Abstract
G-Quadruplex DNA structure has been proven to be a binding target for small molecular organic compounds and hence regarded as a promising pharmacological target. Cisplatin is a widely used chemotherapy drug that targets duplex DNA and was recently shown to react also with G-quadruplex, implying that cisplatin actually may also target G-quadruplex. In this work, we employed magnetic tweezers to investigate the influence of cisplatin on the folding kinetics of single human telomeric G-quadruplex. It was revealed that cisplatin and G-quadruplex interact in two different and competitive ways that depend on cisplatin concentration.
Introduction
G-Quadruplex, an important DNA secondary structure, is stacked by two or more planar guanine tetrads formed by four guanine bases through Hoogsteen hydrogen bonds.1−4 In human telomere sequences, numerous tandem d(TTAGGG) repeats form G-quadruplexes in vivo to protect chromosome ends.5 In gene replication, G-quadruplex plays a vital role in the activity of the telomerases, which maintain the telomere length and are commonly overexpressed in cancer cells. Various small molecules have been reported to be capable of binding to telomere G-quadruplexes through a π–π stacking interaction.6−10 Thus, human telomeric G-quadruplex may be a potential therapeutic target for cancer treatments.7,11,12
Cisplatin has been used as a classical chemotherapy drug for more than 4 decades.13 Its major pharmacological targets have been revealed to be double-stranded DNA (dsDNA).14 Under physiological conditions, cisplatin molecules form positively charged active diaquated intermediates that attack the N7 atoms of guanines or adenines to form monoadducts or diadducts.15,16 DNA damages caused by the adducts give rise to the cell apoptosis process.17,18 The G-quadruplex sequences are guanine-rich, which suggests that G-quadruplex may be a new promising target for cisplatin. Bulk experiments (circular dichroism (CD) spectroscopy, UV-spectroscopically monitored thermal denaturation, and gel electrophoresis) have been used to study the interaction between cisplatin and G-quadruplex. Bombard and Ourliac Garnier19 and Brabec et al.20 observed that cisplatin and transplatin destabilize human telomeric G-quadruplex and H-telo quadruplexes. Viglasky21 showed that cisplatin and transplatin unfold telomeric G-quadruplexes Tel-1 and Tel-2 but do not affect c-myc and platelet-derived growth factor (PDGF)-A DNA quadruplexes. However, details of the interaction between G-quadruplexes and cisplatin remain to be clarified.
Recently single-molecule techniques, such as optical tweezers, magnetic tweezers, and single-molecule fluorescence resonance energy transfer (FRET), have proven to be powerful methods to study G-quadruplex and its interaction with various ligands,22−27 which shed light on the folding kinetics of G-quadruplex. Our previous studies revealed the condensation of dsDNA caused by cisplatin.28,29 In the present research, we used magnetic tweezers to investigate the interaction between G-quadruplex and cisplatin at the single-molecule level and aimed to find out how cisplatin binds to G-quadruplex and affects the folding kinetics of G-quadruplex.
Methods
In our experiments, one human telomeric G-quadruplex sequence 5′-GGGTTAGGGTTAGGGTTAGGGTTA with a lower 699 bp dsDNA handle and an upper 2271 bp dsDNA handle was tethered specifically between an anti-digoxigenin-coated coverslip and a streptavidin-coated super paramagnetic bead (Dynabeads MyOne Streptavidin T1, Invitrogen) (Figure 1A). The kinetics of folding and unfolding of G-quadruplex were measured in the absence or presence of hydrated intermediate of cisplatin in a K+ buffer (100 mM CH3COOK, 10 mM Tris, and 1 mM ethylenediaminetetraacetic acid (EDTA), pH 8.0) at 29 °C.
Figure 1.

Mechanical unfolding of G-quadruplexes without/with cisplatin. (A) Schematic of the magnetic tweezers. G-Quadruplex is tethered between a super-paramagnetic bead and a coverslip. (B) Force–ramp measurements at a constant force-loading rate (lower panel). A typical extension-versus-time curve in more than one stretching cycle is shown (upper panel), where a discontinuous extension jump marks an unfolding event (inset). (C) CD spectra of the G-quadruplex sequence (blue), the G-quadruplex sequence with the 6 bp dsDNA handles on each side (red) and the mutant sequence (green dash) in the working buffer (see text for details). (D,E) Histograms of unfolding force in the absence and the presence of 10 μM cisplatin from 317 and 159 unfolding events, respectively. The force-loading rate was r = 0.2 pN/s. The lines represent the theoretical fits (see text for details).
Magnetic Tweezers
The magnetic tweezers employed in our experiments were from Pico Twist company (Lyon, France), and the main protocol followed that used by Dekker and his co-worker.30,31 In the vertical magnetic tweezers system, all the DNA samples and the buffer were contained by a flow cell. Two neodymium (NdFeB) permanent magnets above the flow cell were used to provide an external magnetic field that exerted a force on each paramagnetic bead and thus stretched the corresponding G-quadruplex sequence. The force was controlled by manipulating the position of the magnets and could achieve up to 20.0 pN in our experiments. The real-time images were recorded using a JAI Giga-Ethernet charge-coupled device (CCD) camera at 60 Hz via a vertical microscope objective (Olympus 100× 1.2, oil immersion). Each bead in the image field was observed as a circular diffraction ring. We tracked the horizontal coordinates of the bead by fitting the center of the diffraction ring with an accuracy of ∼1 nm. In addition, a calibration of the relative vertical coordinate was carried out according to the diffraction ring pattern, which depended on the distance between the bead and the focal plane with an accuracy of ∼10 nm. Smoothing algorithm could improve the vertical resolution to the nanometer level at the expense of time resolution.
DNA Construction for Magnetic Tweezers Experiments
All oligonucleotides were purchased from Invitrogen. All enzymes and plasmids were purchased from New England BioLabs, Inc. (NEB). The dsDNA handles were prepared using polymerase chain reaction (PCR) amplification with pBR322 templates following a standard NEB protocol. The primers were designed using Primer5.0. The PCR products were purified using QIAquick PCR Purification Kit (QIAGEN) and then digested by restriction endonucleases XbaI and KpnI, respectively, to obtain sticky ends that were just complementary to those of the central DNA complex (see Tables S1 and S2). The digested products were further purified using QIAquick PCR Purification Kit. Finally, the two dsDNA handles and the central DNA complex were ligated by ligase T4 following the standard NEB protocol.
Surface Treatment and DNA Tether
The inner surfaces of the coverslips were dealt with 0.2% w/v nitrocellulose (Sigma-Aldrich) and 0.03% w/v polystyrene particles (ACME microspheres) in alcohol and then heated at 150 °C for 5 min.31 The nitrocellulose was slightly melted and polystyrene particles were fixed on the surface as reference beads. Anti-digoxigenin (10 mg/mL, Sigma-Aldrich) was used to treat the surface overnight at 37 °C. Then the surface was dealt with a passivation buffer [10 mg/mL bovine serum albumin (BSA), 1 mM EDTA, 10 mM pH 7.4 phosphate buffers, 10 mg/mL Pluronic F127 surfactant (Sigma-Aldrich), 3 mM NaN3] at room temperature for at least 4 h, to avoid nonspecific adherence of beads to the surface. The DNA constructs were diluted to 50 pM, and mixed with 2× diluted Dynabeads MyOne with a volume ratio of 1:1. The mixture was injected to the flow cell for DNA tethering. After 15 min, the untethered DNA constructs were rinsed away.
Force Calibration
An approximation that the same vertical force was exerted on all the beads recorded by CCD at the same time was introduced. Thus, the force depended only on the vertical position of the magnets. The force can be determined by the horizontal Brownian motion fluctuation (δx) and the vertical extension of DNA (z) as30
| 1 |
In our experiments, we analyzed the Brownian motion of beads in the Fourier space to obtain more accurate results.30 Longer measure time and longer DNA construction also improved the accuracy. λ-DNA molecules (48 502 bp) were used to calibrate the forces under various magnetic positions (zmag) to obtain the force–zmag curve (see Figure S1). A single-exponential function was used empirically to fit the force–zmag relationship.32 In the force–ramp and force–jump experiments, the force was determined by manipulating zmag according to the fitting formula. Considering the nonuniformity of the magnetic moments of the beads and the error of zmag, the relative error of force was estimated to be ∼10%.
Circular Dichroism Spectra Measurements
Circular dichroism (CD) spectra measurements were performed using a Bio-Logic MOS450/AF-CD optical system (Bio-Logic Science Instruments, France). The DNA constructs were dissolved in the working buffer (100 mM CH3COOK, 10 mM Tris and 1 mM EDTA, pH 8.0) to 10 μM and processed, referring to the usual annealing protocol (heated to 95 °C for 10 min and cooled slowly to room temperature). For each measurement, 1.5 mL solution of 1 μM DNA sample was contained in a quartz cell of 1 cm optical path length. The DNA constructs were incubated for 30 min when measuring with cisplatin. Spectra were recorded at each nanometer from 220 to 340 nm. Each curve was the average of 10 measurements and smoothed with a Savitsky–Golay filter.33
DNA Construction for CD Measurements
All oligonucleotides were purchased from Invitrogen. The same sequence 5′-GGGTTAGGGTTAGGGTTAGGGTTA with and without the 6 bp dsDNA handles on each side were employed in CD measurements (see Table S3). As control, a mutant sequence 5′-GGGTTATTATTAGGGTTAGGGTTA that cannot fold to G-quadruplex was also measured.
Cisplatin
Cisplatin was purchased from the Institute of Precious Metal in Kunming, China, as nitrates of hydrated intermediates. It was first dissolved in deionized water (Milli-Q) to a concentration of 3 μM at 37 °C for 24 h, under continuous shaking. Then the solution was diluted to target concentration by the working buffer (100 mM CH3COOK, 10 mM Tris, and 1 mM EDTA, pH 8.0). Chloridion was excluded from the buffer to avoid inactivation of the reactive cisplatin intermediate. According to our previous research,28,29 the dsDNA handles would not shorten under a cisplatin concentration below 100 μM, which is just the case in our present magnetic tweezers experiments. Thus, the interaction between cisplatin and the dsDNA handles was considered not to affect our experiments.
In long-time incubation experiments, the DNA construct was held at 2 pN in a 20 μM cisplatin buffer for certain incubation times, and then the refolding probability was measured.
Results and Discussion
To investigate the effect of cisplatin on the G-quadruplex folding/unfolding, we carried out force–ramp experiments (Figure 1B). At the beginning of each stretching cycle, G-quadruplex was first held at a low force of 2.0 pN for a holding time of 60 s, which is long enough for the G-quadruplex to fold. Then the force was increased linearly at a loading rate of r = 0.2 pN/s from 2.0 to 18.0 pN. The unfolding event of G-quadruplex corresponds to a discontinuous extension jump of Δz = ∼7 nm (Figure 1B, upper panel, see Figure S2 for more detail). When the force reached the 18.0 pN maximum, it was held there for 60 s to assure that no folded G-quadruplex still exists at the beginning of the next stretching cycle.
As mentioned in a previous study,34 the conformation of G-quadruplex would be quite sensitive on the environment. To verify that the DNA constructs indeed formed a G-quadruplex structure in our magnetic tweezers experiments, CD measurements were taken in our working buffer (100 mM CH3COOK, 10 mM Tris, and 1 mM EDTA, pH 8.0). As shown in Figure 1C, the CD spectrum of the G-quadruplex sequence without the dsDNA handles (blue) reveals two positive peaks around 290 and 260 nm and one negative peak around 240 nm, which is a typical CD spectrum of hybrid conformation in K+ buffer. For the G-quadruplex sequence with the 6 bp dsDNA handles (red), the characteristic peaks are also identifiable in the CD spectrum. This indicates that the DNA construct with the dsDNA handles can form G-quadruplexes of hybrid conformation. It is worth noting that the peaks are shifted, especially for the 290 nm peak. We attributed the shift to the CD spectrum of the dsDNA handles in this DNA construct. The green dash curve is the mutant sequence that cannot form G-quadruplex.
Shown in Figure 1D,E are the histograms of unfolding force in the absence and the presence of 10 μM cisplatin, respectively. In the absence of cisplatin, the distribution exhibits a single peak (Figure 1D), which indicates that a single stable G-quadruplex was formed under our buffer conditions. Results of the single-molecule magnetic tweezers experiment are consistent with those of a previous work,35 which reveals that the human telomeric G-quadruplex sequence with a flanking TTA at 3′ end had a major conformation of the hybrid-2 type.
To describe quantitatively the unfolding behavior of single G-quadruplexes at a constant loading rate, we used Bell’s model.36 The unfolding rate (ku) in terms of tension (f) for a biomolecule can be written as
| 2 |
where ku0 is the unfolding rate at f = 0 pN, Δxu is the transition distance between the folding state and the reaction free-energy barrier in the reaction coordinate (extension herein), kB is the Boltzmann constant, and T is the temperature.36 The theoretical prediction26,37,38 of the unfolding force distribution (punfold) at a certain loading rate (r) can be derived as
| 3 |
Accordingly, the peak location of the unfolding
force is given
by 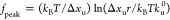 , which
depends on the loading rate r.
, which
depends on the loading rate r.
From the histogram fitted without cisplatin (Figure 1D), the peak position of the unfolding force was found to be located at ∼9.0 pN. In the previous optical tweezers measurements of human telomeric G-quadruplex,22 a higher unfolding force peak around 21 pN was observed at a faster loading rate of r = 5.5 pN/s. Both the previous fast-stretching experimental result and our present result agree with Bell’s model mentioned above. In addition, from the fitting, we obtained the unfolding rate at zero force as ku0 = (5.3 ± 1.5) × 10–3 s–1 (±SD, the same below) and the transition distance as Δxu = 1.00 ± 0.14 nm. It is worth noting that Δxu is much smaller than the extension of the G-quadruplex sequence (∼7 nm). This indicates the high cooperativity of the hybrid-2-type G-quadruplex unfolding under our experiment condition (K+ buffer). When a small part of G-quadruplex is disrupted, the whole structure collapses cooperatively.
Different from the case without cisplatin, two peaks are exhibited in the unfolding force histogram with 10 μM cisplatin (Figure 1E). This means that two structures with different stabilities might be formed by the G-quadruplex sequence. In our magnetic tweezers measurements, these two structures were considered to unfold independently under tension, and a linear combination of two unfolding force distributions p1 (high-force structure) and p2 (low-force structure) defined as p = ap1 + (1 – a)p2 was used to fit the histogram. From the fitting (Figure 1E), we infer that the peak for the low-force structure centered around 9.0 pN, which is the same as that in the case without cisplatin, indicating that the low-force structure is the normal G-quadruplex structure. The other peak centered around a higher force of 15.0 pN, suggesting that G-quadruplex might become more stable because of cisplatin binding. The fraction of the high-force population was determined to be ∼0.58.
For the low-force population, we obtained the unfolding rate at zero force as ku20 = (4.0 ± 1.4) × 10–3 s–1, which is comparable to the unfolding rate without cisplatin, further confirming the cisplatin-free nature of the low-force structure. For the high-force population, we got the unfolding rate at zero force as ku1 = (1.4 ± 1.5) ± 10–4 s–1, which is obviously smaller than ku20, confirming the existence of a more-stable cisplatin-bound state. The transition distances for cisplatin-bound and cisplatin-free states were Δxu1 = 1.83 ± 0.34 nm and Δxu2 = 1.64 ± 0.27 nm, respectively. Both values are close to 1.00 ± 0.14 nm in the absence of cisplatin. This suggests that the binding of cisplatin to G-quadruplex does not shift the position of the reaction energy barrier obviously and the unfolding of cisplatin-bound G-quadruplex also has a high cooperativity. The cisplatin-bound G-quadruplex is also unfolded directly under the stretching force, and no intermediate was observed in the experiments (K+ buffer).
To further study this stable G-quadruplex found in the presence of cisplatin and to reveal the binding kinetics of cisplatin onto G-quadruplexes, we then determined the refolding probabilities at different holding times by force–jump measurements. As shown in Figure 2A, in each cycle, the force was first held at 2.0 pN for a certain holding time to allow G-quadruplex to form, then abruptly raised to 18.0 pN and held at this level for 60 s to let the formed G-quadruplex unfold. The abrupt force rise after low-force holding would avoid the folding events that might occur at the initial low-force region of force–ramp experiments. Here, an unfolding event also corresponds to a discontinuous extension jump of Δz = ∼7 nm (Figure 2A, see Figure S2 for more detail). With a DNA construct, the measurement was repeatedly taken up to 50 times for a given holding time. The refolding probability was then calculated by dividing the number of unfolding events by the number of total repeating cycles.
Figure 2.

Kinetics of G-quadruplex folding without/with cisplatin. (A) Force–jump experiments. A typical DNA extension versus time curve (upper panel) in one stretching cycle. The force jump between 2.0 and 18.0 pN (lower panel) and a discontinuous jump in the DNA extension after the force jumped to 18.0 pN marks an unfolding event (inset). (B) The refolding probabilities of G-quadruplex in the absence and the presence of 10 μM cisplatin versus holding time. The error bars represent standard errors. The lines represent theoretical fits (see text for details). (C) A representative histogram of the unfolding force obtained in a corresponding force–ramp experiment (30 s holding time, 434 unfolding events), from the fitting of which the population ratio of the cisplatin-free and the cisplatin-bound states can be obtained. (D) Refolding probabilities of the cisplatin-free and the cisplatin-bound states.
Figure 2B shows the G-quadruplex refolding probability versus holding time in the absence and the presence of 10 μM cisplatin. Each data point is a weighted average of data from 16–65 DNA constructs. As can be seen clearly, G-quadruplex in the presence of 10 μM cisplatin has a higher probability to refold than in a cisplatin-free buffer.
The G-quadruplex refolding probability in the cisplatin-free buffer could be derived from the simple two-state model,
| 4 |
From the fitting of the data curve in Figure 2B (see “Two-State Model Fitting” in Supporting Information), the refolding and unfolding rates in the cisplatin-free buffer were determined as k1 = (8.2 ± 0.7) × 10–3 s–1 and k2 = (3.6 ± 0.4) × 10–2 s–1. Thus, the refolding rate k1 is smaller than the unfolding rate k2 at a 2.0 pN stretching force. This indicates that the stretching force makes the unfolding state more preferred than the folding state in the cisplatin-free buffer.
The refolding probability in the case with cisplatin should be the sum of the probabilities for cisplatin-bound and cisplatin-free states. To determine the probabilities of the two states at a given holding time, we performed corresponding force–ramp experiments. The histograms of the unfolding force were then fitted by a linear combination of two distributions as before, and with the areas under the fitting curves of two peaks, the probabilities of the two states were obtained (Figure 2C,D).
In the presence of cisplatin, a sequential three-state model might be used to describe the G-quadruplex folding/unfolding dynamics:
| 5 |
As shown in Figure 2D, the refolding probabilities of both cisplatin-bound and cisplatin-free states increased with increasing holding time and reached equilibrium at around 80 s. Their ratio, however, remained almost a constant (∼0.6) from 10 to 120 s (see “Sequential Transition Model Fitting” in Supporting Information). This indicates that in the three-state transition processes, the folding of G-quadruplex (i.e., ssDNA to G4) is the rate-determining step. The process of cisplatin binding to free G-quadruplex (i.e., G4 to G4–cisplatin complex) should be much more rapid and can be regarded as a quasi-equilibrium progress. Thus, we might approximately treat the two G-quadruplex states as a single equivalent state and simplify the three-state model to a two-state one (see “Sequential Transition Model Fitting” in Supporting Information). Then, by fitting the refolding probability data at 10 μM cisplatin in Figure 2B using the equivalent two-state model, we obtained k12 = (9.2 ± 1.7) × 10–3 s–1 and k21 = (6.1 ± 1.8) × 10–2 s–1. As expected, these two values are similar to that of k1 and k2 in the absence of cisplatin.
Considering that the binding of cisplatin to free G-quadruplex is a quasi-equilibrium progress, the rates k23 and k32 have a linear relation k23/k32 = a/(1 – a) (see “Sequential Transition Model Fitting” in Supporting Information). The free-energy difference between the cisplatin-bound and cisplatin-free states at a force of 2.0 pN was estimated as ΔG = ln[a/(1 – a)]/kBT ≈ 0.4kBT in the presence of 10 μM cisplatin. To obtain the rates k23 and k32, experiments with higher temporal resolution need to be carried out.
A series of force–jump measurements were then carried out to obtain the refolding probabilities at different cisplatin concentrations. As shown in Figure 3A, at a holding time of 60 s and a holding force of 2.0 pN, the refolding probability reached the maximum at 10 μM cisplatin and then dramatically decreased at higher cisplatin concentrations. Finally, the refolding event was nearly inhibited completely at 100 μM cisplatin.
Figure 3.

Refolding probabilities versus cisplatin concentration and incubation time. (A) Refolding probabilities at a series of cisplatin concentrations. The refolding probability increased at 10 μM, but dramatically decreased at higher concentrations. Every column is a weighted average of 16 to 45 DNA constructs. The error bars are standard errors (the same as in C). (B) Unfolding force histogram obtained from the corresponding force–ramp experiments with 232, 215, and 121 unfolding events, respectively. At different concentrations of cisplatin, peaks for both cisplatin-free and cisplatin-bound states can be observed. (C) Refolding probabilities at a series of incubation times. The refolding probabilities remained stable in a time scale of 1 h. Every column is a weighted average of 8 to 28 DNA constructs. (D,E) CD spectra of the quadruplex sequence and the mutant sequence (see text for details) at a cisplatin concentration of 100 μM (red), 200 μM (blue), and 500 μM (green).
CD experiments were also conducted for the G-quadruplex and the mutant sequence. As shown in Figure 3D,E, the CD spectra of the G-quadruplex sequence (5′-(GGGTTA)4) and the mutant sequence (5′-GGGTTATTATTAGGGTTAGGGTTA) at 100 μM (red), 200 μM (blue), and 500 μM (green) cisplatin were measured, respectively. For the G-quadruplex sequence (Figure 3D), CD spectra at 100 and 200 μM exhibited the characteristic peaks of hybrid G-quadruplex (positive peaks around 290 and 260 nm, negative peak around 240 nm). However, the CD spectrum at 500 μM did not reveal these peaks. The heights of the character peaks also receded with the increasing cisplatin concentration. As controls, the CD spectra of the mutant sequence (Figure 3E) did not exhibit the characteristic peaks at all cisplatin concentrations. The results of the CD experiments indicated that for the G-quadruplex sequence, hybrid conformation G-quadruplexes obviously formed at a low cisplatin concentration and were gradually destabilized at a high cisplatin concentration. This result agreed with our magnetic tweezers experiments. It is worth noting that the complete destabilization of G-quadruplex occurred at a higher cisplatin concentration in the CD experiments (500 μM) than in the magnetic tweezers experiments (100 μM). We attributed this to the much higher concentration of DNA constructs in CD measurements (∼μM) than in single-molecule magnetic tweezers experiments (∼pM).
Previous studies based on CD spectroscopy, UV-spectroscopically monitored thermal denaturation, and gel-electrophoresis have also reported that cisplatin is capable of binding to the G-quadruplex sequences and destabilizing the G-quadruplex structures.19−21 The adducts formed by cisplatin were proposed to cause the destabilization. Activated cisplatin can attack the N7 atoms of guanines to form adducts by covalent bonds,15,16 which are strong and difficult to rupture. In a G-tetrad of G-quadruplexes, the N7 atoms of guanines are involved in the Hoogsteen hydrogen bonds, which are critical in G-quadruplex folding.1 When the N7 atom in a guanine of the G-quadruplex sequence is bound with cisplatin, should one Hoogsteen hydrogen bond fail to form, the whole G-quadruplex may collapse.
To verify this, corresponding force–ramp experiments were conducted at 10, 20, and 50 μM cisplatin and the unfolding force histograms are shown in Figure 3B. At different cisplatin concentrations, the peaks for both cisplatin-free and cisplatin-bound states can be observed, which revealed that the binding of cisplatin to G-quadruplex reached saturation because the ratio between the populations of the two states was invariable. Thus, it should not be the reversible binding of cisplatin to G-quadruplex that caused the decrease in the refolding probability at high cisplatin concentrations. Our previous studies revealed that the number of cisplatin molecules that form covalent bonds with guanines depends strongly on the cisplatin concentration, which was proposed to be caused by the cationic charges of the cisplatin molecules.39 Thus, we propose here that at low cisplatin concentrations (≤10 μM), only a few cisplatin molecules formed adducts with N7 atoms of guanines in the G-quadruplex sequence. The reversible binding was dominating, and the refolding probability was relatively high. When the cisplatin concentration was increased, more and more cisplatin adducts were formed and, irreversibly, the sequences were unable to fold to G-quadruplex. This caused the decrease in refolding probability at higher cisplatin concentrations. According to population pharmacokinetics, the peak concentration of unbound cisplatin in plasma is usually less than 3000 μg/L.40 The concentration of uptake cisplatin accumulation in the target cells is ∼10 μM at most.18,41 At this relatively low cisplatin concentration, the reversible binding of cisplatin should play a nonignorable role.
To investigate the property of cisplatin adducts, refolding probabilities with 20 μM cisplatin and 60 s holding time at 2.0 pN were measured for different incubation times. As shown in Figure 3C, the refolding probabilities remained invariable in an incubation time range of 10 h. Earlier studies revealed that the process of cisplatin to form a monoadduct with a single guanine is fast and takes <10 min, but the progress to further form a diadduct with another guanine needs a much longer time (in hours).39 The present experiment revealed that a monoadduct formed by a relatively fast reaction is sufficient to avoid G-quadruplex folding.
In summary, we have revealed two ways of interaction between cisplatin and the G-quadruplex sequence as shown in Figure 4. Cisplatin molecules can bind reversibly to G-quadruplexes to form a kind of more stable complexes. On the other hand, they can also bind irreversibly to guanines in the G-quadruplex sequences to avoid folding. These two ways of interactions compete with each other under different cisplatin concentrations in a buffer. At a low concentration, the reversible way dominates and the refolding probability increases with concentration. However, at a high concentration, the irreversible way dominates and the refolding probability decreases and finally vanishes. As a tiny molecular inorganic compound, cisplatin shows promising potential to react with G-quadruplex, which suggests that the interaction between small molecules and G-quadruplex should be investigated.
Figure 4.

Model proposed to describe the interaction between cisplatin and a G-quadruplex sequence. See text for details.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Grant Nos. 11474346 and 11274374), the 973 program of China (Grant No. 2013CB837200), the National Key Research and Development Program (Grant No. 2016YFA0301500).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.6b00044.
Experimental material details and detailed analysis of data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Davis J. T. G-quartets 40 years later: From 5′-GMP to molecular biology and supramolecular chemistry. Angew. Chem., Int. Ed. 2004, 43, 668–698. 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- Burge S.; Parkinson G. N.; Hazel P.; Todd A. K.; Neidle S. Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 2006, 34, 5402–5415. 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochman M. L.; Paeschke K.; Zakian V. A. DNA secondary structures: stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. 10.1038/nrg3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D.; Lipps H. J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015, 43, 8627–8637. 10.1093/nar/gkv862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffitzel C.; Berger I.; Postberg J.; Hanes J.; Lipps H. J.; Pluckthun A. In vitro generated antibodies specific for telomeric guanine-quadruplex DNA react with Stylonychia lemnae macronuclei. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 8572–8577. 10.1073/pnas.141229498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert J. L. Four-stranded nucleic acids: structure, function and targeting of G-quadruplexes. Chem. Soc. Rev. 2008, 37, 1375–1384. 10.1039/b702491f. [DOI] [PubMed] [Google Scholar]
- Neidle S. Human telomeric G-quadruplex: The current status of telomeric G-quadruplexes as therapeutic targets in human cancer. FEBS J. 2010, 277, 1118–1125. 10.1111/j.1742-4658.2009.07463.x. [DOI] [PubMed] [Google Scholar]
- Yang D. Z.; Okamoto K. Structural insights into G-quadruplexes: towards new anticancer drugs. Future Med. Chem. 2010, 2, 619–646. 10.4155/fmc.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat P.; Singh Y.; Defrancq E. Methods for investigating G-quadruplex DNA/ligand interactions. Chem. Soc. Rev. 2011, 40, 5293–5307. 10.1039/c1cs15117g. [DOI] [PubMed] [Google Scholar]
- Le T. V. T.; Han S.; Chae J.; Park H.-J. G-Quadruplex Binding Ligands: from Naturally Occurring to Rationally Designed Molecules. Curr. Pharm. Des. 2012, 18, 1948–1972. 10.2174/138161212799958431. [DOI] [PubMed] [Google Scholar]
- Paeschke K.; Simonsson T.; Postberg J.; Rhodes D.; Lipps H. J. Telomere end-binding proteins control the formation of G-quadruplex DNA structures in vivo. Nat. Struct. Mol. Biol. 2005, 12, 847–854. 10.1038/nsmb982. [DOI] [PubMed] [Google Scholar]
- De Cian A.; Lacroix L.; Douarre C.; Temime-Smaali N.; Trentesaux C.; Riou J.-F.; Mergny J.-L. Targeting telomeres and telomerase. Biochimie 2008, 90, 131–155. 10.1016/j.biochi.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Rosenberg B.; Van Camp L.; Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B.; Van Camp L.; Trosko J. E.; Mansour V. H. Platinum compounds: a new class of potent antitumour agents. Nature 1969, 222, 385–386. 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M. J.; Van der Veer J. L.; Den Hartog J. H. J.; Lohman P. H. M.; Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry 1985, 24, 707–713. 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Takahara P. M.; Frederick C. A.; Lippard S. J. Crystal structure of the anticancer drug cisplatin bound to duplex DNA. J. Am. Chem. Soc. 1996, 118, 12309–12321. 10.1021/ja9625079. [DOI] [Google Scholar]
- Kartalou M.; Essigmann J. M. Recognition of cisplatin adducts by cellular proteins. Mutat. Res., Fundam. Mol. Mech. Mutagen. 2001, 478, 1–21. 10.1016/s0027-5107(01)00142-7. [DOI] [PubMed] [Google Scholar]
- Wang D.; Lippard S. J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discovery 2005, 4, 307–320. 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Ourliac Garnier I.; Bombard S. GG sequence of DNA and the human telomeric sequence react with cis-diammine-diaquaplatinum at comparable rates. J. Inorg. Biochem. 2007, 101, 514–524. 10.1016/j.jinorgbio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Heringova P.; Kasparkova J.; Brabec V. DNA adducts of antitumor cisplatin preclude telomeric sequences from forming G quadruplexes. J. Biol. Inorg. Chem. 2009, 14, 959–968. 10.1007/s00775-009-0508-6. [DOI] [PubMed] [Google Scholar]
- Viglasky V. Platination of telomeric sequences and nuclease hypersensitive elements of human c-myc and PDGF-A promoters and their ability to form G-quadruplexes. FEBS J. 2009, 276, 401–409. 10.1111/j.1742-4658.2008.06782.x. [DOI] [PubMed] [Google Scholar]
- Koirala D.; Dhakal S.; Ashbridge B.; Sannohe Y.; Rodriguez R.; Sugiyama H.; Balasubramanian S.; Mao H. B. A single-molecule platform for investigation of interactions between G-quadruplexes and small-molecule ligands. Nat. Chem. 2011, 3, 782–787. 10.1038/nchem.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala D.; Ghimire C.; Bohrer C.; Sannohe Y.; Sugiyama H.; Mao H. B. Long-Loop G-Quadruplexes Are Misfolded Population Minorities with Fast Transition Kinetics in Human Telomeric Sequences. J. Am. Chem. Soc. 2013, 135, 2235–2241. 10.1021/ja309668t. [DOI] [PubMed] [Google Scholar]
- Li W.; Hou X.-M.; Wang P.-Y.; Xi X.-G.; Li M. Direct measurement of sequential folding pathway and energy landscape of human telomeric G-quadruplex structures. J. Am. Chem. Soc. 2013, 135, 6423–6426. 10.1021/ja4019176. [DOI] [PubMed] [Google Scholar]
- Long X.; Parks J. W.; Bagshaw C. R.; Stone M. D. Mechanical unfolding of human telomere G-quadruplex DNA probed by integrated fluorescence and magnetic tweezers spectroscopy. Nucleic Acids Res. 2013, 41, 2746–2755. 10.1093/nar/gks1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H.; Zeng X.; Xu Y.; Lim C. J.; Efremov A. K.; Phan A. T.; Yan J. Dynamics and stability of polymorphic human telomeric G-quadruplex under tension. Nucleic Acids Res. 2014, 42, 8789–8795. 10.1093/nar/gku581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivalingam A.; Izquierdo M. A.; Marois A. L.; Vyšniauskas A.; Suhling K.; Kuimova M. K.; Vilar R. The interactions between a small molecule and G-quadruplexes are visualized by fluorescence lifetime imaging microscopy. Nat Commun. 2015, 6, 8178. 10.1038/ncomms9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X.-M.; Zhang X.-H.; Wei K.-J.; Ji C.; Dou S.-X.; Wang W.-C.; Li M.; Wang P.-Y. Cisplatin induces loop structures and condensation of single DNA molecules. Nucleic Acids Res. 2009, 37, 1400–1410. 10.1093/nar/gkn933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Sun Z.-Q.; Xie P.; Dou S.-X.; Wang W.-C.; Wang P.-Y. Elastic response and length change of single DNA molecules induced by a combination of cisplatin and transplatin. Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys. 2012, 85, 021918. 10.1103/physreve.85.021918. [DOI] [PubMed] [Google Scholar]
- Vilfan I. D.; Lipfert J.; Koster D. A.; Lemay S. G.; Dekker N. H.. Handbook of Single-Molecule Biophysics; Springer: USA, 2009; pp 371–395. [Google Scholar]
- Cnossen J. P.; Dulin D.; Dekker N. H. An optimized software framework for real-time, high-throughput tracking of spherical beads. Rev. Sci. Instrum. 2014, 85, 103712. 10.1063/1.4898178. [DOI] [PubMed] [Google Scholar]
- Chen H.; Fu H.; Zhu X.; Cong P.; Nakamura F.; Yan J. Improved High-Force Magnetic Tweezers for Stretching and Refolding of Proteins and Short DNA. Biophys. J. 2011, 100, 517–523. 10.1016/j.bpj.2010.12.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitzky A.; Golay M. J. E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. 10.1021/ac60214a047. [DOI] [Google Scholar]
- Long X.; Stone M. D. Kinetic Partitioning Modulates Human Telomere DNA G-Quadruplex Structural Polymorphism. PLoS One 2013, 8, e83420 10.1371/journal.pone.0083420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J.; Carver M.; Yang D. Polymorphism of human telomeric quadruplex structures. Biochimie 2008, 90, 1172–1183. 10.1016/j.biochi.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. Models for specific adhesion of cells to cells. Science 1978, 200, 618–627. 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Evans E.; Ritchie K. Dynamic strength of molecular adhesion bonds. Biophys. J. 1997, 72, 1541–1555. 10.1016/s0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H.; Wu J.; Shao F.; Yan J. Stability and Kinetics of c-MYC Promoter G-Quadruplexes Studied by Single-Molecule Manipulation. J. Am. Chem. Soc. 2015, 137, 2424–2427. 10.1021/ja511680u. [DOI] [PubMed] [Google Scholar]
- Zhang H.-Y.; Liu Y.-R.; Ji C.; Li W.; Dou S.-X.; Xie P.; Wang W.-C.; Zhang L.-Y.; Wang P.-Y. Oxaliplatin and Its Enantiomer Induce Different Condensation Dynamics of Single DNA Molecules. PLoS One 2013, 8, e71556 10.1371/journal.pone.0071556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urien S.; Lokiec F. Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br. J. Clin. Pharmacol. 2004, 57, 756–763. 10.1111/j.1365-2125.2004.02082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately D. P.; Howell S. B. Cellular accumulation of the anticancer agent cisplatin: A review. Br. J. Cancer 1993, 67, 1171–1176. 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



