Abstract

Medical cannabis has been legally available for patients in a number of countries. Licensed producers produce a variety of cannabis strains with different concentrations of phytocannabinoids. Phytocannabinoids in medical cannabis are decarboxylated when subjected to heating for consumption by the patients or when extracted for preparing cannabis derivative products. There is little understanding of the true chemical composition of cannabis extracts, changes occurring during heating of the extracts, and their relevance to pharmacological effects. We investigated the extract from a popular commercial strain of medical cannabis, prior to and after decarboxylation, to understand the chemical profiles. A total of up to 62 compounds could be identified simultaneously in the extract derived from commercial cannabis, including up to 23 phytocannabinoids. Upon heating, several chemical changes take place, including the loss of carboxylic group from the acidic phytocannabinoids. This investigation attempts to reveal the chemical complexity of commercial medical cannabis extracts and the differences in the chemical composition of the native extract and the one subjected to heat. Comprehensive chemical analyses of medical cannabis extracts are needed for standardization, consistency, and, more importantly, an informed employment of this substance for therapeutic purposes.
Introduction
Cannabis spp. plant and its products are controlled drug substances across international borders, and their possession is illegal in many countries. This species of plants produce a distinct group of chemicals called phytocannabinoids.1,2 Due to the psychoactive effects of few phytocannabinoids, Cannabis spp. plants and their derivative products are either regulated or banned. In Canada, cannabis has been available for medicinal purposes since 1999 to patients who obtain a prescription for the same from a physician.3 In the recent years, commercial medicinal cannabis in Canada is produced by licensed producers, who carry a variety of strains with several variations in the phytocannabinoid content.
Cannabis spp. contains a highly complex mixture of compounds, and up to 568 unique molecules are identified in the cannabis to date.1,2 a Among these compounds, Δ9-THC, cannabinol (CBN), and cannabinodiol (CBND) are known to be psychoactive.2 One must note that the concentrations of all of these compounds may not even be at the detectable levels or not present in many commercial cannabis strains; hence, physiological significance of all of these compounds is irrelevant, except those that can be detected. Other cannabinoids such as cannabidiol (CBD) are nonpsychoactive compounds. Cannabinoids exert their physiological effects through a variety of receptors including adrenergic receptors, cannabinoid receptors (CB1 and CB2), and a variety of other recently discovered GPCRs such as GPR55, GPR3, GPR5, among others.2,4 Patients consume medical cannabis, often with little medical evidence, for the treatment of or to seek relief from a variety of clinical conditions including pain, anxiety, epileptic seizures, nausea, appetite stimulation, and so on.5−8 Chemical composition, pharmacological profiling, and complete physiological effects of these medicinal plants, and more importantly the extracts from cannabis, remain to be fully understood.9,10
A small group of commercial suppliers, typically able to produce hundreds of kilograms of medical cannabis annually, are providing medical cannabis to majority of patients, along with some home-grown cannabis as well as some small-scale producers in Canada; a large variety of cannabis plants with large variations in phytocannabinoids content thus exists, and an online store lists up to 542 different strains available as medical cannabis.11 This is a result of the existing legal medical marijuana regulatory system, where each patient or even someone with a license to grow could grow any varieties in small quantities, typically sufficient for a handful of patients’ consumption. These are not necessarily produced by large commercial manufacturers. Cannabis-derived extracts (resins) are becoming popular commercially as well, and many patients prefer to use these extracts, as they do not involve smoking.
Previous studies have analyzed cannabis extracts in the context of understanding the chemical variations and classifying the Cannabis spp. plants.12−14 In a study published by Hazecamp and Fischedick, the authors compared two popular strains of cannabis samples from the coffee shops dispersed geographically in the Netherlands. Samples were collected and analyzed for 28 major compounds; extraction was conducted with organic solvents such as hexanes and ethanol.12 Their data were analyzed by principal component analysis to classify these cannabis samples into distinct chemovar groups. In a separate study, 11 cannabis varieties grown under controlled and similar environmental conditions were analyzed by gas chromatography for 36 compounds and classified these varieties using these compounds profiling.13 Elzinga et al. conducted chemical analyses on 494 cannabis flower samples and 170 extracts, correlating 31 compounds including phytocannabinoids and terpenes.15 In this study, a continuum of chemical composition was identified among various cannabis strains collected from patients in California. There was also variability in the chemical composition of the same strain.
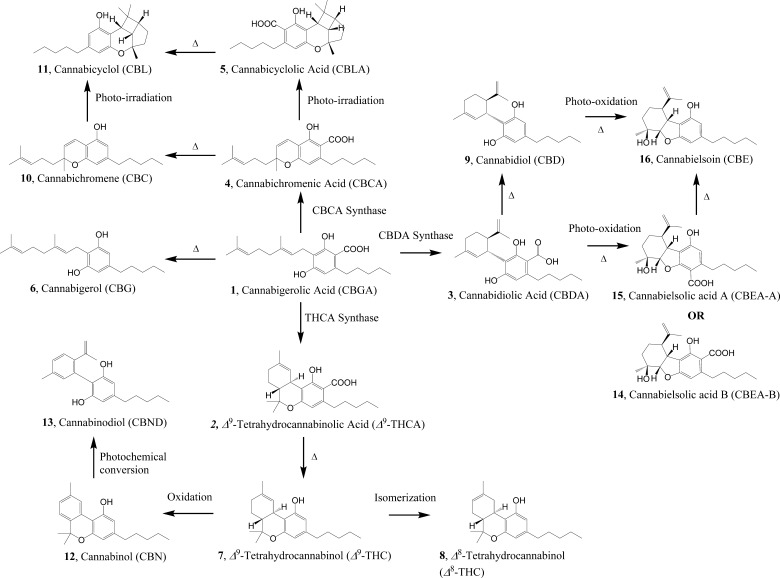
Commercial medical cannabis, in its dried form, is consumed by patients in a number of ways including smoking, vaping, edibles, and infused edible oils. Phytocannabinoids in the dried cannabis generally carry a carboxylic acid moiety and undergo spontaneous loss of this carboxyl moiety when subjected to high temperature (either direct sunlight, smoking, hot oven, and similar heat sources) (Figure 1). Cannabinoid acids generally bind at the cannabinoid receptors, CB1 and CB2, with weaker affinity and exhibit weaker activity,16 but the corresponding decarboxylated phytocannabinoids exhibit higher potency at these receptors.17 Thus, dried cannabis in general is subjected to activation (via decarboxylation) prior to consumption by the patients for maximal in vivo efficacy. Cannabis extracts, on the other hand, are typically produced by either direct infusion into various edible oils at moderate temperatures or by employing supercritical fluid extraction. However, very little is investigated about the chemistry of commercial varieties, the effect of heating on the chemicals in medical cannabis, and their effects on the physiological systems. To understand the effects of heating, the extraction and analytical tools must be also be able to adequately address the chemical changes. To our knowledge, there is no study involving medical cannabis in this context, including from the Canadian market, which is the focus of this investigation. Overall, commercial medical cannabis is significantly underexplored, and it is a potential source for novel bioactive compounds as well.18
Figure 1.
During the biosynthesis of various phytocannabinoids, cannabigerolic acid (1, CBGA) serves as the key branching point for a number of cannabinoids such as Δ9-THC, CBD, cannabielsoin (CBE), cannabichromene (CBC), and cannabicyclol (CBL) families of phytocannabinoids (Figure 1).19−21 CBGA is synthesized from geraniol and C12-polyketides catalyzed by CBGA synthase in Cannabis.22,23 This biosynthetic pathway consists of four types of reactions: enzyme catalysis, thermal reaction, oxidation, and irradiation (Figure 1). The conversion of CBGA into Δ9-THCA, cannabidiolic acid (CBDA), and cannabichromenic acid (CBCA) is catalyzed by the corresponding synthases (Figure 1).19,20 CBCA (4) and CBC (10) can be further converted to cannabicyclolic acid (CBLA) (5) and CBL (11), respectively, when exposed to UV light irradiation.24,25 Δ9-Tetrahydrocannabinol (Δ9-THC) is transformed into CBN (12) through an oxidative aromatization,19 and CBN can in turn be photochemically rearranged into the catechol, cannabinodiol (CBND) (13).26
As Canada is poised to legalize marijuana for recreational purposes with the legislation pending in 2018, and hundreds of thousands of Canadians consume commercially available cannabis strains for the treatment of a variety of ailments, we sought to undertake a chemical approach to investigate the constituents in one particular variety of medicinal cannabis. Most common strains available in Canada tend to carry high concentrations of Δ9-THC with minimal or insignificant amount of CBD, or a balanced amount of Δ9-THC/CBD (approximately 6–12% of each), or high concentration of CBD with insignificant amount of Δ9-THC. In the current study, one of the most popular strains carrying 7–9% (w/w) each of Δ9-THC and CBD in the dried cannabis was considered and obtained from one of the largest commercial vendors in the country. When both CBD and Δ9-THC are produced by the plant, most of the biosynthetic pathway is actively functioning, and one expects to find appropriate intermediates en route to these final products (Figure 1). This is in contrast to a plant that is producing high amount of CBD, where Δ9-THC-related intermediates would be downregulated, and a similar rationale could be applied to a variety that is high in Δ9-THC. In addition, most of the current cannabis industry is focusing on plant extracts (cannabis resin) and is expected to grow as medical cannabis attains more acceptance in the healthcare community, warranting investigations on cannabis resin. Thus, we were interested in identifying as many chemicals as possible simultaneously, and pre- and post-decarboxylation, laying the foundation for studies involving medical cannabis extracts, and relating such changes to the pharmacological effects. Here, we report the extraction of dried medical cannabis, its analyses, and identification of the compounds potentially present in the extract. In this context, biosynthetic pathway for phytocannabinoids is also discussed to establish a relationship with the potential compounds in the cannabis extract. These findings are a stepping stone for further comprehensive chemical analyses of cannabis varieties that are currently made available to the patients and for an objective analysis of their pharmacological effects.
Results and Discussion
Most popular methods for cannabis consumption, especially in the context of decarboxylation and administration of phytocannabinoids include the use of vaporizers with controlled heating or smoking medical cannabis. It is not only very difficult to dose patients in this manner because of the variabilities that might exist in the plant material, but also how each patient inhales. Thus, the extracts of medical cannabis are gaining popularity very quickly, especially in Canada, where a pharmaceutical grade extract preparation is a potential possibility, thus providing one the ability to prepare appropriate dosage forms for administration by patients. Chemical experiments involving activation or decarboxylation are very limited on the extracts, if any. There is no standard process employed in the industry and still rudimentary methods such as “industrial ovens” are widely used to accomplish the decarboxylation. To the best of our knowledge, no true chemical analyses-driven process has been implemented. Thus, we undertook this investigation to consider a popular variety of medical cannabis that produces Δ9-THC and CBD (hence, all of the other intermediates in the biosynthetic pathway) to understand the native cannabis resin and activated cannabis resin.
We extracted dried cannabis for phytocannabinoids using supercritical fluid extraction (SFE), which can be carried out at ambient temperature, so that this native extract contains chemicals that are as close as possible to their natural form in the dried cannabis. The cannabis extract was also subjected to heat (up to 170 °C) to obtain the corresponding decarboxylated cannabis extract. These extracts were analyzed to compare and contrast the chemical changes before and after decarboxylation. Although such decarboxylation and any chemical change is not equivalent to the changes that occur during smoking or vaping, these changes reflect the extraction processes and heat-induced decarboxylations (industrial ovens, etc.) employed in the industry to prepare various cannabis extracts and capsules filled with extracts.
Chemical extraction and decarboxylation processes employed in this study did not involve exposure to light and oxidative conditions (other than the oxygen present in the reaction vessel and in the solvent), thus limiting the potential for the formation of CBN and CBND. CBC (10) is derived from cannabigerol (CBG) (6) in an oxidative cyclization reaction during the biosynthesis.19
Native Cannabis Extract
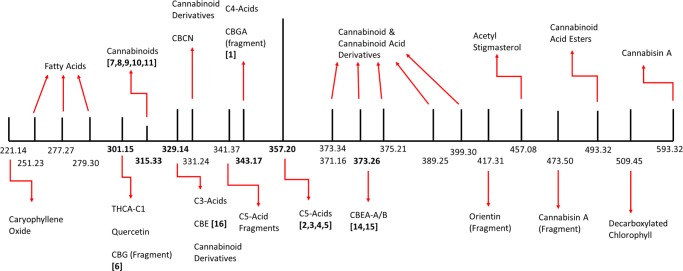
Native cannabis extract was obtained from dried cannabis employing SFE at 25 °C to minimize spontaneous decarboxylation of cannabinoid acids, such as CBGA (1), Δ9-THCA (2), and CBDA (3), catalyzed by the extraction process and to obtain the chemical composition as close as possible to that in the commercial dried cannabis. This extract was analyzed on a ultra performance liquid chromatography (UPLC) equipped with a mass detector in both the negative (ES −ve) and positive (ES +ve) scan modes. Mass spectral analyses revealed 23 unique signals (m/z): 301.15, 329.14, 331.24, 343.17, 357.20, 371.16, 373.26, 375.21, 389.25, 399.30, 417.31, 457.08, 473.50, 493.32, 509.45, 593.32 in the negative mode ([M – H]−), and 220.14, 250.23, 315.33, 341.37, and 373.34 in the positive mode ([M + H]+) (Figure 2 and Table 1). The signals at 371.16 (m/z, ES −ve) and 373.34 (m/z, ES +ve) corresponded to the same molecular weight, thus the total number of unique signals was concluded to be 22 after consolidating both scan modes.
Figure 2.
Simplified schematic of the mass spectral signals from native cannabis resin and the corresponding compounds. Numbers in bold are the mass for compounds identified in the cannabinoid biosynthetic pathway (Figure 1).
Table 1. Signals Identified in Mass Spectra of Native Cannabis Extract (Prior to Decarboxylation Process) and the Corresponding Compoundsa.
| signal # | [M – H]− | [MH]+ | MW | retention time (min) | corresponding compound(s)27,28,32−39 | compound class |
|---|---|---|---|---|---|---|
| 1 | NA | 221.14 | 220.14 | 5–5.5 | β-caryophyllene oxide | sesquiterpene |
| 2 | NA | 251.23 | 250.23 | 1–1.5 | roughanic acid27 | fatty acids |
| 3 | NA | 277.27 | 276.27 | 0–0.5 | stearidonic acid32 | |
| 4 | NA | 279.30 | 278.30 | 0.5–1 | α-linolenic acid | |
| 5 | 301.15 | NA | 302.15 | 0–0.5 | quercetin | flavonols C1-cannabinoid acids |
| 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene33 | ||||||
| Δ9-tetrahydrocannabiorcolic acid | ||||||
| cannabigerol(*) | ||||||
| 9,10-dihydro-2,3,5,6-tetramethoxyphenanthrene-1,4-dione34 | ||||||
| cannabichromevarinic acid(*) | ||||||
| cannabidivarinic acid(*) | ||||||
| Δ9-tetrahydrocannabivarinic acid(*) | ||||||
| cannabielsoin(*) | ||||||
| 6 | NA | 315.33 | 314.33 | 1.5–2 | cannabicitran | neutral cannabinoids |
| Δ8-tetrahydrocannabinol | ||||||
| Δ9-tetrahydrocannabinol | ||||||
| cannabichromene | ||||||
| cannabidiol | ||||||
| cannabicyclol | ||||||
| 7 | 329.14 | NA | 330.14 | 1–1.5 | 9,10-dihydro-2,3,5,6-tetramethoxyphenanthrene-1,4-dione34 | C3-cannabinoid acids and neutral cannabinoid derivatives |
| cannabichromevarinic acid | ||||||
| cannabidivarinic acid | ||||||
| Δ9-tetrahydrocannabivarinic acid | ||||||
| cannabielsoin | ||||||
| 2-geranyl-5-hydroxy-3-n-pentyl-1,4-benzoquinone35 | ||||||
| 10α-hydroxy-Δ9,11-hexahydrocannabinol36 | ||||||
| 10α/β-hydroxy-Δ8-tetrahydrocannabinol36 | ||||||
| 3-hydroxy-Δ4,5-cannabichromene35 | ||||||
| 8α/β-hydroxy-Δ9-tetrahydrocannabinol36 | ||||||
| 9β,10β-epoxyhexahydrocannabinol36 | ||||||
| cannabicoumaronone34 | ||||||
| cannabigerol monomethylether | ||||||
| cannabitriol(*) | ||||||
| cannabichromenic acid(*) | ||||||
| cannabidiolic acid(*) | ||||||
| cannabicyclolic acid(*) | ||||||
| Δ9-tetrahydrocannabinolic acid(*) | ||||||
| 7R-cannabicoumaronic acid35(*) | ||||||
| 5-acetoxy-6-geranyl-3-n-pentyl-1,4-benzoquinone33(*) | ||||||
| cannabigerolic acid monomethyl ether(*) | ||||||
| 8 | 331.24 | NA | 332.24 | 1–1.5 | cannabichromanon | neutral cannabinoid derivatives |
| (±)-6,7-cis/trans-epoxycannabigerol37 | ||||||
| 7-hydroxycannabichromane35 | ||||||
| cannabigerolic acid(*) | ||||||
| cannabitriol(*) | ||||||
| 11-hydroxy-Δ9-tetrahydrocannabinolic acid A(*) | ||||||
| 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol33(*) | ||||||
| 5-acetyl-4-hydroxycannabigerol(*) | ||||||
| 10-ethoxy-9-hydroxy-Δ6a-tetrahydrocannabinol(*) | ||||||
| 9 | NA | 341.37 | 340.37 | 1.5–2 | cannabichromenic acid(*) | C5-cannabinoid acid fragments |
| cannabidiolic acid(*) | ||||||
| cannabicyclolic acid(*) | ||||||
| Δ9-tetrahydrocannabinolic acid(*) | ||||||
| 10 | 343.17 | NA | 344.17 | 1–1.5 | C4-tetrahydrocannabinolic acid | C4-cannabinoid acids and C5-cannabinoid acid fragments |
| cannabigerolic acid(*) | ||||||
| cannabichromenic acid(*) | ||||||
| Δ9-tetrahydrocannabinolic acid(*) | ||||||
| cannabidiolic acid(*) | ||||||
| cannabicyclolic acid(*) | ||||||
| 11 | 357.20 | NA | 358.20 | 0.5–1 | cannabichromenic acid | C5-cannabinoid acids |
| cannabidiolic acid | ||||||
| cannabicyclolic acid | ||||||
| Δ9-tetrahydrocannabinolic acid | ||||||
| 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol33(*) | ||||||
| 10-ethoxy-9-hydroxy-Δ6a-tetrahydrocannabino(*) | ||||||
| 12 | 371.16 | 373.34 | 372.25 | 1–1.5 | 7R-cannabicoumaronic acid35 | cannabinoid derivatives |
| Δ9-tetrahydrocannabinolic acid-A-8-one28 | ||||||
| 5-acetoxy-6-geranyl-3-n-pentyl-1,4-benzoquinone33 | ||||||
| 4-acetoxycannabichromene38 | ||||||
| 13 | 373.26 | NA | 374.26 | 1–1.5 | 11-hydroxy-Δ9-tetrahydrocannabinolic acid A | C5-cannabinoid acids and cannabinoid derivatives |
| α/β-cannabielsoic acid | ||||||
| 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol33 | ||||||
| 10-ethoxy-9-hydroxy-Δ6a-tetrahydrocannabinol | ||||||
| 5-acetyl-4-hydroxycannabigerol35 | ||||||
| 5-methoxycannabigerolic acid37 | ||||||
| cannabigerolic acid monomethyl ether | ||||||
| 14 | 375.21 | NA | 376.21 | 1–1.5 | (±)-6,7-cis/trans-epoxycannabigerolic acid37 | cannabinoid acid derivatives |
| 15 | 389.25 | NA | 390.25 | 0.5–1 | 8b,11-bis-hydroxy-Δ9-tetrahydrocannabinolic acid | |
| 16 | 399.30 | NA | 400.30 | 2.5–3 | Δ9-tetrahydrocannabinolic acid + C2H2O | |
| 17 | 417.31 | NA | 418.31 | 2–2.5 | orientin(*) | flavone fragment |
| 18 | 457.08 | NA | 458.08 | 3–3.5 | acetyl stigmasterol34 | sterol |
| cannabisin A(*) | ||||||
| 19 | 473.50 | NA | 474.50 | 3–3.5 | cannabisin A(*) | lignanamide fragment |
| 20 | 493.32 | NA | 494.32 | 2–2.5 | 4-terpenyl-Δ9-tetrahydro cannabinolate39 | cannabinoid acid esters |
| α-terpenyl-Δ9-tetrahydro cannabinolate39 | ||||||
| bornyl/epi-bornyl-Δ9-tetrahydro cannabinolate39 | ||||||
| α/β-fenchyl-Δ9-tetrahydro cannabinolate39 | ||||||
| 21 | 509.45 | NA | 510.45 | 2–2.5 | chlorophyll (decarboxylated)29 | chlorophyll |
| 22 | 593.32 | NA | 594.32 | 3.5–4 | apigenin-6,8-di-C-β-d-glucopyranoside34 | flavone and lignanamide |
| cannabisin A |
Asterisks (*) indicate fragment ions instead of molecular ions for the corresponding signals.
Dried cannabis contains a wide variety of compounds, including cannabinoids, terpenoids, flavonoids, hydrocarbons, fatty acids, phenols, and other miscellaneous classes of compounds and their metabolites. A comprehensive literature analyses and the above chemical analyses of mass spectral signals led us to identify up to 62 unique compounds in the cannabis extract attributable to the above 22 unique signals based on the corresponding molecular weights of the compounds or the corresponding fragments (Figure 2 and Table 1).
Among these 62 compounds, β-caryophyllene oxide, a sesquiterpene found in cannabis, was observed with its molecular signal at 221.14 Da (m/z, [M + H]+). Roughanic acid, stearidonic acid, and α-linolenic acid, which are polyunsaturated fatty acids, were observed with the molecular weights 251.23, 277.27, and 279.30 Da, respectively (signals 2–4 in Table 1). Stearidonic acid is found in abundance in the seeds of Cannabis spp., and roughanic acid is a component of storage lipids commonly found in a wide variety of plants including cannabis.27 Δ9-Tetrahydrocannabiorcolic acid (C1-Δ9-THCRA), a lighter-chain phytocannabinoid than Δ9-THCA (2) due to its small hydrocarbon tail with one carbon atom instead of five, was observed at 301.15 Da (m/z, [M – H]−). The molecular peak at 301.15 Da (m/z) could also indicate the presence of flavonoid, quercetin, and noncannabinoid, 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene (signal 5 in Table 1).
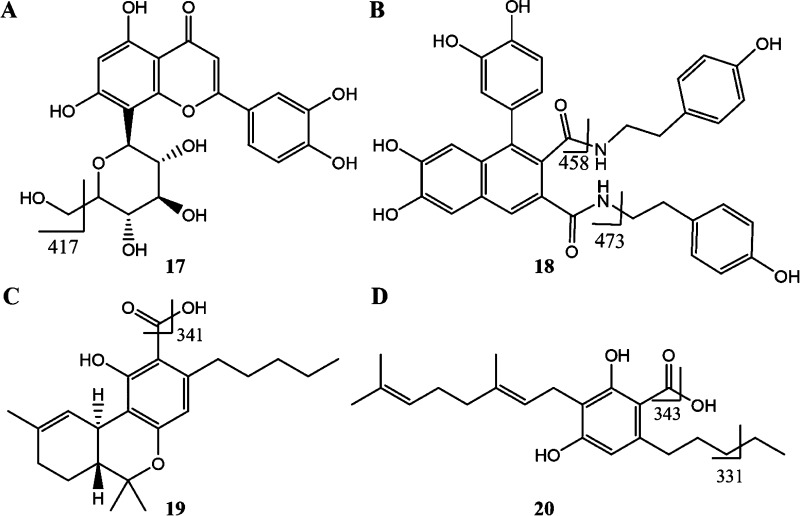
A low-intensity signal at 315.33 Da (m/z, [M + H]+) indicated the potential presence of multiple neutral phytocannabinoids cannabicitran, Δ8-THC, Δ9-THC, CBC, CBD, and the degradation product CBL in relatively small quantities (signal 6 in Table 1). Majority of the phytocannabinoids in the extract were found in the form of C3- or C5-carboxylic acids and in the form of neutral phytocannabinoid derivatives, as expected; these compounds were identified based on the strong signals at 329.14, 331.24, and 357.20 Da (m/z, [M – H]−), corresponding to the molecular weights of 20 cannabinoid derivatives (signals 7, 8, and 11, respectively, in Table 1). The signal at 341.37 Da (m/z, [M + H]+) is due to the fragmentation of the C5-cannabinoid acids, namely, Δ9-THCA, CBDA, CBCA, and CBLA after losing the hydroxyl moiety of the carboxylic acid (mass transition from 357.37 → 340.37 Da; signal 9 in Table 1 and Figure 3). CBGA was not detected in its molecular form, but two signals, at 331.24 Da and 343.13 (m/z, [M – H]−), attributed to the loss of an ethyl group (360 → 331 Da) from the hydrocarbon tail and a hydroxyl moiety from the carboxylic group (360 → 343 Da) of CBGA, respectively, confirmed the presence of CBGA in the cannabis extract (signals 8 and 10, respectively, in Table 1 and Figure 2).
Figure 3.
Fragmentation patterns for (A) orientin (17), (B) cannabisin A (18), (C) Δ9-tetrahydrocannabinolic acid (19), and (D) cannabigerolic acid (20, CBGA).
Further mass spectral analyses led to the identification of the signal at 399.30 (m/z, [M – H]−) and empirical formula for this molecular peak was deduced to be C24H32O5,28 corresponding to the molecular weight of an ethyl aldehyde or acetyl analogue of Δ9-THCA (Figure 2 and signal 16 in Table 1). On the basis of the known metabolism patterns for Δ9-THCA, one may conceive several possible structures for this phytocannabinoid derivative, that is, Δ9-THCA + C2H2O, but further structural analyses will need to be undertaken for further structural elucidation. This compound with the empirical formula Δ9-THCA + C2H2O was first described in 2015 by Nascimento et al.28
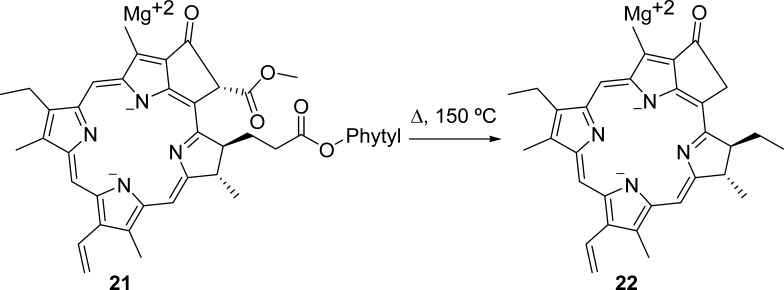
In addition to these derivatives, we identified one cannabinoid monoethyl ether derivative, two derivatives of phytocannabinoid acids, and three miscellaneous cannabinoids in the mass range of 371–399 Da (signals 12–16, ES −ve, Table 1). The mass spectral signal (m/z) at 417.31 could not be attributed to any molecular peak, but a fragment of the flavonoid, orientin, is proposed as a candidate ion with the loss of −CH2OH moiety equivalent to 31 Da (448 → 417 transition, Figure 3A and signal 17 in Table 1). Molecules with m/z higher than 400 Da include acetyl stigmasterol, a terpenoid with a molecular weight of 458.08 (signal 18 in Table 1). The mass spectral signal at 473.50 Da (m/z, [M – H]−) matches the fragment of cannabisin A (signal 19 in Table 1), where the loss of 4-ethylphenolic moiety generates this fragment (594 → 473 Da transition; Figure 3A). Four potential compounds corresponded to the signal at 493.32 Da (m/z, signal 20 in Table 1), where the conjugates of Δ9-THCA with bulky fenchol, terpenol, and borneol moieties resulted in these high-molecular-weight phytocannabinoids derivatives. The signal at 509.45 (m/z, ES −ve) indicated the presence of decarboxylated chlorophyll (signal 21 in Table 1 and Figure 2); such a decarboxylation could occur during the extraction process (Figure 4).29 The mass spectral signal 22 (m/z = 594.52Da, [M – H]−; Table 1) corresponded to the flavonoid derivative, apigenin-6,8-di-C-β-d-glucopyranoside, and the lignanamide derivative, cannabisin A.
Figure 4.
Decarboxylation of chlorophyll (C55H72MgN4O5 → C32H30MgN4O2•).29
Several compounds have been identified to be potentially present in the cannabis (up to 568a) that were not detected in the cannabis extract analyzed in this study, including some members of terpenoids, spermidine alkaloids, amides, lignanamide derivatives, flavonoids, fatty acids, additional cannabinoid esters, and noncannabinoid phenols, in the molecular weight range of 112–815 Da. Part of the reason is that, in our current analysis, we did not conduct any fractionation or enrichment of the extract, thus the natural abundance of all of the compounds determined their detectability. For example, the phytocannabinoid, Δ9-THCA-A-COOH,28 was not detected in the cannabis extract as were lignanamide derivatives, grossamide (MW 624.68), and cannabisins B–D (MW = 594.61–624.68). Similarly, cannabinoid heterodimers, which were known to exist in the mass (m/z) range of 637–717 Da ([M – H]−) were also not detected.28
In this native cannabis extract from the commercial dried cannabis, the most intense signal (m/z) was observed at 357.20 Da [M – H]−, which corresponds to up to five phytocannabinoid carboxylic acids, Δ9-THCA, CBDA, CBCA, and CBLA; there were lower-intensity signals (m/z) at 315.33 Da [M + H]+ (corresponding to Δ9-THC, CBD, CBC, CBL), 329.14 Da [M – H]− (corresponding to CBE) and at 373.26 Da [M – H]− (corresponding to cannabielsoic acid (CBEA)). CBN and CBND were not detected; however, CBE and its acid precursor CBEA appears to be in trace quantities in this native cannabis extract, based on the peak intensities. These two later compounds could have been derived from CBD and CBDA via either photo-oxidation or exposure to heat within the plant tissue, as well.26
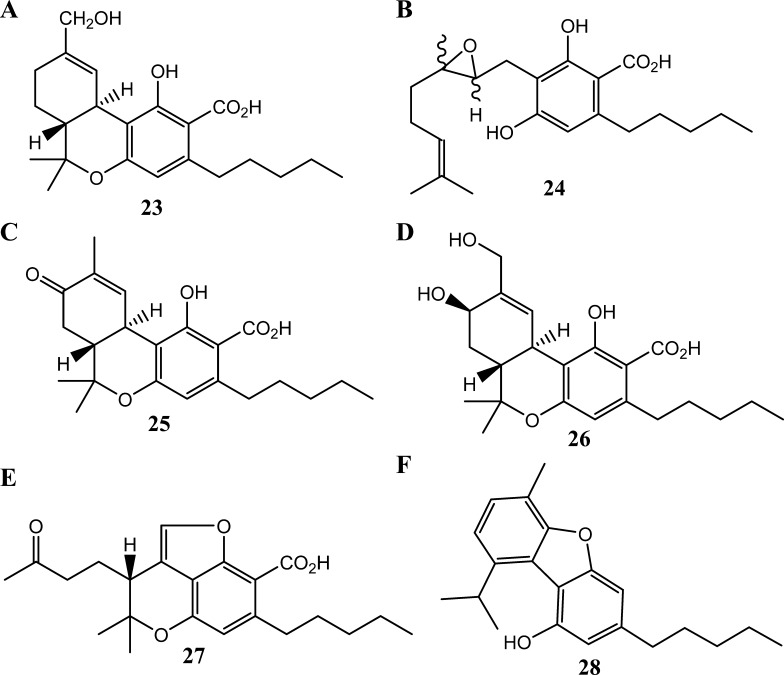
Five signals in the range of 371–399 Da (m/z, [M – H]−; peaks 12–16 in Table 1) may confirm up to 11 known phytocannabinoid derivatives in the extract; these compounds are a result of common modifications including hydroxylation, methoxylation, acetylation, and epoxidation. Additional seven phytocannabinoids with relatively low molecular weight potentially are present in the cannabis extract, which correspond to the two signals in the range of 330–332 Da (m/z, [M – H]−; signals 7 and 8 in Table 1). Depending on the position and nature of the modifications on the cannabinoid ring system, these molecules could either be precursors or metabolites of the active phytocannabinoids. For example, Δ9-THC and Δ9-THCA are commonly modified at positions 8 and 11 (i.e., 11-hydroxy-Δ9-THCA, Δ9-THCA-8-one, and 8β,11-bis-hydroxy-Δ9-THCA),28 whereas CBG and CBGA tend to undergo epoxidation at their 6 and 7 positions (i.e., 6,7-cis/trans-epoxy-CBGA) (Figure 5).
Figure 5.
Chemical structures of the phytocannabinoids, (A) 11-hydroxy-Δ9-tetrahydrocannabinolic acid A (23), (B) (±)-6,7-cis/trans-epoxycannabigerolic acid (24), (C) 8-keto-Δ9-tetrahydrocannabinolic acid A (25), (D) 8β,11-bis-hydroxy-Δ9-tetrahydrocannabinolic acid (26), (E) (−)-7R-cannabicourmaronic acid (27), and (F) cannabifuran.
Activated Cannabis Extract (Post-decarboxylation)
Phytocannabinoids such as compounds 1–3 are decarboxylated when exposed to heat, and the rates of decarboxylation differ for different phytocannabinoids. It is known that the decarboxylated phytocannabinoids are more potent than the corresponding carboxylic acid forms (vide supra). In the current environment, patients use cannabis either by smoking (subjecting the material to higher than 200 °C) or using temperature-controlled vaporizers, exposing cannabis to less than 200 °C in a controlled fashion, or baking, among other methods. All of these methods are employed by the patients individually for their own consumption; in addition, the extracts and resins are produced in commercial settings where heating is employed disproportionately. In either scenario, there is little understanding of the chemical changes and the chemicals. In the current industrial setting in Canada, there is no standard process employed, and still rudimentary methods such as industrial ovens are used to accomplish the decarboxylation, which is also not regulated. No true chemical analyses-driven process is implemented to our knowledge in the commercial processes producing medical cannabis extracts. Thus, decarboxylation (or activation) of phytocannabinoids produced in the plant is an important step for the full efficacy of cannabis extract. Due to the inherent chemical variations in the commercial cannabis strains, one would anticipate quite varied analytical results when the cannabis extracts are subjected to heat. Thus, we were interested in exploring the effect of heat on various chemicals in the native cannabis extract, in addition to the phytocannabinoids carboxylic acids such as 1–3.
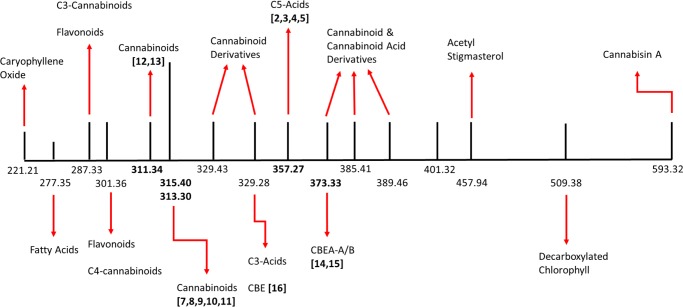
We conducted the chemical comparison of the same commercial medical cannabis strain extract in its native form and after subjecting it to heat to decarboxylate phytocannabinoids carboxylates into their active form. Chromatographic separation with mass spectral detection revealed the chemical composition of this activated cannabis extract (Figure 6). Seventeen unique mass spectral signals (m/z) were identified: 313.30, 329.28, 357.27, 373.33, 389.46, 401.32, 457.94, 509.38, and 593.32 Da in the ES −ve scanning mode, and 221.21, 277.35, 287.33, 301.36, 311.34, 315.40, 329.43, and 385.41 Da in the ES +ve scanning mode (Table 2). The mass spectral signals at 313.30 Da ([M – H]−) and that at 315.40 Da ([M + H]+) were considered to be originating from a single compound, with a molecular weight of 314.35 Da. Thus, 16 unique signals were identified to be representing up to 58 compounds in the decarboxylated cannabis extract, including various phytocannabinoids, flavonoids, terpenes, and miscellaneous compounds.
Figure 6.
Simplified schematic of the mass spectral signals from activated (decarboxylated) cannabis resin and the corresponding compounds. Numbers in bold are the mass for compounds identified in the cannabinoid biosynthetic pathway (Figure 1).
Table 2. Mass Spectral Signals Identified in the Cannabis Extract, after the Decarboxylation Process, as Well as Corresponding Compoundsa.
| signal # | [M – H+] | [M + H+] | MW | retention time (min) | corresponding compounds | compound class |
|---|---|---|---|---|---|---|
| 1 | NA | 221.21 | 220.21 | 0–1.0 | β-caryophyllene oxide | sesquiterpene |
| 2 | NA | 277.35 | 276.35 | 0–0.5 | stearidonic acid32 | fatty acid |
| 3 | NA | 287.33 | 277.33 | 1–1.5 | kaempferol | flavonol and C3-cannabinoids |
| luteolin | ||||||
| 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene36 | ||||||
| 5-methyl-4-pentyl-2,6,2-trihydroxybiphenyl36 | ||||||
| 5-methyl-4-pentylbiphenyl-2,2,6-triol37 | ||||||
| cannabichromevarin | ||||||
| cannabicyclovarin | ||||||
| cannabidivarin | ||||||
| Δ7-cis-iso-tetrahydrocannabivarin | ||||||
| Δ9-tetrahydrocannabivarin | ||||||
| 4 | NA | 301.36 | 300.36 | 2–2.5 | chrysoeriol33 | flavone and C4-cannabinoids |
| C4-cannabidiol | ||||||
| C4-tetrahydrocannabinol | ||||||
| 5 | NA | 311.34 | 310.34 | 3.5–4 | cannabifuran | neutral C5-cannabinoids |
| cannabinol | ||||||
| cannabinodiol | ||||||
| 6 | 313.30 | 315.40 | 314.40 | 2.5–3 | cannabichromene | |
| cannabicitran | ||||||
| cannabicyclol | ||||||
| cannabidiol | ||||||
| Δ8-tetrahydrocannabinol | ||||||
| Δ9-tetrahydrocannabinol | ||||||
| 7 | NA | 329.43 | 328.43 | 0.5–1 | 10-oxo-Δ6a-tetrahydrocannabinol | neutral C5-cannabinoids and neutral cannabinoid derivatives |
| 7R-cannabicourmarone34 | ||||||
| cannabichromanone-D34 | ||||||
| cannabidiol monoethyl ether | ||||||
| 8 | 329.28 | NA | 330.28 | 0.5–1 | 9,10-dihydro-2,3,5,6-tetramethoxyphenanthrene-1,4-dione34 | C3-cannabinoid acids and neutral cannabinoid derivatives |
| cannabichromevarinic acid | ||||||
| cannabidivarinic acid | ||||||
| Δ9-tetrahydrocannabivarinic acid | ||||||
| cannabielsoin | ||||||
| 10α/β-hydroxy-Δ8-tetrahydrocannabinol35 | ||||||
| 10α-hydroxy-Δ9,11-hexahydrocannabinol35 | ||||||
| 2-geranyl-5-hydroxy-3-n-pentyl-1,4-benzoquinone33 | ||||||
| 3-hydroxy-Δ4,5-cannabichromene33 | ||||||
| 8α/β-hydroxy-Δ9-tetrahydrocannabinol35 | ||||||
| 9β,10β-epoxyhexahydrocannabinol35 | ||||||
| cannabicoumaronone34 | ||||||
| cannabigerol monomethyl ether | ||||||
| cannabichromenic acid(*) | ||||||
| cannabicyclolic acid(*) | ||||||
| cannabidiolic acid(*) | ||||||
| Δ9-tetrahydrocannabinolic acid(*) | ||||||
| 10-ethoxy-9-hydroxy-Δ6a-tetrahydrocannabinol(*) | ||||||
| cannabigerolic acid monomethyl ether(*) | ||||||
| 9 | 357.27 | NA | 358.27 | 0.5–1 | cannabichromenic acid | C5-cannabinoid acids |
| cannabicyclolic acid | ||||||
| cannabidiolic acid | ||||||
| Δ9-tetrahydrocannabinolic acid | ||||||
| α/β-cannabielsoic acid(*) | ||||||
| 10-ethoxy-9-hydroxy-Δ6a-tetrahydrocannabinol(*) | ||||||
| 10 | 373.33 | NA | 374.33 | 0.5–1 | 11-hydroxy-Δ9-tetrahydrocannabinolic acid-A28 | cannabinoid acid derivatives |
| α/β-cannabielsoic acid | ||||||
| 10-ethoxy-9-hydroxy-Δ6a-tetrahydrocannabino | ||||||
| 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol33 | ||||||
| 5-acetyl-4-hydroxycannabigerol35 | ||||||
| 5-methoxycannabigerolic acid37 | ||||||
| cannabigerolic acid monomethyl ether | ||||||
| 11 | NA | 385.41 | 384.41 | 1–1.5 | sesquicannabigerol34 | cannabinoid derivative |
| 12 | 389.46 | NA | 390.46 | 0.5–1 | 8b,11-bis-hydroxy-Δ9-tetrahydrocannabinolic acid28 | cannabinoid acid derivative |
| 13 | 401.32 | NA | 402.32 | 2.5–3 | C24H32O528 | cannabinoid acid derivative |
| 14 | 457.94 | NA | 458.94 | 2–2.5 | acetyl stigmasterol34 | sterol |
| cannabisin A(*) | ||||||
| 15 | 509.38 | NA | 510.38 | 1.5–2 | chlorophyll (decarboxylated)29 | chlorophyll |
| 16 | 593.32 | NA | 594.32 | 3–3.5 | apigenin-6,8-di-C-β-d-glucopyranoside34 | flavone and lignanamide |
| cannabisin A |
Asterisks (*) indicate fragment ions instead of molecular ions for the corresponding signals.
Among these potential 58 compounds, up to 36 compounds are found to be in the native cannabis extract (prior to decarboxylation) as well, and did not change due to the decarboxylation process (Table 1 vs Table 2). The remaining 22 compounds in the decarboxylated cannabis extract were not present in the native cannabis extract (prior to decarboxylation), and admittedly, most of these new compounds are the decarboxylated forms of phytocannabinoid acids (Table 3, right column). Among the other categories of compounds in the activated cannabis extract, sesquiterpene, β-caryophyllene oxide, corresponded to the signal at 221.21 Da ([M + H]+; signal 1 in Table 2). Similarly, stearidonic acid was found at 277.35 Da (m/z, [M – H]−; signal 2 in Table 2). Two fatty acids, roughanic acid and α-linolenic acid, were observed in the native cannabis extract but were absent after subjecting the extract to heat for decarboxylation.
Table 3. Changes in the Chemical Composition of the Cannabis Extract.
| unique compounds present in the native cannabis extract | unique compound present in the activated cannabis extract |
|---|---|
| roughanic acid (fatty acid) | kaempferol (flavonol) |
| α-linolenic acid (fatty acid) | luteolin (flavonol) |
| quercetin (flavonoid) | 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene (noncannabinoid) |
| 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene (noncannabinoid) | 5-methyl-4-pentyl-2,6,2-trihydroxybiphenyl (noncannabinoid) |
| Δ9-tetrahydrocannabiorcolic acid (C1-cannabinoid acid) | 5-methyl-4-pentylbiphenyl-2,2,6-triol (noncannabinoid) |
| cannabigerol (C5-neutral cannabinoid) | cannabichromevarin (C3-neutral cannabinoid) |
| cannabichromanon (neutral cannabinoid) | cannabicyclovarin (C3-neutral cannabinoid) |
| cannabigerovarinic acid (C5-cannabinoid acid) | cannabidivarin (C3-neutral cannabinoid) |
| 6,7-cis/trans-epoxycannabigerol (cannabinoid derivative) | Δ7-cis-iso-tetrahydrocannabivarin (C3-neutral cannabinoid) |
| 7-hydroxycannabichromane (cannabinoid derivative) | Δ9-tetrahydrocannabivarin (C3-neutral cannabinoid) |
| C4-tetrahydrocannabinolic acid (C4-cannabinoid acid) | chrysoeriol (flavone) |
| cannabitriol (neutral cannabinoid) | C4-cannabidiol (C4-neutral cannabinoid) |
| cannabiripsol (neutral cannabinoid) | C4-tetrahydrocannabinol (C4-neutral cannabinoid) |
| cannabigerolic acid (C5-cannabinoid acid) | cannabifuran (neutral cannabinoid) |
| 7R-cannabicoumaronic acid (cannabinoid acid) | cannabinol (C5-neutral cannabinoid) |
| tetrahydrocannabinolic acid-8-one (cannabinoid acid derivative) | cannabinodiol (C5-neutral cannabinoid) |
| 5-acetoxy-6-geranyl-3-n-pentyl-1,4-benzoquinone (noncannabinoid) | 10-oxo-Δ6a-tetrahydrocannabinol (cannabinoid derivative) |
| 4-acetoxycannabichromene (cannabinoid derivative) | 7R-cannabicourmarone (neutral cannabinoid) |
| cannabielsoic acid-α/β (cannabinoid acid) | cannabichromanone-D(neutral cannabinoid) |
| 6,7-trans/cis-epoxycannabigerolic acid (cannabinoid acid derivative) | cannabidiol monoethylether (cannabinoid derivative) |
| Δ9-tetrahydrocannabinolic acid + C2H2O (cannabinoid acid derivative) | cannabielsoic acid-A/B (cannabinoid acid) |
| orientin (flavone) | sesquicannabigerol (cannabinoid derivative) |
| 4-terpenyl-Δ9-tetrahydrocannabinolate (cannabinoid acid ester) | |
| α-terpenyl-Δ9-tetrahydrocannabinolate (cannabinoid acid ester) | |
| bornyl/epi-bornyl-Δ9-tetrahydrocannabinolate (cannabinoid acid ester) | |
| α/β-fenchyl Δ9-tetrahydrocannabinolate (cannabinoid acid ester) |
Three C3-cannabinoids, cannabichromevarin (CBCV), cannabidivarin (CBDV), and Δ9-THCV, were attributed to the mass spectral signal ([M + H+]) at 287.33 Da (signal 3 in Table 2). These compounds are structurally similar to CBC, CBD, and Δ9-THC (i.e., C5-cannabinoids), respectively, except that the side chain is an n-propyl moiety (C3) rather than an n-pentyl group (C5). Unlike C5-cannabinoids, which behave as agonists at CB1 and CB2 receptors, C3-cannabinoids such as Δ9-THCV act as antagonists at CB1 receptor.27 Although C3-cannabinoid acids (CBCVA, Δ9-THCVA, and CBDVA) could potentially be present in both native and activated cannabis extracts, decarboxylated C3-cannabinoids, that is, CBCV, Δ9-THCV, and CBDV were only found in the activated cannabis extract, as expected.
C4-Δ9-THC, a butyl analogue of Δ9-THC, was observed at 301.36 Da (m/z, [M + H]+; signal 4 in Table 2), and its precursor, C4-Δ9-THCA, was found only in the native cannabis extract. The mass spectral signal at 301.36 Da (m/z, [M + H]+) can also be attributed to another C4-cannabinoid, C4-CBD, but there was no comparable precursor, that is, C4-CBDA in the native cannabis extract (i.e., prior to decarboxylation); hence, the presence of C4-CBD was discounted in the activated cannabis extract from the current cannabis strain.
The molecular ion at 311.34 Da (m/z, [M + H]+; signal 5 in Table 2) corresponded to three potential cannabinoids: cannabinol (12), cannabinodiol (13), and cannabifuran (28).30 The first two compounds, 12 and 13 (CBN and CBND, respectively), are derivatives of Δ9-THC (7) and derived from the oxidation and photochemical conversion reactions, respectively (Figure 1). It is unlikely that the heating in alcohol promoted oxidation and/or photochemical conversion of Δ9-THC into CBN and CBND. Also, cannabifuran (28; Figure 5) is derived from the oxidative cyclization of cannabidiol (signal 5 in Table 2). On the basis of the current data, any or all of these compounds might comprise this mass signal. Additionally, (−)-7R-cannabicourmarone1 with a signal at 329.43 Da (signal 7 in Table 2) suggested the decarboxylation of (−)-7R-cannabicourmaronic acid in the cannabis extract (m/z, [M + H]+; signal 12 in Table 1 and Figure 5).1 Six additional compounds, cannabichromanone D (a neutral cannabinoid), 10-oxo-Δ6a-tetrahydrocannabinol, cannabidiol monoethylether, sesquicannabigerol, flavonoids kaempferol (an antioxidant), and luteolin, were identified in the activated cannabis extract that were not present in the native extract, and these were attributed to signals 5 (m/z = 311.34 Da, [M + H]+), 6 (m/z = 315.40 Da, [M + H]+), and 9 (m/z = 357.27, [M – H]−) (Table 2). This is an interesting observation because one would expect these compounds to be present in the native cannabis extract; it could not have been due to the extraction process, but it is possible that they were present in a derivative form in the native extract and were released upon heating.
The identities of the compounds matching the mass spectral signal at 401.32 Da (m/z, [M – H]−; signal 13 in Table 2) could not be attributed to any known compound in cannabis; however, the molecular weight (402.32 Da) corresponding to this signal suggested the empirical formula C24H34O5. Interestingly, this empirical formula has two additional hydrogens than a known compound described in the literature, C24H32O5 (Δ9-THCA + C2H2O), and was also identified in the native cannabis extract (signal 16 in Table 1).20 Its presence in the native cannabis extract obtained through SFE suggested a chemical structure akin to THCA derivative in the activated cannabis extract, that is, Δ9-THCA + C2H4O. This empirical formula suggests a previously unknown hydroxyethyl derivative of Δ9-THCA. Such a transformation may have occurred during the heating of cannabis extract (decarboxylation process) in ethanol; however, we could not rule out the possibility that this mass spectral signal could be that of a molecular fragment of a heavier compound.
Upon comparison of the chemical composition of the cannabis extracts prior to and after decarboxylation, signals corresponding to the molecular weights of 26 compounds were not found in the decarboxylated cannabis extract (Table 3, left column). Absence of these compounds in the decarboxylated cannabis extract can be mainly attributed to the decarboxylation of acidic cannabinoids, or the loss of an ester moiety from cannabinoid esters. Likewise, cannabinoid acids and their derivatives could be susceptible to degradation when heated, although most of their direct degradation products were not observed in the decarboxylated cannabis extract in the current experiments.
In this investigation, we considered one popular cannabis strain, and thus the chemical changes cannot be generalized to all of the strains of medical cannabis; these are limited to those strains that carry a balanced Δ9-THC and CBD compounds (7–9% each of Δ9-THC and CBD). Overall, one must view the extracts from each strain or variety of medical cannabis as unique substances because many chemicals get concentrated in the extract. The present study is limited by its use of only one variety of medical cannabis, albeit it is a popular variety. To limit any process-related bias, we employed the SFE method at ambient temperature for the extraction to facilitate the identification of compounds prior to heat exposure. We used supercritical fluid (liquid CO2) and ethanol as co-solvents for the extraction of as many chemicals as possible. Analysis using a liquid chromatography coupled with mass spectral identification provided the ability to identify most of the compounds, including those that do not carry a chromophore.
When one correlates the above chemical changes, effect of controlled heating, changes in the chemical composition in addition to decarboxylated phytocannabinoids, and attempt to correlate the pharmacological effects, it is imperative to think that inconsistency in the extracts and decarboxylation could have profound effects for the patient. Additional research in this direction is certainly warranted to lead toward safe products derived from the extracts of medical cannabis. Here, we disclose the simultaneous identification of up to 62 chemicals including phytocannabinoids, terpenes, fatty acids, and other common phytochemicals from one commercial strain of medical cannabis available in Canada. We also disclosed the chemical changes that occurred when one subjects the cannabis extract to controlled, common heating conditions, and its relevance to the biosynthesis of phytocannabinoids. As medical cannabis gains more acceptance in the society and healthcare practitioners become comfortable with the therapeutic benefits of this plant product, the need for standardization and consistency of the chemical constituents beyond Δ9-THC and CBD in the medical cannabis strains is immediate. Furthermore, basic and clinical sciences supporting proper dosage forms yielding adequate pharmacological activity and outlining the potential adverse effects and risks of cannabis consumption are also urgently needed; but these are immensely dependent on the chemical constituents of the extracts consumed by the patients. Healthcare practitioners would benefit from predictable dosing, a better understanding of the pharmacological activity and knowledge of the common adverse events. Given the inconsistency and misrepresentation of cannabis in the marketplace in general, including in Canada, new metered dosing modalities would be welcomed by healthcare practitioners and patients. The comprehensive chemical analyses such as those presented in the current investigation, including the chemical profiling of activation of phytocannabinoids and other chemical transformations in various medical cannabis strain would help facilitate the adoption of medical cannabis extract-based products by the wider medical community.
Experimental Section
General
Dried cannabis (50 g) was obtained from a Canadian licensed producer in a commercial package, and this supply was used for all of the experiments. Supercritical fluid extraction (SFE) was performed on a Jasco SFE/SFC system consisting of a fluid delivery module (CO2 pump and two solvent pumps), photodiode array detector (PDA), column oven, autosampler, fraction collector, and an automated back-pressure regulator. Decarboxylation of the phytocannabinoids in the cannabis extract was performed by heating it in a microwave reactor. Carbon dioxide (SFE grade) was obtained from Praxair. Cerilliant standards for CBD and Δ9-THC were purchased from Sigma-Aldrich as Certified Reference Standards. All of the samples were analyzed in triplicate, and triple deuterated Δ9-THC was used as an internal standard. Water (Milli-Q) and methanol (HPLC grade) were used in chromatography. All of the other solvents were reagent grade and used as such without further purification.
Extraction
Dried cannabis (1.0 g) was macerated and transferred to an SFE extraction vessel. This vessel was placed in the extraction column in the SFE and subjected to extraction with supercritical CO2 (solvent A) and ethanol (solvent B). The PDA detector was set in the rage of 200–600 nm and the back-pressure regulator was set to 12 MPa. The SFE conditions are as follows: flow rate = 10 mL/min (CO2 and slave pumps) and 1 mL/min (make-up pump); temperature = 25 °C; gradient conditions: 100–50% solvent A and 0–50% solvent B from 0 → 25 min. The extraction was performed in two additional cycles on the sample, and all of the fractions were combined to afford 277 mg of a green, sticky resin.
Decarboxylation
Decarboxylation of phytocannabinoid carboxylic acids was conducted by subjecting the cannabis extract to high temperature. A 5 mL size vial was charged with cannabis extract (27.7 mg) suspended in ethanol (2 mL). The vial was sealed and subjected to microwave irradiation for 10 min at 140–170 °C. This was concentrated to dryness at 35 °C to obtain the activated (decarboxylated) cannabis extract as a resin (21.2 mg).31
UPLC–MS Analyses
Cannabis extracts were analyzed on a Waters ACQUITY UPLC H-Class System equipped with Quaternary Solvent Manager, Sample Manager FTN, and Acquity UPLC BEH column (2.1 × 50 mm2, C18, 1.7 μm particle size). The injection plate and the column were maintained at 15 and 40 °C, respectively. The sample injection volume was 2 μL, and the flow rate was 0.6 mL/min. The mobile phase consisted of solvent A (0.1% HCOOH/H2O) and solvent B (0.1% HCOOH/MeOH), and a gradient between the two phases was established as follows: an initial gradient of 30% solvent A/70% solvent B to 100% solvent B from 0 to 4.5 min, 100% solvent B from 4.5 to 5 min, change to 70% of B from 5 to 5.2 min and then continued at 30% solvent A/70% solvent B till the end of the run at 6 min. A Waters MS3100 mass spectrometer was used as the detector and set to the m/z range of 60–2000 Da. Unique mass spectral signals were identified by comparing the mass spectrum for the blank solution and that for the resin solution.
Acknowledgments
L.P.K. gratefully acknowledges the financial support from Canada Foundation for Innovation, Ontario Research Fund, University Health Network, and Scientus Pharma (formerly CannScience Innovations, Inc.). H.A.C. is supported by a Merit Award from the Department of Anesthesia, University of Toronto.
Glossary
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- CBD
cannabidiol
- CBN
cannabinol
- CBDL
cannabinodiol
- CBC
cannabichromene
- CBL
cannabicyclol
- CBE
cannabielsoin
- CBG
cannabigerol
- THV
tetrahydrocannabivarin
- CBDV
cannabidivarin
- CBCV
cannabichromevarin
- THCA
tetrahydrocannabidiolic acid
- CBDA
cannabidiolic acid
- CBNA
cannabinolic acid
- CBCA
cannabichromenic acid
- CBLA
cannabicyclolic acid
- CBEA
cannabielsoic acid
- CBGA
cannabigerolic acid
- THCVA
tetrahydrocannabivarinic acid
- C1-THCRA
tetrahydrocannabiorcolic acid
- C4-THCA
C4-tetrahydrocannabinolic acid
- CBND
cannabinodiol
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00996.
Mass spectra for the cannabis extracts prior to and after decarboxylation (PDF)
The authors declare the following competing financial interest(s): L.P.K. and H.A.C. serve on the scientific and medical advisory board of Scientus Pharma, Inc. and receive a consulting fee.
Footnotes
Ross, S. A., personal communication.
Supplementary Material
References
- Hanuš L. O.; Meyer S. M.; Munoz E.; Taglialatela-Scafiti O.; Appendino G. Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. 10.1039/c6np00074f. [DOI] [PubMed] [Google Scholar]
- Handbook of Cannabis; Pertwee R. G. E., Ed.; Oxford University Press: Oxford, 2014. [Google Scholar]
- Lucas P. G. Regulating compassion: an overview of Canada’s federal medical cannabis policy and practice. Harm. Reduct. J. 2008, 5, 5. 10.1186/1477-7517-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone E.; Pomatto V.; Cerri F.; Campantico E.; Mackie K.; Delpero M.; Guastalla A.; Dati C.; Bovolin P.; Franzoni M. F. Cannabinoid receptors are widely expressed in goldfish: molecular cloning of a CB2-like receptor and evaluation of CB1 and CB2 mRNA expression profiles in different organs. Fish Physiol. Biochem. 2013, 39, 1287–1296. 10.1007/s10695-013-9783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal A. M.; Slade D.; Gul W.; El-Alfy A. T.; Ferreira D.; Elsohly M. A. Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications. Recent Pat. CNS Drug Discovery 2009, 4, 112–136. 10.2174/157488909788453031. [DOI] [PubMed] [Google Scholar]
- Styrczewska M.; Kulma A.; Ratajczak K.; Amarowicz R.; Szopa J. Cannabinoid-like anti-inflammatory compounds from flax fiber. Cell. Mol. Biol. Lett. 2012, 17, 479–499. 10.2478/s11658-012-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer E. J.; Campbell V. A. Phytocannabinoids, CNS cells and development: a dead issue?. Drug Alcohol Rev. 2010, 29, 91–98. 10.1111/j.1465-3362.2009.00102.x. [DOI] [PubMed] [Google Scholar]
- Manduca A.; Campolongo P.; Trezza V. Cannabinoid modulation of mother-infant interaction: is it just about milk?. Rev. Neurosci. 2012, 23, 707. 10.1515/revneuro-2012-0074. [DOI] [PubMed] [Google Scholar]
- Radwan M. M.; ElSohly M. A.; El-Alfy A. T.; Ahmed S. A.; Slade D.; Husni A. S.; Manly S. P.; Wilson L.; Seale S.; Cutler S. J.; Ross S. A. Isolation and Pharmacological Evaluation of Minor Cannabinoids from High-Potency Cannabis sativa. J. Nat. Prod. 2015, 78, 1271–1276. 10.1021/acs.jnatprod.5b00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S. A.; Ross S. A.; Slade D.; Radwan M. M.; Khan I. A.; ElSohly M. A. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199. 10.1016/j.phytochem.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List of medical cannabis strains at http://www.medicalmarijuanastrains.com/, as of Jun 30, 2017.
- Hazekamp A.; Fischedick J. T. Cannabis - from cultivar to chemovar. Drug Test Anal. 2012, 4, 660–667. 10.1002/dta.407. [DOI] [PubMed] [Google Scholar]
- Fischedick J. T.; Hazekamp A.; Erkelens T.; Choi Y. H.; Verpoorte R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. 10.1016/j.phytochem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Peschel W. Quality Control of Traditional Cannabis Tinctures: Pattern, Markers, and Stability. Sci. Pharm. 2016, 84, 567–584. 10.3390/scipharm84030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga S.; Fischedick J.; Podkolinski R.; Raber J. C. Cannabinoids and Terpenes as Chemotaxonomic Markers in Cannabis. Nat. Prod. Chem. Res. 2015, 3, 181. 10.4172/2329-6836.1000181. [DOI] [Google Scholar]
- Hazekamp A.Medicinal Use of Cannabis: a Review. In Comprehensive Natural Products Chemistry, 2nd ed.; Elsevier: Oxford, U.K., 2008; pp1033–1084. [Google Scholar]
- Fischedick J.; Van Der Kooy F.; Verpoorte R. Cannabinoid receptor 1 binding activity and quantitative analysis of Cannabis sativa L. smoke and vapor. Chem. Pharm. Bull. 2010, 58, 201–207. 10.1248/cpb.58.201. [DOI] [PubMed] [Google Scholar]
- Owens B. Drug development: The treasure chest. Nature 2015, 525, S6–S8. 10.1038/525S6a. [DOI] [PubMed] [Google Scholar]
- De Backer B.; Maebe K.; Verstraete A. G.; Charlier C. Evolution of the content of THC and other major cannabinoids in drug-type cannabis cuttings and seedlings during growth of plants. J. Forensic Sci. 2012, 57, 918–922. 10.1111/j.1556-4029.2012.02068.x. [DOI] [PubMed] [Google Scholar]
- Morimoto S.; Komatsu K.; Taura F.; Shoyama Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998, 49, 1525–1529. 10.1016/S0031-9422(98)00278-7. [DOI] [PubMed] [Google Scholar]
- Hanuš L. O.; Meyer S. M.; Muñoz E.; Taglialatela-Scafati O.; Appendino G. Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. 10.1039/C6NP00074F. [DOI] [PubMed] [Google Scholar]
- Marijuana and the Cannabinoids; El-Sohly M., Ed.; Humana Press: Totowa, NJ, 2007. [Google Scholar]
- Taura F.; Tanaka S.; Taguchi C.; Fukamizu T.; Tanaka H.; Shoyama Y.; Morimoto S. Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett. 2009, 583, 2061–2066. 10.1016/j.febslet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Shoyama Y.; Oku R.; Yamauchi T.; Nishioka I. Cannabis. VI. Cannabicyclolic Acid. Chem. Pharm. Bull. 1972, 20, 1927–1930. 10.1248/cpb.20.1927. [DOI] [Google Scholar]
- Shoyama Y.; Fujita T.; Yamauchi T.; Nishioka I. Cannabichromenic acid, a genuine substance of cannabichromene. Chem. Pharm. Bull. 1968, 16, 1157–1158. 10.1248/cpb.16.1157. [DOI] [PubMed] [Google Scholar]
- ElSohly M. A.; Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Biochemistry & Molecular Biology of Plants; Buchanan B. B., Gruissem W., Jones R. L., Eds.; American Society of Plant Physiologists: Rockville, Maryland, 2000. [Google Scholar]
- Nascimento I. R.; Costa H. B.; Souza L. M.; Soprani L. C.; Merlo B. B.; Romão W. Chemical identification of cannabinoids in street marijuana samples using electrospray ionization FT-ICR mass spectrometry. Anal. Methods 2015, 7, 1415–1424. 10.1039/C4AY02355B. [DOI] [Google Scholar]
- Helaja J.; Tauber A. Y.; Abel Y.; Tkachenko N. V.; Lemmetyinen H.; Kilpeläinen I.; Hynninen P. H. Chlorophylls. IX. The first phytochlorin–fullerene dyads: synthesis and conformational studies. J. Chem. Soc., Perkin Trans. 1 1999, 2403–2408. 10.1039/a904817k. [DOI] [Google Scholar]
- Friedrich-Fiechtl J.; Spiteller G. Neue cannabinoide-1. Tetrahedron 1975, 31, 479–487. 10.1016/0040-4020(75)85016-2. [DOI] [Google Scholar]
- Kotra L. P.; Lewis M. M.; Wasilewski E.; Grover H. U.S. Provisional Patent # 62/356262, June 29, 2016.
- Mölleken H.; Theimer R. R. Survey of minor fatty acids in Cannabis sativa L. fruits of various origins. J. Intl. Hemp Assoc. 1997, 4, 13–17. [Google Scholar]
- Radwan M. M.; ElSohly M. A.; Slade D.; Ahmed S. A.; Wilson L.; El-Alfy A. T.; Khan I. A.; Ross S. A. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry 2008, 69, 2627–2633. 10.1016/j.phytochem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handbook of Cannabis; Pertwee R. G., Ed.; Oxford University Press: Oxford, 2014. [Google Scholar]
- Radwan M. M.; Elsohly M. A.; Slade D.; Ahmed S. A.; Khan I. A.; Ross S. A. Biologically active cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2009, 72, 906–911. 10.1021/np900067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan M. M.; ElSohly M. A.; El-Alfy A. T.; Ahmed S. A.; Slade D.; Husni A. S.; Manly S. P.; Wilson L.; Seale S.; Cutler S. J.; Ross S. A. Isolation and Pharmacological Evaluation of Minor Cannabinoids from High-Potency Cannabis sativa. J. Nat. Prod. 2015, 78, 1271–1276. 10.1021/acs.jnatprod.5b00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan M. M.; Ross S.; Slade D.; Ahmed S.; Zulfiqar F.; ElSohly M. Isolation and characterization of new Cannabis constituents from a high potency variety. Planta Med. 2008, 74, 267–272. 10.1055/s-2008-1034311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizpurua-Olaizola O.; Omar J.; Navarro P.; Olivares M.; Etxebarria N.; Usobiaga A. Identification and quantification of cannabinoids in Cannabis sativa L. plants by high performance liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 7549–7560. 10.1007/s00216-014-8177-x. [DOI] [PubMed] [Google Scholar]
- Ahmed S. A.; Ross S. A.; Slade D.; Radwan M. M.; Zulfiqar F.; Matsumoto R. R.; Xu Y.-T.; Viard E.; Speth R. C.; Karamyan V. T.; ElSohly M. A. Cannabinoid ester constituents from high-potency Cannabis sativa. J. Nat. Prod. 2008, 71, 536–542. 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.