Abstract
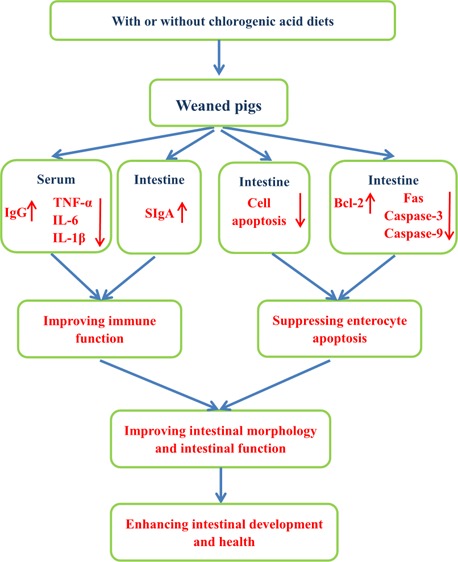
Chlorogenic acid (CGA) is a naturally occurring polyphenol in the human diet and plants, exhibiting antioxidant and anti-inflammatory activities. This study was conducted to investigate the effects of CGA on intestinal development and health in weaned pigs. Twenty-four weaned pigs were randomly assigned to two treatments and fed with a basal diet or a basal diet supplemented with 1000 mg/kg CGA. After a 14 d trial, samples were collected. Compared with the control group, CGA supplementation decreased the serum tumor necrosis factor-α, interleukin-6, and interleukin-1βIL-6 concentrations and elevated the serum immunoglobulin G and jejunal secretory immunoglobulin A concentrations. Meanwhile, jejunal villus height, duodenal and jejunal villus width, and jejunal and ileal villus height/crypt depth were increased by CGA. CGA not only decreased the number of duodenal and jejunal cells in the G0G1 phase but also increased the number of jejunal and ileal cells in the S phase. The percentages of late and total apoptotic cells in jejunum and the ratio of B-cell lymphoma-2-assiciated X protein to B-cell lymphoma-2 (Bcl-2) in duodenum and jejunum were also decreased by CGA supplementation. Finally, CGA upregulated the expression level of Bcl-2 in duodenum and jejunum, whereas it downregulated the expression levels of caspase-3 in duodenum and jejunum, caspase-9 in jejunum, as well as Fas in jejunum and ileum. This study suggested that the beneficial effects of CGA on intestinal development and health are partially due to improvement in immune defense and suppression in excessive apoptosis of intestinal epithelial cells in weaned pigs.
Introduction
Weaning is an unavoidable stressful event, exposing young infants to multiple stressful factors and often resulting in tremendous changes in gastrointestinal physiology and immunology,1,2 because the intestine of young infants is still not completely mature.1 Previous evidence in infants indicates that weaning results in physiological inflammation of the gut and subsequently causes the epithelial crypt hyperplasia.1 Furthermore, a recent study showed that weaning could also damage the intestine by contributing to oxidative stress and eventually led to enterocyte apoptosis and cell cycle arrest in the small intestine of weaned pigs.3 It is well-known that the intestine plays important roles in the digestion and absorption of nutrients, and the gut-associated lymphoid tissue is the largest immune organ in the body.4 Therefore, how to alleviate the negative effects of weaning stressors has become an urgent problem for the healthy development of animals. Nowadays, there is increasing interest in ameliorating the damage of animals or poultry induced by stress through dietary supplementation with certain plant polyphenols.5−7
Chlorogenic acid (CGA) is one of the most abundant dietary polyphenols formed by esterification of caffeic acid and quinic acid, which is the major active ingredient found in various fruits and vegetables such as pear, apple, and potatoes8 and other daily drinks including coffee and tea.9 Accumulating evidence has demonstrated that CGA possesses numerous health-promoting properties, including antioxidant, antibacterial, and antiinflammatory effects.10−12 In addition, studies have shown that CGA efficiently inhibited the apoptosis induced by acetaminophen and methylmercury in vivo and in vitro models,11,12 respectively. Earlier work by Ruan et al. (2014)13 showed that CGA has been used as a feed supplement to protect the intestinal morphology in LPS-challenged weaned rats. Therefore, it has received considerable attention as a functional ingredient to attenuate the stress-induced damages in animals and human beings.14−16 However, until now, there is extremely limited information about the effects of CGA supplementation on intestinal development and health in weaned pigs. It is well-known that intestinal inflammation and unbalanced enterocyte cellular processes in the weaning period are tightly linked to intestinal injury.1,17 Therefore, we speculated that CGA can enhance the intestinal development and health of weaned pigs by improving the immune function and suppressing the enterocyte apoptosis. This study was conducted to verify this hypothesis.
Results
Serum Immunoglobulin and Cytokines
Compared with the CON group, the serum immunoglobulin G (IgG) concentration was increased (P < 0.05), whereas the serum tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) concentrations were decreased (P < 0.05) in pigs fed with the CGA-supplemented diet (Table 1). However, no significant difference in serum immunoglobulin A (IgA) and interleukin-10 (IL-10) was observed between pigs on the CGA group and the CON group.
Table 1. Effects of CGA on the Serum Immunoglobulin and Cytokine Concentrations in Weaned Pigsa.
| treatments |
|||
|---|---|---|---|
| items | CON | CGA | p-value |
| IgA (μg/mL) | 99.41 ± 9.19 | 117.2 ± 7.13 | 0.18 |
| IgG (μg/mL) | 419.1 ± 18.09b | 492.10 ± 19.02a | 0.03 |
| TNF-α (pg/mL) | 714.90 ± 69.07a | 544.70 ± 30.48b | 0.04 |
| IL-6 (ng/mL) | 1.19 ± 0.05a | 1.06 ± 0.04b | 0.03 |
| IL-10 (pg/mL) | 171.00 ± 37.92 | 190.70 ± 48.15 | 0.75 |
| IL-1β (pg/mL) | 243.90 ± 17.89a | 171.60 ± 15.93b | 0.01 |
Results expressed in means ± SEM, n = 8 pigs/group. CON, pigs receiving a basal diet and CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA. IgA, immunoglobulin A; IgG, immunoglobulin G; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-10, interleukin-10; and IL-1β, interleukin-1β. a,bMean values within a row with unlike superscript letters were significantly different (P < 0.05).
Secretory IgA Concentrations in the Small Intestine
As shown in Table 2, dietary CGA supplementation had no effect on the duodenal secretory IgA (SIgA) concentration but resulted in a greater (P < 0.05) SIgA concentration in the jejunum. Moreover, dietary CGA supplementation tended (P < 0.10) to increase the concentration of SIgA in the ileum.
Table 2. Effects of CGA on the Concentration of Intestinal SIgA in Weaned Pigsa.
| treatments |
|||
|---|---|---|---|
| items (μg/mL) | CON | CGA | p-value |
| duodenum | 67.69 ± 1.62 | 69.05 ± 1.91 | 0.59 |
| jejunum | 50.38 ± 3.26b | 71.13 ± 2.03a | <0.01 |
| ileum | 70.52 ± 1.08 | 81.09 ± 5.55 | 0.08 |
Results expressed in means ± SEM, n = 8 pigs/group. CON, pigs receiving a basal diet; CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA; and SIgA, secretory immunoglobulin A. a,bMean values within a row with unlike superscript letters were significantly different (P < 0.05).
Intestinal Morphology
The small intestinal morphology of weaned pigs was presented in Table 3 and Figure 1. In the duodenum, dietary CGA supplementation did not affect the villus height and crypt depth but increased (P < 0.05) the villus width and tended (P < 0.10) to increase the villous height/crypt depth ratio (VCR). In the jejunum, pigs in the CGA group had higher (P < 0.05) villus height, villus width, and VCR than those in the CON group. In the ileum, the VCR was increased (P < 0.05) by CGA supplementation. However, no significant effect of dietary CGA supplementation was detected on villus height, villus width, and crypt depth.
Table 3. Effects of CGA on the Intestinal Mucosa Morphology in Weaned Pigsa.
| treatments |
|||
|---|---|---|---|
| items | CON | CGA | p-value |
| Duodenum | |||
| villus height (μm) | 257.30 ± 27.32 | 316.76 ± 32.36 | 0.14 |
| villus width (μm) | 108.34 ± 12.51b | 143.37 ± 17.61a | 0.04 |
| crypt depth (μm) | 209.94 ± 10.74 | 182.57 ± 12.83 | 0.13 |
| VCR | 1.26 ± 0.15 | 1.77 ± 0.21 | 0.08 |
| Jejunum | |||
| villus height (μm) | 277.18 ± 14.24b | 341.29 ± 9.82a | <0.01 |
| villus width (μm) | 110.72 ± 2.98b | 133.58 ± 8.56a | 0.03 |
| crypt depth (μm) | 178.60 ± 9.25 | 163.30 ± 10.08 | 0.29 |
| VCR | 1.59 ± 0.15b | 2.14 ± 0.13a | 0.03 |
| Ileum | |||
| villus height (μm) | 284.45 ± 22.91 | 325.33 ± 15.00 | 0.17 |
| villus width (μm) | 129.15 ± 9.66 | 111.2 ± 5.45 | 0.14 |
| crypt depth (μm) | 155.70 ± 15.02 | 135.40 ± 8.90 | 0.27 |
| VCR | 1.87 ± 0.15b | 2.45 ± 0.17a | 0.03 |
Results expressed in means ± SEM, n = 8 pigs/group. CON, pigs receiving a basal diet; CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA; and VCR, villous height/crypt depth ratio. a,bMean values within a row with unlike superscript letters were significantly different (P < 0.05).
Figure 1.
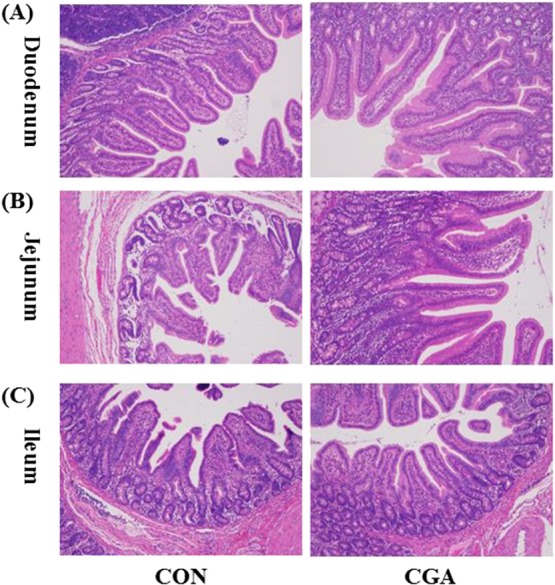
Histological evaluation of duodenal (A), jejunal (B), and ileal (C) tissues (H & E; ×100) after exposure to CGA. CON, pigs receiving a basal diet; CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA.
Apoptotic Percentage
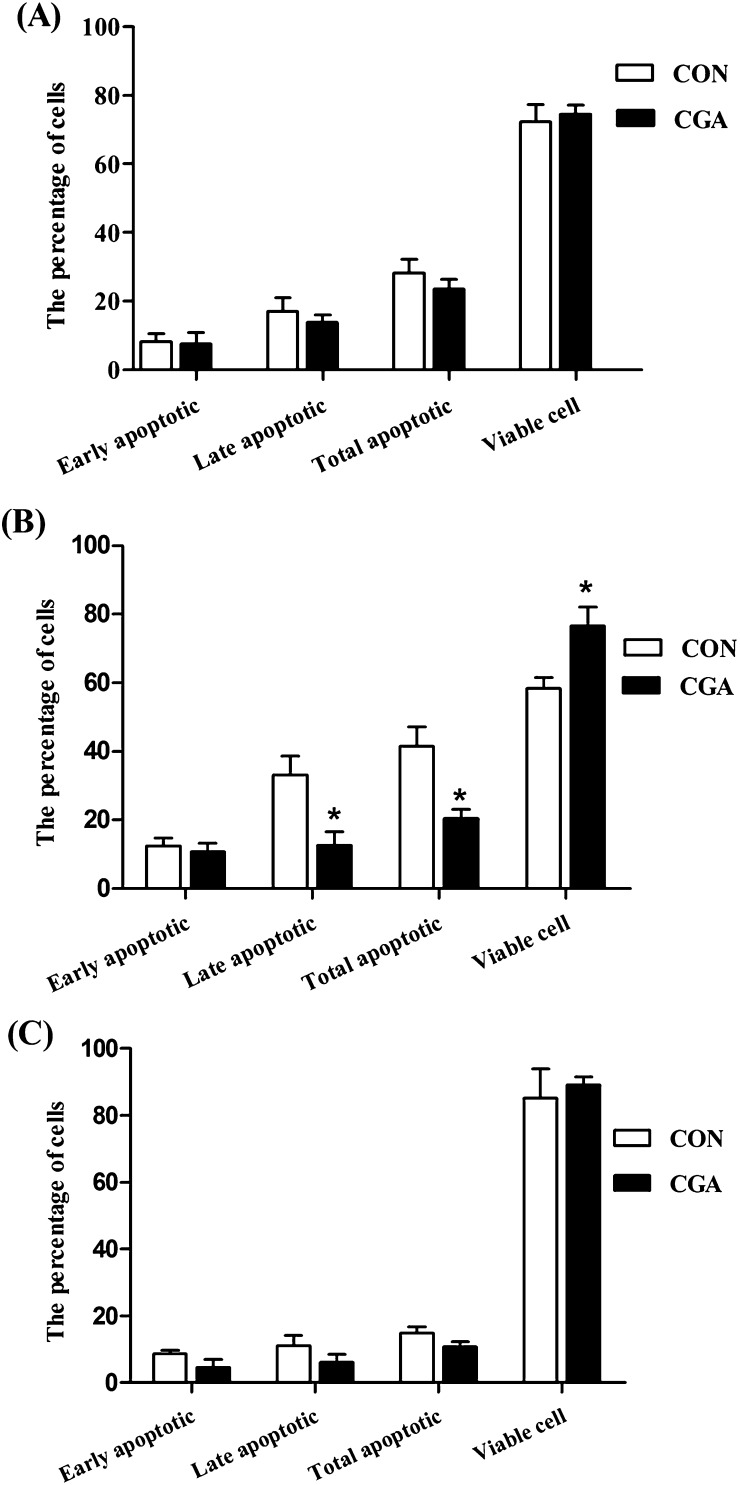
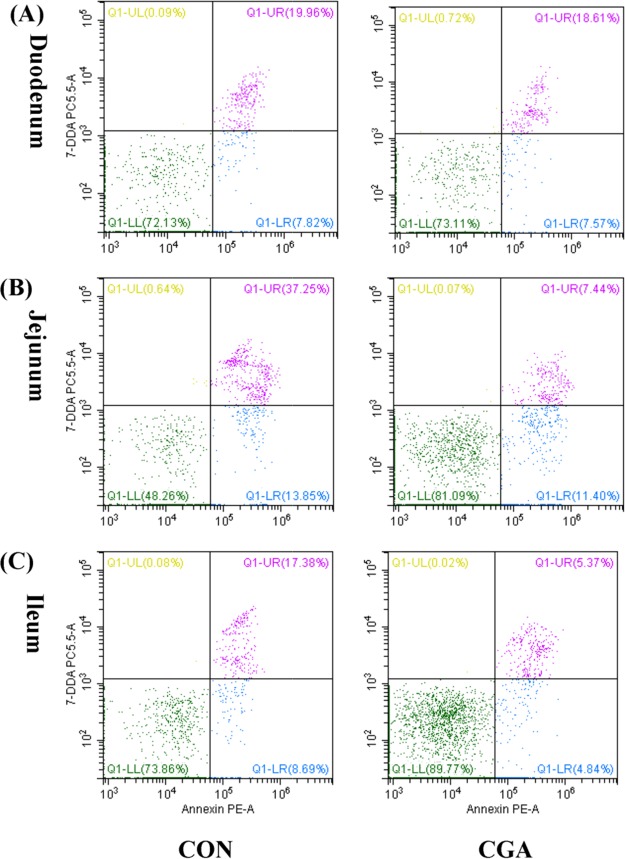
The percentages of apoptotic cells in the duodenum of weaned pigs were not significantly affected by dietary treatment (Figures 2 and 3). Compared with the CON group, CGA decreased (P < 0.05) the percentages of late apoptotic cells and total apoptotic cells in the jejunum. Furthermore, pigs on the CGA group had an increased (P < 0.05) viable cells percentage and tended (P < 0.10) to have a decreased ileal late apoptotic percentage than those on the CON group. However, no significant effects of dietary CGA supplementation were observed on the early apoptotic percentage in jejunum, as well as the early and total apoptotic percentages in ileum.
Figure 2.
Effects of CGA on the percentages of apoptotic cells in the duodenum (A), jejunum (B), and ileum (C) of weaned pigs. 30 000 cells were used in each acquisition reading. CON, pigs receiving a basal diet and CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA. The values shown represent the means and SEM, n = 8; *P < 0.05 means significant difference between CON and CGA groups.
Figure 3.
Evaluation of duodenal (A), jejunal (B), and ileal (C) cell apoptosis by flow cytometry in weaned pigs after exposure to CGA. 30 000 cells were used in each acquisition reading. Frames were divided into 4 quadrants: Q1-UL represents necrotic cells; Q1-UR represents late apoptotic and early necrotic cells; Q1-LR represents early apoptotic cells; and Q1-LL represents normal cells. CON, pigs receiving a basal diet and CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA.
Cell Cycle Distribution
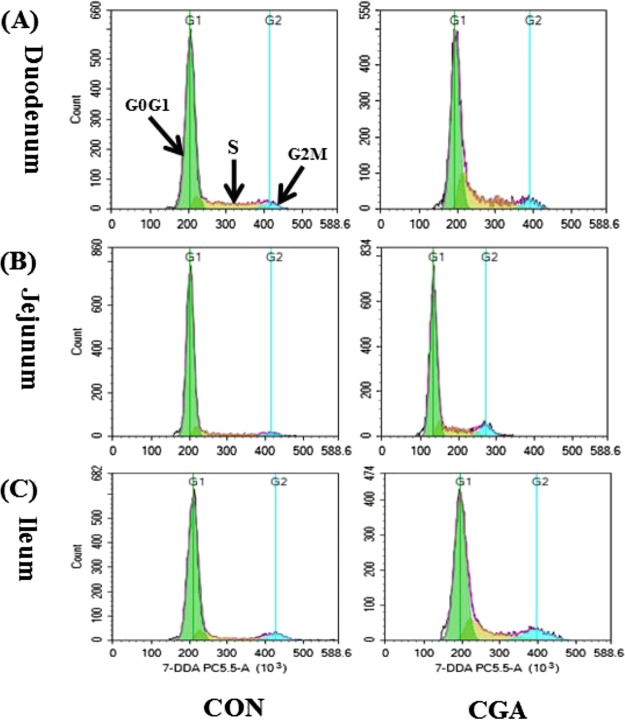
The cell cycle phase distribution in the duodenum, jejunum, and ileum of weaned pigs were presented in Table 4 and Figure 4. In the duodenum, pigs fed on the CGA diet decreased (P < 0.05) the number of cells in the G0G1 phase compared with those on the CON diet. In the jejunum, CGA supplementation decreased (P < 0.05) the number of cells in the G0G1 phase as well as increased (P < 0.05) the number of cells in the S phase and the cell proliferation index (PI) compared with those on the CON group. In the ileum, the number of cells in the S phase was increased (P < 0.05) by CGA supplementation. Meanwhile, pigs fed with the CGA diet tended (P < 0.10) to have an increased PI value compared with those on the CON diet.
Table 4. Effects of CGA on the Cell Cycle Phase Distribution in the Intestine of Weaned Pigsa.
| treatments |
|||
|---|---|---|---|
| items | CON | CGA | p-value |
| Duodenum | |||
| G0G1 phase cells (%) | 80.41 ± 3.01a | 72.28 ± 5.36b | 0.04 |
| S phase cells (%) | 14.55 ± 2.31 | 18.04 ± 3.69 | 0.16 |
| G2M phase cells (%) | 6.12 ± 1.02 | 8.96 ± 4.20 | 0.24 |
| PI | 20.42 ± 2.19 | 27.18 ± 5.73 | 0.07 |
| Jejunum | |||
| G0G1 phase cells (%) | 80.26 ± 2.43a | 68.68 ± 5.82b | 0.01 |
| S phase cells (%) | 11.64 ± 3.34b | 17.65 ± 2.28a | 0.02 |
| G2M phase cells (%) | 8.21 ± 2.84 | 8.96 ± 1.37 | 0.80 |
| PI | 19.80 ± 2.95b | 28.00 ± 2.24a | <0.01 |
| Ileum | |||
| G0G1 phase cells (%) | 63.84 ± 3.75 | 68.96 ± 5.22 | 0.39 |
| S phase cells (%) | 21.86 ± 2.50b | 28.63 ± 4.59a | 0.04 |
| G2M phase cells (%) | 6.30 ± 0.57 | 10.01 ± 1.86 | 0.10 |
| PI | 37.89 ± 4.09 | 29.02 ± 3.77 | 0.08 |
Results expressed in means ± SEM, n = 8 pigs/group. CON, pigs receiving a basal diet; CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA; and PI, proliferating index. a,bMean values within a row with unlike superscript letters were significantly different (P < 0.05).
Figure 4.
Evaluation of the duodenal (A), jejunal (B), and ileal (C) cell cycle by flow cytometry in weaned pigs after exposure to CGA. CON, pigs receiving a basal diet and CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA.
Apoptosis-Related Gene mRNA Levels
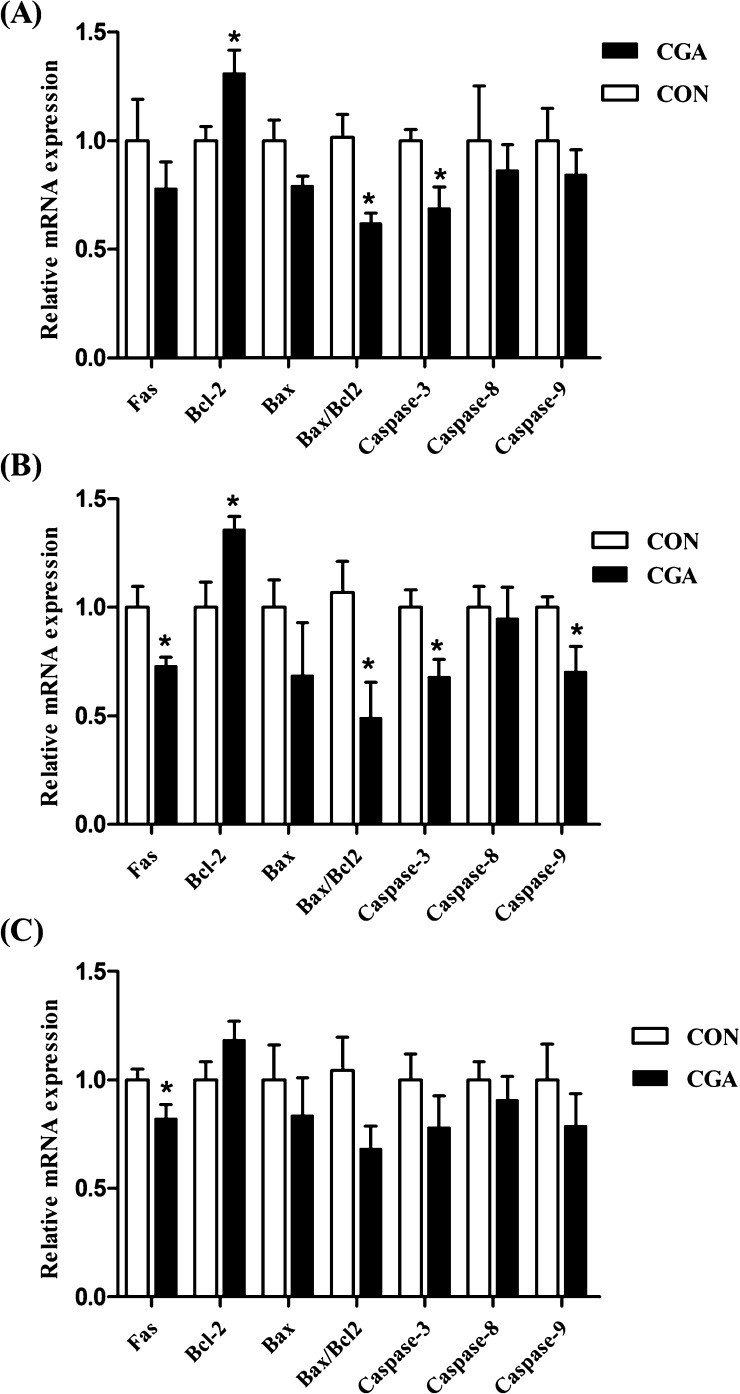
The mRNA expression levels of apoptosis-related genes of weaned pigs are presented in Figure 5. In the duodenum, the mRNA expression level of caspase-3 was decreased (P < 0.05), and the mRNA expression level of B-cell lymphoma-2 (Bcl-2) was increased (P < 0.05) by dietary CGA supplementation. Meanwhile, pigs supplemented with CGA decreased (P < 0.05) the ratio of B-cell lymphoma-2-associated X protein (Bax) to Bcl-2. In the jejunum, the mRNA expression levels of Fas, caspase-3, and caspase-9 were decreased (P < 0.05), whereas the mRNA expression level of Bcl-2 was increased (P < 0.05) by dietary CGA supplementation. Furthermore, the ratio of Bax to Bcl-2 was lower (P < 0.05) in pigs fed with the CGA diet than those in the CON diet. In the ileum, dietary CGA supplementation had no effect on the mRNA expression levels of Bax, Bcl-2, caspase-3, caspase-8, and caspase-9 but resulted in higher (P < 0.05) mRNA expression level of Fas.
Figure 5.
Effects of CGA on mRNA levels of apoptosis-related genes in the duodenum (A), jejunum (B), and ileum (C) of weaned pigs. CON, pigs receiving a basal diet; CGA, pigs receiving a basal diet supplemented with 1000 mg/kg CGA; Bcl-2, B-cell lymphoma-2; and Bax, B-cell lymphoma-2-associated X protein. The values shown represent the means and SEM, n = 8; *P < 0.05 means significant difference between CON and CGA groups.
Discussion
To achieve the optimal herd performance, early weaning has become a conventional method used in the pig industry. After weaning, young pigs usually encounter three aspects of stressors, including nutrition, psychology, and environment changes, which frequently result in pathogen invasion, intestinal inflammation and intestinal dysfunction, and subsequently jeopardize their health severely.18 Therefore, efficiency strategy is urgently needed. CGA, one of the most representative polyphenols, has been shown to possess various biological effects,14−16 which promote the application of CGA in animal production.
Numerous studies in vivo and in vitro have confirmed that CGA possesses strong anti-inflammatory effects.10,19,20 Cytokines play important roles in the immune and inflammatory responses.21 Among these, TNF-α, IL-1β, and IL-6 are the most important cytokines released from various porcine immune cells, and the systemic TNF-α concentration is considered as an indicator to monitor the severity of systemic immunity in stress-challenged pigs.22,23 Previous studies showed that the levels of inflammatory cytokines were increased at the early weaning process of pigs and subsequently induced the intestinal damage.24,25 In the present study, we found that CGA could decrease the serum TNF-α, IL-1β, and IL-6 concentrations in weaned pigs, which is in accordance with the results of the previous study in LPS-challenged rodents,26 indicating that dietary CGA supplementation may alleviate the overstimulation of the systemic immunity and early immune response in weaned pigs. IgG is one of the main components of serum, which is considered as the important indicator to diagnose immunological disorders.4 In the present study, we found that the serum IgG concentration in the pigs fed with the CGA-supplemented diet was improved. On the contrary, Ying et al. (2009)20 reported that there was no significant effect of CGA on the serum IgA, IgG and IgM in weaned pigs. A previous study reported that the efficacy of the feed additive might be partly depended on the health status of weaned pigs.27 Therefore, a possible explanation is that the piglets used in our experiment may be in a vulnerable health status because of the weaning stress, indicating the availability of the CGA supply under the current experimental conditions.
SIgA, secreted by intestinal lamina propria plasmacytes, acts as the first line of specific defence and plays an important role in the mucosal immune system of the intestine.28 It has been reported that the concentration of SIgA in intestine reduced with the increasing number of bacteria adhered to the mucosa.29 Therefore, the SIgA concentration in intestine is closely related with the intestinal mucosal immunity. Previous studies reported that the weaning stress could easily trigger intestinal inflammation in pigs.30 In the present study, the concentration of SIgA in the jejunum was higher in pigs fed with the CGA diet than those fed with the CON diet, suggesting that the intestinal mucosal immunity of weaned pigs could be enhanced by CGA.
An integrated intestinal villus–crypt morphological structure is very important in the digestion and absorption of nutrients and to the intestinal immune barrier.31 Obvious intestinal morphological changes, characterized by villous shedding, villus atrophy, and crypt hyperplasia, could result in pathogenic bacteria invasion, influence the digestion and absorption of nutrients, and consequently lead to the stunted growth.31 Thus, maintaining the integrity of the intestinal mucosal morphology is the premise of body health. However, the weaning stress has been reported to damage the intestinal mucosal integrity of pigs.32 In the present study, the small intestinal mucosal morphology has been elevated by improving the villus height and the villus width, which is in line with the previous result in LPS-challenged weaned rodents.13 Moreover, in the present study, we found that the VCR, an important parameter in evaluating the nutrient digestion and absorption capacity,33 was significantly increased in the jejunum and ileum of pigs fed the CGA-supplemented diet. Therefore, we speculated that CGA can improve the intestinal development partly by enhancing the integrity of the intestinal mucosal morphology.
The villus atrophy and crypt hyperplasia of the small intestine are partly attributed to the decreased proliferation and differentiation of villus epithelial cells.34 Cell apoptosis, a physiological process, plays a critical role in maintaining the epithelial turnover of the intestinal mucosa by eliminating abnormal or damaged cells.3,35 However, excessive apoptosis inversely breaks the tissue homeostasis in intestinal epithelium and finally leads to the intestinal mucosal barrier damage and gastrointestinal disorders.35 Thus, it has been reported that the balance between the cell proliferation and cell death, mainly the apoptosis, was closely associated with the tissue growth.36 However, the weaning stress has been reported to induce the small intestinal enterocyte apoptosis and cell cycle arrest in pigs by causing oxidative stress, achieved by disrupting the physiologic equilibrium of the oxidant and antioxidant.3 The results of the current study showed that dietary CGA supplementation decreased the percentages of late and total stage apoptotic cells in the jejunum, which are in accordance with the results of previous studies in acetaminophen-challenged mice11 and MeHg-challenged PC12 cells,12 suggesting that CGA could suppress the weaning stress-induced excessive apoptosis of the small intestinal epithelial cells, which might be the partial reason why CGA could promote the improvement of the intestinal morphology in weaned pigs. It is well-known that the cell proliferation is regulated by cyclin-dependent kinases, which regulate the progression of cells from the G0/G1 phase into the S phase of the cell cycle.37 In the present study, we observed that CGA elevated the cell proliferation of small intestinal epithelial cells by decreasing the proportion of the G0G1 phase, as well as increasing the proportion of the S phase and the PI value. Numerous studies in vitro and in vivo have confirmed that CGA is a potential polyphenolic antioxidant.12,19 In addition, Ying et al. (2009)20 reported that CGA could enhance the antioxidant ability of weaned pigs by improving the levels of GSH-Px and CAT and decreasing the content of MDA, which may partly explain why CGA can regulate the negative effects of the weaning stress on the intestinal epithelial cell proliferation and apoptosis in piglets.
To further explore the mechanism underlying the suppression effect of CGA on the enterocyte apoptosis in weaned pigs, the expression levels of apoptotic and antiapoptotic genes were evaluated. Generally, the apoptosis response is regulated by either the intrinsic pathway (mitochondria-dependent apoptosis pathway) or the extrinsic pathway (Fas-dependent apoptosis pathway) in cells.3 In detail, the intrinsic pathway is mediated by mitochondria and mainly represented by the activation of caspase-9,38 whereas the extrinsic pathway is mediated by the activation of membrane death receptors, especially Fas, and mainly characterized by the activation of caspase-8.39 Caspase-3 is an executioner caspase, which could be activated by these two pathways and thus initiates the process of apoptosis.39,40 Weaning could induce the enterocyte apoptosis by increasing the expressions of apoptotic genes and decreasing the antiapoptotic genes.3 Previous studies in vivo and in vitro reported that CGA could inhibit the apoptosis by downregulating the expression levels of antiapoptotic genes.11,12 Similarly, our present study found that the mRNA expression levels of Fas, caspase-3, and caspase-9 in the small intestine were downregulated by CGA, which might partly explain the antiapoptosis effect of CGA in weaned pigs. In addition, except for the cytokines, a higher level of caspase-3 is also a marker of the inflammatory response in the intestine.41 Therefore, our result further supported the notion that dietary supplementation with CGA is effective in preventing the intestinal inflammatory response in weaned pigs.
The Bcl-2 family includes pro-apoptotic Bax and antiapoptotic Bcl-2, which are the main regulatory proteins involved in the intrinsic apoptosis pathway and play critical roles in stress-induced apoptosis.42,43 The Bax protein could form a heterodimer with Bcl2 and then induce apoptosis.44 In particular, previous studies have confirmed that the ratio of Bax/Bcl-2 acts as an essential regulated factor in determining the cell susceptibility to apoptosis, and a decreased Bax/Bcl-2 ratio is the essential factor in the suppression of apoptosis.45,46 In the present study, dietary CGA supplementation decreased the ratio of Bax/Bcl-2 in the duodenum and jejunum, which further explained the beneficial effect of CGA on rendering the intestinal epithelial cells resistant to excessive apoptosis in weaned pigs. Taken together, our present study provided the first evidence in a weaned pig model that CGA could suppress the excessive apoptosis of intestinal epithelial cells. However, the specific mechanisms need further research.
In conclusion, to the best of our knowledge, this study provides new insights into the beneficial effects of dietary CGA supplementation on the intestinal morphology and intestinal health, and the mechanisms of action might be associated with an improvement in immune defense and the suppression in excessive apoptosis of intestinal epithelial cells.
Materials and Methods
Animal Care and Experimental Design
The experimental protocol used in this study was approved by the Animal Care Advisory Committee of the Sichuan Agricultural University. Twenty-four DLY (Duroc × Landrace × Yorkshire) weaned pigs (6.83 ± 0.10 kg) were randomly allocated to 2 treatment groups with 12 replicates per treatment group and 1 pig per replicate. The two treatment groups were as follows: the CON group, in which pigs were fed a basal diet and the CGA group, in which pigs were fed the basal diet supplemented with 1000 mg/kg CGA. The CGA was provided by China Animal Husbandry Industry Co. Ltd. (Beijing, China). The basal diet was formulated to meet or exceed the National Research Council (NRC 2012)47 recommendations for the nutrient requirements of piglets. Ingredient composition and nutrient levels were presented in Table 5. All pigs were housed in individual metabolism cages (0.7 × 1.5 m) and were given ad libitum access to fresh water and feed. Room temperature was maintained at 25–28 °C, and the relative humidity was controlled at 55–65%. The experimental period lasted for 14 days.
Table 5. Ingredient Composition and Nutrient Levels of Basal Diets (Air-Dry Basis, %).
| ingredient | % | nutrient concentrationsa | % |
|---|---|---|---|
| corn | 28.00 | CP | 20.36 |
| extruded corn | 28.00 | ME (MJ/kg) | 14.83 |
| soybean meal | 10.00 | Ca | 0.82 |
| extruded soybean | 7.00 | total P | 0.61 |
| fish meal | 5.00 | available P | 0.43 |
| whey powder | 7.00 | lysine | 1.37 |
| soybean protein concentrate | 8.00 | methionine | 0.45 |
| soybean oil | 2.16 | methionine + cystine | 0.74 |
| sucrose | 2.50 | threonine | 0.81 |
| limestone | 0.70 | tryptophan | 0.21 |
| dicalcium phosphate | 0.45 | ||
| salt | 0.30 | ||
| l-lysine HCl | 0.28 | ||
| dl-methionine | 0.12 | ||
| l-threonine | 0.04 | ||
| choline chloride | 0.10 | ||
| vitamin premixb | 0.05 | ||
| mineral premixc | 0.30 |
Values are calculated.
The premix provides the following per kilogram of the diet: vitamin A, 6000 IU; vitamin D3, 400 IU; vitamin E, 10 IU; vitamin K3, 2 mg; vitamin B1, 0.8 mg; vitamin B2, 6.4 mg; vitamin B6, 2.4 mg; vitamin B12, 12 μg; folic acid, 0.2 mg; nicotinic acid, 14 mg; and d-pantothenic acid, 10 mg.
The premix provides the following per kilogram of the diet: Fe (as ferrous sulfate), 130 mg; Cu (as copper sulfate), 80 mg; Mn (as manganese sulfate), 60 mg; Zn (zinc sulfate), 120 mg; I (potassium iodide), 0.3 mg; and Se (as sodium selenite), 0.35 mg.
Blood and Intestinal Sample Collections
At the end of the trial, eight pigs with the average body weight of each treatment were randomly selected after 12 h fasting for sample collection. Blood samples were collected from the anterior vena cava, and serum samples were then collected after centrifugation at 3000g for 15 min at 4 °C and stored at −80 °C for further analysis. The same 16 pigs were euthanized with an intravenous injection of chlorpromazine hydrochloride (3 mg/kg BW), and then the abdomen was immediately opened to remove the small intestine. About 2.0 cm segments of the middle of duodenum, jejunum, and ileum were quickly isolated, gently flushed with ice-cold phosphate-buffered saline (PBS), and then preserved in PBS for flow cytometry. This was followed by isolating and fixing the tissues of duodenum, jejunum, and ileum in 4% paraformaldehyde solution for histological analyses. After that, the mucosa of duodenum, jejunum, and ileum was collected by scraping using a sterile glass slide, snap-frozen in liquid nitrogen, and then stored at −80 °C until the further analysis.
Measurement of Serum Immunoglobulin Subsets and Cytokines
The levels of serum immunoglobulin subsets (IgA and IgG) and cytokines (TNF-α, IL-6, IL-10, and IL-1β) were measured by the sandwich ELISA kits (Beijing winter song Boye Biotechnology Co. Ltd., Beijing, China) according to the manufacturer’s instructions. Absorbance (450 nm) was determined using a BioTek Synergy HT microplate reader (BioTek Instruments, VT). The minimum detectable levels were 1.0 μg/mL and 1.0 pg/mL for immunoglobulin subsets and cytokines, respectively.
Analysis of Mucosa Secretory IgA
About 1 g of the frozen mucosa sample of the small intestine (duodenum, jejunum, and ileum) was weighed, homogenized in ice-cold physiological saline (1:9, wt/vol), and centrifuged at 2500g for 10 min at 4 °C, and then the supernatant was collected for the determination of the mucosa SIgA. The concentrations of SIgA in duodenum, jejunum, and ileum were determined using the sandwich ELISA kits (Beijing winter song Boye Biotechnology Co. Ltd., Beijing, China) according to the manufacturer’s instructions. The minimum detectable level was 1.0 μg/mL.
Small Intestinal Morphology Analysis
The morphology of duodenum, jejunum, and ileum was conducted according to our previous report.34 Briefly, paraformaldehyde-fixed small intestine samples were dehydrated with normal saline, embedded in paraffin wax, and then cut into ∼5 μm transverse sections, followed by staining with hematoxylin and eosin and sealing with a neutral resin size. Intestinal mucosal morphology including villus height, crypt depth, and villus width was determined with image processing and analysis system (Image-Pro Plus 6.0, Media Cybernetics, Inc., Rockville, MD, USA). A minimum of 10 well-orientated villi and their adjoined crypts from each intestinal segment were measured in triplicate.
Apoptosis of Small Intestinal Epithelial Cell by Flow Cytometry
The epithelial cells of duodenal, jejunal, and ileal were isolated to measure the proportion of apoptotic cells, which were assessed by flow cytometry with the PE Annexin V apoptosis detection kit (BD Biosciences, San Diego, CA, USA) as previously described.48 Briefly, the excised fresh mucosal layer of duodenum, jejunum, and ileum were immediately isolated and then ground and filtered to form a cell suspension. The cells were carefully washed twice with the ice-cold PBS and suspended in PBS at the density of 1 × 106 cells/mL. After that, a total of 100 μL of the cell suspension was taken and placed into 5 mL streaming tubes, and then 5 μL PE Annexin V and 5 μL 7-AAD were added. The mixture was incubated for 15 min in the dark. Finally, 400 μL of Annexin V binding buffer (1×) was added into the reaction tubes and then mixed thoroughly. The apoptotic cells were examined using CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA) within 1 h and 30 000 cells were used in each acquisition reading.
Cell Cycle of the Small Intestinal Epithelial Cell by Flow Cytometry
Likewise, a total of 100 μL of the cell suspension was taken and placed into a 5 mL streaming tubes, and then 1 mL Triton X-100 was added. The mixture was incubated for 10 min at 4 °C and then centrifuged. After washing twice with PBS, 5 μL of 7-AAD was added and then the mixture was incubated for 30 min at 4 °C in the dark. Subsequently, 400 μL of PBS was added and then mixed thoroughly. The cell cycle distribution was analyzed using a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA) within 1 h. The PI was carried out by the formula: PI = (S + G2M)/(G0G1 + S + G2M) × 100%.
Total RNA Extraction and Gene Expression Analysis
Total RNA of the small intestinal mucosa (duodenum, jejunum, and ileum) was isolated using the TRIzol reagent (TaKaRa, Dalian, China) following the manufacturer’s protocols. The integrity of RNA was checked by formaldehyde gel electrophoresis. The concentration and purity of RNA were determined from OD 260/280 readings (ratio = 1.8–2.0) using a spectrophotometer (Beckman Coulter, DU 800; Beckman Coulter Inc). Reverse transcription was performed using the PrimeScript RT reagent kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The primers were synthesized commercially by Life Technologies Limited and are shown in Table 6.
Table 6. Primer Sequences Used for Real-Time PCR.
| genea | accession no. | primer sequencesb (5′–3′) | size, bp |
|---|---|---|---|
| Bcl-2 | XM_021099593.1 | F: GCTACTTACTGCCAAAGGGA | 161 |
| R: TTCAGGCGGAGCTGTAAGAG | |||
| Bax | XM_013998624.2 | F: GACGCTGGACTTCCTTCGAG | 334 |
| R: GTGGCCCGAGAGAGGTTTATT | |||
| Fas | NM_213839 | F: TGATGCCCAAGTGACTGACC | 103 |
| R: GCAGAATTGACCCTCACGAT | |||
| caspase-3 | NM_214131.1 | F: GGAATGGCATGTCGATCTGGT | 351 |
| R: ACTGTCCGTCTCAATCCCAC | |||
| caspase-8 | XM_021074714.1 | F: TCTGCGGACTGGATGTGATT | 165 |
| R: TCTGAGGTTGCTGGTCACAC | |||
| caspase-9 | XM_013998997.2 | F: AATGCCGATTTGGCTTACGT | 195 |
| R: CATTTGCTTGGCAGTCAGGTT | |||
| GAPDH | NM_001206359.1 | F: TCGGAGTGAACGGATTTGGC | 147 |
| R: TGCCGTGGGTGGAATCATAC |
Bcl-2, B-cell lymphoma-2; Bax, B-cell lymphoma-2-associated X protein; and GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
F, forward and R, reverse.
Quantitative real-time polymerase chain reaction (PCR) was conducted to analyze the mRNA expression levels of Fas, Bax, Bcl-2, caspase-3, caspase-8, and caspase-9 in the small intestinal mucosa using the CFX-96 real-time PCR detection system (Bio-Rad) and SYBR Premix Ex Taq II (Tli RNaseH Plus) reagents (TaKaRa, Dalian, China). The PCR reaction was run in a 10 μL reaction volume, which contained 5 μL of SYBR Premix Ex Taq II (Tli RNaseH Plus), 0.2 μL of ROX reference dye II (50×), 0.4 μL of each primer, 1 μLl of the cDNA sample, and 3 μLl of double-distilled H2O. The PCR cycling parameters were as follows: initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 5 min. A melting curve analysis was performed following each real-time quantitative PCR assay to verify the specificity of the reactions. Each sample was tested simultaneously in triplicate on the same PCR plate. The relative levels of the mRNA expression of target genes were calculated using the 2–ΔΔCT method,49 and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the house-keeping gene.
Statistical Analysis
The normality test was performed by the Shapiro–Wilk test, and the analyses were performed by T-test using the statistical program SAS 9.4 (SAS Inst., Inc., Cary, NC, USA). Each pig was considered as an experimental unit. The results were expressed as mean values and using scanning electron microscopy (SEM) images. Statistical significance and a tendency toward difference were considered as P < 0.05 and P < 0.10, respectively.
Acknowledgments
The present study was financially supported by the Special Fund for Agro-Scientific Research in the Public Interest (201403047) and the Youth Innovation teams of Animal Feed Biotechnology of the Sichuan Province (2016TD0028).
Glossary
Abbreviations
- Bax
B-cell lymphoma-2-assiciated X protein
- Bcl-2
B-cell lymphoma-2
- CGA
chlorogenic acid
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IL-6
interleukin-6
- IL-10
interleukin-10
- IL-1β
interleukin-1β
- PI
proliferating index
- SIgA
secretory immunoglobulin A
- TNF-α
tumor necrosis factor-α
- VCR
villous height/crypt depth ratio
Author Contributions
§ J.C. and H.X. are contribute equally.
The authors declare no competing financial interest.
References
- Cummins A. G.; Thompson F. M. Effect of breast milk and weaning on epithelial growth of the small intestine in humans. Gut 2002, 51, 748–754. 10.1136/gut.51.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J. M.; Opapeju F. O.; Pluske J. R.; Kim J. C.; Hampson D. J.; Nyachoti C. M. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Cai X.; Guo Q.; Chen X.; Zhu S.; Xu J. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Br. J. Nutr. 2013, 110, 1938–1947. 10.1017/s0007114513001608. [DOI] [PubMed] [Google Scholar]
- Hu L.; Peng X.; Chen H.; Yan C.; Liu Y.; Xu Q. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur. J. Nutr. 2017, 56, 1753–1765. 10.1007/s00394-016-1223-z. [DOI] [PubMed] [Google Scholar]
- Cirillo G.; Curcio M.; Vittorio O.; Iemma F.; Restuccia D.; Spizzirri U. G. Polyphenol conjugates and human health: a perspective review. Crit. Rev. Food Sci. Nutr. 2016, 56, 326–337. 10.1080/10408398.2012.752342. [DOI] [PubMed] [Google Scholar]
- Hu Q.; Li S.; Zhang Y.; Zhuo Z.; Feng J. Phytosterols on growth performance, antioxidant enzymes and intestinal morphology in weaned piglets. J. Sci. Food Agric. 2017, 97, 4629–4634. 10.1002/jsfa.8333. [DOI] [PubMed] [Google Scholar]
- Relja B.; Töttel E.; Breig L.; Henrich D.; Schneider H.; Marzi I.; Lehnert M. Plant polyphenols attenuate hepatic injury after hemorrhage/resuscitation by inhibition of apoptosis, oxidative stress, and inflammation via NF-kappaB in rats. Eur. J. Nutr. 2012, 51, 311–321. 10.1007/s00394-011-0216-1. [DOI] [PubMed] [Google Scholar]
- Dao L.; Friedman M. Chlorogenic acid content of fresh and processed potatoes determined by ultraviolet spectrophotometry. J. Agric. Food Chem. 1992, 40, 2152–2156. 10.1021/jf00023a022. [DOI] [Google Scholar]
- Clifford M. N.; Marks S.; Knight S.; Kuhnert N. Characterization by LC-MS(n) of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agric. Food Chem. 2006, 54, 4095–4101. 10.1021/jf060536p. [DOI] [PubMed] [Google Scholar]
- Ali N.; Rashid S.; Nafees S.; Hasan S. K.; Shahid A.; Majed F.; Sultana S. Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: An experimental approach. Chem.-Biol. Interact. 2017, 272, 80–91. 10.1016/j.cbi.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Ji L.; Jiang P.; Lu B.; Sheng Y.; Wang X.; Wang Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013, 24, 1911–1919. 10.1016/j.jnutbio.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Li Y.; Shi W.; Li Y.; Zhou Y.; Hu X.; Song C. Neuroprotective effects of chlorogenic acid against apoptosis of PC12 cells induced by methylmercury. Environ. Toxicol. Pharmacol. 2008, 26, 13–21. 10.1016/j.etap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Ruan Z.; Liu S.; Zhou Y.; Mi S.; Liu G.; Wu X. Chlorogenic acid decreases intestinal permeability and increases expression of intestinal tight junction proteins in weaned rats challenged with LPS. PLoS One 2014, 9, e97815 10.1371/journal.pone.0097815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzl C.; Knasmüller S.; Wagner K.-H.; Elbling L.; Huber W.; Kager N.; et al. Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol. Nutr. Food Res. 2010, 54, 1722–1733. 10.1002/mnfr.201000048. [DOI] [PubMed] [Google Scholar]
- Thom E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. 10.1177/147323000703500620. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Huang H.; Yang T.; Ye Y.; Shan J.; Yin Z. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 2010, 41, 746–752. 10.1016/j.injury.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Zhu L. H.; Xu J. X.; Zhu S. W.; Cai X.; Yang S. F.; Chen X. L.; Guo Q. Gene expression profiling analysis reveals weaning-induced cell cycle arrest and apoptosis in the small intestine of pigs. J. Anim. Sci. 2014, 92, 996–1006. 10.2527/jas.2013-7551. [DOI] [PubMed] [Google Scholar]
- Owusu-Asiedu A.; Nyachoti C. M.; Marquardt R. R. Response of early-weaned pigs to an enterotoxigenic (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody, zinc oxide, fumaric acid, or antibiotic. J. Anim. Sci. 2003, 81, 1790–1798. 10.2527/2003.8171790x. [DOI] [PubMed] [Google Scholar]
- Palócz O.; Pászti-Gere E.; Gálfi P.; Farkas O. Chlorogenic Acid Combined with Lactobacillus plantarum 2142 Reduced LPS-Induced Intestinal Inflammation and Oxidative Stress in IPEC-J2 Cells. PLoS One 2016, 11, e0166642 10.1371/journal.pone.0166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying L.; Wang Z. S.; Zhou A. G. Effects of hesperidin and chlorogenic acid on performance, antioxidance and immunity of weaned piglets. Chin. J. Vet. Sci. 2009, 29, 1233–1236. [Google Scholar]
- Praveena P. E.; Periasamy S.; Kumar A. A.; Singh N. Cytokine profiles, apoptosis and pathology of experimental Pasteurella multocida serotype A1 infection in mice. Res. Vet. Sci. 2010, 89, 332–339. 10.1016/j.rvsc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Jesmok G.; Lindsey C.; Duerr M.; Fournel M.; Emerson T. Jr. Efficacy of monoclonal antibody against human recombinant tumor necrosis factor in E. coli-challenged swine. Am. J. Pathol. 1992, 141, 1197–1207. [PMC free article] [PubMed] [Google Scholar]
- Maeda K.; Schoeniger L. O.; Shimada M.; Winchurch R. A.; Buchman T. G.; Robotham J. L. Regulation of acute phase gene expression following surgery and endotoxin administration in the anesthetized pig. Anesthesiology 1993, 79, 1324–1337. 10.1097/00000542-199312000-00024. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R.; Boivin M.; Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765–2778. 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pié S.; Lallès J. P.; Blazy F.; Laffitte J.; Sève B.; Oswald I. P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Ruifeng G.; Yunhe F.; Zhengkai W.; Ershun Z.; Yimeng L.; Minjun Y.; Xiaojing S. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur. J. Pharmacol. 2014, 729, 54–58. 10.1016/j.ejphar.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zheng P.; Yu B.; He J.; Yu J.; Mao X. Dietary spray-dried chicken plasma improves intestinal barrier function and modulates immune status in weaning piglets. J. Anim. Sci. 2016, 94, 173–184. 10.2527/jas.2015-9530. [DOI] [PubMed] [Google Scholar]
- Ushida K.; Kameue C.; Tsukahara T.; Fukuta K.; Nakanishi N. Decreasing traits of fecal immunoglobulin A in neonatal and weaning piglets. J. Vet. Med. Sci. 2008, 70, 849–852. 10.1292/jvms.70.849. [DOI] [PubMed] [Google Scholar]
- Rodrigues A. C. P.; Cara D. C.; Fretez S. H. G. G.; Cunha F. Q.; Vieira E. C.; Nicoli J. R.; Vieira L. Q. Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. J. Appl. Microbiol. 2000, 89, 404–414. 10.1046/j.1365-2672.2000.01128.x. [DOI] [PubMed] [Google Scholar]
- McCracken B. A.; Spurlock M. E.; Roos M. A.; Zuckermann F. A.; Gaskins H. R. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J. Nutr. 1999, 129, 613–619. 10.1093/jn/129.3.613. [DOI] [PubMed] [Google Scholar]
- Cera K. R.; Mahan D. C.; Cross R. F.; Reinhart G. A.; Whitmoyer R. E. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J. Anim. Sci. 1988, 66, 574–584. 10.2527/jas1988.662574x. [DOI] [PubMed] [Google Scholar]
- Montagne L.; Boudry G.; Favier C.; Le Huërou-Luron I. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007, 97, 45–57. 10.1017/s000711450720580x. [DOI] [PubMed] [Google Scholar]
- Montagne L.; Pluske J. R.; Hampson D. J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. 10.1016/s0377-8401(03)00163-9. [DOI] [Google Scholar]
- Chen J. L.; Zheng P.; Zhang C.; Yu B.; He J.; Yu J. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Physiol. Anim. Nutr. 2016, 101, 1137. 10.1111/jpn.12627. [DOI] [PubMed] [Google Scholar]
- Guchelaar H. J.; Vermes A.; Vermes I.; Haanen C. Etoposide phosphate, the water soluble prodrug of etoposide. Pharm. World Sci. 1997, 19, 119–125. 10.1023/a:1008654316572. [DOI] [PubMed] [Google Scholar]
- Ray R. M.; Johnson L. R. Regulation of intestinal mucosal growth by amino acids. Amino Acids 2014, 46, 565–573. 10.1007/s00726-013-1565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo R. A.; Poon R. Y. C. Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle 2003, 2, 315–323. 10.4161/cc.2.4.468. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001, 15, 2922–2933. [PubMed] [Google Scholar]
- Budihardjo I.; Oliver H.; Lutter M.; Luo X.; Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.; Wang L.; Zhang W.; Yang Z.; Ding B.; Zhu H. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 2012, 43, 1233–1242. 10.1007/s00726-011-1191-9. [DOI] [PubMed] [Google Scholar]
- Reed J. C.; Miyashita T.; Takayama S.; Wang H.-G.; Sato T.; Krajewski S. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J. Cell. Biochem. 1996, 60, 23–32. . [DOI] [PubMed] [Google Scholar]
- Yuan Z.; Liu S.; Yao J.; Zeng Q.; Tan S.; Liu Z. Expression of Bcl-2 genes in channel catfish after bacterial infection and hypoxia stress. Dev. Comp. Immunol. 2016, 65, 79–90. 10.1016/j.dci.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Yamagata K.; Izawa Y.; Onodera D.; Tagami M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell. Biochem. 2017, 1–11. 10.1007/s11010-017-3171-1. [DOI] [PubMed] [Google Scholar]
- Raisova M.; Hossini A. M.; Eberle J.; Riebeling C.; Orfanos C. E.; Geilen G. C.; Wieder T.; Sturm I. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Invest. Dermatol. 2001, 117, 333–340. 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- Sedlak T. W.; Oltvai Z. N.; Yang E.; Wang K.; Boise L. H.; Thompson C. B. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc. Natl. Acad. Sci. U.S.A. 1995, 92, 7834–7838. 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, 2012. [Google Scholar]
- Zhang X.; Dong S.; Qin Y.; Bian X. Protective effect of erythropoietin against myocardial injury in rats with sepsis and its underlying mechanisms. Mol. Med. Rep. 2015, 11, 3317–3329. 10.3892/mmr.2015.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 2001, 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]