Abstract
The uniqueness of Doxil can be attributed, to a large extent, to its intraliposomal doxorubicin-sulfate nanorod crystal. We re-examine these nanocrystal features and their mechanism of the formation by studying pegylated liposomal doxorubicins (PLDs) of the same lipid composition, size distribution, and extraliposome medium that were prepared at different ammonium sulfate (AS) concentrations. This study includes a comparison of the thermotropic behavior, morphology, and in vitro ammonia-induced doxorubicin release (relevant to Doxil’s in vivo performance) of these PLDs. In this study, we confirm that a transmembrane ammonium gradient is critical for doxorubicin remote loading, and we demonstrate that the intraliposomal concentration of sulfate counteranions and ammonium ions determine to a large extent the physical state and stability of the PLDs’ remote loaded doxorubicin. “Fully-developed” intraliposome doxorubicin-sulfate nanorod crystals (as defined by cryogenic transmission electron microscopy imaging) develop only when the ammonium sulfate (AS) concentration used for PLD preparation is ≥150 mM. Less than 10% of PLDs prepared with 100 mM AS show fully developed nanorod crystals. Intraliposomal AS concentration ≥200 mM is required to support the stable nanocrystallization in PLDs. The presence of nanocrystals and their melting enthalpy and phase transition co-operativity strongly affect the ammonia-induced doxorubicin release of PLDs. A quick, biphasic release occurs for PLDs that lack the nanorod crystals or have crystals of poor crystallinity, whereas PLDs prepared with ≥200 mM AS show a monophasic, zero-order slow release. This study also demonstrates that after remote loading, residual intraliposomal ammonium concentration and the transmembrane pH gradient related to it also play an important role in doxorubicin-sulfate intraliposomal crystallization and ammonia-induced doxorubicin release.
Introduction
Doxil (pegylated liposomal doxorubicin, (PLD)) is the first FDA-approved nanodrug (1995).1 It is based on two concepts: (i) the prolonged circulation time afforded by poly(ethylene glycol) (PEG) corona on the nanoliposomes’ surface by 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(poly(ethylene glycol))-2000] (DSPE-PEG2k) lipopolymer2,3 and (ii) the transmembrane ammonium sulfate (AS) gradient ([AS]liposome ≫ [AS]medium4,5) that enables the highly efficient and stable remote loading of doxorubicin required for human treatment (∼50 mg/m2).1,4,5 This remote loading also not only plays a role in achieving high encapsulation and retention of doxorubicin during long-term storage and blood circulation after injection but also simultaneously allows for the ammonium-induced drug release at the tumor site. This release is induced by ammonia, which is continuously produced in tumors due to the unique tumor metabolic pathway, glutaminolysis.6
For Doxil, the well-established remote loading mechanism is based on continuous “escape” (efflux) of ammonia gas (formed by the pH-dependent dissociation of the intraliposomal NH4+ to neutral ammonia plus a proton), which creates a transmembrane pH gradient (pHliposome ≪ pHmedium).1,5 The unionized doxorubicin that diffuses from the medium to the intraliposomal aqueous phase along the gradient becomes protonated and forms intraliposome-insoluble doxorubicin-sulfate (dox-sulfate) salt. Consumption of extra protons by doxorubicin elevates the intraliposomal pH and renews the dissociation of NH4+ to NH3 and H+, enabling the continuation of the loading cycle until all of the doxorubicin in the medium is exchanged with NH4+. When the intraliposome doxorubicin concentrations are increased, the dox-sulfate-insoluble salt assembles into nanorod crystals.1,5 Namely, the transmembrane ammonium gradient serves as the “driving force” for triggering the cycle in which doxorubicin is almost entirely remotely and stably loaded into PLDs, mostly in the form of dox-sulfate nanorod crystals.1,4,5,7
In the past studies, we demonstrated by cryogenic transmission electron microscopy (cryo-TEM)4 and X-ray diffraction (XRD)4,8 the crucial role of sulfate counteranion in the formation of dox-sulfate nanocrystals. However, the actual impact of intraliposome ammonium and sulfate ion concentrations on the formation and properties of these intraliposomal nanocrystals, as well as on the functional performance of PLDs, remained unclear. The present study is aimed to fill in this gap. Recently, we reported the application of “high-sensitivity” differential scanning calorimetry (DSC) in the physicochemical characterization of liposomal nanodrugs, such as Doxil, and showed, for the first time, the thermotropic behavior of intraliposome dox-sulfate rod nanocrystals in Doxil treatment.9 Following the heating scan, Doxil exhibits two distinct endotherms attributed to the phase transition of membrane lipids at ∼52 °C and the melting of intraliposomal dox-sulfate nanorod crystals at ∼70 °C. Both phase transitions are reversible. Recently, we achieved a high-resolution structure of Doxil’s membrane and its dox-sulfate nanorod crystals using XRD.10 We also developed a functional in vitro doxorubicin release assay.6
In this study, we combine high-sensitivity DSC with structural characterization by cryo-TEM11 and the functional in vitro ammonia induced doxorubicin release assay to re-examine the independent role of ammonium and sulfate ions in the formation and properties of dox-sulfate nanocrystals in PLDs. We also studied how the nanocrystals’ properties affect the PLDs’ ammonia-induced doxorubicin release profiles in vitro. Our results strongly suggest that the individual concentration of each of these two ions, ammonium and sulfate, and their mole ratio play a very important role in Doxil’s unique stable loading and its ammonia-triggered release. These results shed new light on the remote-loading mechanism driven by the transmembrane ammonium gradient employed in the design and optimization of other remotely loaded liposomal nanodrugs.
Results and Discussion
This study focuses on the role of ammonium and sulfate ions used in the remote loading of doxorubicin and in the formation and properties of the intraliposomal dox-sulfate nanorod crystals in PLDs. To better understand and quantify the interaction between doxorubicin and sulfate in the intraliposomal aqueous phase, we first studied the effect of ammonium and sulfate ions on the doxorubicin precipitation in bulk aqueous solutions mimicking the pH and ionic strength of the intraliposomal aqueous phase. Next, we studied the doxorubicin sulfate nanocrystal formation inside PLDs using two different groups of “transmembrane ammonium gradients”. In the first group, AS solutions in different concentrations with the ammonium-to-sulfate mole ratio fixed at 2:1 were used; in the second group, AS mixed with either sodium sulfate (SS) or ammonium chloride solutions were used to study the effect of the “mole” ratio of ammonium-to-sulfate on nanocrystal formation. The morphology features of the resulting PLDs and intraliposomal nanocrystals were characterized by cryo-TEM,11 and their thermotropic behaviors were studied by high-sensitivity DSC.9 We studied the effects of the above properties on the liposome performance (as expressed by ammonia-induced doxorubicin release kinetics) to better understand all of the aspects of ammonium and sulfate ion involvement.
Precipitation Titration of Doxorubicin and Ammonium Sulfate or Sodium Sulfate
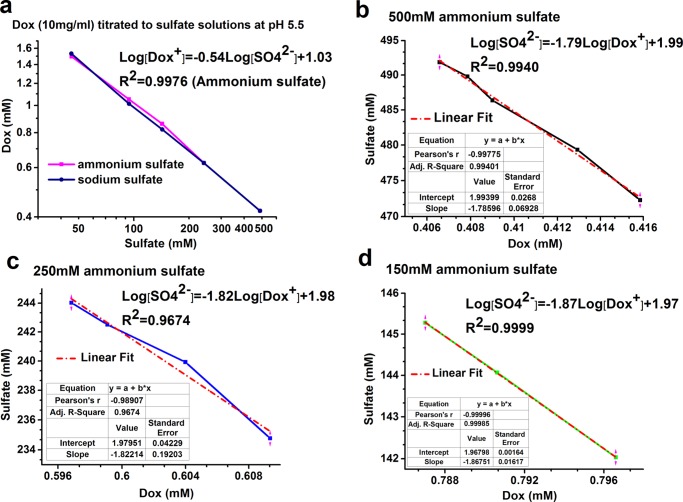
Doxorubicin self-aggregation is a well-established phenomenon.12 It is thermodynamically driven by stacking of the planner aromatic rings of doxorubicin. High ionic strength levels in the medium facilitate self-aggregation. Doxorubicin dimers are observed at a concentration of as low as 1 μM, whereas large aggregates are formed at higher concentrations.12 In this study, we followed the formation of large dox-sulfate aggregates in the sulfate solutions (AS or sodium sulfate) by precipitation titration and used the turbidity visible to the naked eye as the endpoint of titration. For the most part, the titration curves of AS and sodium sulfate (Figure 1a) almost overlap, suggesting that sulfate ion concentration is the main factor in the precipitation, whereas the cationic ion (ammonium or sodium) has no or only negligible effect. In Figure 1b–d, the linear curves for the ammonium sulfate solutions in all of the three concentrations were of very similar slopes. The calculated Ksp (solubility product) of doxorubicin sulfate (Ksp = [Dox+]2[SO42–]) is ∼1.1 × 10–7 M3 similar to the values previously reported.7,13
Figure 1.
Doxorubicin sulfate precipitation titration. (a) Doxorubicin (10 mg/mL) titrated with AS or sodium sulfate solutions at pH 5.5; (b–d) doxorubicin in varied concentrations titrated with 500, 250, and 150 mM AS (pH 5.5), respectively. All of the curves are displayed in log scale.
Effect of Intraliposomal Ammonium and Sulfate Concentrations on the Encapsulation Efficiency of PLDs
All of the blank small unilamellar vesicles (SUVs) (pegylated nanoliposomes before doxorubicin loading) and the related PLDs used in this study were of identical lipid composition and similar size (Table 1, data not shown for blank SUVs). All of the PLDs were of 16 mg/mL total lipids and 2 mg/mL total doxorubicin. All of the PLDs used in this study had a similar diameter in the range of 75–85 nm (with polydispersity index (PDI) of 0.05–0.07). Remote loading did not significantly change the size distribution as measured by dynamic light scattering (DLS). The mole ratio of doxorubicin to lipid used in the remote loading was maintained at 0.16. The PLDs prepared with an AS concentration of ≥200 mM achieved an encapsulation efficiency of almost 100%. Only the PLDs prepared with 150 mM AS and the mixture of AS with ammonium chloride showed a loading efficiency below 90% (Table 1).
Table 1. Properties of PLDs with Different Intraliposomal Ammonium and Sulfate Concentrations.
| concn (mM) | size (nm) by DLS | PDI | ΔpH | loading efficiency (%) | [Dox] apparent (mM) | [Dox] inside liposome (mM)a | [SO42–] total (mM) | [NH4+] total (mM) | [NH4+] residual (mM)b | |
|---|---|---|---|---|---|---|---|---|---|---|
| gradient composition | ||||||||||
| ammonium sulfate (AS) | 100 | 82.1 | 0.06 | N.D. | 90.2 | 3.11 | 182.9 | 100 | 200 | 17.1 |
| 150 | 81.2 | 0.07 | 0.5 | 77.3 | 2.67 | 157.1 | 150 | 300 | 142.9 | |
| 200 | 76.9 | 0.05 | 1.2 | 91.7 | 3.16 | 185.9 | 200 | 400 | 214.1 | |
| 250 | 78.3 | 0.06 | 1.7 | 93.3 | 3.22 | 189.4 | 250 | 500 | 310.6 | |
| 300 | 78.4 | 0.05 | 1.8 | 92.5 | 3.19 | 187.6 | 300 | 600 | 412.4 | |
| mixture solutions | ||||||||||
| AS + ammonium chloride | 100 | 84.2 | 0.07 | N.D. | 63.3 | 2.18 | 128.2 | 100 | 350 | 221.8 |
| 150 | ||||||||||
| AS + sodium sulfate (SS) | 100 | 86.4 | 0.07 | N.D. | 90.1 | 3.11 | 182.9 | 250 | 200 | 17.1 |
| 150 |
Trapped aqueous volume of 16 mg/mL PLD total lipids measured based on intraliposomal sulfate amounts measured by ion chromatography (see Methods section) is 1.7% of total dispersion volume.
[NH4+]residual = [NH4+]total – [Dox]inside liposome, considering 1:1 mole exchange ratio of doxorubicin to ammonium during remote loading.
Effect of Intraliposome Ammonium and Sulfate Concentrations on the Thermotropic Behavior and Morphology of Blank Liposomes and PLDs
The effect of intraliposomal ammonium and sulfate concentrations on the thermotropic behavior of blank SUVs was characterized (Figure S1, Supporting Information). All of the blank liposomes show similar thermograms having only a membrane lipid endotherm phase transition at ∼53 °C with low enthalpy (∼1–1.5 kcal/mol) and poor co-operativity (ΔT1/2 of ∼13 °C, Table S1, Supporting Information), similar to what we have recently demonstrated for cholesterol-rich (∼38 mol %) hydrogenated soybean phosphatidylcholine (HSPC)/cholesterol SUV membrane.9
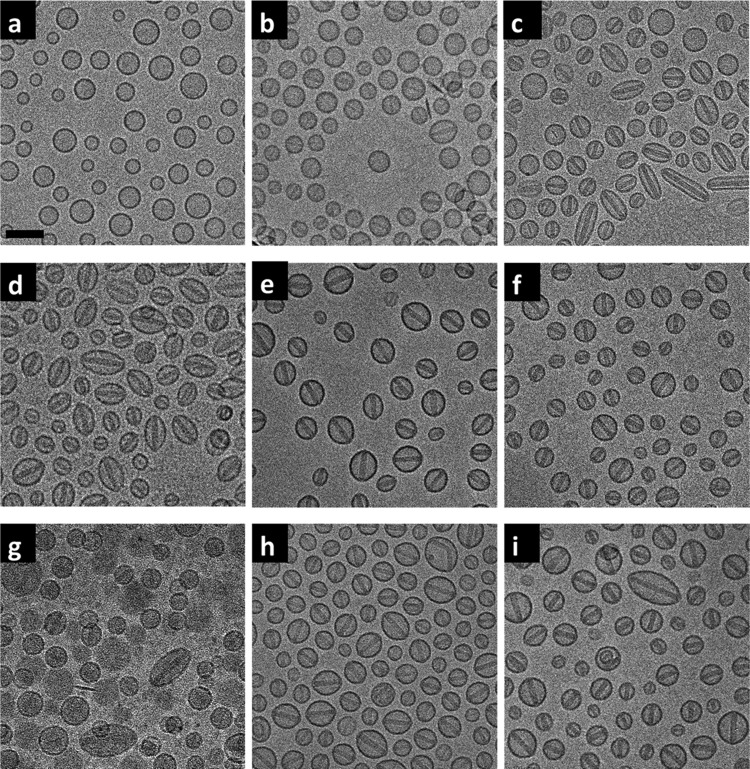
Remote loading of doxorubicin had a marked impact on PLD morphology as imaged by cryo-TEM (Figure 2). Morphology of the blank liposomes and the PLDs was determined by their aspect ratio (the measured major axis divided by the measured minor axis). Several hundreds of liposomes were measured for each analysis (resolution limit = 2 nm, Table 2). An aspect ratio of 1.06 was set as the threshold at which PLDs change from spherical to ellipsoid. All of the blank liposomes are spherical (as exemplified in Figure 2a for blank liposomes prepared with 250 mM AS), with the PLD aspect ratio dependent on the AS concentration used for their preparation. PLDs prepared with 100 mM AS retained a spherical shape (Figure 2b). Most PLDs prepared with 100 mM AS lack obvious nanocrystals, although some did contain nanocrystals with low electron density displaying lower contrast in the cryo-TEM images (Figure 2b); hence, we subsequently refer to these nanocrystals as being not “fully developed”. Conversely, nanorod crystals that are clearly imaged due to their high electron density and hence higher contrast in the cryo-TEM images (Figure 2c–f and Table 2) are defined as fully developed.
Figure 2.
Cryo-TEM of the blank SUV (prepared with 250 mM ammonium sulfate) and the PLDs prepared with different ammonium and sulfate concentrations. (a) Blank SUV. (b–f) PLDs prepared with 100, 150, 200, 250, and 300 mM AS, respectively. (g, h) PLDs prepared with a mixture of 100 mM AS and 150 mM ammonium chloride and a mixture of 100 mM AS and 150 mM sodium sulfate. (i) Lipodox JKL 3911. Scale bar = 100 nm.
Table 2. Quantitative Cryo-TEM Analysis of Blank 250 mM AS SUV and PLDs Prepared at Different Ammonium and Sulfate Concentrationsa.
| gradients | major axis (nm)b | minor axis (nm)b | aspect ratio | PLDs with fully developed nanocrystals (%) | nanocrystal width (nm) |
|---|---|---|---|---|---|
| blank 250 mM AS | 68 ± 2 | 67 ± 2 | 1.02 ± 0.03 | ||
| PLD 100 mM AS | 68 ± 2 | 64 ± 2 | 1.06 ± 0.04 | 10 | 20 ± 2 |
| PLD 150 mM AS | 76 ± 2 | 62 ± 2 | 1.22 ± 0.07 | 90 | 21 ± 1 |
| PLD 200 mM AS | 74 ± 2 | 59 ± 2 | 1.24 ± 0.09 | 100 | 22 ± 1 |
| PLD 250 mM AS | 69 ± 2 | 62 ± 2 | 1.13 ± 0.08 | 100 | 21 ± 2 |
| PLD 300 mM AS | 67 ± 2 | 63 ± 2 | 1.06 ± 0.01 | 100 | 21 ± 1 |
| PLD (100 mM AS+ 150 mM NH4Cl)c | 75 ± 2 | 66 ± 2 | 1.14 ± 0.03 | ||
| PLD (100 mM AS + 150 mM Na2SO4) | 69 ± 2 | 64 ± 2 | 1.09 ± 0.05 | 100 | 22 ± 1 |
| PLD 250 mM AS (Lipodox Lot#JKL 3911) | 72 ± 2 | 64 ± 2 | 1.11 ± 0.06 | 100 | 20 ± 2 |
Liposomes (200–700) were measured for each analysis with resolution limit of 2 nm. Data of aspect ratio shown as mean ± SD.
9.6 nm was added to each axis value determined by cryo-TEM image analysis based on the small-angle X-ray scattering (SAXS) characterization of PEG layer thickness (4.8 ± 1.2 nm).10
Cryo-TEM images did not display sufficient contrast to obtain enough PLDs for the manual measurement.
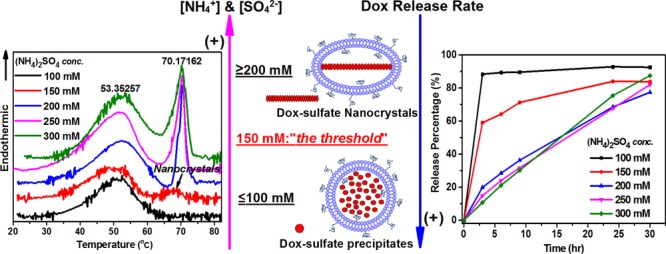
As the AS concentration is increased to 150 mM, PLDs begin to show a mixed morphology of spherical and ellipsoid shapes (Figure 2c); when the PLDs are prepared at 200 and 250 mM, they become mostly ellipsoid (Figure 2d–f and Table 2). At the same time, nanorod crystals defined as fully developed were observed in most (≥90%) of the PLDs when the AS concentration used for PLD preparation was ≥150 mM (Figure 2c–f and Table 2). We therefore regard this concentration as the threshold for the formation of PLDs with fully developed intraliposomal dox-sulfate nanocrystals. All of the intraliposomal nanorod crystals in the PLDs that display fully developed intraliposomal nanorod crystals share a similar diameter of 21 ± 2 nm, whereas crystal length varied according to the long axis of the ellipsoid PLDs. The results are in a agreement with the previous X-ray diffraction measurement data10 (nanocrystal diameter of 17.6 ± 2.1 nm). The lack of effect of AS concentration on nanocrystal diameter strongly suggests that the diameter of the nanocrystal is determined to a large extent by the drug-to-lipid ratio used for the remote loading, as long as the ammonium gradient enables >90% encapsulation and there is sufficient sulfate to induce complete crystallization of all of the intraliposomal doxorubicin.
PLDs prepared with 300 mM AS keep their spherical shape in spite of the prevalent presence of intraliposomal nanocrystals (Table 2). Although the intraliposomal nanocrystals show similar morphology under cryo-TEM to those in the PLDs prepared with 150, 200, and 250 mM AS concentrations, they did show a distinctly higher melting enthalpy (as shown in Table 3). This suggests that the nanocrystals formed in PLDs prepared with 300 mM AS are arranged in a tighter structure, and hence more doxorubicin is packed in the same crystal volume. Therefore, there is no need for the crystal to expand in length and impose a change in the spherical shape of the PLDs. We are presently studying if the reason for this is related to an increase in the amount of bound water.
Table 3. Thermodynamic Parameters Obtained from First Heating Scan of the PLDs Prepared with Different Ammonium and Sulfate Concentrationsa.
| membrane
lipid (normalized to 12.2 mM HSPC) |
dox-sulfate nanocrystals (normalized to doxorubicin concnb) |
|||||
|---|---|---|---|---|---|---|
| gradients | Tm (°C) | ΔT1/2 (°C) | ΔH (kcal/mol) | Tm (°C) | ΔT1/2 (°C) | ΔH (kcal/mol) |
| 100 mM AS | 52.43 ± 0.34 | 12.96 ± 0.39 | 1.02 ± 0.05 | |||
| 150 mM AS | 50.50 ± 0.33 | 14.46 ± 0.43 | 1.24 ± 0.06 | 67.98 ± 0.09 | 6.72 ± 0.15 | 0.52 ± 0.01 |
| 200 mM AS | 50.19 ± 0.33 | 15.81 ± 0.47 | 1.46 ± 0.07 | 70.20 ± 0.09 | 2.36 ± 0.05 | 1.78 ± 0.03 |
| 250 mM AS | 49.70 ± 0.32 | 15.81 ± 0.47 | 1.87 ± 0.09 | 69.86 ± 0.08 | 2.51 ± 0.06 | 1.82 ± 0.03 |
| 300 mM AS | 51.81 ± 0.34 | 16.84 ± 0.51 | 2.30 ± 0.12 | 69.85 ± 0.08 | 4.05 ± 0.09 | 2.62 ± 0.05 |
| 100 mM AS + 150 mM NH4Clc | 50.27 ± 0.33 | 14.44 ± 0.43 | 1.73 ± 0.09 | |||
| 100 mM AS + 150 mM Na2SO4 | 49.48 ± 0.32 | 14.30 ± 0.43 | 1.20 ± 0.06 | 68.99 ± 0.08 | 3.01 ± 0.07 | 1.22 ± 0.02 |
| 250 mM AS (Lipodox Lot#JKL 6054) | 51.84 ± 0.34 | 13.64 ± 0.40 | 1.53 ± 0.08 | 68.82 ± 0.09 | 2.84 ± 0.06 | 1.63 ± 0.03 |
| 250 mM AS (Lipodox Lot#JKL 3911) | 52.40 ± 0.34 | 13.94 ± 0.42 | 1.68 ± 0.08 | 69.19 ± 0.09 | 2.50 ± 0.06 | 1.51 ± 0.03 |
Data presented as mean ± SD.
Enthalpy of dox-sulfate nanocrystals is normalized to the doxorubicin concentrations listed in Table 1. For the two batches of Lipodox, the enthalpy of the doxorubicin sulfate is normalized to 3.45 mM doxorubicin.
Endotherm was manually selected and calculated in the temperature range from 15 to 70 °C.
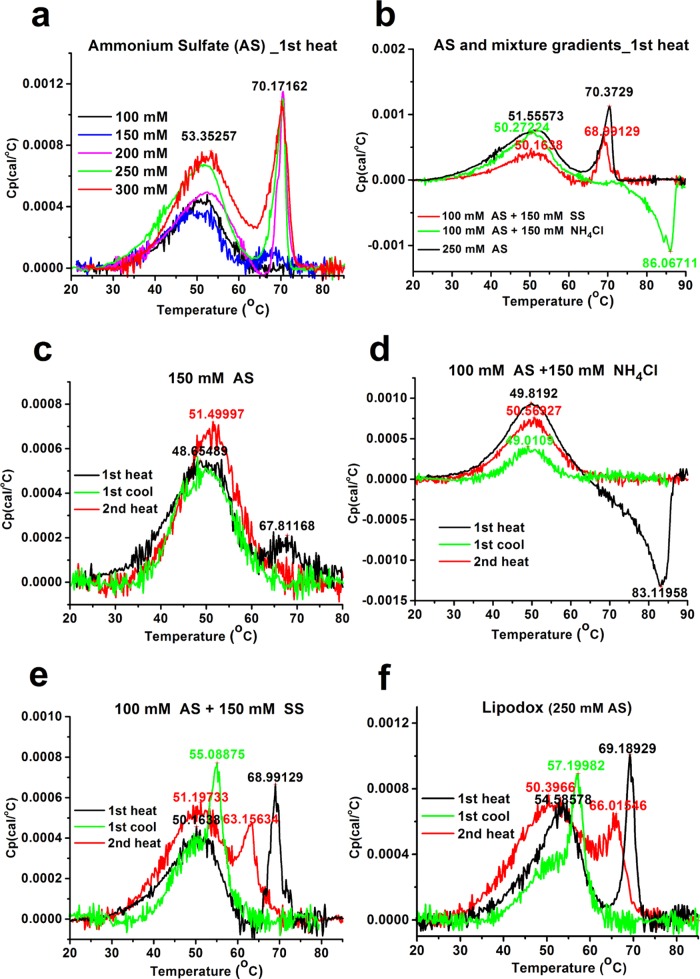
There is a strong correlation between the PLD’s morphology and the thermotropic behavior of the PLDs’ dox-sulfate nanorod crystals. In a previous study, we revealed that dox-sulfate nanocrystals in Doxil exhibit a sharp reversible melting endotherm in the first heating scan with Tm at ∼70 °C.9 Not surprisingly, no nanocrystal melting endotherm was found for PLDs prepared with 100 mM AS that lack fully developed intraliposomal nanocrystals (Figure 3a, black line, Table 2 and Figure 2b). In contrast, PLDs prepared with 150 mM AS, the “threshold concentration” for the formation of fully developed nanocrystals, exhibit intraliposomal nanocrystal melting endotherm (Figure 3a, blue line). However, the much lower melting enthalpy (0.52 kcal/mol) and lower melting co-operativity (ΔT1/2 of 6.72 °C) compared with those prepared with ≥200 mM AS (enthalpy of 1.78–2.62 kcal/mol and ΔT1/2 of 2.6–4.0 °C, Table 3) suggest that crystallinity degree of the nanocrystals formed at this threshold is much lower. Although the lower enthalpy may also be explained by the smaller fraction of doxorubicin present in the nanocrystalline form, the lower co-operativity (broadened ΔT1/2) proves that the “quality” of the nanocrystal is the main contributor to the difference in enthalpy. In addition, the phase transition of the intraliposomal nanocrystals in PLDs with 150 mM AS is irreversible (Figure 3c) and occurs only in the first heating scan. This irreversibility may be explained by weaker forces that “hold” together the loosely packed nanocrystals and enable the larger and quicker release of doxorubicin from the liposomes occurring during the first heating scan above the nanocrystals’ Tm. As a result, insufficient doxorubicin remains inside liposomes after the first heating scan to enable detectable recrystallization during the cooling scan. On the other hand, all of the nanocrystals in the PLDs prepared with ≥200 mM AS show a reversible melting/recrystallization process (Figure S1, Supporting Information and ref (9)), as was observed for Lipodox (PLDs prepared with 250 mM AS). The physical state of the intraliposomal nanocrystals above the intraliposome dox-sulfate nanocrystal melting transition in PLDs that exhibit reversible dox-sulfate phase transition is not yet clear. Further investigation with other methods such as XRD are required. We observed that the nanocrystals that exhibit reversible phase transition also have a much higher melting enthalpy (Table 3), namely, more energy is required to enable the meting. Indeed, they are expected to a have better resistance to leakage during DSC scanning, as described in our previous study.9
Figure 3.
Thermograms of PLDs prepared with different ammonium and/or sulfate concentrations in the first heating scan (a, b) and the overlapped thermograms of PLDs prepared with 150 mM AS (c), 100 mM AS and 150 mM ammonium chloride (d), 100 mM AS and 150 mM sodium sulfate (SS) (e) and 250 mM AS (Lipodox Lot#JKL 3911) (f) in cycled DSC scanning.
Of special interest are the PLDs prepared with the mixture of the two salts. PLDs prepared with the mixture of 100 mM AS and 150 mM ammonium chloride show only 63% loading efficiency (Table 1), much lower than those prepared with 100 mM AS, despite theoretically having a larger transmembrane ammonium gradient (350 vs 200 mM). These unexpected results may be explained by the presence of 150 mM chloride in the intraliposomal aqueous phase. The chloride ion has a much higher permeability coefficient than that of the sulfate ion, and it may also be released from liposomes in the form of HCl, “stealing” protons from the intraliposomal doxorubicin and aqueous phase.14 Similar to the other PLDs with low encapsulation efficiency, PLDs prepared with the mixture of ammonium chloride and AS also show that only a small fraction of these PLDs have fully developed nanocrystals (Figure 2g and Table 2). These PLDs also lack any dox-sulfate melting endotherm (Figure 3b, green). The exotherm (upon heating) with Tm at 86 °C (Figure 3b, green) suggests an as yet unexplained metastable process. Only the reversible phase transition of the membrane lipids was observed in the cycled DSC scanning (Figure 3d), whereas PLDs prepared with a mixture of 100 mM AS + 150 mM sodium sulfate share a similar morphology (Figure 2h) and thermotropic behavior (Figure 3e) to PLDs prepared with ≥200 mM AS and to Lipodox (Figure 3f). These findings strongly support the critical role of intraliposomal sulfate concentration in the crystallization of doxorubicin sulfate to form nanorod crystals. The reversible phase transition of these nanocrystals also supports the lack of drug leakage at temperatures above the Tm of the membrane lipids and the nanocrystals. However, the intraliposomal nanocrystals in PLDs prepared with the AS and sodium sulfate mixture recrystallize and remelt at ∼55 and 63 °C, respectively (Figure 3e), both 3 °C lower than Lipodox (PLDs with 250 mM AS) (Figure 3f). The melting enthalpy of the nanocrystals is also somewhat lower (1.22 kcal/mol compared with 1.51 kcal/mol, Table 3), despite having an identical intraliposomal sulfate concentration (250 mM). These results suggest that in addition to the sulfate counteranion, the intraliposome ammonium ions that remain after doxorubicin remote loading (Table 1) also contribute to the properties of the dox-sulfate nanorod crystals (further discussed below).
In Vitro Ammonia-Induced Doxorubicin Release of PLDs
Based on the product information and certificates of analysis,15 it is clear that Doxil exhibits a good stability and resists doxorubicin leakage upon long-term storage at 2–8 °C. Plasma pharmacokinetic studies in animals and humans show that Doxil is also resistant to leakage in blood circulation.16,17 We previously demonstrated that ammonia/ammonium, which are continuously generated in tumors due to the metabolic pathway of glutaminolysis, may induce doxorubicin release at sufficient levels to achieve therapeutic efficacy.6 The ammonia-induced release of PLDs was recently reconfirmed by other researchers.18 Therefore, we use it here as a functional assay that may predict the in vivo rate of doxorubicin release in tumor tissues.
The mechanism of ammonia-induced doxorubicin release is a reverse process of remote loading.5,6 It is based on an exchange of ammonia gas that diffuses from the “release-medium” across the liposome membrane into the intraliposomal aqueous phase, concomitantly with the efflux of intraliposomal uncharged doxorubicin into the medium.6 To be released, the doxorubicin molecule needs to disassociate from intraliposomal doxorubicin sulfate nanorod crystals and donate its proton to the liposome-internalized ammonia, after which the unprotonated (neutral) doxorubicin molecule diffuses across the liposome membrane into the medium.
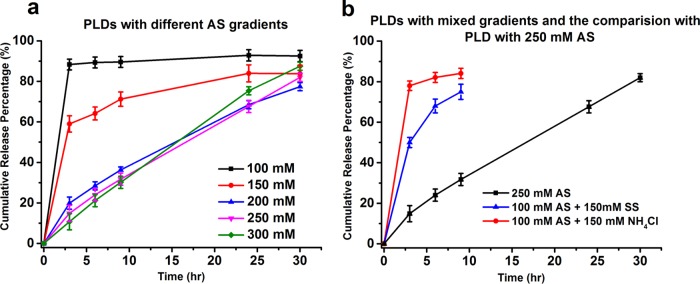
The in vitro release profiles of the PLDs clearly demonstrate the relationship between the release rate and intraliposomal doxorubicin sulfate nanocrystal properties. The presence of fully developed nanocrystals determines the kinetic order and rate of doxorubicin release (Figure 4). This result suggests that doxorubicin in the nanocrystal has a reduced tendency (compared to doxorubicin that is not part of the nanocrystal) to donate its proton to the liposome-internalized ammonia. Therefore, PLDs that lack a significant level of fully developed nanocrystals, including those prepared with 100 mM AS or with the mixture of 100 mM AS and 150 mM ammonium chloride, showed a biphasic release kinetics with a rapid release phase in which ≥80% doxorubicin is released within the first 3 h (Figure 4a, black and 4b, red line).
Figure 4.
In vitro release of the PLDs prepared with different intraliposomal AS concentrations (a) and with the two mixed transmembrane ammonium or sulfate gradients with the comparison to PLDs prepared in 250 mM AS (b).
An additional factor is the degree of nanocrystal crystallinity, which in our study is quantified by the melting enthalpy and the co-operativity of the nanocrystal melting endotherm. A lower melting enthalpy suggests a weaker interaction between doxorubicin molecules in the nanocrystals, therefore less energy is required for doxorubicin molecules to dissociate from the nanocrystals and a faster release rate is expected. This explains the faster release of doxorubicin from PLDs prepared with 150 mM AS than those prepared with ≥200 mM AS. Despite the prevalent presence of intraliposomal nanocrystals in at least 90% of the PLDS (Figure 2c) and a clear nanocrystal melting endotherm in the first heat scan, PLDs prepared with 150 mM AS show a two-phase release-kinetics profile with a fast release of 60% doxorubicin in the first 3 h (Figure 4a, red line). In addition, the phase transition of intraliposomal nanocrystals in 150 mM AS PLDs is irreversible, possibly due to lower resistance to the drug leakage that occurs during the DSC heat scans (as discussed above). In comparison, PLDs with dox-sulfate nanocrystals having a melting enthalpy and co-operativity close to that of Lipodox and Doxil/Caelyx (including PLDs prepared with 200 and 300 mM AS) also show similar slow and almost linear one-phasic, zero-order release profiles (Figure 4a, blue and green).
The third factor that affects the nanocrystal properties and doxorubicin release is the residual ammonium concentration inside PLDs after doxorubicin remote loading. The intraliposomal residual ammonium acts like a proton buffer. A higher intraliposomal residual ammonium concentration is expected to generate a higher transmembrane pH gradient (Table 1). Therefore, the physicochemical properties of the PLDs vary with the intraliposomal residual ammonium concentration, despite having a similar intraliposomal sulfate concentration and drug-to-lipid mole ratio. Meanwhile, the higher residual intraliposomal ammonium concentration after remote loading, the slower the proton removal and release rate of doxorubicin. This explains the faster release of PLDs prepared with the mixture of 100 mM AS and 150 mM sodium sulfate as compared with that of 250 mM AS (Figure 4b, blue and black lines). The sulfate concentration in both PLDs is identical, whereas the residual ammonium concentration in the PLDs prepared with 250 mM AS is 18-fold higher (310 vs 17 mM; Table 1).
Conclusions
Our results demonstrate the roles of ammonium and sulfate ions in the remote loading of Doxil and their different and complimentary contribution to remote loading stability and PLD performance (as followed through the ammonia-induced doxorubicin release). The individual concentration of each of the two ions and the ratio between them have a profound effect on the morphology, physical state of intraliposomal doxorubicin sulfate salt, and the thermotropic behavior of the PLDs. The lessons learned from this study may enable significant improvement in the design and characterization of liposome-based nanodrugs remotely loaded with other APIs.
Materials and Methods
Materials
Hydrogenated soybean phosphatidylcholine (HSPC), cholesterol, and DSPE-PEG2k were obtained from Lipoid (Ludwigshafen, Germany). For more details on these lipids, see Wei et al.9 Doxorubicin hydrochloride was obtained from Sicor, Rho, Italy. Ammonium sulfate, ammonium chloride, sodium sulfate, and USP grade l-histidine were obtained from Merck (Darmstadt, Germany). Analytical grade sucrose was obtained from Bio-Lab Ltd. (Jerusalem, Israel). All of the other reagents used were of analytical grade or better. High-purity water of conductivity 18.2 MΩ was prepared using the Barnstead Nanopure ultrapure water purification system (Thermo Fisher Scientific, Waltham, MA) and referred as deionized distilled water. Lipodox is a product of Sun Pharma (Gujarat, India). All of the DSC studies of Lipodox were performed before the product expiration date.
Methods
Precipitation Titration of Doxorubicin to Ammonium Sulfate or Sodium Sulfate
Doxorubicin hydrochloride (10 mg/mL) was added dropwise to 2 mL ammonium sulfate or sodium sulfate solutions (pH 5.5) in concentrations of 100, 150, 250, and 500 mM under vigorous magnetic stirring. The point at which the sulfate solution turned turbid or “hazy” was regarded as the titration endpoint, and the total volume of the doxorubicin solution added was recorded and translated into final doxorubicin concentration. This concentration was regarded as the minimum concentration necessary for precipitation.
“Reverse titration” was carried out with doxorubicin at various concentrations: 2, 5, 7.5, 10, 15, 20, and 25 mM doxorubicin solutions were added dropwise to 150, 250, or 500 mM ammonium sulfate solution (pH 5.5), respectively. The volume of doxorubicin used to reach the initial precipitation in each counterion solution was recorded and translated into final doxorubicin concentration. The first points of titration were excluded from the calculation as being of too low doxorubicin or ammonium sulfate concentrations.
Preparation of Doxorubicin-Loaded Liposomes (PLDs) in Different Ammonium and Sulfate Concentrations
Two groups of blank SUVs having membrane lipid composition identical to that of Doxil and Lipodox, but differing in their intraliposomal concentrations of ammonium or sulfate ions, were prepared as previously described.5,10 In summary, in the first group, ammonium sulfate solutions of different concentrations (100, 150, 200, 250, and 300 mM, ammonium-to-sulfate mole ratio = 2:1) were used. In the second group, an ammonium sulfate solution (100 mM) mixed with sodium sulfate or ammonium chloride (both at 150 mM) was used. Namely, in the second group, the ratio of ammonium to sulfate varied. Osmolality of the above solutions was measured using the freezing point depression method (Advanced Micro-Osmometer, model 3320, Advanced Instruments, MA). The transmembrane ion gradients were created by diafiltration using Labscale TFF system (Millipore, MA) against 10% sucrose. The desired PLDs prepared by adding doxorubicin hydrochloride solution to these SUVs to a final doxorubicin concentration of 2 to ∼16 mg/mL total lipids followed by incubation at 60 °C for 30 min. Histidine buffer (pH 6.5, to a final 10 mM concentration) was added after cooling. The PLD dispersion’s osmolality is 350–360 mOsm/kg.8 The blank liposomes and PLDs were stored at 2–8 °C until use.
Liposome Characterization
All of the liposomal dispersions used in this study were characterized for phospholipid concentrations by the modified Bartlett method19 and/or high-performance liquid chromatography with evaporative light-scattering detection; size and size distribution via dynamic light scattering (DLS)20 by a Malvern Zetasizer Nano ZS (Worcestershire, U.K.); and drug encapsulation using the cationic exchanger Dowex 50 assay.21,22
Transmembrane pH gradients of the liposomes were measured by [14C]-methylamine distribution as previously described.23 In brief, [14C]-methylamine (23.8 μCi/mL) was added to freshly prepared SUVs before and after doxorubicin loading. The pH of the extraliposomal medium was maintained at pH 6.5 by 50 mM histidine saline. After 20 min incubation at 55 °C, the liposome dispersions were diluted with 50 mM histidine saline and divided equally into two parts. One part of the liposome solution was ultracentrifuged using Amicon Ultra-15 tubes using a filter of 100 K molecular weight cutoff (Millipore, MA) for the separation of the unencapsulated (extraliposome medium) from the encapsulated [14C]-methylamine. The medium collected after ultracentrifugation and the other part of the original liposome dispersion (before centrifugation) were first treated with Opti-Fluor (Perkin Elmer, Waltham, MA) and stored overnight at 2–8 °C before scintillation counting of the radioactivity by a β-counter. The ratio of [14C]-methylamine between the liposomes and the extraliposomal medium used to calculate the transmembrane pH gradient is
The trapped volume of the resulting PLDs was measured in the intraliposomal sulfate concentration and analyzed by ion chromatography24 on an ICSep AN2 (anion exchange column, Transgenomic, Omaha, NE) with carbonate/bicarbonate eluent and conductivity detection. For 16 mg/mL of lipids present in Lipodox and Doxil, it amounted to 1.7% of the total volume.
Cryo-TEM Characterization of PLDs Prepared with Different Ammonium and Sulfate Concentrations
Direct imaging of the liposomes was performed by cryo-TEM.25 Vitrified specimens were prepared on a copper grid coated with a perforated lacey carbon 300 mesh (Ted Pella, CA). Prior to specimen preparation, the grids were plasma-etched to increase their hydrophilicity. Typically, a drop (3–4 μL) of the solution was applied to a grid in a controlled environment (25 °C and 100% relative humidity), and blotted with filter paper to form a thin liquid film of solution. The automatically blotted sample was immediately plunged into liquid ethane (Leica Microsystems, Wetzlar, Germany). The vitrified specimens were transferred into liquid nitrogen for storage. The samples were studied using an FEI Tecnai 12 G2 TWIN TEM (FEI, Oregon), which was operated at 120 kV in low-dose mode to minimize electron beam radiation damage with a Gatan 626 cryo-holder maintained at −183 °C. Images recorded using a Gatan charge-coupled device camera (model 794) and analyzed by Digital Micrograph software (version 3.1).
The structure of the PLDs were analyzed from the cryo-TEM images using MATLAB software programmed by Sivan Peretz-Damari in Prof. Oren Regev’s laboratory.11 Briefly, 200–700 well-separated PLDs were analyzed for each sample. The lengths of the major (long) axis and the minor (short) axis of each liposome were determined by the image analysis, and the aspect ratio was calculated as the PLDs’ shape index. A PEG layer that does not have sufficient mass-thickness contrast cannot be determined by cryo-TEM imaging. Therefore, we added the 9.6 nm to the long axis and the short axis (2 times the 4.8 ± 1.2 nm thickness of the PEG layer on the external liposome surface as previously determined by us using SAXS8,10).
The percentage of the vesicles having fully developed intraliposomal doxorubicin sulfate nanorod crystals was manually counted using 300–600 PLD particles. The fully developed nanorod crystals are defined as those that are clearly imaged because they demonstrate a high electron density and hence a higher contrast in the cryo-TEM images (Figure 2c–f and Table 2).
The width of the intraliposomal doxorubicin sulfate nanorod crystals was manually measured using Digital Micrograph software (Gatan, CA). The average and SD of the crystal width were calculated based on the measurement of 50 PLDs for each of the PLDs prepared at the different ammonium sulfate concentrations (see above).
Resolution of the PLDs’ axis and intraliposome nanocrystal width measurement was 1 nm.
DSC Measurements
The blank SUVs and PLDs prepared with different ammonium and sulfate concentrations were scanned using high-sensitivity MicroCal VP-DSC system (Malvern, Worcestershire, U.K.). Based on our previous experience with Doxil and Lipodox,9 scanning was carried out at a slow rate of 1.0 °C/min to achieve conditions close to the thermodynamic equilibrium.26 The samples were vacuum degassed by the MicroCal ThermoVac system (Malvern, Worcestershire, U.K.). The samples and the reference (10% sucrose in 10 mM histidine buffer pH 6.5) were scanned in a cycle of heat–cool–reheat from 15 to 90 °C, using a filtering period of 10 s and the feedback mode of “Mid”. The sucrose/histidine buffer used as a reference for all of the liposomal samples was identical to the PLD storage medium. For more details on DSC measurements and the justification of measurements’ conditions, see Wei et al.9
Calculation of the thermodynamic parameters was carried out using Microcal LLC DSC workstation software (Malvern, Worcestershire, U.K.). Initially, the original thermograms were corrected by baseline subtraction and normalized to lipid or doxorubicin concentrations. The “lipid-related” endotherms/exotherms were normalized to phosphatidylcholine (PC) concentration, whereas the ones related to dox-sulfate nanocrystals were normalized to “apparent” (as actual intraliposomal doxorubicin concentration is difficult to calculate due to dox-sulfate intraliposome crystallization) intraliposome doxorubicin concentration (Table 1). The normalized thermograms were analyzed by one- or two-peak nonlinear modeling at the smallest chi-square or reduced chi-square values. The thermodynamic parameters were calculated from the obtained endotherms/exotherms in the thermograms: the model with the closest Tm to the Tm of the lipids or the nanocrystals was selected for membrane or intraliposome crystals, respectively, and the enthalpy (ΔH) and ΔT1/2 (a measure of phase transition co-operativity) were calculated correspondingly. Tm was determined by the workstation from the normalized thermograms. The excellent repeatability and reproducibility of the DSC measurements for PLDs were demonstrated previously in Figure S1 and Table S1 of Wei et al.9
In Vitro Release of Doxorubicin from PLDs with Different Ammonium and Sulfate Concentrations
In vitro release was carried out based on the procedure described by Silverman and Barenholz6 and scaled up to a standard pharmaceutical dissolution tester Vision G2 Classic 6 (Hanson, Chatsworth, CA). A medium of normal saline, buffered with 20 mM histidine to pH 7.3 ± 0.1, was used. Ammonium sulfate (50 mM) served as the doxorubicin release inducer. Dowex 50WX4 cation-exchange resin (Sigma-Aldrich, St. Louis, MO) was employed as a “sink” for the released drug as well as for differentiating between liposomal and “free” doxorubicin.10
Acknowledgments
The authors wish to thank Michael Raslin and Alex Lyskin of our group for the HSPC analysis and commercial PLDs’ PC species. This study was supported by the following: (1) the Barenholz Fund; (2) a postdoctoral fellowship jointly sponsored by the Planning and Budgeting Committee of Council for Higher Education in Israel and the Hebrew University of Jerusalem to Xiaohui Wei; and (3) The National Natural Science Foundation of China to Xiaohui Wei (No. 81202475).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01235.
Thermodynamic parameters of the blank SUVs prepared with different ammonium and sulfate concentrations;
the overlapped thermograms of PLDs prepared with 200 and 300 mM ammonium sulfate gradients in cycled DSC scanning to demonstrate the reversibility of phase transitions for both the membrane lipids and the intraliposomal doxorubicin sulfate nanorod crystals (PDF)
The authors declare the following competing financial interest(s): Yechezkel Barenholz is one of the inventors of two already expired (March 2010) patents relevant to Doxil: (1) Barenholz, Y., and Haran, G. “Method of Amphipathic Drug Loading in Liposomes by pH Gradient”. U.S. Patent 5,192,549, March 9, 1993. U.S. Patent 5,244,574, September 14, 1993; (2) Barenholz Y., and Haran, G. “Liposomes: Efficient Loading and Controlled Release of Amphipathic Molecules”, U.S. Patent 5,316,771, May 31, 1994. The Hebrew University received royalties from Doxil sales until the patents expired. The Barenholz Fund established with a portion of these royalties are used to support research in the Barenholz laboratory, including this study. Dima Shamrakov and Sioma Nudeman are employees of Ayana Ltd., a start-up founded by Yechezkel Barenholz and Yissum Hebrew University TTO. Ayana Ltd. works on the development of liposomal drugs including liposomal doxorubicin.
Supplementary Material
References
- Barenholz Y. Doxil—the first FDA-approved nano-drug: lessons learned. J. Controlled Release 2012, 160, 117–134. 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Allen T. M.; Papahadjopoulos D.. Sterically Stabilized (‘Stealth’) Liposomes: Pharmacokinetics and Therapeutic Advantages. In Liposome Technology; Gregoriadis G., Ed.; CRC Press: Boca Raton, FL, 1993; Vol. 3, pp 59–72. [Google Scholar]
- Torchilin V. P.; Omelyanenko V. G.; Papisov M. I.; Bogdanov A. A.; Trubetskoy V. S.; Herron J. N.; Gentry C. A. Poly (ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim. Biophys. Acta, Biomembr. 1994, 1195, 11–20. 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Lasic D. D.; Frederik P.; Stuart M.; Barenholz Y.; McIntosh T. Gelation of liposome interior A novel method for drug encapsulation. FEBS Lett. 1992, 312, 255–258. 10.1016/0014-5793(92)80947-F. [DOI] [PubMed] [Google Scholar]
- Haran G.; Cohen R.; Bar L. K.; Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta, Biomembr. 1993, 1151, 201–215. 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- Silverman L.; Barenholz Y. In vitro experiments showing enhanced release of doxorubicin from Doxil in the presence of ammonia may explain drug release at tumor site. Nanomedicine 2015, 11, 1841–1850. 10.1016/j.nano.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Lasic D. D.; Čeh B.; Stuart M. C. A.; Guo L.; Frederik P. M.; Barenholz Y. Transmembrane gradient driven phase transitions within vesicles: lessons for drug delivery. Biochim. Biophys. Acta, Biomembr. 1995, 1239, 145–156. 10.1016/0005-2736(95)00159-Z. [DOI] [PubMed] [Google Scholar]
- Varga Z.; Berényi S.; Szokol B.; Őrfi L.; Kèri G.; Peták I.; Hoell A.; Bóta A. A closer look at the structure of sterically stabilized liposomes: a small-angle X-ray scattering study. J. Phys. Chem. B 2010, 114, 6850–6854. 10.1021/jp9109207. [DOI] [PubMed] [Google Scholar]
- Wei X.; Cohen R.; Barenholz Y. Insights into composition/structure/function relationships of Doxil gained from “high-sensitivity” differential scanning calorimetry. Eur. J. Pharm. Biopharm. 2016, 104, 260–270. 10.1016/j.ejpb.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Schilt Y.; Berman T.; Wei X.; Barenholz Y.; Raviv U. Using solution X-ray scattering to determine the high-resolution structure and morphology of PEGylated liposomal doxorubicin nanodrugs. Biochim. Biophys. Acta, Gen. Subj. 2016, 1860, 108–119. 10.1016/j.bbagen.2015.09.012. [DOI] [PubMed] [Google Scholar]
- https://www.mathworks.com/matlabcentral/fileexchange/47852-liposome-analysis?requestedDomain=www.mathworks.com.
- Barenholz Y.; Amselem S.; Goren D.; Cohen R.; Gelvan D.; Samuni A.; Golden E. B.; Gabizon A. Stability of liposomal doxorubicin formulations: problems and prospects. Med. Res. Rev. 1993, 13, 449–491. 10.1002/med.2610130404. [DOI] [PubMed] [Google Scholar]
- Csuhai E.; Kangarlou S.; Xiang T.-X.; Ponta A.; Bummer P.; Choi D.; Anderson B. D. Determination of Key Parameters for a Mechanism-Based Model to Predict Doxorubicin Release from Actively Loaded Liposomes. J. Pharm. Sci. 2015, 104, 1087–1098. 10.1002/jps.24307. [DOI] [PubMed] [Google Scholar]
- Deamer D. W.; Bramhall J. Permeability of lipid bilayers to water and ionic solutes. Chem. Phys. Lipids 1986, 40, 167–188. 10.1016/0009-3084(86)90069-1. [DOI] [PubMed] [Google Scholar]
- http://www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/human/002049/WC500112957.pdf.
- http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000089/WC500020175.pdf.
- Gabizon A.; Shmeeda H.; Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin. Clin. Pharmacokinet. 2003, 42, 419–436. 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- Fugit K. D.; Xiang T.; Choi D. H.; Kangarlou S.; Csuhai E.; Bummer P. M.; Anderson B. D. Mechanistic model and analysis of doxorubicin release from liposomal formulations. J. Controlled Release 2015, 217, 82–91. 10.1016/j.jconrel.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Shmeeda H.; Even-Chen S.; Honen R.; Cohen R.; Weintraub C.; Barenholz Y. Enzymatic assays for quality control and pharmacokinetics of liposome formulations: comparison with nonenzymatic conventional methodologies. Methods Enzymol. 2003, 367, 272–292. 10.1016/S0076-6879(03)67017-5. [DOI] [PubMed] [Google Scholar]
- Barenholz Y.; Amselem S.. Quality Control Assays in the Development and Clinical Use of Liposome-Based Formulations. In Liposome Technology, 2nd ed.; Gregoriadis G., Ed.; CRC Press: Boca Raton, FL, 1993; Vol. 1, pp 527–616. [Google Scholar]
- Amselem S.; Gabizon A.; Barenholz Y. Optimization and upscaling of doxorubicin-containing liposomes for clinical use. J. Pharm. Sci. 1990, 79, 1045–1052. 10.1002/jps.2600791202. [DOI] [PubMed] [Google Scholar]
- Druckmann S.; Gabizon A.; Barenholz Y. Separation of liposome-associated doxorubicin from non-liposome-associated doxorubicin in human plasma: implications for pharmacokinetic studies. Biochim. Biophys. Acta, Biomembr. 1989, 980, 381–384. 10.1016/0005-2736(89)90329-5. [DOI] [PubMed] [Google Scholar]
- Abraham S. A.; Edwards K.; Karlsson G.; MacIntosh S.; Mayer L. D.; McKenzie C.; Bally M. B. Formation of transition metal–doxorubicin complexes inside liposomes. Biochim. Biophys. Acta, Biomembr. 2002, 1565, 41–54. 10.1016/S0005-2736(02)00507-2. [DOI] [PubMed] [Google Scholar]
- Fritz K.; Yamamura S. S. Rapid microtitration of sulfate. Anal. Chem. 1955, 27, 1461–1464. 10.1021/ac60105a030. [DOI] [Google Scholar]
- Talmon Y. Transmission electron microscopy of complex fluids: the state of the art. Ber. Bunsen-Ges. 1996, 100, 364–372. 10.1002/bbpc.19961000322. [DOI] [Google Scholar]
- Biltonen R. L.; Lichtenberg D. The use of differential scanning calorimetry as a tool to characterize liposome preparations. Chem. Phys. Lipids 1993, 64, 129–142. 10.1016/0009-3084(93)90062-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.