners were blinded to treatment group allocation.
Prior to enrollment, an investigator not involved with data collection or treatment used a random-number generator to create a randomization list and prepared individual, sequentially numbered index cards that indicated group assignment. The cards were then folded and placed in sealed envelopes. Participants were randomly assigned to one of two treatment groups: dry needling (DN) or sham (SH) needling group. All treatments were performed by an experienced physical therapist (SK) who was trained in dry needling and blinded to the baseline assessment outcomes. After the baseline examination, the treating investigator opened the envelope which indicated the treatment group assignment. Participants in both groups made a total of two visits to the clinic; once for the baseline examination and intervention and then again at a 72-hour follow-up appointment.
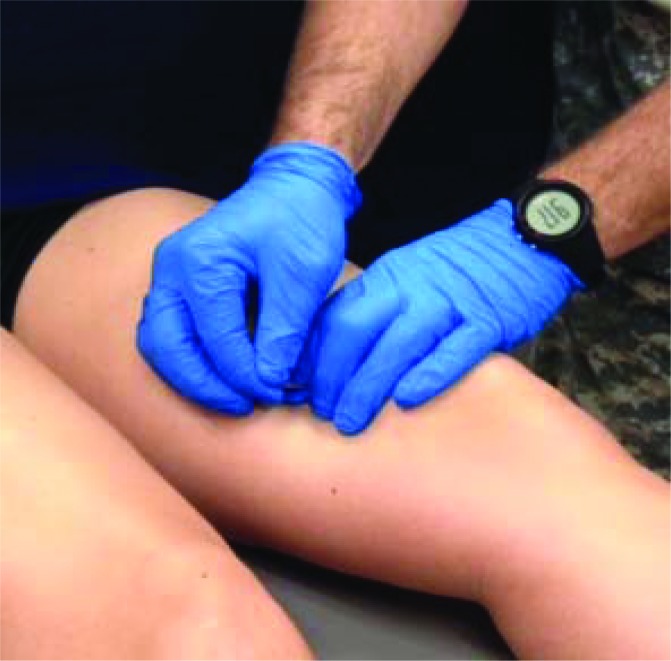
Individuals in the DN group received a single session of dry needling therapy using a standardized protocol. Participants lay in the supine position with a vertical drape positioned at the level of their waist so that they were blinded to the treatment they received. The needling procedure started with systematic manual palpation of three superficial quadriceps femoris muscles (vastus medialis, rectus femoris, vastus lateralis) ipsilateral to the symptomatic knee. This was performed to determine the presence or absence of perceived trigger points, operationally defined as palpable and painful nodules in the muscle tissue and considered present when active or latent,17 and to guide needle placement. Two trigger points were identified for treatment in each of the three targeted quadriceps femoris muscles, for a total of six needle insertions per thigh. Upon the rare occasion that two trigger points were not identifiable in a specific muscle, DN was provided to the most painful location as determined by palpation. The needling technique included the insertion or a sterile, disposable 0.25 x 40 mm stainless steel acupuncture needle (Seirin Corporation, Shizuoka, Japan) into three of four the quadriceps femoris muscles (vastus medialis, rectus femoris, vastus lateralis) (Figure 1). The “clean technique” was used throughout the treatment procedure, which included hand washing, use of latex-free examination gloves, and skin-surface preparation with an alcohol wipe.44 Needle insertions lasted approximately 5-10 seconds using “sparrow pecking” (in-and-out motion) and “coning” (small redirections of needle angle) techniques in an attempt to elicit as many local twitch responses as possible.
Figure 1.
Dry needling technique. Participants were supine with a dark drape positioned at their waist to blind them from the treatment.
The SH group received a simulated needling intervention that was developed and validated by Sherman and colleagues.45 The procedures performed for the SH group were identical to those described for the DN group, with the exception that a small, sharp object in a needle guide tube was used in place of the acupuncture needle. Firm pressure was applied to the skin of subjects in the SH group to simulate the sensation of an acupuncture needle, but the skin was never punctured. The same sparrow pecking and coning techniques and treatment durations used in the DN group were simulated in the SH group.
Immediately following the treatment session, participants repeated each of the functional tests (step-up, step-down, squat) and rated the pain experienced during each activity on the NPRS. All participants were instructed in a basic home exercise program of isometric quadriceps femoris contractions and quadriceps stretching exercises to be performed daily until they returned for the follow-up appointment. Participants returned to the clinic 72 hours following the initial session and completed a second AKPS and LEFS, as well as a Global Rating of Change (GROC) questionnaire.46,47 The functional activities and pain assessments were also repeated, as well as selected muscle strength, length and ROM measures. During the follow up session, participants were asked which treatment they believe they received. Following their response, the investigators revealed to each participant which treatment was actually received.
STATISTICAL ANALYSIS
A priori power analysis was performed using G*Power 3.48 Estimated sample size was based on having at least 80% power to detect a minimal clinically important difference (MCID) in the LEFS of 8 points38,39 between groups, assuming a standard deviation of 10 points, alpha of 0.05 and 10% attrition at follow up. Enrolling 60 subjects (30 per group) was planned to additionally give adequate power for analysis of secondary outcomes and adequate precision to correlational estimates.
Statistical analyses were performed using IBM SPSS Version 21 software (Chicago, IL). Descriptive statistics were performed on demographic and clinical history characteristics of the sample. Treatment effects were estimated using separate, random intercept and slope linear mixed models for each repeated measure outcome variable. Time, treatment group, and time by treatment group interaction were modeled as fixed effects. Age, sex, and length of symptoms were evaluated for additional fixed effect covariates based on their potential to affect prognosis. For each model, the covariance structure (autoregressive, unstructured, scaled identity) was used based on best model fit and ability of the model to reach convergence. Consistent with the intention to treat principle, missing data points were estimated in the mixed model analyses using restricted maximum likelihood ratio estimation with 100 iterations; therefore all participants randomized to a treatment group were included in the analyses of outcome for that group. Alpha level was 0.05 for all analyses.
RESULTS
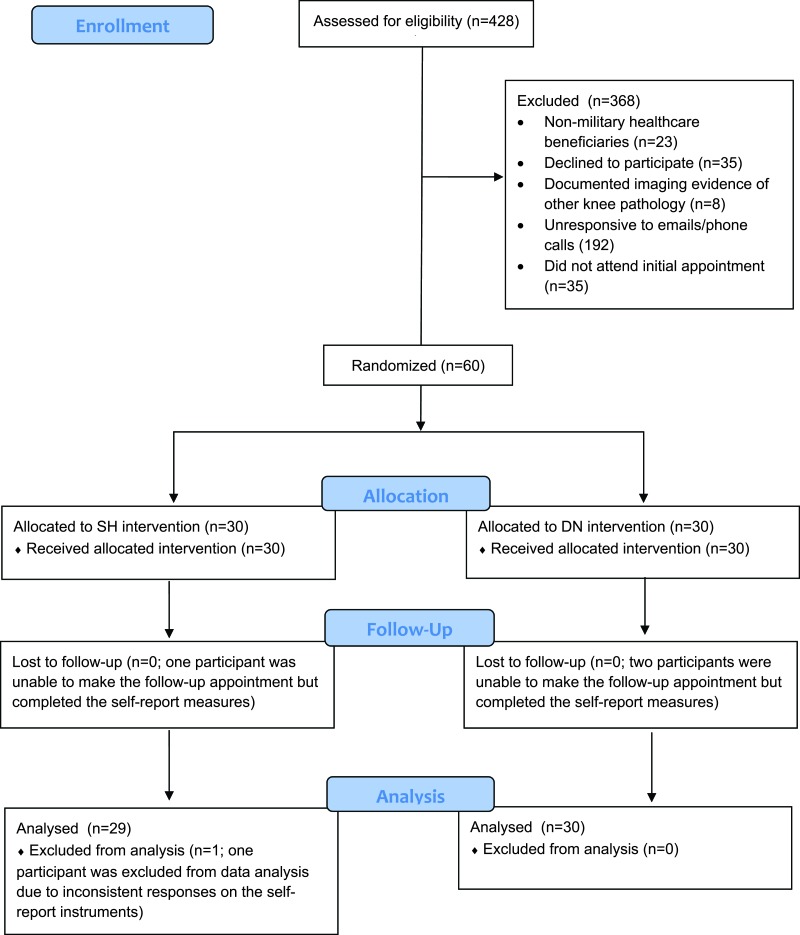
Three of the 60 enrolled participants were unable to attend their 72-hour follow-up appointments, but they were able to complete the self-report instruments either telephonically or via email. One participant was excluded from data analysis due to inconsistent responses on his self-report outcome measures. Therefore, data from 59 participants were available and included in the final analysis (Figure 2). Of those 59 individuals, 30 were randomized to the DN group and 29 to the SH group. Baseline demographic and self-reported variables for all participants are shown in Table 1.
Figure 2.
Flow of participant recruitment and exclusion.
Table 1.
Baseline demographic and self-reported variables
| Variable | DN Group (n = 30) | SH Group (n = 30) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (y) | 30.3 (5.5) | 31.1 (5.1) |
| Gender (% male) | 56.7 | 66.7 |
| Body mass index | 26.4 (4.4) | 26.8 (3.2) |
| Symptom duration (months) | 27.4 (29.7) | 53.0 (66.8) |
| LEFS | 61.9 (10.0) | 60.4 (12.2) |
| Kujala | 76.3 (9.8) | 70.6 (9.7) |
| NPRS squat | 3.9 (2.2) | 3.3 (2.3) |
| NPRS up stairs | 1.6 (1.7) | 1.6 (1.6) |
| NPRS down stairs | 2.2 (1.6) | 1.6 (1.5) |
DN = dry needling group; SH = sham group; LEFS = Lower Extremity Functional Scale; NPRS = Numeric Pain Rating Scale
Primary results of the between-group comparisons are depicted in Table 2. Overall, both groups exhibited a clinically meaningful reduction in pain with the functional activities (step-up, step-down, squat) immediately post-treatment and at 72 hours, based on a 30% reduction42 in the NPRS scores. But the differences between the groups on the NPRS scores immediately following treatment and at 72 hours were not significant (p = 0.22, 0.31). There were no significant or clinically meaningful differences between the groups or over time within each group based on the LEFS, Kujala or GROC scores. The minimal clinically important difference for the LEFS has been reported to be 8 to 9 points,38,39 10 to 13 points for the Kujala scale,37,39 and 3 points for the GROC.46
Table 2.
Mean and standard deviation (SD) of each self-report instrument over time
| DN | Sham | Adj. Difference* | |
|---|---|---|---|
| Self-report instrument: | Mean (SD) | Mean (SD) | Mean (95% CI) LEFS |
| LEFS | |||
| Baseline | 61.9 (10.0) | 60.4 (12.2) | |
| 72 hours | 66.7 (12.8) | 63.2 (12.3) | 1.5 (-6.0, 3.1) |
| Kujala | |||
| Baseline | 76.3 (9.8) | 70.6 (9.7) | |
| 72 hours | 82.8 (11.0) | 76.6 (12.6) | 1.2 (-3.9, 6.3) |
| NPRS step-up | |||
| Baseline | 1.6 (1.7) | 1.4 (1.4) | |
| Immediate post-treatment | 1.3 (1.5) | 1.2 (1.2) | 0.0 (-0.5, 0.5) |
| 72 hours | 1.0 (1.6) | 1.0 (1.2) | 0.0 (-0.7, 0.7) |
| NPRS step-down | |||
| Baseline | 2.2 (1.6) | 1.4 (1.8) | |
| Immediate post-treatment | 1.4 (1.4) | 0.9 (1.1) | 0.0 (-0.5, 0.5) |
| 72 hours | 1.2 (1.4) | 0.8 (1.1) | 0.0 (-0.7, 0.7) |
| NPRS squat | |||
| Baseline | 3.9 (2.2) | 3.1 (2.3) | |
| Immediate post-treatment | 2.7 (2.2) | 2.0 (1.8) | 0.2 (-0.6, 0.9) |
| 72 hours | 2.2 (2.1) | 1.6 (1.8) | 0.1 (-0.9, 1.0) |
| GROC | |||
| 72 hours | 2.2 (2.3) | 1.3 (2.7) | 1.0 (-0.4, 2.3) |
DN = dry needling group; SH = sham group; LEFS = Lower Extremity Functional Scale; NPRS = Numeric Pain Rating Scale
Adjusted for baseline difference in each outcome (except the GROC, done only at 72 hours)
Based on analysis using Fisher's Exact Test, the number of participants who correctly versus incorrectly guessed the treatment they received was not significantly different between groups (p = 0.41). Seventeen (63%) of the participants in the SH group thought they had received the DN treatment, four (15%) correctly stated they had received the SH treatment, and six (22%) were unsure. Similarly, twenty (71%) of the participants in the DN group correctly identified the treatment they received, two (7%) believed they were in the SH group, and four (14%) were unsure. The remaining individuals either did not attend their follow-up appointment (n = 3) or simply failed to respond to the question regarding perceived treatment (n = 3).
None of the participants experienced serious adverse events or had to discontinue the study due to study-related procedures. Furthermore, no participants reported any minor transient side effects such as lightheadedness, nausea, or fatigue after receiving the DN or sham treatments.
DISCUSSION
PFPS remains a complex and significant clinical problem. Despite its high prevalence among active individuals1-6 and recent innovations in rehabilitation,9-12,26 the etiology of PFPS remains poorly understood. A number of abnormal biomechanical and neuromuscular factors may contribute to increased patellofemoral joint reaction forces, which in turn ultimately lead to pain and dysfunction.9-12,26 Because the etiology of PFPS is multifactorial in nature, and due to the variations in the clinical presentation of patients, numerous treatment strategies have been proposed for the condition. Dry needling is a therapeutic intervention that has been growing in both popularity and supportive research evidence.13-16 DN has been investigated extensively in populations of individuals with low back pain,13,30,31,49,50 neck pain,15,51-54 and shoulder pain,15,16,55-58 while more limited research has been published regarding its effects on less prevalent musculoskeletal conditions such as plantar fascitis,59 Achilles tendinopathy60 and temporomandibular joint dysfunction.61 No previous investigation has examined the effects of DN on symptoms in subjects with PFPS. Therefore, the purpose of the study was to determine if a single session of DN to the quadriceps femoris muscles was more effective at reducing pain and disability than a sham treatment in individuals with PFPS. There were no significant differences in outcomes existed between the groups at any time following the intervention, suggesting that a single session of DN was not more effective than a sham treatment at reducing short-term pain and disability in individuals with PFPS when used as an isolated treatment approach.
While no significant differences were found between groups in any outcome, point estimates of treatment effects consistently favored DN over SH. Therefore, it is certainly possible that DN is effective for some patients with the condition and not effective for others. This would align with many practitioners’ clinical experience as well as other evidence suggesting that there are sub-groups of patients with PFPS who respond best to specific interventions based on the identification of distinct clinical characteristics.34,40,62-67 Koppenhaver and colleagues recently identified several baseline examination factors that were associated with clinical improvement after DN in subjects with LBP.30 Future studies of individuals with PFPS should determine if there are variables from the history and physical examination that are associated with or predictive of a successful response to treatment with DN.
The current study specifically investigated the short-term response of individuals with PFPS to a single session of DN or a sham treatment. A possible shortcoming of the study was the fact that the participants’ response to the intervention was measured only as far out as 72 hours. The follow up periods reported in most randomized trials of patients with PFPS tend to be on the order of weeks or months.28,29,68-71 Although clinically meaningful reductions in pain were seen in both groups during the study period, the difference between the two groups was not significant, and the differences in the LEFS, Kujala and GROC scores did not reach clinically meaningful levels. In addition to the short follow up period, the study participants were seen for just a single treatment session. Previous investigations of DN for the treatment of other prevalent musculoskeletal disorders included multiple treatment sessions, with many occurring over a period of several weeks.14,53,59,72,73 Therefore, the single treatment session and brief follow-up period that used in the current study may not reflect typical clinical practice and also made it difficult to draw any strong conclusions about the effectiveness of the intervention, particularly given that PFPS is considered to be a chronic problem with a high recurrence rate.8,74-76 Future investigations should consider the use of multiple treatment sessions to more accurately replicate what is done in a clinical setting. The intervention was also limited to just DN and the authors recognize that using a single treatment modality may not be representative of a typical clinical strategy for the management of patients with PFPS. Clinical experts report that the strongest research evidence shows that multimodal or combined interventions result in the most robust and consistent therapeutic effects for individuals with anterior knee pain.9,26 Therefore, recommendations for future studies in PFPS populations include the investigation of DN in conjunction with other interventions such as quadriceps and gluteal strengthening, stretching, patellofemoral joint mobilization, and taping.77
Despite these limitations, the current investigators believe that the approach was a reasonable initial venture into examining the specific, isolated effects of one session of DN as an intervention for persons with PFPS. No known published report has examined the use of dry needling for the treatment of PFPS. Two previous investigations28,29 described the use of acupuncture as an intervention for anterior knee pain, with conflicting results. Jensen and colleagues28 treated traditional acupuncture points that included the low back, vastus medialis, vastus lateralis and peripatellar regions. The authors treated their subjects twice a week for 4 weeks and reported that acupuncture reduced pain better than a control at five months post-intervention.28 Naslund et al described sensory stimulation of acupuncture sites just proximal and distal to the knee in subjects with idiopathic anterior knee pain.29 Subjects were randomized into deep (treatment) and minimal superficial (control) groups and treated twice a week for a total of 15 treatments. The investigators reported a clinically meaningful reduction in pain in all subjects at a six-month follow up, with no significant difference between the two groups.29 However, the treatment techniques used in those studies and the proposed mechanisms for traditional acupuncture are substantially different than DN, making it difficult to draw comparisons with the results of the current study.
The current investigation focused treatment on the quadriceps femoris muscles because patients with PFPS characteristically present with weakness and poor motor control of that muscle group.11,22-25,78 Restoration of quadriceps muscle strength and function are predictive of a successful rehabilitation outcome for patients with PFPS.26,27 Travell and Simons described trigger points in three of the four quadriceps femoris muscles which, when palpated, could generate the peripatellar and anterior knee pain that is characteristic of PFPS.21 They proposed that treatment of these trigger points may be an effective way to diminish the pain associated with PFPS and to help restore quadriceps muscle function.21 Based on their work, the intervention used in this study focused on the vastus medialis, rectus femoris, and vastus lateralis muscles. However, because addressing proximal impairments has been shown in recent studies9,12,26 to be an important component in the successful rehabilitation of patients with PFPS, future investigations should consider DN treatment of hip and trunk muscles that have been linked to the disorder. Specific target muscles that should be considered in future needling studies of patients with PFPS are the gluteal, hip lateral rotator,11,26,68,79-83 and trunk muscles.84-86
CONCLUSIONS
The authors believe that this was the first study to examine the effects of DN in a population of individuals with PFPS. Both the DN and SH groups experienced clinically meaningful reductions in pain during the study period. However, the differences were not statistically significant, and there were no differences between the groups in terms of the LEFS, Kujala or GROC scores. There were a number of possible shortcomings in the study design, and recommendations for future randomized trials include investigating the effects of DN on patellofemoral pain and disability include multiple treatment sessions, alternative needling sites, longer follow up periods, and DN treatment in conjunction with other interventions such as specific therapeutic exercises, manual therapy, and taping. Additionally, future studies should attempt to identify the characteristics of individuals with PFPS who respond successfully to treatment with DN. Until a clinically relevant subgroup is established, DN should not be considered as an isolated intervention for patients with PFPS.
REFERENCES
- 1.van Middelkoop M van Linschoten R Berger MY Koes BW Bierma-Zeinstra SM. Knee complaints seen in general practice: active sport participants versus non-sport participants. BMC Musculoskel Disord. 2008;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood L Muller S G. P. The epidemiology of patellofemoral disorders in adulthood: a review of routine general practice morbidity recording. Prim Health Care Res Dev. 2011;12:157-164. [DOI] [PubMed] [Google Scholar]
- 3.Blond L Hansen L. Patellofemoral pain syndrome in athletes: a 5.7-year retrospective follow-up study of 250 athletes. Acta Orthopaedica Belgica. 1998;64(4):393-400. [PubMed] [Google Scholar]
- 4.Taunton JE Ryan MB Clement DB McKenzie DC Lloyd-Smith DR Zumbo BD. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhon DI. A physical therapist experience, observation, and practice with an infantry brigade combat team in support of Operation Iraqi Freedom. Mil Med. 2010;175(6):442-447. [DOI] [PubMed] [Google Scholar]
- 6.Songer TJ LaPorte RE. Disabilities due to injury in the military. Am J Sports Med. 2000;18(3 Suppl):33-40. [DOI] [PubMed] [Google Scholar]
- 7.Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Rel Res. 2005(436):100-110. [DOI] [PubMed] [Google Scholar]
- 8.Stathopulu E Baildam E. Anterior knee pain: a long-term follow-up. Rheumatol. 2003;42(2):380-382. [DOI] [PubMed] [Google Scholar]
- 9.Crossley KM van Middelkoop M Callaghan MJ Collins NJ Rathleff MS Barton CJ. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 2: recommended physical interventions (exercise taping, bracing, foot orthoses and combined interventions). Br J Sports Med. 2016;50(14):844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis IS Powers CM. Patellofemoral pain syndrome: proximal, distal, and local factors, an international retreat, April 30-May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther. 2010;40(3):A1-16. [DOI] [PubMed] [Google Scholar]
- 11.Powers CM Bolgla LA Callaghan MJ Collins N Sheehan FT. Patellofemoral pain: proximal, distal, and local factors, 2nd International Research Retreat. J Orthop Sports Phys Ther. 2012;42(6):A1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witvrouw E Callaghan MJ Stefanik JJ, et al. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. Br J Sports Med. 2014;48(6):411-414. [DOI] [PubMed] [Google Scholar]
- 13.Furlan AD van Tulder MW Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database of Systematic Reviews. 2005(1):CD001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kietrys DM Palombaro KM Azzaretto E, et al. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2013;43(9):620-634. [DOI] [PubMed] [Google Scholar]
- 15.Liu L Huang QM Liu QG, et al. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96(5):944-955. [DOI] [PubMed] [Google Scholar]
- 16.Ong J Claydon LS. The effect of dry needling for myofascial trigger points in the neck and shoulders: a systematic review and meta-analysis. J Bodywork Movement Ther. 2014;18(3):390-398. [DOI] [PubMed] [Google Scholar]
- 17.Dommerholt J. Dry needling - peripheral and central considerations. J Man Manip Ther. 2011;19(4):223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalichman L Vulfsons S. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med. 2010;23(5):640-646. [DOI] [PubMed] [Google Scholar]
- 19.Vulfsons S Ratmansky M Kalichman L. Trigger point needling: techniques and outcome. Curr Pain Headache Rep. 2012;16(5):407-412. [DOI] [PubMed] [Google Scholar]
- 20.Lavelle ED Lavelle W Smith HS. Myofascial trigger points. Anesth Clin. 2007;25(4):841-851, vii-iii. [DOI] [PubMed] [Google Scholar]
- 21.Simons D Travell J Simons L. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. 2nd ed: Williams & Wilkins; 1999. [Google Scholar]
- 22.Cowan SM Bennell KL Hodges PW Crossley KM McConnell J. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2001;82(2):183-189. [DOI] [PubMed] [Google Scholar]
- 23.Cowan SM Hodges PW Bennell KL Crossley KM. Altered vastii recruitment when people with patellofemoral pain syndrome complete a postural task. Arch Phys Med Rehabil. 2002;83(7):989-995. [DOI] [PubMed] [Google Scholar]
- 24.Crossley K Bennell K Green S Cowan S McConnell J. Physical therapy for patellofemoral pain: a randomized, double-blinded, placebo-controlled trial. Am J Sports Med. 2002;30(6):857-865. [DOI] [PubMed] [Google Scholar]
- 25.Powers CM. Rehabilitation of patellofemoral joint disorders: a critical review. J Orthop Sports Phys Ther. 1998;28(5):345-354. [DOI] [PubMed] [Google Scholar]
- 26.Barton CJ Lack S Hemmings S Tufail S Morrissey D. The ‘Best Practice Guide to Conservative Management of Patellofemoral Pain’: incorporating level 1 evidence with expert clinical reasoning. Br J Sports Med. 2015;49(14):923-934. [DOI] [PubMed] [Google Scholar]
- 27.Pattyn E Mahieu N Selfe J Verdonk P Steyaert A Witvrouw E. What predicts functional outcome after treatment for patellofemoral pain? Med Sci Sports Exerc. 2012;44(10):1827-1833. [DOI] [PubMed] [Google Scholar]
- 28.Jensen R Gothesen O Liseth K Baerheim A. Acupuncture treatment of patellofemoral pain syndrome. J Altern Complement Med. 1999;5(6):521-527. [DOI] [PubMed] [Google Scholar]
- 29.Naslund J Naslund UB Odenbring S Lundeberg T. Sensory stimulation (acupuncture) for the treatment of idiopathic anterior knee pain. J Rehabil Med. 2002;34(5):231-238. [DOI] [PubMed] [Google Scholar]
- 30.Koppenhaver SL Walker MJ Smith RW, et al. Baseline examination factors associated With clinical improvement after dry needling in individuals with low back pain. J Orthop Sports Phys Ther. 2015;45(8):604-612. [DOI] [PubMed] [Google Scholar]
- 31.Koppenhaver SL Walker MJ Su J, et al. Changes in lumbar multifidus muscle function and nociceptive sensitivity in low back pain patient responders versus non-responders after dry needling treatment. Man Ther. 2015;20(6):769-76. [DOI] [PubMed] [Google Scholar]
- 32.Altman R AE Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American Rheumatism Association. Arthritis Rheumatol. 1986;29(8):1039-1049. [DOI] [PubMed] [Google Scholar]
- 33.Magee DJ. Orthopedic Physical Assessment. 6th ed. New York: Saunders; 2013. [Google Scholar]
- 34.Iverson CA Sutlive TG Crowell MS, et al. Lumbopelvic manipulation for the treatment of patients with patellofemoral pain syndrome: development of a clinical prediction rule. J Orthop Sports Phys Ther. 2008;38(6):297-309; discussion 309-212. [DOI] [PubMed] [Google Scholar]
- 35.Petrone MR Guinn J Reddin A Sutlive TG Flynn TW Garber MP. The accuracy of the Palpation Meter (PALM) for measuring pelvic crest height difference and leg length discrepancy. J Orthop Sports Phys Ther. 2003;33(6):319-325. [DOI] [PubMed] [Google Scholar]
- 36.Crossley KM Bennell KL Cowan SM Green S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Arch Phys Med Rehabil. 2004;85(5):815-822. [DOI] [PubMed] [Google Scholar]
- 37.Kujala UM Jaakkola LH Koskinen SK Taimela S Hurme M Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163. [DOI] [PubMed] [Google Scholar]
- 38.Binkley JM Stratford PW Lott SA Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383. [PubMed] [Google Scholar]
- 39.Watson CJ Propps M Ratner J Zeigler DL Horton P Smith SS. Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J Orthop Sports Phys Ther. 2005;35(3):136-146. [DOI] [PubMed] [Google Scholar]
- 40.Sutlive TG Mitchell SD Maxfield SN, et al. Identification of individuals with patellofemoral pain whose symptoms improved after a combined program of foot orthosis use and modified activity: a preliminary investigation. Phys Ther. 2004;84(1):49-61. [PubMed] [Google Scholar]
- 41.Abbott JH Schmitt J. Minimum important differences for the patient-specific functional scale, 4 region-specific outcome measures, and the numeric pain rating scale. J Orthop Sports Phys Ther. 2014;44(8):560-564. [DOI] [PubMed] [Google Scholar]
- 42.Farrar JT Young JP Jr. LaMoreaux L Werth JL Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158. [DOI] [PubMed] [Google Scholar]
- 43.Price DD Bush FM Long S Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56(2):217-226. [DOI] [PubMed] [Google Scholar]
- 44.Baima J Isaac Z. Clean versus sterile technique for common joint injections: a review from the physiatry perspective. Curr Rev Musculoskel Med. 2008;1(2):88-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherman KJ Hogeboom CJ Cherkin DC Deyo RA. Description and validation of a noninvasive placebo acupuncture procedure. J Altern Complement Med. 2002;8(1):11-19. [DOI] [PubMed] [Google Scholar]
- 46.Jaeschke R Singer J Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407-415. [DOI] [PubMed] [Google Scholar]
- 47.Juniper EF Guyatt GH Willan A Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47(1):81-87. [DOI] [PubMed] [Google Scholar]
- 48.Faul F Erdfelder E Lang AG Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191. [DOI] [PubMed] [Google Scholar]
- 49.Rainey CE. The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: a case report. Int J Sports Phys Ther. 2013;8(2):145-161. [PMC free article] [PubMed] [Google Scholar]
- 50.Tough EA White AR Cummings TM Richards SH Campbell JL. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomised controlled trials. Eur J Pain. 2009;13(1):3-10. [DOI] [PubMed] [Google Scholar]
- 51.Cagnie B Castelein B Pollie F Steelant L Verhoeyen H Cools A. Evidence for the use of ischemic compression and dry needling in the management of trigger points of the upper trapezius in patients with neck pain: a systematic review. Am J Phys Med Rehabil. 2015;94(7):573-583. [DOI] [PubMed] [Google Scholar]
- 52.Cerezo-Tellez E Lacomba MT Fuentes-Gallardo I Mayoral Del Moral O Rodrigo-Medina B Gutierrez Ortega C. Dry needling of the trapezius muscle in office workers with neck pain: a randomized clinical trial. J Man Manip Ther. 2016;24(4):223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerezo-Tellez E Torres-Lacomba M Fuentes-Gallardo I, et al. Effectiveness of dry needling for chronic nonspecific neck pain: a randomized, single-blinded, clinical trial. Pain. 2016;157(9):1905-1917. [DOI] [PubMed] [Google Scholar]
- 54.Segura-Orti E Prades-Vergara S Manzaneda-Pina L Valero-Martinez R Polo-Traverso JA. Trigger point dry needling versus strain-counterstrain technique for upper trapezius myofascial trigger points: a randomised controlled trial. J Br Med Acupunct Soc. 2016;34(3):171-177. [DOI] [PubMed] [Google Scholar]
- 55.Calvo-Lobo C Pacheco-da-Costa S Martinez-Martinez J Rodriguez-Sanz D Cuesta-Alvaro P Lopez-Lopez D. Dry needling on the infraspinatus latent and active myofascial trigger points in older adults with nonspecific shoulder pain: a randomized clinical trial. J Geriatr Phys Ther. 2018;41(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clewley D Flynn TW Koppenhaver S. Trigger point dry needling as an adjunct treatment for a patient with adhesive capsulitis of the shoulder. J Orthop Sports Phys Ther. 2014;44(2):92-101. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh YL Kao MJ Kuan TS Chen SM Chen JT Hong CZ. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil. 2007;86(5):397-403. [DOI] [PubMed] [Google Scholar]
- 58.Koppenhaver S Embry R Ciccarello J, et al. Effects of dry needling to the symptomatic versus control shoulder in patients with unilateral subacromial pain syndrome. Man Ther. 2016;26:62-69. [DOI] [PubMed] [Google Scholar]
- 59.Eftekharsadat B Babaei-Ghazani A Zeinolabedinzadeh V. Dry needling in patients with chronic heel pain due to plantar fasciitis: A single-blinded randomized clinical trial. Med J Islamic Republic Iran. 2016;30:401. [PMC free article] [PubMed] [Google Scholar]
- 60.Yeo A Kendall N Jayaraman S. Ultrasound-guided dry needling with percutaneous paratenon decompression for chronic Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2112-2118. [DOI] [PubMed] [Google Scholar]
- 61.Blasco-Bonora PM Martin-Pintado-Zugasti A. Effects of myofascial trigger point dry needling in patients with sleep bruxism and temporomandibular disorders: a prospective case series. Acupunct Med. 2017;35(1):69-74. [DOI] [PubMed] [Google Scholar]
- 62.Barton CJ Menz HB Crossley KM. Clinical predictors of foot orthoses efficacy in individuals with patellofemoral pain. Med Sci Sports Exerc. 2011;43(9):1603-1610. [DOI] [PubMed] [Google Scholar]
- 63.Crowell MS Wofford NH. Lumbopelvic manipulation in patients with patellofemoral pain syndrome. J Man Manip Ther. 2012;20(3):113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang BY Shih YF Chen WY Ma HL. Predictors for identifying patients with patellofemoral pain syndrome responding to femoral nerve mobilization. Arch Phys Med Rehabil. 2015;96(5):920-927. [DOI] [PubMed] [Google Scholar]
- 65.Lesher JD Sutlive TG Miller GA Chine NJ Garber MB Wainner RS. Development of a clinical prediction rule for classifying patients with patellofemoral pain syndrome who respond to patellar taping. J Orthop Sports Phys Ther. 2006;36(11):854-866. [DOI] [PubMed] [Google Scholar]
- 66.Vicenzino B Collins N Cleland J McPoil T. A clinical prediction rule for identifying patients with patellofemoral pain who are likely to benefit from foot orthoses: a preliminary determination. Br J Sports Med. 2010;44(12):862-866. [DOI] [PubMed] [Google Scholar]
- 67.Watari R Kobsar D Phinyomark A Osis S Ferber R. Determination of patellofemoral pain sub-groups and development of a method for predicting treatment outcome using running gait kinematics. Clin Biomech. 2016;38:13-21. [DOI] [PubMed] [Google Scholar]
- 68.Ferber R Bolgla L Earl-Boehm JE Emery C Hamstra-Wright K. Strengthening of the hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Athl Train. 2015;50(4):366-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mason M Keays SL Newcombe PA. The effect of taping, quadriceps strengthening and stretching prescribed separately or combined on patellofemoral pain. Physiother Res Intl. 2011;16(2):109-119. [DOI] [PubMed] [Google Scholar]
- 70.Mills K Blanch P Dev P Martin M Vicenzino B. A randomised control trial of short term efficacy of in-shoe foot orthoses compared with a wait and see policy for anterior knee pain and the role of foot mobility. Br J Sports Med. 2012;46(4):247-252. [DOI] [PubMed] [Google Scholar]
- 71.Osorio JA Vairo GL Rozea GD, et al. The effects of two therapeutic patellofemoral taping techniques on strength, endurance, and pain responses. Phys Ther Sport. 2013;14(4):199-206. [DOI] [PubMed] [Google Scholar]
- 72.France S Bown J Nowosilskyj M Mott M Rand S Walters J. Evidence for the use of dry needling and physiotherapy in the management of cervicogenic or tension-type headache: a systematic review. Cephalalgia. 2014;34(12):994-1003. [DOI] [PubMed] [Google Scholar]
- 73.Haser C Stoggl T Kriner M, et al. Effect of dry needling on thigh muscle strength and hip flexion in elite soccer players. Med Sci Sports Exerc. 2017;49(2):378-383. [DOI] [PubMed] [Google Scholar]
- 74.Collins NJ Bierma-Zeinstra SM Crossley KM van Linschoten RL Vicenzino B van Middelkoop M. Prognostic factors for patellofemoral pain: a multicentre observational analysis. Br J Sports Med. 2013;47(4):227-233. [DOI] [PubMed] [Google Scholar]
- 75.Lankhorst NE van Middelkoop M Crossley KM, et al. Factors that predict a poor outcome 5-8 years after the diagnosis of patellofemoral pain: a multicentre observational analysis. Br J Sports Med. 2016;50(14):881-886. [DOI] [PubMed] [Google Scholar]
- 76.Nimon G Murray D Sandow M Goodfellow J. Natural history of anterior knee pain: a 14- to 20-year follow-up of nonoperative management. J Ped Orthop 1998;18(1):118-122. [PubMed] [Google Scholar]
- 77.Collins NJ Bisset LM Crossley KM Vicenzino B. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2012;42(1):31-49. [DOI] [PubMed] [Google Scholar]
- 78.Bolgla LA Earl-Boehm J Emery C Hamstra-Wright K Ferber R. Comparison of hip and knee strength in males with and without patellofemoral pain. Phys Ther Sport. 2015;16(3):215-221. [DOI] [PubMed] [Google Scholar]
- 79.Baldon Rde M Piva SR Scattone Silva R Serrao FV. Evaluating eccentric hip torque and trunk endurance as mediators of changes in lower limb and trunk kinematics in response to functional stabilization training in women with patellofemoral pain. Am J Sports Med. 2015;43(6):1485-1493. [DOI] [PubMed] [Google Scholar]
- 80.Barton CJ Lack S Malliaras P Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):207-214. [DOI] [PubMed] [Google Scholar]
- 81.Bolgla LA Malone TR Umberger BR Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285-296. [PMC free article] [PubMed] [Google Scholar]
- 82.Hamstra-Wright KL Earl-Boehm J Bolgla L Emery C Ferber R. Individuals with patellofemoral pain have less hip flexibility than controls regardless of treatment outcome. Clin J Sport Med. 2017;27(2):97-103. [DOI] [PubMed] [Google Scholar]
- 83.Thomson C Krouwel O Kuisma R Hebron C. The outcome of hip exercise in patellofemoral pain: A systematic review. Man Ther. 2016;26:1-30. [DOI] [PubMed] [Google Scholar]
- 84.Bazett-Jones DM Cobb SC Huddleston WE O’Connor KM Armstrong BS Earl-Boehm JE. Effect of patellofemoral pain on strength and mechanics after an exhaustive run. Med Sci Sports Exerc. 2013;45(7):1331-1339. [DOI] [PubMed] [Google Scholar]
- 85.Nakagawa TH Moriya ET Maciel CD Serrao FV. Trunk, pelvis, hip, and knee kinematics, hip strength, and gluteal muscle activation during a single-leg squat in males and females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2012;42(6):491-501. [DOI] [PubMed] [Google Scholar]
- 86.Noehren B Pohl MB Sanchez Z Cunningham T Lattermann C. Proximal and distal kinematics in female runners with patellofemoral pain. Clin Biomech. 2012;27(4):366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]