Abstract
To improve intraocular transparency of collagen matrices, hydroxypropyl methylcellulose (HPMC) was introduced for the first time into cross-linked collagen to form collagen–HPMC composite membranes. Light transmittance and refractive indices of the membranes are enhanced by incorporation of HPMC in comparison to the control of cross-linked collagen membranes. Maximum light transmittance of the collagen–HPMC membrane was up to 92%. In addition, their permeability of nutrients such as glucose, tryptophan, and NaCl was superior or comparable to that of human corneas. In vitro results demonstrated that the collagen–HPMC membrane supported adhesion and proliferation of human corneal epithelial cells (HCECs), showing good cytocompatibility to HCECs. The corneas maintained a smooth surface and clear stroma postoperatively after 7 months of implantation of collagen–HPMC membranes into the corneas of rabbits. The good intraocular biocompatibility was verified by maintaining a high optical clarity for over 6 months after transplantation. Hematoxylin and eosin staining results showed the growth of stromal keratocytes into the collagen–HPMC implants, indicating the ability of the collagen–HPMC membrane to induce corneal cell regeneration. Taken together, the collagen–HPMC membrane might be a promising candidate for use in corneal repair and regeneration.
1. Introduction
Corneal damage or disease is the second leading cause of blindness worldwide. According to WHO, there is an estimate of 36 million blind people globally, and 217 million people have suffered from impaired vision.1 More than 5% of these patients are left blind because of corneal diseases.2 Transplantation with cornea donors is the only available treatment currently. Because of the lack of human donor corneas, however, there are only 40 000 patients receiving corneal transplantation each year in the United States.3 The situation would be worse in the developing countries. The increasing demand of high-quality corneal tissues as well as the shortage of donor corneas has raised a concern to develop corneal substitutes. In the field of corneal substitute development, many strides have been made by developing keratoprosthesis. A variety of keratoprostheses are now in clinical use or trials.4−8 However, because of the existing problems of keratoprosthesis, such as retroprosthetic membrane formation, corneal melting, optic deposition, extrusion, and retinal detachment, none of them is widely accepted.9 On the other hand, Griffith et al.9 suggested that an ideal corneal substitute, from a regenerative medicine field, should be biodegradable and should promote postoperative endogenous host tissue reconstruction. Complications of keratoprosthesis and donor cornea rejection are expected to be conquered by this regenerative cornea. Therefore, a biodegradable and biocompatible material, which could induce corneal tissue regeneration, for example, corneal cells and nerves, is more desirable for use in corneal reconstruction.
Collagen is the predominant extracellular matrix (ECM) component of the cornea.10 ECM provides templates on the organogenesis process and also serves as a reconstruction template during wound healing.11 Therefore, collagen is one of the excellent candidates for corneal repair and regeneration. Collagen possesses various desirable features as the ECM component of the cornea. It exhibits biodegradability, low antigenicity, and excellent biocompatibility because of weak toxicity and low immunoreaction.12−14 It also has the ability to induce normal tissue regeneration.10 Because of these excellent characteristics, collagen is widely used in ophthalmology as suture material, bandage lenses, punctual plugs, or viscous solutions during surgery.15 One of the most important ophthalmic applications of collagen is the grafts for corneal reconstruction.
Many strides have been made in the use of collagen-based matrices for corneal regeneration in recent years, and encouraging results were achieved. Previous studies conducted by Griffith et al. have shown that cross-linked collagen patches produced from porcine or bovine collagen promoted corneal cell and nerve regeneration in the rabbit and pig models.16−18 These patches also demonstrated good biocompatibility.16−18 Another collagen 2-methacryloyloxyethyl phosphorylcholine hydrogel produced by Liu et al.19 could induce corneal cell and nerve in-growth in vitro. This collagen-based hydrogel also promoted the regeneration of the corneal epithelium and stroma in the corneas of mini-pigs. What is more, allowing nerve regeneration in vivo with the aid of these complexes is an important step toward the way of successful corneal reconstruction. However, all these matrices had opacity problems when implanted in corneas. That is, these collagen or collagen-based matrices remained opaque or hazy in corneas at least for several weeks after transplantation and did not restore full transparency until 3–6 months after implantation.16,17,19,20 Apart from tissue reconstruction, visual function is our goal to fulfill while implanting corneal patches or substitutes into the corneas of patients. A patient’s visual function can be restored right after transplantation, instead of waiting for 6–12 months, which is highly desired by patients. Therefore, we aim to develop corneal scaffolds that can maintain high transparency in vivo during the process of promoting the regeneration of corneal tissues.
Hydroxypropyl methylcellulose (HPMC) is a semisynthetic, biodegradable cellulose derivative, which is widely used in a broad range of applications, for example, as thickeners, water binders, film-forming agents, surfactants,21 and in the fields of pharmaceuticals22−25 and foods.26,27 HPMC has shown good biocompatibility and nontoxicity to the human body. Trojani et al.28 reported the silated HPMC-based hydrogel used for three-dimensional culture of osteogenic cells. It was able to support osteoblastic survival, proliferation, and differentiation, representing a potential tissue engineering basis for bone repair. Moreover, HPMC has already had its applications in ophthalmology. As a bioadhesive polymer, for example, HPMC has been used as ophthalmic viscosurgical devices during cataract surgery.28,29 The abovementioned studies have demonstrated the intraocular biocompatibility of HPMC and its potential for application in ocular surface reconstruction. In addition, HPMC has excellent film-forming capacity,21,30 making it easy to be incorporated with other polymers such as collagen, hyaluronic acid, and chitosan to form composite membranes.
To improve the in vivo transparency of collagen membranes, HPMC will be an excellent additive for cross-linked collagen to produce collagen–HPMC composite membranes. The addition of HPMC in the collagen membrane is expected to regulate collagen fibril spacing and therefore improve the transparency of collagen. In the present work, we used type I collagen to produce a collagen network by cross-linking with 1-ethyl-3-(3-dimethyl aminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS).31 HPMC was then added to form the collagen–HPMC composite membrane. The optical characteristics as well as nutrient diffusion of composite membranes were measured. The in vitro biocompatibility of composite membranes to corneal epithelial cells was also studied. We reported the postoperative results of composite membrane implantation into the corneas of rabbits so as to evaluate the feasibility of these composite membranes as grafts or patches for corneal regeneration. To the best of our knowledge, this work is the first report to introduce HPMC as an additive into collagen membranes to improve its in vivo transparency.
2. Results and Discussion
2.1. Fabrication and Optical Properties of Collagen-Based Membranes
Unlike other tissue transplantations, corneal transplantation has a special requirement: corneal patches or substitutes should be able to maintain transparency and suitable refractive indices when implanted in vivo. Although type I collagen is widely studied as a corneal substitute, collagen fibrils tend to aggregate, forming opaque or semitransparent membranes at neutral pH.20 The low transparency of this material in vivo is a significant drawback for clinical application in corneal regeneration.16,17,19,20
To produce transparent membranes suitable for application as corneal patches, it is essential to improve the optical properties of the collagen membrane. In this work, this goal was achieved through the addition of HPMC to collagen. Collagen–HPMC composite membranes and cross-linked collagen membranes were successfully manufactured by chemical cross-linking with EDC–NHS.31 The optical properties of both membranes were measured.
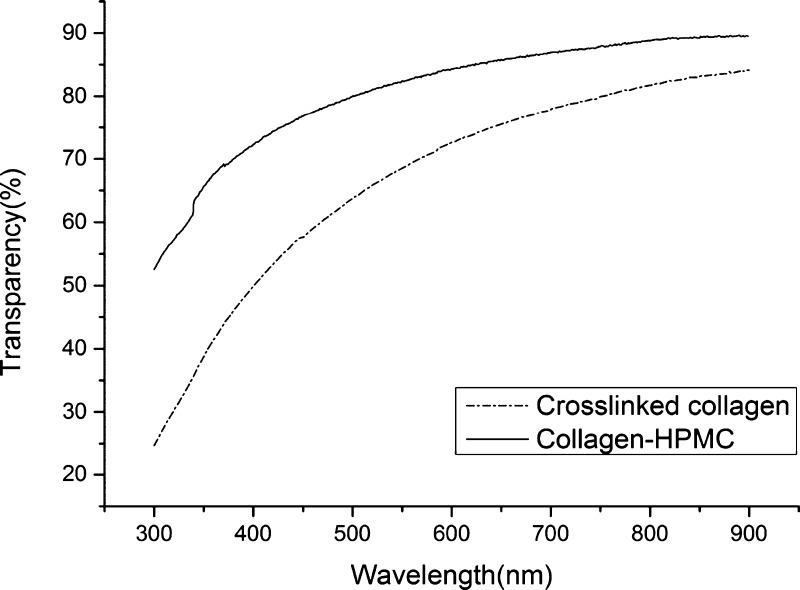
Figure 1 shows the light transmittance curve of the collagen–HPMC composite membrane and the cross-linked collagen membrane. The light transmittance of both the membranes increased with the increase of wavelength. However, the transparency of the collagen–HPMC membrane improved strikingly when compared with that of the cross-linked collagen membrane. In the case of the human cornea, the light transmittance was 80% at the wavelength of 430 nm and increased with the increase of wavelength.32 The transparency of the cross-linked collagen membrane was only 56% at 430 nm but that of the collagen–HPMC membrane reached 78%, which is similar to that of the human cornea. With increasing wavelength, the light transmittance of the collagen–HPMC membrane increased and reached a maximum of 92%, which is higher than that of the cross-linked collagen membrane. The light transmittance of the collagen–HPMC membrane in the wavelength range of 300–900 nm is comparable to that of the human cornea.33 The refractive index of the collagen membrane also increased to approximately 1.34 after incorporating HPMC (Table 1), which is close to that of the human cornea, 1.37–1.38.34 We also noted that the refractive index of the cross-linked collagen membrane in our study was identical with the results in the previous study conducted by Griffith et al.35
Figure 1.
Transparency of the cross-linked collagen and collagen–HPMC membranes in the wavelength range of 300–900 nm.
Table 1. Physical Properties of Cross-linked Collagen and Collagen–HPMC Membranesa.
| permeability |
|||||
|---|---|---|---|---|---|
| materials | water content (%) | glucose diffusivity (cm2/s) | tryptophan diffusivity (cm2/s) | NaCl diffusivity (cm2/s) | refractive index |
| collagen–HPMC | 83.4 | 5.29 ± 0.3 × 10–6 | 1.86 ± 0.3 × 10–6 | 8.03 ± 0.4 × 10–6 | 1.3375 |
| cross-linked collagen | 76.5 | 3.88 ± 0.3 × 10–6 | 1.05 ± 0.3 × 10–5 | 1.22 ± 0.8 × 10–5 | 1.3350 |
| human cornea | 81b | 2.4 × 10–6c | >10–6d | >10–6e | 1.373–1.380f |
It could be seen from the abovementioned results that because of the addition of HPMC, light transmittance and refractive index of the composite membrane were enhanced and similar to those of the human cornea. The improvement of transparency may be due to HPMC functioning as a regulator, which regulated the collagen fibril spacing in the composite membrane. From a previous study,36 Fourier transform infrared spectra results showed that there were intermolecular hydrogen bond interactions between collagen and HPMC in collagen–HPMC blend membranes. In addition, scanning electron microscopy and atomic force microscopy images showed a more homogeneous and compact structure of the collagen–HPMC blend membrane than the collagen membrane, indicating good compatibility and miscibility of collagen and HPMC.36,37 The intermolecular hydrogen bond interactions may regulate the collagen fibril formation and arrangement, resulting in the improvement of membrane transparency.
2.2. Equilibrium Water Content
The equilibrium water content of membranes is an important factor for their application to corneal reconstruction. Appropriate water content is essential for the human cornea to deliver nutrients to the corneal cells. The water content of the human cornea is 81%.38 In our study, the equilibrium water contents of the cross-linked collagen and collagen–HPMC membranes are 76.5 and 83.4%, respectively (Table 1). The water-uptake capability of the collagen–HPMC membrane is similar to that of the human cornea and is able to meet the requirement for use of corneal substitutes.
2.3. Glucose, Tryptophan, and NaCl Permeability
Nutrients are essential for the cornea to remain transparent and hydrated and to perform routine metabolism. As an avascular tissue, the cornea intakes nutrients through the tear film, vitreous humor, and stromal matrix by diffusion.39 Besides glucose, other nutrient molecules such as tryptophan and NaCl are also found in the cornea. Determining the permeability of glucose, tryptophan, and NaCl through the corneal matrix could reflect the permeability of the cornea to nutrients. Therefore, the permeability of nutrients such as glucose, tryptophan, and NaCl was evaluated because the membrane permeability is critical for use as corneal regeneration.
The permeability of the cross-linked collagen and collagen–HPMC membranes to glucose, tryptophan, and NaCl is summarized in Table 1. The glucose diffusion coefficients for the collagen–HPMC and cross-linked collagen membranes are 5.29 ± 0.3 × 10–6 and 3.88 ± 0.3 × 10–6 cm2/s, respectively, which are superior to those of the human corneal stroma, which is 2.4 × 10–6 cm2/s.40 The tryptophan diffusion coefficients for the collagen–HPMC and cross-linked collagen membranes are within the range of those for human corneal stroma,41 which are 1.86 ± 0.3 × 10–6 and 1.05 ± 0.3 × 10–5 cm2/s, respectively. The NaCl diffusion coefficients for the collagen–HPMC and cross-linked collagen membranes were 8.03 ± 0.4 × 10–6 and 1.22 ± 0.8 × 10–5 cm2/s, respectively, both of which meet the requirement of the human cornea for NaCl permeability.42 Meanwhile, we noted that the addition of HPMC into the collagen improved the permeability of membranes to glucose, whereas it slowed down the permeability of tryptophan and NaCl. The effect of HPMC on the permeability of composite membranes may be related to the interaction between nutrient molecules and HPMC. Both membranes have good permeability to nutrients and are suitable for corneal regeneration.
2.4. In Vitro Biocompatibility and Performance
Figure 2 shows the morphology of human corneal epithelial cells (HCECs) cultured on cross-linked collagen and collagen–HPMC membranes at day 3. Both of them supported the attachment and proliferation of HCECs. The attachment and proliferation of HCECs on collagen–HPMC membranes showed no significant variation (p < 0.05) from those on cross-linked membranes. As could be seen from Figure 2A, HCECs appeared healthy, and no cytotoxic effects were observed when seeded on collagen–HPMC membranes. Moreover, HCECs reached confluence on collagen–HPMC membranes over 4 days (Figure 2C), indicating that the collagen–HPMC membrane is favorable for HCEC overgrowth.
Figure 2.
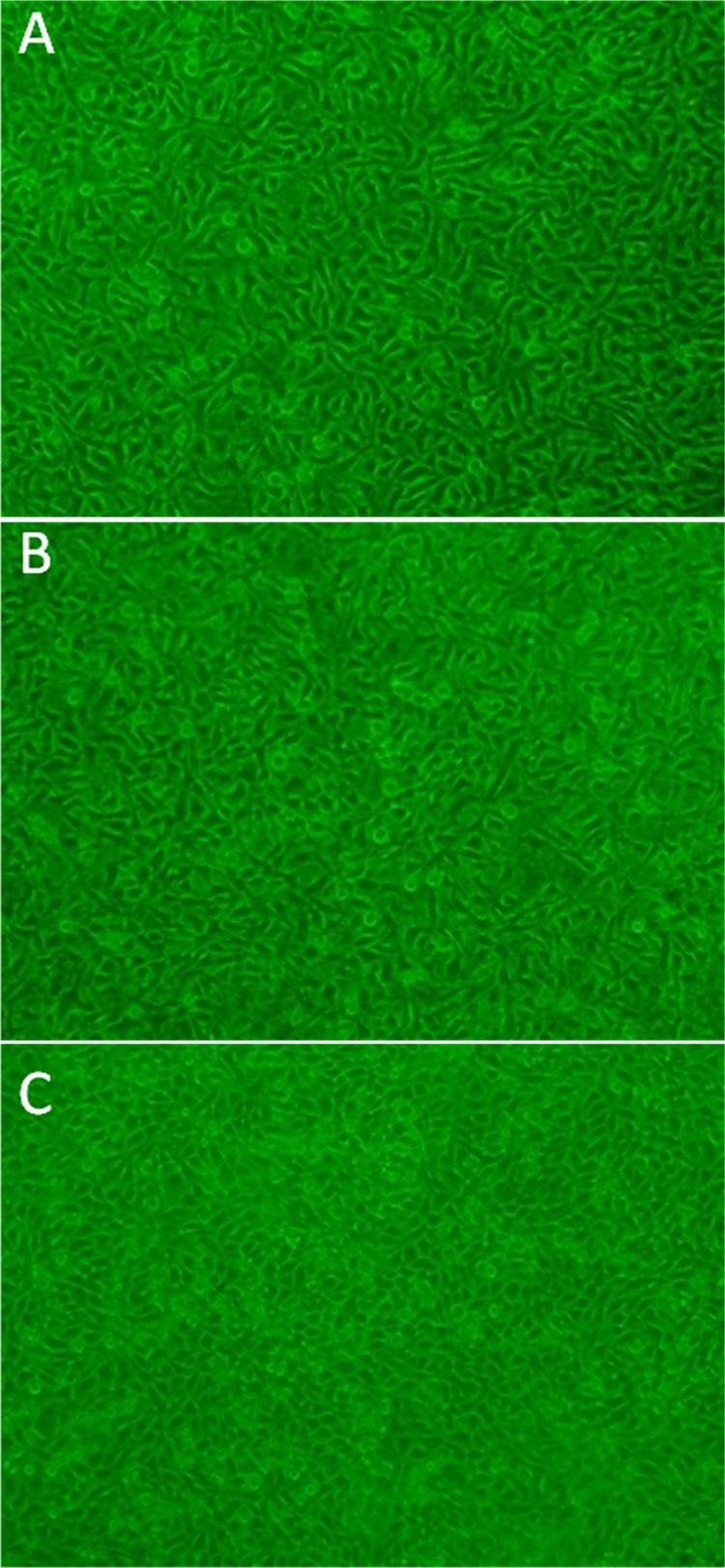
Morphological comparison of HCECs cultured on membrane surfaces. (A) Collagen–HPMC membrane and (B) cross-linked collagen membrane; (C) in vitro HCEC attachment and growth to confluence over 4 days on the collagen–HPMC membrane.
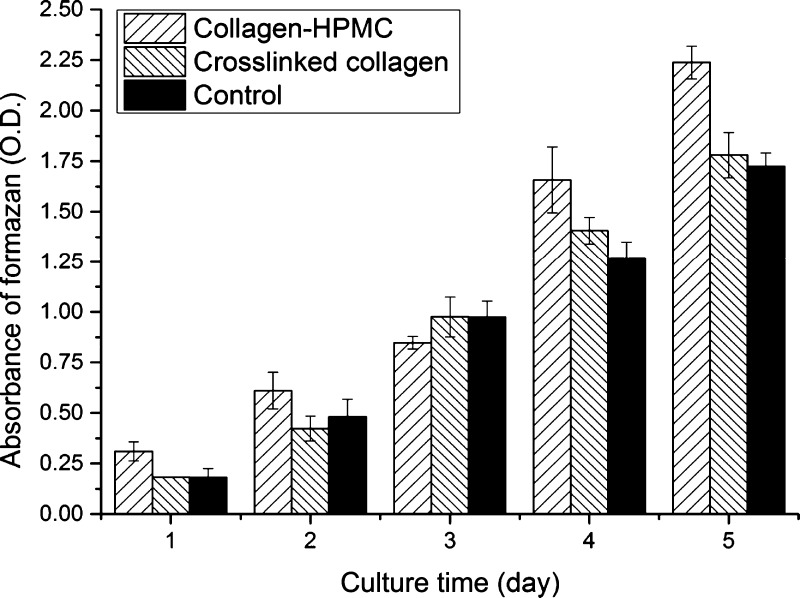
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to examine the viability and proliferation of HCECs on the cross-linked collagen and collagen–HPMC membranes. Figure 3 shows the viability of HCECs cultured on two different membranes at 1–5 consecutive days. As seen from Figure 3, cells on the collagen–HPMC membrane showed great increase in cell viability on days 4 and 5, and its cell viability for 1, 2, 4, and 5 days was significantly higher (p < 0.05) than those on the cross-linked collagen and cyclic citrullinated peptide (CCP). Cell viability on the cross-linked collagen was similar with that on CCP, showing no significant variation. According to MTT results, the reproductive activity of HCECs on the collagen–HPMC membranes was better than that on the cross-linked membranes, indicating that the incorporation of HPMC into collagen could have some positive effects on the membrane cytocompatibility, and these collagen–HPMC membranes may have potential application as corneal patches for corneal regeneration.
Figure 3.
Viability and proliferation of HCECs cultured on collagen–HPMC and cross-linked collagen membranes by MTT assay (MTT measured in units of optical density at 490 nm).
2.5. Implantation and Postoperative Evaluation
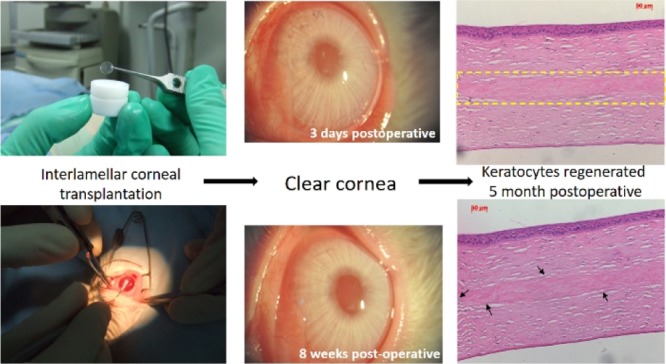
To demonstrate the intraocular compatibility of our composite membranes as grafts for corneal regeneration, interlamellar corneal transplantation has been performed to assess the degree of composite membranes to promote corneal regeneration in rabbits. The corneas as well as implants remained clear right after transplantation (Figure 4A) and maintained optical clarity for over 6 months postoperatively. Meanwhile, no adverse inflammatory or immune reactions were observed in the operative rabbit corneas after implantation (Figure 4). The corneal surfaces of the rabbits were smooth, and stroma and implants remained clear throughout the time. No inflammation and hyperemia were observed 1 month after implantation. Only a mild rejection reaction was observed 2 weeks after transplantation, mainly because of the suture. Neovascularization occurred at 1 month postoperatively but disappeared 1 week after. A slit-lamp examination at 6 months postoperatively indicates that the operative rabbit corneas remained clear and smooth (data not shown).
Figure 4.
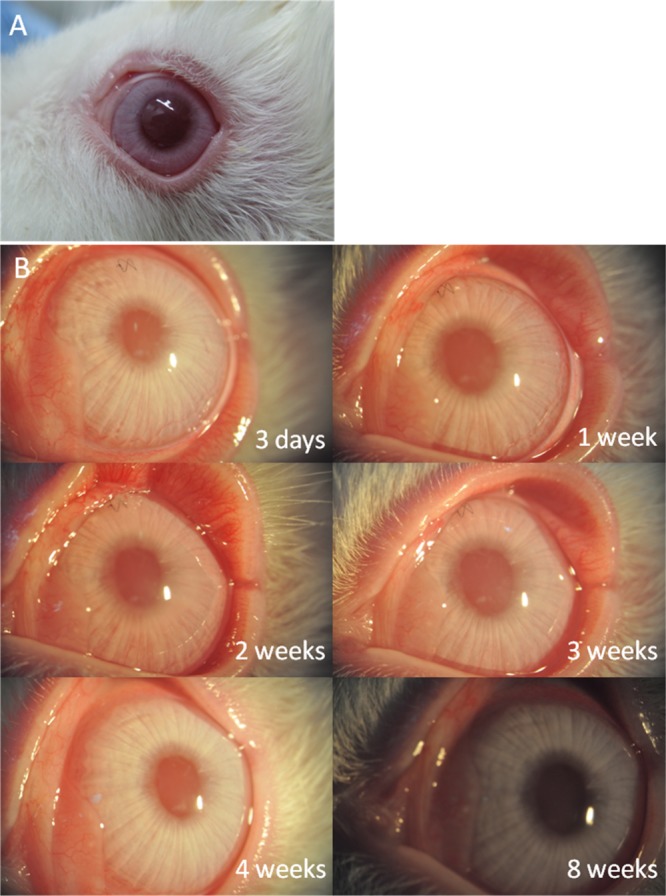
(A) Right after transplantation, the cornea and collagen–HPMC implants were clear, no adverse inflammatory or immune reactions were observed, (B) postoperative observation of interlamellar corneal transplantation at different time intervals.
Figure 5 shows the hematoxylin and eosin (H&E) sections of collagen–HPMC implanted corneas. As could be seen from the representative images, there was good biocompatibility and connection between the implant and stroma (Figure 5A,B). No inflammatory cells, such as macrophages, monocytes, or new vessels, were observed. The implant was almost degraded at 7 months postoperatively without causing adverse inflammatory or immune reactions (Figure 5C,D). More interestingly, some keratocytes were observed to grow into the superficial lamellae of the implant during 5–7 months postoperatively (Figure 5,D), indicating that the new corneal tissue regeneration may be developed as degradation takes place. According to cornea implantation results, these collagen–HPMC membranes have excellent intraocular biocompatibility while implanted into rabbit corneas and may have the ability to promote a generation of new stromal keratocytes.
Figure 5.
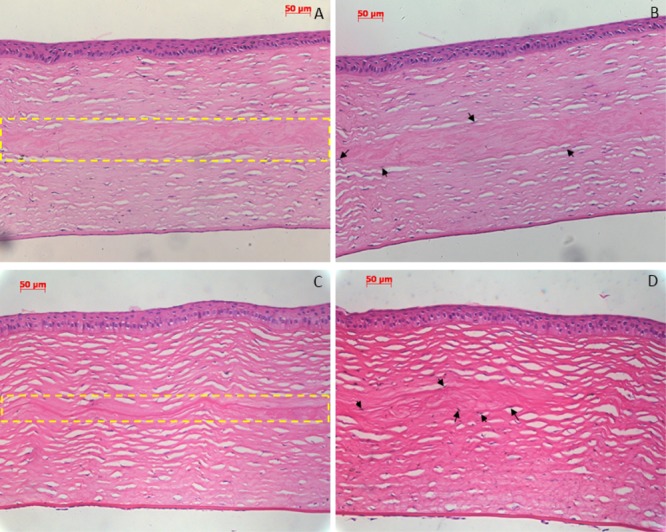
H&E staining sections of the rabbit cornea after implantation. (A,B) Postoperative 5 months: there was good biocompatibility and connection between the implant and stroma [(A) inside the yellow dashed frame]; no inflammatory cells and new vessels were observed; and some keratocytes began to grow into the superficial lamellae of implant (B). (C,D) Postoperative 7 months: implant was almost degraded without causing adverse inflammatory or immune reactions [(C) inside the yellow dashed frame], only a little residuary implant was observed, and some keratocytes began to grow into the superficial lamellae of the implant (D). Arrow indicates new keratocytes grown into the superficial lamellae of the implant.
3. Conclusions
We introduced HPMC into cross-linked collagen for the first time to form a novel corneal substitute with excellent transparency. The optical properties of the composite membranes were equivalent to those of the human cornea, and the composite membranes implanted in rabbits maintained a high optical clarity for over 6 months after transplantation. Collagen–HPMC membranes also reproduced key features of the human cornea, such as appropriate water content and sufficient permeability to nutrients. Collagen–HPMC membranes were capable of supporting the adhesion and proliferation of HCECs, showing good cytocompatibility to human corneal cells in vitro. These membranes displayed good compatibility with corneal tissues after implanting in rabbits in the intrastromal surgical model and were able to induce repopulation of the stromal keratocytes, indicating the potential ability to promote corneal tissue regeneration. The abovementioned results demonstrated that the collagen–HPMC composite membrane is a promising material for corneal regeneration. The collagen–HPMC composite membrane may have future clinical applications as patches in corneal repair and reconstruction. A more comprehensive surgical model is needed to further evaluate the feasibility of the composite membranes as grafts for corneal regeneration, and postoperative observation is currently ongoing.
4. Experimental Section
4.1. Materials
Type I bovine collagen was produced by our laboratory. EDC and NHS were purchased from Shanghai GL Biochem, China. HPMC (METHOCEL E series) was supplied by Dow Wolff Cellulosics, Shanghai, China. Acetic acid and sodium hydroxide were obtained from Tianjin Chemical Reagent No. 1 Plant, China. Phosphate-buffered saline (PBS, pH = 7.6) was prepared by our laboratory. Deionized water (YN-ZD-Z, Boxun, Shanghai) was used throughout.
4.2. Preparation of the Collagen–HPMC Composite Membrane
Type I collagen (900 mg) was dissolved in a 3% (v/v) acetic acid solution to obtain a concentration of 6 g L–1. HPMC (18 mg) was dissolved in 10 mL of deionized water and was then added dropwise to the abovementioned solution at 4 °C. After stirring with an electromagnetic stirrer for 1 h, calculated volumes of EDC and NHS solutions were then added to cross-link the collagen. This mixture required continuous stirring for 12 h or longer at 4 °C. Then, the pH of the abovementioned mixed solution was adjusted to 5 ± 0.5 by injecting microliter quantities of 1 M aqueous NaOH,9 followed by thorough mixing at 4 °C. The final homogeneous solution was immediately dispensed into polypropylene molds (35 mm diameter) and dried to form collagen–HPMC composite membranes at room temperature. All samples were removed from the molds after soaking in deionized water for half an hour. The resulting membranes were rinsed with deionized water repeatedly and dried (the thickness of films was about 50 μm). For comparison purposes, cross-linked collagen membranes were prepared without the addition of HPMC in the same manner.
4.3. Optical Properties
Light transmittance of the collagen–HPMC composite membranes and cross-linked collagen membranes in the wavelength of 350–900 nm was measured by a UV3802 ultraviolet–visible spectrophotometer (Shanghai UNICO) at room temperature. Before the test, all samples with dimensions of 1 × 3 cm2 were equilibrated in PBS and then stuck on a quartz colorimetric vessel. Refractive indices of collagen–HPMC composite membranes and cross-linked collagen membranes equilibrated in PBS were recorded on an Abbe refractometer (bromonaphthalene as the calibration agent, 2 W, Shanghai Optical Instrument Factory, Shanghai) at room temperature.
4.4. Equilibrium Water Content
The collagen–HPMC composite and cross-linked collagen membranes were immersed in PBS overnight at 4 °C. All samples were removed from PBS, the surface water was gently blotted off, and then, the wet weights of the samples were immediately recorded. The samples of known weight were then vacuum-dried to constant weight. Water content (Wt) was calculated according to the following equation:
where W0 and W denote the weights of dry and PBS-equilibrated samples, respectively.
4.5. Glucose, Tryptophan, and NaCl Permeability
Diffusion permeability studies were carried out at room temperature.43 The experimental procedure was the same as stated in a previous study.44 Briefly, the collagen–HPMC composite membrane or cross-linked collagen membrane was placed between two glass chambers, one assigned as a diffusion chamber with a buffer of 2% glucose, 0.05% tryptophan, and 0.9% NaCl and the other assigned as a receptor chamber with distilled water. The membrane was fixed between the two chambers without leakage. Solutions in both the chambers were stirred continuously while taking samples from the receptor chamber at 1 h intervals. Well-established methods were used to measure the concentrations of glucose, tryptophan, and NaCl,44 followed by a calculation of their permeability values using a previous method.45
4.6. Cell Culture
The HCEC line from the State Key Lab of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, China, was used for the in vitro cytocompatibility study. HCECs were cultured in a CO2 incubator with 5% CO2/95% air at 37 °C. A supplemented Dulbecco’s modified Eagle’s medium was used to grow the cells, as stated in a previous study.32
4.7. Morphological Observation of HCECs
The collagen–HPMC composite membranes and cross-linked collagen membranes were washed three times in PBS under aseptic conditions, sterilized by ultraviolet radiation for 2 h, and washed three times in PBS again. The sterile cross-linked collagen and collagen–HPMC composite membranes were attached on the bottom of the cell culture plate, and 2 mL of HCECs were consequently seeded on the surface of each membrane at a density of 5 × 104 cells/mL. After cell culture on the surface of these membranes for 3 days, the cell morphology was observed under an inverted phase contrast microscope.
4.8. Cell Viability and Proliferation Assay (MTT Assay)
MTT assay was performed to evaluate the cell viability and proliferation on the cross-linked collagen and collagen–HPMC composite membranes over a 5 day cell culture. The experimental procedure was the same as stated in a previous study.32
4.9. In Vivo Biocompatibility and Performance
4.9.1. Implantation
Collagen–HPMC composite membranes, 100 μm thick and 6 mm in diameter, were implanted into the corneas of New Zealand white rabbits. Briefly, all implants were sterilized with ethylene oxide. Before implantation, the implants were equilibrated in PBS and rinsed in a solution of normal saline. Under general anesthesia, the rabbit corneas were excised to the middle of the stromal layer in the central area of the cornea, and the grafts of the collagen–HPMC composite membranes were buried into the recipient corneal beds by using two interrupted 10–0 nylon sutures for closing the incision. Animals were not given steroids postoperatively but only antibiotics (tobramycin) over the first postoperative week. Sutures were removed at 3 weeks postoperatively.
4.10. Clinical and Histopathological Evaluation
Follow-ups were conducted daily on each animal for 7 days postoperatively and then weekly. Clinical examination mainly focused on slit-lamp biomicroscopy (Topcon corporation, Japan) to evaluate the corneas for optical clarity. Inflammation such as excessive redness or swelling was evaluated if any, and neovascularization and degradation of grafts were evaluated using a slit-lamp biomicroscopy instrument. At postoperative 5 and 7 months, three rabbits were euthanized each time, and the corneal specimens were stained with H&E for routine histopathological examination.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (51232002 and 51603073), Guang Zhou Important Scientific and Technological Special Project (201508020123), and Guangdong Scientific and Technological Project (2014B090907004).
The authors declare no competing financial interest.
References
- WHO . Vision Impairment and Blindness. http://www.who.int/mediacentre/factsheets/fs282/en/ (updated October, 2017).
- WHO . Global Data on Visual Impairments 2010. http://www.who.int/blindness/GLOBALDATAFINALforweb.pdf?ua=1 (2012).
- Niederkorn J. Y. Corneal Transplantation and Immune Privilege. Int. Rev. Immunol. 2013, 32, 57–67. 10.3109/08830185.2012.737877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.; Khoueir Z.; Tsikata E.; Chodosh J.; Dohlman C. H.; Chen T. C. Long-term Visual Outcomes and Complications of Boston Keratoprosthesis Type II Implantation. Ophthalmology 2017, 124, 27–35. 10.1016/j.ophtha.2016.07.011. [DOI] [PubMed] [Google Scholar]
- de Oliveira L. A.; Magalhães F. P.; Hirai F. E.; de Sousa L. B. Experience with Boston Keratoprosthesis Type 1 in the Developing World. Can. J. Ophthalmol. 2014, 49, 351–357. 10.1016/j.jcjo.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Ferreiro A. V. S.; Bellido L. M. Keratoprosthesis in Cornea and Ocular Surface Diseases. Arch. Soc. Esp. Oftalmol. 2013, 88, 327–328. 10.1016/j.oftale.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Griffith M.; Polisetti N.; Kuffova L.; Gallar J.; Forrester J.; Vemuganti G. K.; Fuchsluger T. A. Regenerative Approaches as Alternatives to Donor Allografting for Restoration of Corneal Function. Ocul. Surf. 2012, 10, 170–183. 10.1016/j.jtos.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Laattala K.; Huhtinen R.; Puska M.; Arstila H.; Hupa L.; Kellomäki M.; Vallittu P. K. Bioactive Composite for Keratoprosthesis Skirt. J. Mech. Behav. Biomed. Mater. 2011, 4, 1700–1708. 10.1016/j.jmbbm.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Deng C.; Li F.; Hackett J. M.; Chaudhry S. H.; Toll F. N.; Toye B.; Hodge W.; Griffith M. Collagen and Glycopolymer Based Hydrogel for Potential Corneal Application. Acta Biomater. 2010, 6, 187–194. 10.1016/j.actbio.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Huang Y.-X.; Li Q.-H. An Active Artificial Cornea with the Function of Inducing New Corneal Tissue Generation in vivo-A New Approach to Corneal Tissue Engineering. Biomed. Mater. 2007, 2, S121–S125. 10.1088/1748-6041/2/3/S07. [DOI] [PubMed] [Google Scholar]
- Yannas I. V. Synthesis of Organs: in vitro or in vivo?. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 9354–9356. 10.1073/pnas.180313497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H.; Singla A.; Lee Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. 10.1016/S0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- Timpl R.Immunology of the Collagens. In Extracellular Matrix Biochemistry; Piez K. A., Reddi A. H., Eds.; Elsevier: New York, 1984; pp 159–190. [Google Scholar]
- Linsenmeyer T. F.Immunology of Purified Collagens and Their Use in Localization of Collagen Types in Tissue. In Collagen in Health and Disease; Weiss J. B., Jayson M. I. V., Eds.; Churchill Livingstone: Edinburgh, 1982; pp 244–268. [Google Scholar]
- DeVore D. P.Collagen as an Ophthalmic Biomaterial. In Encyclopedic Handbook of Biomaterials and Bioengineering; Wise D. L., Trantolo D. J., Altobelli D. E., Yaszemski M. J., Gresser J. D., Schwartz E. R., Eds.; Marcel Dekker: New York, 1995; pp 1233–1260. [Google Scholar]
- Li F.; Carlsson D.; Lohmann C.; Suuronen E.; Vascotto S.; Kobuch K.; Sheardown H.; Munger R.; Nakamura M.; Griffith M. Cellular and Nerve Regeneration within a Biosynthetic Extracellular Matrix for Corneal Transplantation. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 15346–15351. 10.1073/pnas.2536767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Gan L.; Carlsson D. J.; Fagerholm P.; Lagali N.; Watsky M. A.; Munger R.; Hodge W. G.; Priest D.; Griffith M. A Simple, Cross-linked Collagen Tissue Substitute for Corneal Implantation. Invest. Ophthalmol. Visual Sci. 2006, 47, 1869–1875. 10.1167/iovs.05-1339. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Griffith M.; Watsky M. A.; Forrester J. V.; Kuffová L.; Grant D.; Merrett K.; Carlsson D. J. Properties of Porcine and Recombinant Human Collagen Matrices for Optically Clear Tissue Engineering Applications. Biomacromolecules 2006, 7, 1819–1828. 10.1021/bm060160o. [DOI] [PubMed] [Google Scholar]
- Liu W.; Deng C.; McLaughlin C. R.; Fagerholm P.; Lagali N. S.; Heyne B.; Scaiano J. C.; Watsky M. A.; Kato Y.; Munger R.; Shinozaki N.; Li F.; Griffith M. Collagen–phosphorylcholine Interpenetrating Network Hydrogels as Corneal Substitutes. Biomaterials 2009, 30, 1551–1559. 10.1016/j.biomaterials.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Liu W.; Merrett K.; Griffith M.; Fagerholm P.; Dravida S.; Heyne B.; Scaiano J. C.; Watsky M. A.; Shinozaki N.; Lagali N.; Munger R.; Li F. Recombinant Human Collagen for Tissue Engineered Corneal Substitutes. Biomaterials 2008, 29, 1147–1158. 10.1016/j.biomaterials.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Bodvik R.; Dedinaite A.; Karlson L.; Bergström M.; Bäverbäck P.; Pedersen J. S.; Edwards K.; Karlsson G.; Varga I.; Claesson P. M. Aggregation and Network Formation of Aqueous Methylcellulose and Hydroxypropylmethylcellulose Solutions. Colloids Surf., A 2010, 354, 162–171. 10.1016/j.colsurfa.2009.09.040. [DOI] [Google Scholar]
- Lee J.-S.; Kim H. W.; Chung D.; Lee H. G. Catechin-loaded Calcium Pectinate Microparticles Reinforced with Liposome and Hydroxypropylmethylcellulose: Optimization and in vivo Antioxidant Activity. Food Hydrocolloids 2009, 23, 2226–2233. 10.1016/j.foodhyd.2009.05.005. [DOI] [Google Scholar]
- Janssens S.; Denivelle S.; Rombaut P.; Van den Mooter G. Influence of Polyethylene Glycol Chain Length on Compatibility and Release Characteristics of Ternary Solid Dispersions of Itraconazole in Polyethylene Glycol/hydroxypropyl Methylcellulose 2910 E5 Blends. Eur. J. Pharm. Sci. 2008, 35, 203–210. 10.1016/j.ejps.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Kiil S.; Dam-Johansen K. Controlled Drug Delivery from Swellable Hydroxypropylmethylcellulose Matrices: Model-based Analysis of Observed Radial Front Movements. J. Controlled Release 2003, 90, 1–21. 10.1016/S0168-3659(03)00122-6. [DOI] [PubMed] [Google Scholar]
- Kavanagh N.; Corrigan O. I. Swelling and Erosion Properties of Hydroxypropylmethylcellulose (Hypromellose) Matrices—Influence of Agitation Rate and Dissolution Medium Composition. Int. J. Pharm. 2004, 279, 141–152. 10.1016/j.ijpharm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Pastor C.; Sánchez-González L.; Marcilla A.; Chiralt A.; Cháfer M.; González-Martínez C. Quality and Safety of Table Grapes Coated with Hydroxypropylmethylcellulose Edible Coatings Containing Propolis Extract. Postharvest Biol. Biotechnol. 2011, 60, 64–70. 10.1016/j.postharvbio.2010.11.003. [DOI] [Google Scholar]
- Sánchez-González L.; Pastor C.; Vargas M.; Chiralt A.; González-Martínez C.; Cháfer M. Effect of Hydroxypropylmethylcellulose and Chitosan Coatings with and without Bergamot Essential oil on Quality and Safety of Cold-stored Grapes. Postharvest Biol. Biotechnol. 2011, 60, 57–63. 10.1016/j.postharvbio.2010.11.004. [DOI] [Google Scholar]
- Trojani C.; Weiss P.; Michiels J.; Vinatier C.; Guicheux J.; Daculsi G.; Gaudray P.; Carle G.; Rochet N. Three-dimensional Culture and Differentiation of Human Osteogenic Cells in an Injectable Hydroxypropylmethylcellulose Hydrogel. Biomaterials 2005, 26, 5509–5517. 10.1016/j.biomaterials.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Maltese A.; Borzacchiello A.; Mayol L.; Bucolo C.; Maugeri F.; Nicolais L.; Ambrosio L. Novel Polysaccharides-based Viscoelastic Formulations for Ophthalmic Surgery: Rheological Characterization. Biomaterials 2006, 27, 5134–5142. 10.1016/j.biomaterials.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Sánchez-González L.; Chiralt A.; González-Martínez C.; Cháfer M. Effect of Essential Oils on Properties of Film Forming Emulsions and Films Based on Hydroxypropylmethylcellulose and Chitosan. J. Food Eng. 2011, 105, 246–253. 10.1016/j.jfoodeng.2011.02.028. [DOI] [Google Scholar]
- Li W.; Long Y.; Liu Y.; Long K.; Liu S.; Wang Z.; Wang Y.; Ren L. Fabrication and Characterization of Chitosan–collagen Crosslinked Membranes for Corneal Tissue Engineering. J. Biomater. Sci., Polym. Ed. 2014, 25, 1962–1972. 10.1080/09205063.2014.965996. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Guo L.; Ren L.; Yin S.; Ge J.; Gao Q.; Luxbacher T.; Luo S. A Study on the Performance of Hyaluronic Acid Immobilized Chitosan Film. Biomed. Mater. 2009, 4, 035009. 10.1088/1748-6041/4/3/035009. [DOI] [PubMed] [Google Scholar]
- Beems E. M.; Van Best J. A. Light Transmission of the Cornea in Whole Human Eyes. Exp. Eye Res. 1990, 50, 393–395. 10.1016/0014-4835(90)90140-P. [DOI] [PubMed] [Google Scholar]
- Patel S.; Marshall J.; Fitzke F. W. III Refractive Index of the Human Corneal Epithelium and Stroma. J. Refract. Surg. 1995, 11, 100–105. 10.3928/1081-597X-19950301-09. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Griffith M.; Watsky M. A.; Forrester J. V.; Kuffová L.; Grant D.; Merrett K.; Carlsson D. J. Properties of Porcine and Recombinant Human Collagen Matrices for Optically Clear Tissue Engineering Applications. Biomacromolecules 2006, 7, 1819–1828. 10.1021/bm060160o. [DOI] [PubMed] [Google Scholar]
- Ding C.; Zhang M.; Li G. Preparation and Characterization of Collagen/hydroxypropyl Methylcellulose (HPMC) Blend Film. Carbohydr. Polym. 2015, 119, 194–201. 10.1016/j.carbpol.2014.11.057. [DOI] [PubMed] [Google Scholar]
- Ding C.; Zhang M.; Li G. Rheological Properties of Collagen/hydroxypropyl Methylcellulose (COL/HPMC) Blended Solutions. J. Appl. Polym. Sci. 2014, 131, 40042. 10.1002/app.40042. [DOI] [Google Scholar]
- Righe B. Eye Contact. Chem. Br. 1992, 28, 241–244. [Google Scholar]
- Bock R. H.; Maumenee A. E. Corneal Fluid Metabolism. Arch. Ophthalmol. 1953, 50, 282–285. 10.1001/archopht.1953.00920030289003. [DOI] [PubMed] [Google Scholar]
- Pettit D. K.; Knight P. M. Glucose Permeability of Potential Intrastromal Implants. Invest. Ophthalmol. Visual Sci. 1985, 26, S151. [Google Scholar]
- Yao Z.; Wu H.; Han B.; Liu W. The Properties of Chitosan-hyaluronic Acid Blend Membrane. High Technol. Lett. 2004, 14, 95–99. [Google Scholar]
- Cai L.; Liu Z.; Cui Y.; Li X.; Zhang Y.; Han H. Synthesis of Copolymer Hydrogel for Cornea Contact Lens and Study on its Ion Penetration Capability. Mater. Rev. 2006, 20, 134–136. 10.3321/j.issn:1005-023X.2006.12.037. [DOI] [Google Scholar]
- Wang J. M.; Ren L.; Long Y. Y.; Liu S.; Wang Y. J.; Ji P. H.; Lin M. K.; Xi L. The Diffusion Properties of Chitosan-collagen Blend Membrane. J. Funct. Mater. 2012, 43, 1–4. 10.3969/j.issn.1001-9731.2012.20.004. [DOI] [Google Scholar]
- Yaoa Z.-A.; Wu H.-G. Characterization of Chitosan-chondroitin Sulfate Blended Membranes and Effects on the Growth of Corneal Cells. Adv. Mat. Lett. 2010, 1, 67–74. 10.5185/amlett.2010.4113. [DOI] [Google Scholar]
- Thacharodi D.; Rao K. P. Propranolol Hydrochloride Release Behaviour of Crosslinked Chitosan Membranes. J. Chem. Technol. Biotechnol. 1993, 58, 177–181. 10.1002/jctb.280580211. [DOI] [PubMed] [Google Scholar]