Abstract
Conjugation of CpG to an antigen induces a stronger immune response compared to that of the mixture. This study compares the in vitro immunostimulatory activity of CpG conjugated via either its 5′ or 3′ end to the model antigen ovalbumin (OVA). CpG modified with an amine at either the 5′ or 3′ end was conjugated to OVA via a stable bis-aryl hydrazone bond. Similar levels of CpG conjugation to OVA were observed for both conjugates on the basis of the absorbance at 360 nm for the formation of the bis-aryl hydrazone bond, which determined 2.8 ± 0.3 CpGs linked per OVA. Both the 5′ and 3′ CpG–OVA conjugates had similar size-exclusion chromatography elution profiles. The immunostimulatory properties of the conjugates were determined by dendritic cells (DCs) and T-cells isolated from mice. The activation of DCs was determined by the upregulation of activation markers CD86 and CD40. T-cells were co-cultured with stimulated DCs, and the immunogenicity was determined by measuring T-cell proliferation and interferon γ production. Both the CpG 5′- and 3′-linked conjugates induced the same level (p > 0.5) of DC activation markers, which were significantly higher than those of the untreated control. Similarly, T-cell assays showed no significant difference (p > 0.5) between the 5′ and 3′ conjugates with respect to T-cell proliferation and interferon γ production. The 5′ and 3′ conjugates induced T-cell activation significantly higher than the mixture of CpG and OVA. This study showed that the end at which CpG is conjugated to an antigen has no influence on the generation of a T-cell-based immune response in vitro.
Introduction
The generation of a cellular T-helper (TH) 1 immune response is important for the development of vaccination strategies. The co-delivery of an adjuvant and an antigen to antigen-presenting cells (APCs) has been shown to induce strong cellular immune responses and, therefore, has the potential to develop enhanced immunotherapies.1,2 Co-delivery of an antigen and an adjuvant for inducing cellular immune responses has been achieved by formulating the antigen and adjuvant into or onto a particle3−5 or by directly conjugating the antigen and adjuvant to each other.6−9
Oligodeoxynucleotides (ODN), containing unmethylated CpG motifs, are a class of adjuvants that are being investigated in preclinical mouse models and more recently in clinical trials for immunotherapy.10,11 CpGs activate APCs by binding to toll-like receptor 9 (TLR9) in the endosome of the APC. This interaction induces a downstream signaling cascade that leads to the upregulation of proinflammatory genes, including NF-κB, which induces the upregulation of various activation markers on the APC for T-cell binding.12−14 Co-delivery of an antigen with CpG has been shown in vitro and in vivo to enhance antigen presentation to T-cells, the activation and differentiation of T-cells into TH1 and cytotoxic T lymphocytes (CTL), and the cytotoxicity of effector T-cells compared with delivery as a mixture.2,4,5,8,15,16The enhanced immune response observed when co-delivering an antigen and CpG is believed to be due to their simultaneous internalization by an APC. When delivered as a mixture, only one of the two agents might be internalized, leading to either an activated APC that does not present the antigen to T-cells or an APC that may present the antigen, however, without being fully activated, thereby not being able to induce a full T-cell response.
For co-delivery using the conjugation strategy, CpG can be conjugated to an antigen by modification with a functional group either on its 5′ or 3′ end, thereby blocking one end of the ODN. Additionally, conjugating the CpG ODN via either its 5′ or 3′ end to the antigen will result in the CpG motif being at a different distance from the respective free ODN terminus, which may influence its activation of TLR9 in the APC. Previous studies have shown that the end of the CpG that is modified for conjugation can influence the immunological activity of the CpG.17−21 For example, Agrawal et al. have performed various studies that blocked CpG at either the 5′ or 3′ end and showed that CpGs with a free 5′ end were more effective in inducing an immune response in vitro quantified by NF-κB activity and cytokine production.22−24 An in vivo assay based on the enlargement of the spleen and the production of proinflammatory cytokines indicated that having the 5′ end of CpG free was also more effective.19,22,24,25 These results suggest that the 5′ end of CpG is required to induce a greater TLR9 activation in APCs. However, for an efficient immunotherapeutic vaccine, the generation of a cellular immune response by the activation of a cytotoxic T-cell response is required. The objective of this study was to investigate whether conjugation via the 5′ or 3′ end of CpG has an effect on T-cell activation and proliferation in vitro. This was assessed by activating the DCs with CpGs conjugated to OVA antigen via either the 5′ or 3′ end and then co-culturing the DCs with T-cells. These studies showed the linkage of CpG to OVA by either the 5′ or 3′ end induced the same effective T-cell response.
Results and Discussion
Synthesis and Characterization of Conjugates
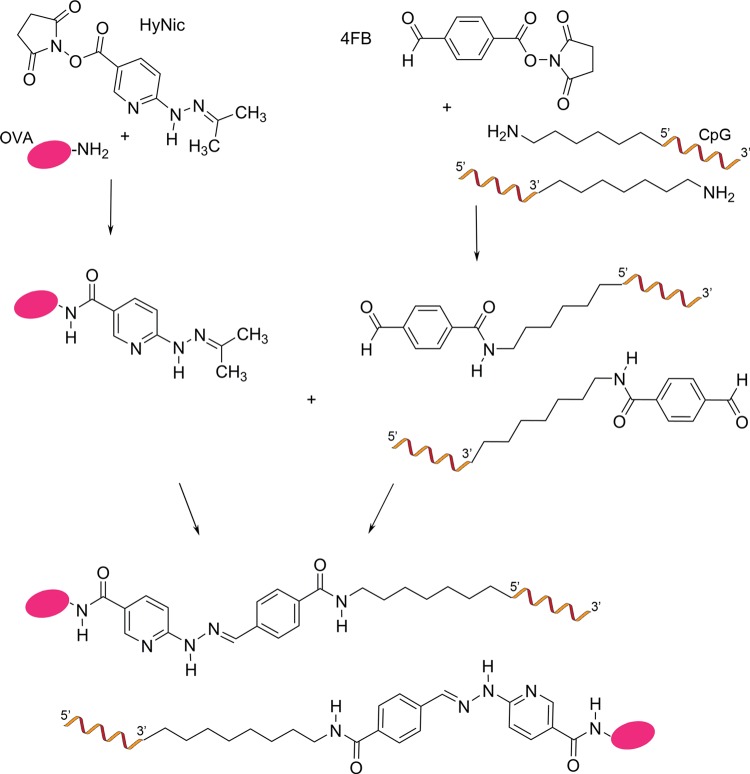
To determine whether conjugating the 5′ or 3′ end of CpG to an antigen affects the induction of a cellular immune response, CpG was custom-synthesized with an amine on either the 5′ or 3′ end, which enabled conjugation to the model antigen, OVA, using the bis-aryl-hydrazone-linking strategy.26 The bis-aryl hydrazone bond was chosen for the conjugation of CpG to OVA, as it has been reported to be biologically stable.2,26−28 The conjugation strategy is shown in Figure 1, in which the terminal amine of the CpG was modified to an aromatic aldehyde by reacting it with the activated ester of the linker succinimidyl 4-formylbenzoate (4FB) and the unreacted linker was removed by spin filtration. The substitution of the CpG with the 4FB linker was 100% complete, as determined by reacting the 4FB-modified CpG with 2-hydrazinopyridine and measuring the formation of the bis-aryl hydrazone bond at 360 nm. Before conjugation, the OVA monomer was purified from OVA aggregates by preparative size-exclusion chromatography (SEC). The lysine residues on OVA were modified to an aromatic hydrazine group by reaction with the activated ester of the linker succinimidyl 6-hydrazinonicotinate acetone hydrazone (HyNic), and the unreacted linker was removed by spin filtration. The average molar substitution ratio of OVA with the HyNic linker was 6.1 ± 1.6, which is determined by reacting the HyNic-modified OVA with 2-sulfobenzaldehdye and measuring the formation of the bis-aryl hydrazone bond at 345 nm. The HyNic-modified OVA was then reacted with either the 5′ or 3′ 4FB-modified CpG at a molar ratio of CpG to OVA of 4:1 to form a stable covalent bis-aryl hydrazone bond. The CpG–OVA conjugate was purified from unconjugated CpG using preparative SEC. The conjugation ratio of CpG to OVA was determined by measuring the formation of the bis-aryl hydrazone bond by the change in absorption at 354 nm. The 5′ and 3′ CpG–OVA conjugates were calculated to have the same level of conjugation of 2.8 ± 0.3 CpG per OVA.
Figure 1.
General scheme for the preparation of 5′ and 3′ CpG–OVA conjugates.
The level of CpG conjugated to the OVA antigen observed in this study was similar to that described in previous studies, which ranged between two and three CpGs per antigen.4,6,7,29−31
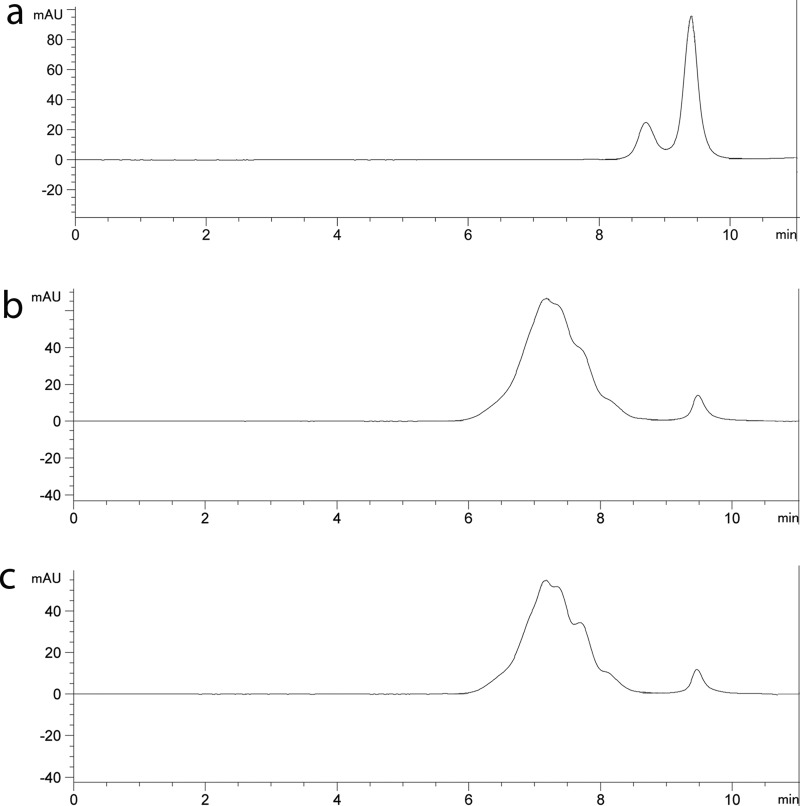
The yield of each conjugate obtained was calculated on the basis of protein recovery; the 5′ and 3′ CpG–OVA conjugates had similar yields of 64 and 67%, respectively. The 5′ CpG–OVA and 3′ CpG–OVA conjugates were characterized for homogeneity by analytical SEC. Figure 2a shows the elution profile for a mixture of CpG (9.4 ± 0.1 min) and OVA (8.7 ± 0.1 min). The 5′ and 3′ CpG–OVA conjugates generated in this study eluted earlier (6–8 min) than the free CpG and OVA. This earlier elution time confirmed the conjugation of CpG to OVA (Figure 2b,c). The elution profiles of both conjugates were similar; however, they contained subpopulations of conjugates, indicating a heterogeneous population of CpG–OVA. The observed heterogeneity of OVA conjugates would be expected as OVA contains 20 lysine residues, which are the primary target for amine modification of proteins by activated esters.32,33 SEC analysis of the conjugates identified a small peak at 9.5 min, which indicates residual unconjugated CpG that was not removed by the previous purification step. The amount of unconjugated CpG was estimated to represent approximately 3% of the CpG bound to the OVA.
Figure 2.
Elution profiles of conjugates on SEC. A 25 μL sample of (a) a mixture of CpG (4.7 μM) and OVA (1.7 μM), (b) 5′ CpG–OVA conjugate (equivalent to 4.7 μM CpG and 1.7 μM OVA), and (c) 3′ CpG–OVA conjugate (equivalent to 4.7 μM CpG and 1.7 μM OVA) was injected onto a Yarra-2000 SEC column equilibrated in phosphate buffer at a flow rate of 0.35 mL/min, and the absorbance was recorded at 260 nm.
These characterization studies showed that the 5′ and 3′ CpG–OVA conjugates produced in this study have similar properties and are, therefore, suitable for comparative immune studies in vitro.
APC Activation by Conjugates
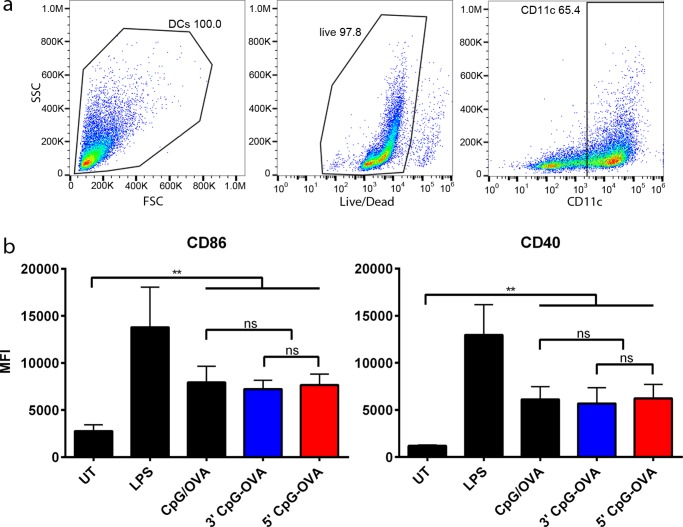
To evaluate the immunological response generated by the 5′ and 3′ CpG–OVA conjugates, they were tested in cell-culture studies. Bone-marrow-derived dendritic cells (BMDCs) were pulsed with either the 5′ and 3′ CpG–OVA conjugates or a mixture of CpG and OVA. The concentration of the 5′ and 3′ CpG–OVA conjugates was adjusted to contain 0.2 μM CpG and 79 nM OVA to be equivalent to the concentrations of controls used. After 24 h, the level of upregulation of the activation markers, MHC-II, CD86, and CD40, was measured by flow cytometry. Figure 3a shows the gating strategy used, where following doublet discrimination (data not shown), live CD11c+ cells were identified. The mean florescence intensity (MFI) of MHC-II, CD86, and CD40 was used to determine the level of upregulation of these markers. Figure 3b shows that the 5′ and 3′ CpG–OVA conjugates and the mixture of CpG and OVA induced a significant upregulation of CD86 and CD40 compared to that of untreated cells. There were no statistical differences in the upregulation of the markers (Figure 3b) and the upregulation of the MHC-II activation marker (data not shown) for all three treatments.
Figure 3.
BMDC activation with 5′ and 3′ CpG–OVA conjugates. BMDCs were incubated for 24 h with a mixture of CpG (0.2 μM) and OVA (79 nM), 3′ CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA), 5′ CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA), or LPS (2.5 μg/mL) as a positive control. (a) Gating strategy on BMDCs to remove dead cells and gate on CD11c+ cells. (b) Expression of activation markers MHC-II, CD86, and CD40 expressed in mean fluorescent intensity (MFI). Bars represent three independent experiments ± SEM; statistical significance was determined by one-way ANOVA with Dunnett’s post hoc test; **p < 0.01, ns = nonsignificant.
These results suggest that the co-delivery by conjugation of CpG to OVA at the 5′ or 3′ end was not more effective in activating BMDCs than the mixture of CpG and OVA. Additionally, these results suggest that even when conjugated through a stable linker to OVA, CpG can activate APCs, and the linking strategy does not restrict the activation of TLR9, as shown in previous studies.2,6,7,34 The activation of APCs, such as BMDCs, with the expression of activation markers is necessary for a T-cell response. For T-cells to get activated and differentiated into effector cells, activated DCs process antigen and present it on MHC-I and MHC-II molecules at the same time as displaying co-stimulatory receptors. T-cells differentiate into effector cells following binding to the displayed antigen, co-stimulatory receptors, and through recognition of proinflammatory cytokines released by DCs and T-cells.35−41
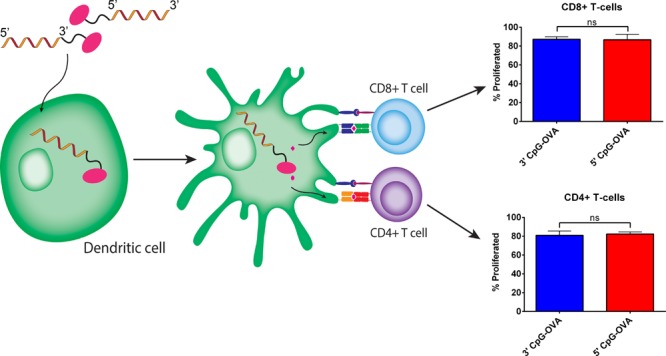
T-Cell Proliferation in Response to Conjugates
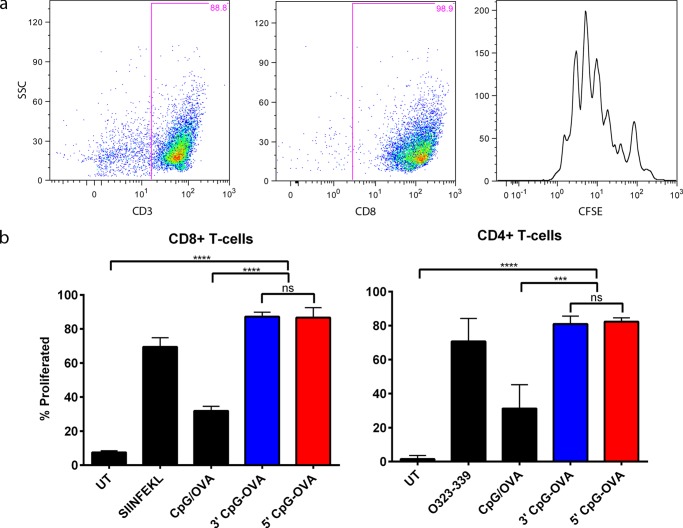
To generate a strong cellular immune response directed against cancer cells, naive CD8+ and CD4+ T-cells need to proliferate and differentiate into effector CTL and TH1 cells. Having established the activation of BMDCs with all three treatments, which is necessary for T-cell differentiation, it was then analyzed whether conjugation on the 5′ end versus the 3′ end of CpG has an impact on T-cell proliferation. To test this, a DC–T-cell co-culture was performed. BMDCs pulsed with the test treatments were co-cultured with sorted carboxyfluorescein succinimidyl ester (CFSE)-stained CD8+ and CD4+ T-cells. Figure 4a shows the gating strategy for T-cells, following doublet discrimination (data not shown); T-cells were gated on CD3 followed by gating on CD8 or CD4 (data not shown). The cell dye binds to intracellular proteins, and as T-cells divide during proliferation, the concentration of CFSE is twofold diluted in progeny cells. This dilution in dye and, thereby, proliferation of the cells are detectable by flow cytometry. The proliferation peaks of the CD8+ and CD4+ (data not shown) T-cells were visualized on a histogram plot, and the percent proliferation was calculated using the flow cytometry software FlowJo. The percent T-cell proliferation was compared between treatments, as shown in Figure 4b. Both the 5′ and 3′ CpG–OVA conjugates induced the same percentage of proliferation of CD8+ T-cells (p > 0.5), which was significantly higher than the proliferation induced by the mixture of CpG and OVA and the untreated control. In CD4+ T-cells, both the 5′ and 3′ CpG–OVA conjugates also induced the same level of proliferation (p > 0.5), which was a significantly higher level of T-cell proliferation compared to that of the mixture of CpG and OVA and the untreated control. These studies showed that both the 5′ and 3′ CpG–OVA conjugates were significantly more effective than their mixture in stimulating the proliferation of the CD8+ and CD4+ T-cells, and there was no difference in the level of proliferation between the 5′ and 3′ conjugates.
Figure 4.
Proliferation of CD8 and CD4 T-cells following 5′ and 3′ CpG–OVA conjugate treatment. BMDCs were pulsed with a mixture of CpG (0.2 μM) and OVA (79 nM), 3′ CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA), 5′ CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA), or a control of either SIINFEKL (2.6 μM) or OVA323-339 (1.4 μM) peptide. Sorted, CFSE-stained CD8+ or CD4+ T-cells were co-cultured with pulsed BMDCs at a ratio of 1:10. (a) Gating strategy to identify the proliferation peaks of CD8 T-cells. (b) Percent proliferated CD8+ and CD4+ T-cells after incubation with activated BMDC for 72 h. Bars represent the mean of three independent experiments ± SEM; statistical significance was determined by one-way ANOVA Dunnett’s post hoc test; ****p < 0.0001, ***p < 0.001, ns = nonsignificant.
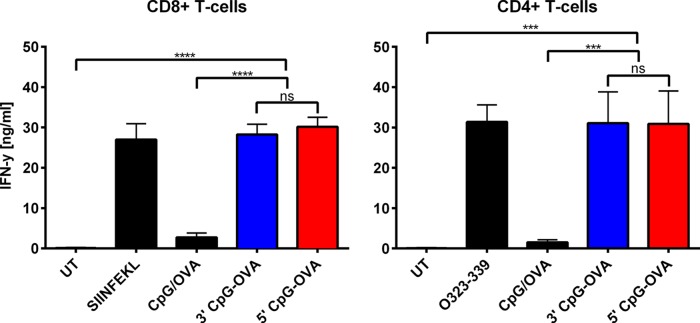
IFN-γ Production by T-Cells
In addition to proliferation of T-cells, the production of the proinflammatory cytokine IFN-γ is a key indicator for the generation of an effective anticancer immune response.42 IFN-γ production by T-cells was measured following co-culture of activated BMDCs with T-cells. BMDCs were first pulsed with the various treatments, as described in the previous T-cell proliferation studies, and then co-cultured with CD8+ and CD4+ T-cells from OT-I and OT-II mice. The level of IFN-γ produced in the co-culture after 72 h is shown in Figure 5. Both the 5′ and 3′ CpG–OVA conjugates induced statistically the same level of IFN-γ production in CD8+ T-cells (p > 0.5), which was significantly higher than the amount of IFN-γ produced in response to the mixture of CpG and OVA. For CD4+ T-cells, the IFN-γ produced by the conjugates was also significantly higher than that produced in response to the mixture of CpG and OVA, and there was also no statistical difference in the level of IFN-γ produced by either the 5′ or 3′ CpG–OVA conjugate (p > 0.5). These studies show that there was no statistical difference between the IFN-γ produced in response to the 5′ and 3′ CpG–OVA conjugates for either CD8+ or CD4+ T-cells.
Figure 5.
IFN-γ production by conjugate-treated T-cells. BMDCs were pulsed with a mixture of CpG (0.2 μM) and OVA (79 nM), 3′ CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA), 5′ CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA), or a control of either SIINFEKL (2.6 μM) or OVA323-339 (1.4 μM) peptide. CD8+ or CD4+ T-cells from OT-I and OT-II mice were co-cultured with pulsed BMDCs at a ratio of 1:10. Cell-culture supernatants were analyzed for IFN-γ levels by ELISA. Bars represent the mean of three independent experiments ± SEM; statistical significance was determined by one-way ANOVA Dunnett’s post hoc test; ****p < 0.0001, ***p < 0.001, ns = nonsignificant.
Conjugates of antigen and adjuvant have the potential to induce a strong cellular immune response that can be used for the development of immunotherapeutic vaccines. Activation of APCs by CpG–OVA conjugates is the first step to initiate a strong cellular immune response. The in vitro BMDC assay showed that the BMDCs were activated to the same extent with class B CpG conjugated to OVA via either the 5′ or 3′ end or unconjugated class B CpG in the mixture with OVA. Previous studies have shown that the incubation of BMDCs with increasing concentrations of CpG in a titration study has not increased the upregulation of the activation marker CD86.4 No difference in the BMDC activation between the 5′ and 3′ conjugates and the mixture of CpG and OVA may therefore be due to a similar upregulation of activation markers on BMDCs by CpG from a certain threshold concentration, as discussed previously.2
The class B CpG-1668 has been reported to specifically activate murine cells and has been used in previous CpG–antigen conjugates.3,6−8,29,34,43−46 It was therefore selected for this study to evaluate the immune response toward the 5′- and 3′-linked conjugates in murine cells in in vitro cultures of BMDCs and T-cells.
Previous studies have reported a reduction of TLR9 activation by CpG conjugated via the 5′ end compared with the 3′-conjugated or unconjugated CpG.17−21 TLR9 activation was determined by a direct quantification of NF-κB activity following the incubation of conjugated CpGs with J774 macrophage, 293XL, and Human Embryonic Kidney 293 cell lines expressing TLR9.17−21 Additionally, the proliferation of total splenocytes and the production of cytokines IL-12, IL-6, MIP-1α, and TNF-α following incubation with total splenocytes in vitro17−20 and measuring cytokine production and spleen weight following injection of the CpGs in vivo19 were used to determine the reduced immunostimulatory effect of 5′-conjugated CpG.
In this study, the difference in BMDC activation was measured through upregulation of activation markers. An explanation for the difference in results may therefore be that the 5′-conjugated CpG induces a lower level of TLR activation than a 3′-conjugated or free CpG, as shown previously; however, the difference in TLR9 activation does not translate to a difference in the upregulation of activation molecules. However, to induce a T-cell-mediated cellular immune response, only the upregulation of co-stimulatory markers on DCs that bind to T-cells as well as the presentation of the antigen on MHC-I and MHC-II is critical, but not the total amount of NK-κB produced following TLR9 activation.
The in vitro T-cell assays showed that the 5′ or 3′ CpG–OVA conjugate induced a significantly higher proliferation and IFN-γ production than the mixture of CpG and OVA. This may be due to the co-delivery of antigen and adjuvant in the conjugates compared with the mixture.2Within the conjugates, both CpG and OVA are internalized into the same DC, allowing for DC activation and antigen epitope presentation. DCs incubated with the mixture may only take up one of the components, thereby not being able to induce a full T-cell response.
A direct comparison of class B CpG linked via either the 5′ or the 3′ end to the model tumor antigen OVA in this study showed that for the induction of a cellular immune response in vitro conjugation to the 5′ end of CpG is of no disadvantage compared with the conjugation to the 3′ end. Although a range of investigative studies have shown that conjugation to the 5′ end of CpG lowers the activation of TLR9, previous studies that linked CpG onto either the antigen or the delivery agents mainly used the 5′ end for conjugation.4,6,29,34,47,48 A reason for this may be that the CpG ODNs are synthesized from the 3′ end to the 5′ end. Attaching a functional group for conjugation to the ODN to the 5′ end can be done after synthesis of the ODN. However, to have a functional group on the 3′ end, the synthesis is started with the functional group, followed by attaching of the ODN base by base onto it until the full CpG ODN is synthesized.49 The modification of the 5′ end of CpG with a functional group for conjugation is therefore cheaper and commercially more readily available than the modification of the 3′ end, which may be the reason for more co-delivery studies using the 5′ end for conjugation. The studies investigating the conjugation of CpG to adjuvant reported an enhanced antitumor immune response generated by the 5′-linked CpG conjugates compared to that generated by the mixtures of the CpG and antigen or delivery agent. The results shown here suggest that conjugation via the 3′ end may have led to similar strong antitumor immune responses to those of the 5′-linked conjugates.
In conclusion, this study showed that conjugation of the class B CpG-1668 to the model antigen OVA via either the 5′ or 3′ end has no effect on the induction of a cellular immune response, as measured by the upregulation of activation markers on isolated murine DCs as well as subsequent T-cell proliferation and IFN-γ production. These results were generated using in vitro assays of isolated DCs and T-cells; however, it remains to be determined what happens in more a complex in vivo system. Additionally, although this work shows that using the murine-specific CpG-1668 the 5′- and 3′-linked CpG–OVA conjugates have the same immunostimulatory activity, this effect has not yet been determined for human-specific CpGs, such as CpG-2006 and CpG-2007, in a human system. These reported findings have implications for the design of new immunotherapies that co-deliver CpG and an antigen through conjugation and have the potential to be further developed for use in clinical trials.
Experimental Procedures
Conjugation
CpG-1668 (5′-TCCATGACGTTCCTGATGCT-3′) with a phosphorothioate backbone modified with a 5′ amine (5′ NH2–CpG) or 3′ amine (3′ CpG–NH2) with a purity of 97–99% was obtained from GeneWorks Pty Ltd (Hindmarsh, SA, Australia). OVA (Sigma-Aldrich, Saint Louis, MO) was reconstituted in phosphate-buffered saline (PBS) and purified by preparative SEC (Superdex 200 10/300 GL; GE Healthcare Bio-Sciences, Uppsala, SE) using PBS as the elution buffer. The OVA monomer was collected, concentrated with a Vivaspin 2 spin filter (3 kDa MWCO, GE Healthcare Ltd., Buckinghamshire, U.K.), and the protein concentration was measured at 280 nm using a NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA). 5′ NH2–CpG and 3′ CpG–NH2 were modified with a 30-fold molar excess of succinimidyl 4FB (Solulink Inc., San Diego, CA) in 0.1 M sodium phosphate, 0.15 M NaCl, pH 8 buffer for 2 h at room temperature. The unreacted linker was removed by buffer exchange in 0.1 M sodium phosphate, 0.15 M NaCl, pH 6.0 buffer using a Vivaspin 500 spin filter (Amicon Ultra 3K, Cork, Ireland). A 4FB-modified CpG (2 μL) was reacted with 0.5 mM 2-hydrazinopyridine·2HCl (2-HP, Solulink) at 37 °C. After 30 min, the absorbance at 360 nm (extinction coefficient (EC) at 360 nm = 24 500 M–1 cm–1)26 was measured using a Nanodrop 1000, and the molar substitution ratio of CpG with the 4FB linker was determined.
OVA was modified with a 30-fold molar excess of succinimidyl 6-hydrazinonicotinate acetone hydrazone (HyNic, Solulink) in 0.1 M sodium phosphate, 0.15 M NaCl, pH 8 for 2 h at room temperature. Excess linker was removed by buffer exchange with PBS using a Vivaspin 2 spin filter. The HyNic-modified OVA (2 μL) was reacted with a 0.5 mM solution of 2-sulfobenzaldehdye (2-SBA, Solulink) at 37 °C. After 30 min, the absorbance at 345 nm (EC at 345 nm = 28 500 M–1 cm–1)26 was measured using a Nanodrop 1000, and the molar substitution ratio of OVA with the HyNic linker was determined.
The modified OVA was then reacted with the modified CpG at a 1:4 molar ratio for 2 h at room temperature to form a stable bis-aryl hydrazone bond. The 5′ CpG–OVA and 3′ CpG–OVA conjugates were purified by preparative SEC (Superdex 200 10/300 GL) and concentrated using a Vivaspin 2 spin filter. The conjugates were measured at a wavelength of 360 nm (EC at 354 nm = 29 000 M–1 cm–1)26 to quantify the amount of the stable bis-aryl hydrazone bond formed and determine the conjugation ratio. The protein concentration of the conjugate was measured by Quant-iT Protein Assay Kit (Thermo Fisher Scientific).
Analytical SEC
Analytical SEC was performed on an Agilent Technologies 1290 Infinity Chromatograph with an automatic sampler and a diode array absorbance detector (Agilent Technologies, Santa Clara, CA). The column Yarra 3u SEC-2000 (Phenomenex Inc., Torrance, CA) was equilibrated in 0.1 M sodium phosphate, 0.025% (w/v) NaN3, pH 7.4 buffer, and the column temperature was maintained at 25 °C. The elution of the sample was performed at a flow rate of 0.35 mL/min, and the data were analyzed using the Agilent ChemStation software.
Animals: Source and Ethics
Specific-pathogen-free C57BL/6, OT-I, and OT-II mice were sourced from the Hercus Taieri Research Unit, University of Otago, Dunedin, New Zealand. The experiments were conducted in accordance with the ethical guidelines established by the University of Otago Animal Ethics Committee (AEC ET10/13, AEC 53/14, and AEC 09/14). All animals were euthanized by carbon dioxide euthanasia or cervical dislocation.
Generation and Activation of BMDC
BMDCs from the C57BL/6 mice were prepared as described by Inaba et al.50 Briefly, femurs and tibiae were isolated from euthanized mice, and bone marrow was flushed out of the bones. After red blood cells were lysed with ammonium chloride, the BM cells were cultured for 6 days in cIMDM containing 5% FCS and 20 ng/mL granulocyte/macrophage colony-stimulating factor (mGM-CSF) (R&D Systems). On day 6, either PBS, a mixture of CpG (0.2 μM) and OVA (79 nM), LPS (2.5 μg/mL), or a CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA) was added to 1 × 106 cells/mL of BMDCs. After incubation for 24 h, the BMDCs were stained with LIVE/DEAD Fixable Near-IR Dead Cell Stain (Life Technologies, Eugene, Oregon), blocked with CD16/CD32 Fc-blocking antibody (clone 2.4G2, BD Pharmingen, San Jose, CA), and then labeled with the following antibodies (BioLegend, San Diego, CA): CD11c-APC (clone N418) to distinguish DCs, CD86-PE (clone GL-1), CD40-PE/Cy-7 (clone 3123), and MHC-II-FITC (clone M5/144.15.2) as activation markers. Fluorescence was measured using a Gallios flow cytometer (Beckman Coulter, Brea, CA) with three lasers (405, 488, and 633 nm) and 10 color configurations and analyzed using Kaluza software (Beckman Coulter, Brea, CA). One-way ANOVA with Dunnett’s post hoc test was performed using GraphPad Prism version 6.0b.
BMDC–T-Cell Co-Culture for Proliferation
Spleens were isolated from OT-I and OT-II mice, passed through a cell strainer, and treated with ammonium chloride to lyse red blood cells. The remaining white blood cells were sorted for CD8a (Ly-2) and CD4 (L3T4) T-cells with MicroBeads (Miltenyi, Bergisch Gladbach, Germany). The sorted T-cells were stained with CFSE (Invitrogen) (20 μM). Then, they were added to BMDCs activated for 24 h with either PBS, a mixture of CpG (0.2 μM) and OVA (79 nM), a CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA), or activated for 3 h with SIINFEKL (2.6 μM) or OVA323-339 (1.4 μM) at a BMDC–T-cell ratio of 1:10. After 72 h, the cells were stained with LIVE/DEAD Fixable Near-IR Dead Cell Stain, treated with CD16/CD32 Fc-blocking antibody, and then labeled with the following antibodies (BioLegend): CD3-PE-CF594 (clone 145-2C11) to distinguish T-cells and CD8α-APC (clone 53–6.7) or CD4-APC (clone RM4-5). Fluorescence was measured using a Gallios flow cytometer and analyzed using FlowJo software version 8.8.6 (TreeStar Inc., Ashland, OR). Percent proliferation of T-cells was calculated using the “Proliferation” feature in FlowJo, which assesses the proliferation peaks of CFSE-stained cells. One-way ANOVA with Dunnett’s post hoc test was performed using GraphPad Prism version 6.0b.
BMDC–T-Cell Co-Culture for IFN-γ Production
Spleens were isolated from OT-I and OT-II mice, passed through a cell strainer, and treated with ammonium chloride to lyse red blood cells. The remaining white blood cells were co-cultured with BMDCs activated for 24 h with either PBS, a mixture of CpG (0.2 μM) and OVA (79 nM), a CpG–OVA conjugate (equivalent to 0.2 μM CpG and 79 nM OVA), or activated for 3 h with SIINFEKL (2.6 μM) or OVA323-339 (1.4 μM) at a BMDC–T-cell ratio of 1:10. After 72 h, the cell-culture supernatants were harvested and the IFN-γ levels were measured by ELISA.
Acknowledgments
This work was supported by the University of Otago Doctoral Scholarship.
Glossary
Abbreviations
- 4FB
succinimidyl 4-formylbenzoate
- APC
antigen-presenting cell
- BMDC
bone-marrow-derived dendritic cell
- CFSE
carboxyfluorescein succinimidyl ester
- CpG
cytosine–phosphate–guanosine
- CTL
cytotoxic T lymphocyte
- EC
extinction coefficient
- ELISA
enzyme-linked immunosorbent assay
- HyNic
succinimidyl 6-hydrazinonicotinate acetone hydrazone
- IFN
interferon
- IL
interleukin
- MHC
major histocompatibility complex
- ODN
oligodeoxynucleotide
- OVA
ovalbumin
- SEC
size-exclusion chromatography
- TH
T-helper cell
- TLR
toll-like receptor
- UT
untreated
The authors declare no competing financial interest.
References
- Paulis L. E.; Mandal S.; Kreutz M.; Figdor C. G. Dendritic Cell-Based Nanovaccines for Cancer Immunotherapy. Curr. Opin. Immunol. 2013, 25, 389–395. 10.1016/j.coi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Kramer K.; Shields N. J.; Poppe V.; Young S. L.; Walker G. F. Intracellular Cleavable CpG Oligodeoxynucleotide - Antigen Conjugate Enhances Anti-Tumour Immunity. Mol. Ther. 2017, 25, 62–70. 10.1016/j.ymthe.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudette T. T.; Bachelder E. M.; Cohen J. A.; Obermeyer A. C.; Broaders K. E.; Fréchet J. M. J.; Kang E.-S.; Mende I.; Tseng W. W.; Davidson M. G.; et al. In Vivo Studies on the Effect of Co-Encapsulation of CpG DNA and Antigen in Acid-Degradable Microparticle Vaccines. Mol. Pharm. 2009, 6, 1160–1169. 10.1021/mp900038e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Titta A.; Ballester M.; Julier Z.; Nembrini C.; Jeanbart L.; van der Vlies A. J.; Swartz M. A.; Hubbell J. A. Nanoparticle Conjugation of CpG Enhances Adjuvancy for Cellular Immunity and Memory Recall at Low Dose. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 19902–19907. 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamachari Y.; Salem A. K. Innovative Strategies for Co-Delivering Antigens and CpG Oligonucleotides. Adv. Drug Delivery Rev. 2009, 61, 205–217. 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit A.; Schmitz F.; O’Keeffe M.; Staib C.; Busch D. H.; Wagner H.; Huster K. M. Protective CD8 T Cell Immunity Triggered by CpG-Protein Conjugates Competes with the Efficacy of Live Vaccines. J. Immunol. 2005, 174, 4373–4380. 10.4049/jimmunol.174.7.4373. [DOI] [PubMed] [Google Scholar]
- Kreutz M.; Giquel B.; Hu Q.; Abuknesha R.; Uematsu S.; Akira S.; Nestle F. O.; Diebold S. S. Antibody-Antigen-Adjuvant Conjugates Enable Co-Delivery of Antigen and Adjuvant to Dendritic Cells in Cis but Only Have Partial Targeting Specificity. PLoS One 2012, 7, e40208 10.1371/journal.pone.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbáth M.; Szekeres Z.; Kövesdi D.; Papp K.; Erdei A.; Prechl J. Coadministration of Antigen-Conjugated and Free CpG: Effects of in Vitro and in Vivo Interactions in a Murine Model. Immunol. Lett. 2014, 160, 178–185. 10.1016/j.imlet.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Zom G. G.; Khan S.; Britten C. M.; Sommandas V.; Camps M. G. M.; Loof N. M.; Budden C. F.; Meeuwenoord N. J.; Filippov D. V.; van der Marel G. A.; et al. Efficient Induction of Antitumor Immunity by Synthetic Toll-like Receptor Ligand-Peptide Conjugates. Cancer Immunol. Res. 2014, 2, 756–764. 10.1158/2326-6066.CIR-13-0223. [DOI] [PubMed] [Google Scholar]
- Gérard C.; Baudson N.; Ory T.; Segal L.; Louahed J. A Comprehensive Preclinical Model Evaluating the Recombinant PRAME Antigen Combined With the AS15 Immunostimulant to Fight Against PRAME-Expressing Tumors. J. Immunother. 2015, 38, 311–320. 10.1097/CJI.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrsdörfer B.; Weiner G. J. CpG Oligodeoxynucleotides as Immunotherapy in Cancer. Update Cancer Ther. 2008, 3, 27–32. 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman D. M.; Barnhart K. M.; Conover J. CpG Motifs as Immune Adjuvants. Vaccine 1999, 17, 19–25. 10.1016/S0264-410X(98)00151-0. [DOI] [PubMed] [Google Scholar]
- Bode C.; Zhao G.; Steinhagen F.; Kinjo T.; Klinman D. M. CpG DNA as a Vaccine Adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai Y.; Takeuchi O.; Akira S. TLR9 as a Key Receptor for the Recognition of DNA. Adv. Drug Delivery Rev. 2008, 60, 795–804. 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Wagner H.; Heit A.; Schmitz F.; Bauer S. Targeting Split Vaccines to the Endosome Improves Vaccination. Curr. Opin. Biotechnol. 2004, 15, 538–542. 10.1016/j.copbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Datta S. K.; Takabayashi K.; Raz E. The Therapeutic Potential of Antigen-Oligonucleotide Conjugates. Ann. N. Y. Acad. Sci. 2003, 1002, 105–111. 10.1196/annals.1281.022. [DOI] [PubMed] [Google Scholar]
- Lenert P.; Goeken A. J.; Ashman R. F. Extended Sequence Preferences for Oligodeoxyribonucleotide Activity. Immunology 2006, 117, 474–481. 10.1111/j.1365-2567.2006.02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W.; Yamazaki T.; Nishida Y.; Hanagata N. Nuclease-Resistant Immunostimulatory Phosphodiester CpG Oligodeoxynucleotides as Human Toll-like Receptor 9 Agonists. BMC Biotechnol. 2011, 11, 88. 10.1186/1472-6750-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putta M. R.; Zhu F.; Li Y.; Bhagat L.; Cong Y.; Kandimalla E. R.; Agrawal S. Novel Oligodeoxynucleotide Agonists of TLR9 Containing N3-Me-dC or N1-Me-dG Modifications. Nucleic Acids Res. 2006, 34, 3231–3238. 10.1093/nar/gkl430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.; Zhu F.-G.; Bhagat L.; Wang H.; Kandimalla E. R.; Zhang R.; Agrawal S. Potent CpG Oligonucleotides Containing Phosphodiester Linkages: In Vitro and in Vivo Immunostimulatory Properties. Biochem. Biophys. Res. Commun. 2002, 297, 83–90. 10.1016/S0006-291X(02)02127-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lin A.; Sui Q.; Zhang C.; Tian Z.; Zhang J. Phosphorothioate Modification of the TLR9 Ligand CpG ODN Inhibits poly(I:C)-Induced Apoptosis of Hepatocellular Carcinoma by Entry Blockade. Cancer Lett. 2014, 355, 76–84. 10.1016/j.canlet.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Putta M. R.; Zhu F.-G.; Wang D.; Bhagat L.; Dai M.; Kandimalla E. R.; Agrawal S. Peptide Conjugation at the 5′-end of Oligodeoxynucleotides Abrogates Toll-like Receptor 9-Mediated Immune Stimulatory Activity. Bioconjugate Chem. 2010, 21, 39–45. 10.1021/bc900425s. [DOI] [PubMed] [Google Scholar]
- Kandimalla E. R.; Bhagat L.; Yu D.; Cong Y.; Tang J.; Agrawal S. Conjugation of Ligands at the 5′-end of CpG DNA Affects Immunostimulatory Activity. Bioconjugate Chem. 2002, 13, 966–974. 10.1021/bc0200374. [DOI] [PubMed] [Google Scholar]
- Yu D.; Zhao Q.; Kandimalla E. R.; Agrawal S. Accessible 5′-End of CpG-Containing Phosphorothioate Oligodeoxynucleotides Is Essential for Immunostimulatory Activity. Bioorg. Med. Chem. Lett. 2000, 10, 2585–2588. 10.1016/S0960-894X(00)00537-0. [DOI] [PubMed] [Google Scholar]
- Putta M. R.; Bhagat L.; Wang D.; Zhu F.-G.; Kandimalla E. R.; Agrawal S. Immune-Stimulatory Dinucleotide at the 5′-End of Oligodeoxynucleotides Is Critical for TLR9-Mediated Immune Responses. ACS Med. Chem. Lett. 2013, 4, 302–305. 10.1021/ml300482z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S-4FB and S-HyNic Cross-Linkers; Solulink Inc.: San Diego, CA. http://www.solulink.com/.
- Hermanson G. T.Bioconjugate Techniques; Elsevier, 2013. [Google Scholar]
- Kozlov I. A.; Melnyk P. C.; Stromsborg K. E.; Chee M. S.; Barker D. L.; Zhao C. Efficient Strategies for the Conjugation of Oligonucleotides to Antibodies Enabling Highly Sensitive Protein Detection. Biopolymers 2004, 73, 621–630. 10.1002/bip.20009. [DOI] [PubMed] [Google Scholar]
- Maurer T.; Heit A.; Hochrein H.; Ampenberger F.; O’Keeffe M.; Bauer S.; Lipford G. B.; Vabulas R. M.; Wagner H. CpG-DNA Aided Cross-Presentation of Soluble Antigens by Dendritic Cells. Eur. J. Immunol. 2002, 32, 2356–2364. . [DOI] [PubMed] [Google Scholar]
- Heit A.; Huster K. M.; Schmitz F.; Schiemann M.; Busch D. H.; Wagner H. CpG-DNA Aided Cross-Priming by Cross-Presenting B Cells. J. Immunol. 2004, 172, 1501–1507. 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- Slütter B.; Soema P.; Ding Z.; et al. Conjugation of Ovalbumin to Trimethyl Chitosan Improves Immunogenicity of the Antigen. J. Controlled Release 2010, 143, 207–214. 10.1016/j.jconrel.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Nisbet A. D.; Saundry R. H.; Moir A. J. G.; Fothergill L. A.; Fothergill J. E. The Complete Amino-Acid Sequence of Hen Ovalbumin. Eur. J. Biochem. 1981, 115, 335–345. 10.1111/j.1432-1033.1981.tb05243.x. [DOI] [PubMed] [Google Scholar]
- Huntington J. A.; Stein P. E. Structure and Properties of Ovalbumin. J. Chromatogr. B: Biomed. Sci. Appl. 2001, 756, 189–198. 10.1016/S0378-4347(01)00108-6. [DOI] [PubMed] [Google Scholar]
- Heit A.; Maurer T.; Hochrein H.; Bauer S.; Huster K. M.; Busch D. H.; Wagner H. Cutting Edge: Toll-Like Receptor 9 Expression Is Not Required for CpG DNA-Aided Cross-Presentation of DNA-Conjugated Antigens but Essential for Cross-Priming of CD8 T Cells. J. Immunol. 2003, 170, 2802–2805. 10.4049/jimmunol.170.6.2802. [DOI] [PubMed] [Google Scholar]
- Lee K.-H.; Holdorf A. D.; Dustin M. L.; Chan A. C.; Allen P. M.; Shaw A. S. T Cell Receptor Signaling Precedes Immunological Synapse Formation. Science 2002, 295, 1539–1542. 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- Grakoui A.; et al. The Immunological Synapse: A Molecular Machine Controlling T Cell Activation. Science 1999, 285, 221–227. 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Dustin M. L.; Tseng S.-Y.; Varma R.; Campi G. T Cell-Dendritic Cell Immunological Synapses. Curr. Opin. Immunol. 2006, 18, 512–516. 10.1016/j.coi.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Shahinian A.; Pfeffer K.; Lee K.; Kundig T.; Kishihara K.; Wakeham A.; Kawai K.; Ohashi P.; Thompson C.; Mak T. Differential T Cell Costimulatory Requirements in CD28-Deficient Mice. Science 1993, 261, 609–612. 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Voigt H.; Schrama D.; Eggert A. O.; Vetter C. S.; Müller-Blech K.; Reichardt H. M.; Andersen M. H.; Becker J. C.; Lühder F. CD28-Mediated Costimulation Impacts on the Differentiation of DC Vaccination-Induced T Cell Responses. Clin. Exp. Immunol. 2006, 143, 93–102. 10.1111/j.1365-2249.2005.02972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M.; Lillemeier B. F.; Kuhns M. S.; Chen D. S.; Davis M. M. T Cells Use Two Directionally Distinct Pathways for Cytokine Secretion. Nat. Immunol. 2006, 7, 247–255. 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- Pulecio J.; Petrovic J.; Prete F.; Chiaruttini G.; Lennon-Dumenil A.-M.; Desdouets C.; Gasman S.; Burrone O. R.; Benvenuti F. Cdc42-Mediated MTOC Polarization in Dendritic Cells Controls Targeted Delivery of Cytokines at the Immune Synapse. J. Exp. Med. 2010, 207, 2719–2732. 10.1084/jem.20100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J.; Briere F.; Caux C.; Davoust J.; Lebecque S.; Liu Y. J.; Pulendran B.; Palucka K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Khan S.; Bijker M. S.; Weterings J. J.; Tanke H. J.; Adema G. J.; van Hall T.; Drijfhout J. W.; Melief C. J. M.; Overkleeft H. S.; van der Marel G. A.; et al. Distinct Uptake Mechanisms but Similar Intracellular Processing of Two Different Toll-like Receptor Ligand-Peptide Conjugates in Dendritic Cells. J. Biol. Chem. 2007, 282, 21145–21159. 10.1074/jbc.M701705200. [DOI] [PubMed] [Google Scholar]
- Hartmann S.; Nuhn L.; Palitzsch B.; Glaffig M.; Stergiou N.; Gerlitzki B.; Schmitt E.; Kunz H.; Zentel R. CpG-Loaded Multifunctional Cationic Nanohydrogel Particles as Self-Adjuvanting Glycopeptide Antitumor Vaccines. Adv. Healthcare Mater. 2015, 4, 522–527. 10.1002/adhm.201400460. [DOI] [PubMed] [Google Scholar]
- Shirota H.; Sano K.; Kikuchi T.; Tamura G.; Shirato K. Regulation of Murine Airway Eosinophilia and Th2 Cells by Antigen-Conjugated CpG Oligodeoxynucleotides as a Novel Antigen-Specific Immunomodulator. J. Immunol. 2000, 164, 5575–5582. 10.4049/jimmunol.164.11.5575. [DOI] [PubMed] [Google Scholar]
- Zhang X.-Q.; Dahle C. E.; Weiner G. J.; Salem A. K. A Comparative Study of the Antigen-Specific Immune Response Induced by Co-Delivery of CpG ODN and Antigen Using Fusion Molecules or Biodegradable Microparticles. J. Pharm. Sci. 2007, 96, 3283–3292. 10.1002/jps.20978. [DOI] [PubMed] [Google Scholar]
- Cho H. J.; Takabayashi K.; Cheng P. M.; Nguyen M. D.; Corr M.; Tuck S.; Raz E. Immunostimulatory DNA-Based Vaccines Induce Cytotoxic Lymphocyte Activity by a T-Helper Cell-Independent Mechanism. Nat. Biotechnol. 2000, 18, 509–514. 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- Daftarian P.; Sharan R.; Haq W.; Ali S.; Longmate J.; Termini J.; Diamond D. J. Novel Conjugates of Epitope Fusion Peptides with CpG-ODN Display Enhanced Immunogenicity and HIV Recognition. Vaccine 2005, 23, 3453–3468. 10.1016/j.vaccine.2005.01.093. [DOI] [PubMed] [Google Scholar]
- Wilkins T.GeneworksPtyLtd. Personal communication, Sept 16, 2016.
- Inaba K.; Inaba M.; Romani N.; Aya H.; Deguchi M.; Ikehara S.; Muramatsu S.; Steinman R. M. Generation of Large Numbers of Dendritic Cells from Mouse Bone Marrow Cultures Supplemented with Granulocyte/macrophage Colony-Stimulating Factor. J. Exp. Med. 1992, 176, 1693–1702. 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]