ABSTRACT
Objective:
To evaluate the impact of the use of the molecular test for Mycobacterium tuberculosis and its resistance to rifampin (Xpert MTB/RIF), under routine conditions, at a referral hospital in the Brazilian state of Bahia.
Methods:
This was a descriptive study using the database of the Mycobacteriology Laboratory of the Octávio Mangabeira Specialized Hospital, in the city of Salvador, and georeferencing software. We evaluated 3,877 sputum samples collected from symptomatic respiratory patients, under routine conditions, between June of 2014 and March of 2015. All of the samples were submitted to sputum smear microscopy and the Xpert MTB/RIF test. Patients were stratified by gender, age, and geolocation.
Results:
Among the 3,877 sputum samples evaluated, the Xpert MTB/RIF test detected M. tuberculosis in 678 (17.5%), of which 60 (8.8%) showed resistance to rifampin. The Xpert MTB/RIF test detected M. tuberculosis in 254 patients who tested negative for sputum smear microscopy, thus increasing the diagnostic power by 59.9%.
Conclusions:
The use of the Xpert MTB/RIF test, under routine conditions, significantly increased the detection of cases of tuberculosis among sputum smear-negative patients.
Keywords: Tuberculosis/diagnosis, Molecular diagnostic techniques, Sputum
RESUMO
Objetivo:
Avaliar o impacto do teste rápido molecular automatizado Xpert MTB/RIF, utilizado para a detecção de Mycobacterium tuberculosis e sua resistência à rifampicina, em condições de rotina, em um hospital de referência no estado da Bahia.
Métodos:
Estudo descritivo retrospectivo utilizando o banco de dados do Laboratório de Micobacteriologia do Hospital Especializado Octávio Mangabeira, localizado na cidade de Salvador, e um programa de georreferenciamento. Entre junho de 2014 e março de 2015, foram incluídas no estudo 3.877 amostras de escarro coletadas de pacientes sintomáticos respiratórios em condições de rotina. Todas as amostras coletadas foram submetidas tanto à baciloscopia quanto a Xpert MTB/RIF. Os pacientes foram estratificados por sexo, idade e georreferenciamento.
Resultados:
Das 3.877 amostras de escarro analisadas, Xpert MTB/RIF detectou a presença de M. tuberculosis em 678 pacientes (17,5%). Desses, 60 (8,8%) apresentaram resistência à rifampicina. O Xpert MTB/RIF detectou 254 pacientes com baciloscopia negativa, representando um acréscimo diagnóstico de 59,9%.
Conclusões:
A implantação do Xpert MTB/RIF, sob condições de rotina, teve um impacto significativo no aumento da detecção de casos de tuberculose em pacientes com baciloscopia negativa.
Descritores: Tuberculose/diagnóstico, Técnicas de diagnóstico molecular, Escarro
INTRODUCTION
In 2010, the World Health Organization endorsed the use of the Xpert MTB/RIF molecular test (Cepheid Inc., Sunnyvale, CA, USA) for the diagnosis of tuberculosis. The Xpert MTB/RIF test is a molecular test, based on polymerase chain reaction, which detects Mycobacterium tuberculosis DNA and, simultaneously, resistance to rifampin, within two hours. 1 Following the recommendation of the World Health Organization, many countries have incorporated this technology into the tuberculosis diagnostic routine, replacing sputum smear microscopy. 2 Although many studies have shown the high sensitivity and specificity of this test in the diagnosis of tuberculosis and in the detection of rifampin resistance, 3 - 8 it is relevant to evaluate its routine use in local programs, considering that many logistical and health system barriers can influence the impact of this test on patient care. In Brazil, limited access to health care and poor patient perception of the symptoms were identified as important factors for delayed diagnosis and consequently for a delay in the initiation of treatment. 9 Until July of 2014, the diagnosis of tuberculosis in Brazil was based on the clinical-radiological profile and on phenotypic tests (sputum smear microscopy and culture for mycobacteria). 10 However, the Brazilian scientific community had for some time emphasized the need for the incorporation of new diagnostic technologies into the Brazilian public health system, including genotypic tests for pulmonary and extrapulmonary tuberculosis. 11 - 13 A survey involving tuberculosis experts worldwide demonstrated the high acceptability of new rapid tests for the diagnosis of tuberculosis and the widespread use of the Xpert MTB/RIF test (reported by 46.7% of interviewees). 14
A pragmatic clinical trial, conducted in two Brazilian cities (Rio de Janeiro and Manaus), showed the feasibility of the routine use of the Xpert MTB/RIF test in the National Tuberculosis Control Program in a country of continental dimensions and immense regional differences in the organization and quality of health care services. In that study, Durovni et al. 15 observed a 59% increase in the rate of cases with laboratory confirmation and a reduction in the time to treatment initiation (from 11 to 8 days). In addition, some studies conducted in Brazil have demonstrated that the Xpert MTB/RIF test is a cost-effective diagnostic strategy for tuberculosis. 16 , 17
In September of 2013, the Xpert MTB/RIF test was approved by the National Committee for Health Technology Incorporation for use in the Brazilian Unified Health Care System. 18 The use of this test in Brazil was initiated by the Brazilian National Ministry of Health (NMH) in July of 2014, and the Rapid Molecular Testing Network for Tuberculosis was created. Since then, the National Tuberculosis Control Program has distributed 160 Xpert MTB/RIF systems throughout the country, priority being given to all state capitals and the Federal District of Brasília, as well as to host cities of prisons, border towns, and municipalities with more than 130 cases of tuberculosis per year. 19
In 2014, 69,262 new cases of tuberculosis were reported in Brazil, with an incidence coefficient of 33.5 cases/100,000 population. Among all Brazilian states, Bahia ranks third in terms of the burden of tuberculosis, with 4,833 new cases reported in 2014 (32 cases/100,000 population). 20 The state of Bahia received 5 GeneXpert MTB/RIF systems from the NMH in 2014, 3 of which were allocated to the Hospital Especializado Otávio Mangabeira (HEOM, Octávio Mangabeira Specialized Hospital), located in the city of Salvador. In the context of operational research, the present study evaluated the impact of using the Xpert MTB/RIF test under routine conditions at a referral center for tuberculosis in Bahia.
METHODS
The research was conducted in the mycobacteriology laboratory of the HEOM, a tertiary referral hospital for tuberculosis and belonging to the state public network. The HEOM has hospital wards and an outpatient care clinic, serving patients from the capital and from the state at large. Due to the operational difficulties of making a diagnosis based on laboratory test results within the local primary care network, approximately 37% of tuberculosis cases in Salvador are diagnosed at the HEOM laboratory (State Tuberculosis Control Program, unpublished data). The HEOM laboratory makes Xpert MTB/RIF test results available on the day of collection, and there is a routine return flow of these results to the various hospital sectors.
This was a laboratory-based retrospective descriptive study, conducted in the context of operational research, under routine conditions. The information was obtained using the database of the HEOM mycobacteriology laboratory and stored in the Microsoft Excel program.
The sample consisted of patients who underwent the Xpert MTB/RIF test and sputum smear microscopy, from the same sputum sample, between June 10, 2014 and March 31, 2015. The study focused on the performance of the two different methodologies, carried out under routine laboratory conditions, following algorithms recommended by the NMH 19 in the scenario of a referral center in Bahia.
Tuberculosis cases were georeferenced as to their spatial distribution and demographic incidence. The objective was to process the data as geographic information, to support the planning and health management of the referral center in question. We used the geodetic reference system currently in use in Brazil, known as the Geocentric Reference System for the Americas 2000, which allows the direct use of the Global Navigation Satellite Systems technology. To generate the thematic map, we used the kernel method. On the map, the point intensity of certain phenomena is plotted across the study region. 21
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, whereas categorical variables were expressed as absolute and relative frequencies. The agreement between the results of the semiquantitative Xpert MTB/RIF test and sputum smear microscopy was calculated using weighted kappa statistics. Data were analyzed using the GraphPad Prism program, version 5.01 (GraphPad Inc., San Diego, CA, USA).
Ethical aspects
This study was approved by the Research Ethics Committee of the Bahia School of Medicine and Public Health of the Bahia Foundation for the Advancement of Science (Protocol no. 119/2008, addendum in 2011). Data confidentiality and anonymity of the patients were assured in the design and database handling.
RESULTS
During the study period (between June of 2014 and March of 2015), 19,117 tests (sputum smear microscopy, culture for mycobacteria, and the Xpert MTB/RIF test) were performed in the HEOM mycobacteriology laboratory for the diagnosis of suspected cases and follow-up of cases of tuberculosis. We identified 3,877 patients in whom the Xpert MTB/RIF test and sputum smear microscopy were performed simultaneously in the same sputum sample. The mean age of the study population was 41.5 ± 15.4 years, and males predominated (accounting for 67.1% of the sample).
Among the 3,877 patients evaluated, positive results on sputum smear microscopy and the Xpert MTB/RIF test were observed in 424 (10.9%) and 678 (17.5%), respectively (Table 1). The Xpert MTB/RIF test detected positivity for tuberculosis in 254 patients who tested negative on sputum smear microscopy, a diagnostic gain of 59.9%.
Table 1. Distribution of Xpert MTB/RIF test and sputum smear microscopy results at Octávio Mangabeira Specialized Hospital, in the city of Salvador, Brazil, between June of 2014 and March of 2015 (N = 3,877).a .
| Result | Sputum smear microscopy | Xpert MTB/ RIF test |
|---|---|---|
| Positive | 424 (10.9) | 678 (17.5) |
| Negative | 3,453 (89.1) | 3,199 (82.5) |
| Total | 3,877 (100%) | 3,877 (100%) |
Values expressed as n (%).
A positive result on the Xpert MTB/RIF test is available on its platform in four levels of semiquantitative detection: very low, low, medium, and high. However, the result of the sputum smear microscopy is categorized in the routine of the facility as negative, positive (1-9 bacilli/100 fields examined), 1+, 2+, or 3+. Table 2 shows the correlation between the semiquantitative Xpert MTB/RIF test results and the bacillary load in the sputum smear microscopy examination. All 424 sputum samples testing positive on sputum smear microscopy also tested positive on the Xpert MTB/RIF test. Of the 189 Xpert MTB/RIF test results classified as very low positivity, 175 (92.6%) tested negative on sputum smear microscopy. Of the 245 Xpert MTB/RIF test results classified as low positivity, 169 (68.9%) were categorized as 1+ on sputum smear microscopy. Of the 57 Xpert MTB/RIF test results classified as medium positivity, 54 (94.5%) were categorized as 1+ or 2+ on sputum smear microscopy. Of the 187 Xpert MTB/RIF test results classified as high positivity, 142 (75.9%) were categorized as 3+ on sputum smear microscopy. The statistical analysis showed a strong correlation between the semiquantitative Xpert MTB/RIF test results and the sputum smear microscopy results (weighted kappa = 0.82).
Table 2. Correlation between the results of the semiquantitative Xpert MTB/RIF test and those of sputum smear microscopy (N = 678).a,*.
| Sputum smear microscopy | Xpert MTB/RIF test | ||||
|---|---|---|---|---|---|
| Results | Very low | Low | Medium | High | Total |
| Negative | 175 (92.6) | 76 (31) | 3 (5.3) | 0 | 254 (37.5) |
| Positiveb | 14 (7.4) | 0 | 0 | 0 | 14 (2.0) |
| 1+ | 0 | 169 (69) | 15 (26.3) | 0 | 184 (27.1) |
| 2+ | 0 | 0 | 39 (68.4) | 45 (24.1) | 84 (12.4) |
| 3+ | 0 | 0 | 0 | 142 (75.9) | 142 (30.0) |
| Total | 189 (100) | 245 (100) | 57 (100) | 187 (100) | 678 (100) |
Values expressed as n (%). b1-9 bacilli/100 fields). *Weighted kappa = 0.82 (strong agreement).
The reports of 175 cases in which the Xpert MTB/RIF test result was positive and the sputum smear microscopy result was negative were later reviewed in the Brazilian Case Registry Database. Of those 175 cases, 146 (83.4%) were new cases and 29 (16.6%) were identified as cases of retreatment (presence of one or more reports prior to the date of the two tests). Of the 29 cases of retreatment, 10 had positive cultures recorded, confirming active tuberculosis, records of the culture results being unavailable for the remaining 19. Among the 175 patients with positive results on the Xpert MTB/RIF test and negative results on sputum smear microscopy, there were 19 cases (10.9%) in which it was not possible to confirm the presence of active tuberculosis.
Among the 424 patients with positive sputum smear microscopy results, the Xpert MTB/RIF test result was classified as “undetectable”, suggesting the presence of nontuberculous mycobacteria, in 9 (2.1%).
Of the 678 cases confirmed by the Xpert MTB/RIF test (new cases and cases of retreatment), 60 (8.8%) presented rifampin resistance (Table 3). Patients infected with rifampin-resistant strains were referred to the HEOM tertiary referral outpatient clinic for follow-up.
Table 3. Resistance to rifampin detected by the Xpert MTB/RIF test at Octávio Mangabeira Specialized Hospital, in the city of Salvador, Brazil, between June of 2014 and March of 2015 (N = 678).
| Response to rifampin | n | % |
|---|---|---|
| Resistant | 60 | 8.8 |
| Sensitive | 618 | 91.2 |
| Total | 678 | 100 |
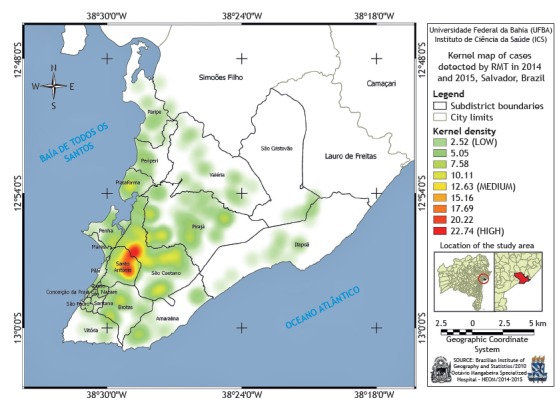
Analysis of the spatial distribution of the incidence of tuberculosis in the study population by subdistricts of the city of Salvador showed a higher concentration of cases in the eastern region of the city, in the subdistricts of Santo Antônio and São Caetano (Figure 1).
Figure 1. Map of the city of Salvador, Brazil, generated with the kernel method, indicating the spatial distribution of tuberculosis cases diagnosed by a rapid molecular test (RMT)-the Xpert MTB/RIF test-during the study period.
DISCUSSION
In the present study, conducted under routine laboratory conditions at a tertiary referral hospital for tuberculosis in Bahia, the Xpert MTB/RIF test detected 254 cases of tuberculosis that tested negative on sputum smear microscopy, representing a 59.9% gain in the rate of biologically confirmed diagnosis. Wide variability in the proportion of diagnostic gain was observed in several published studies comparing the two methods. 22 - 25 In a study conducted in Rwanda, in which both methods were applied in the same group of patients, Ngbonziza et al. found a 32.3% gain in diagnostic power, 22 whereas Cowan et al. reported a 69.0% diagnostic gain in a study conducted in Mozambique. 23 In a population of 401 HIV-infected individuals in Cambodia, Auld et al. 24 reported a diagnostic gain of only 26.0% attributed to the use of the Xpert MTB/RIF test. Evaluating routine program data in 18 countries, Ardizzoni et al. 25 observed that the use of the Xpert MTB/RIF test resulted in a mean relative diagnostic gain of 42.3% over the use of sputum smear microscopy. However, those authors pointed out the wide variation in the diagnostic gain, which ranged from 9.7% to 110%, among various countries. 25 Those differences were attributed to different epidemiological scenarios among the countries evaluated and the heterogeneity among the studies in terms of the methodologies used.
In a clinical trial conducted in two Brazilian cities where the incidence of tuberculosis is high (Rio de Janeiro and Manaus), during the trial period prior to the incorporation of the Xpert MTB/RIF test into the public health care system, Durovni et al. 15 reported that the use of the new test resulted in a 59% increase in rate of bacteriologically confirmed diagnosis. The authors compared baseline data (collected when diagnosis was performed using conventional sputum smear microscopy in two samples) with those collected after the Xpert MTB/RIF test had replaced sputum smear microscopy. Therefore, the methodology used differed from that used in the present study, in which the same group of patients performed concomitant tests (sputum smear microscopy and Xpert MTB/RIF). Although the present study was carried out under routine conditions and with a different methodology, our results corroborate those of Durovni et al. 15
There are some potential explanations for the wide variability across studies in terms of the proportional diagnostic gain achieved with the Xpert MTB/RIF test in comparison with sputum smear microscopy. First, epidemiological contexts differ among regions, with heterogeneous rates of tuberculosis incidence. Another plausible explanation is that the sensitivity of sputum smear microscopy varies among laboratories and geographic locations, as well as that the quality of the results of the examination is highly dependent on the training of the professionals responsible. Therefore, in laboratories where the sensitivity of sputum smear microscopy is low, the relative diagnostic gain achieved with the Xpert MTB/RIF test might be artificially high. Our study was carried out at a tertiary referral center for tuberculosis, where the laboratory professionals are periodically trained by the NMH to perform sputum smear microscopy.
Although there have been few studies of the topic, the semiquantitative results of the Xpert MTB/RIF test estimate the bacterial load by measuring the real-time polymerase chain reaction threshold cycle. The bacterial load can be an elementary biomarker for evaluation of disease severity, risk of transmission, and therapeutic response. 26 In our study, we observed a strong correlation between the sputum smear microscopy results and those of the semiquantitative Xpert MTB/RIF test (weighted kappa = 0.82), similar to that observed in a multicenter study. 27 In the absence of culture results (as was the case for a considerable proportion of cases in the present study) or in the waiting period for the release of the test results, the Xpert MTB/RIF semiquantitative test result (especially in cases with low positivity) can be useful in the identification of cases of active tuberculosis in patients not previously treated for tuberculosis with consistent clinical and radiological findings. In the present study, we found that 175 (92.6%) of the 189 Xpert MTB/RIF tests in which the results were classified as very low positivity were in samples that tested negative on sputum smear microscopy. It is likely that the quantification of the Xpert MTB/RIF test results represents an important tool in the early identification of the subset of potentially infectious patients, even before the culture result for mycobacteria is known. However, in cases of retreatment, these data should be interpreted with caution, and it is advisable to wait for the result of the culture before initiating treatment, because the Xpert MTB/RIF test could produce a false positive result.
Resistance to rifampin was observed in 8.8% of the cases evaluated in the present study. In the previously mentioned pragmatic clinical trial conducted in Brazil, Durovni et al. 15 found that rate to be 3.8% overall (3.3% among new cases and 7.5% among cases of retreatment). The greater proportion of cases of rifampin resistance found in our study can be explained by certain factors. First, there were methodological differences between the two studies, which also had different designs. Second, because our study was performed at a tertiary referral hospital for tuberculosis, it is possible that there was a selection bias, although our hospital also conducts tests for the primary care network. However, data from the NMH Rapid Molecular Testing Network for Tuberculosis monitoring during the first year of its implementation (between June of 2014 and May of 2015) showed higher proportions of rifampin resistance, similar to those found in our study, in Brazil as a whole and in the state of Bahia-in 4.6% and 7.2% of new cases, respectively, and in 13.9% and 17.7% of cases of retreatment, respectively. 28
The Xpert MTB/RIF test has high specificity for the detection of rifampin resistance (98%), already well established in previous studies. 6 , 29 Trajman et al. 30 showed that the test has a high positive predictive value for rifampin resistance (90.2%), even in countries where the prevalence of drug-resistant tuberculosis is relatively low. Those authors also demonstrated that 82% of the rifampin-resistant cases detected by the Xpert MTB/RIF test were confirmed as cases of multidrug-resistant tuberculosis in a phenotypic sensitivity test. 30 Therefore, although our sample might not have been representative of the true proportion of resistance in the state, the detection of rifampin resistance in 8.8% of the cases indicates that the Xpert MTB/RIF test can facilitate the early identification of cases of multidrug-resistant tuberculosis in Brazil.
The highest density of cases detected in the georeferencing of the subdistricts of Santo Antônio and São Caetano, in the city of Salvador, can be attributed to the proximity of these regions to the HEOM. Another aspect to be considered is the fact that these two subdistricts are located in the historical center and São Caetano/Valéria health districts, respectively, which present high rates of tuberculosis incidence.
Our study has a number of limitations. Such limitations include the retrospective design, as well as the fact that the study was laboratory-based and was carried out at a tertiary referral hospital for tuberculosis. In addition, some weaknesses in data collection at the beginning of the implementation of the Xpert MTB/RIF test at the HEOM prevented us from adequately identifying new cases and cases of retreatment, constituting a limitation related to drawing comparisons with other studies. In settings with a high prevalence of tuberculosis, one of the major limitations of the Xpert MTB/RIF test is the possibility of a false-positive result in patients who have had active disease and been cured, because the genetic material can be detected in the sputum of such individuals. Another important limitation of our study is the small proportion of cultures performed, thus preventing us from resolving questions related to the presence or absence of active tuberculosis, especially in cases of retreatment. However, our review of the reports of the 175 cases with positive results on the Xpert MTB/RIF test and negative results on sputum smear microscopy, in the Brazilian Case Registry Database, showed that there were only 19 cases (10.9%) in which it was not possible to confirm active tuberculosis, which could theoretically correspond to the possibility of false-positive results on the Xpert MTB/RIF test. Especially in cases of retreatment, a positive result on the Xpert MTB/RIF test (which could represent a false-positive result) should be interpreted with caution and the importance of culturing mycobacteria should be emphasized in tuberculosis training for health professionals. Nevertheless, we should also draw attention to cases of positive sputum smear microscopy results and a result of “undetectable” on the Xpert MTB/RIF test in the same sputum sample (as occurred in 2.1% of our cases), which could represent the presence of nontuberculous mycobacteria, indicating the need to continue research with culture and species identification.
In conclusion, the introduction of the use of the Xpert MTB/RIF test under routine conditions contributed significantly to the increased detection of tuberculosis cases in patients with negative sputum smear microscopy results, thereby increasing the rate of treatment of active tuberculosis in patients not diagnosed by sputum smear microscopy.
Study carried out at the Octávio Mangabeira Specialized Hospital, Bahia State Health Department, Salvador (BA) Brazil.
Financial support: None
REFERENCES
- 1.World Health Organization . Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Policy statement. Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 2.World Health Organization . Global tuberculosis report 2014. Switzerland: World Health Organization; 2014. [Google Scholar]
- 3.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Nicol MP. Xpert MTB/RIF assay development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6(9):1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang K, Lu W, Wang J, Zhang K, Jia S, Li F. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay a meta-analysis. J Infect. 2012;64(6):580–588. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance a multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carriquiry G, Otero L, González-Lagos E, Zamudio C, Sánchez E, Nabeta P. A diagnostic accuracy study of Xpert MTB/RIF in HIV-positive patients with high clinical suspicion of pulmonary tuberculosis in Lima, Peru. PLoS One. 2012;7(9):e44626. doi: 10.1371/journal.pone.0044626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon C, Cattamanchi A, Davis JL, Worodria W, den Boon S, Kalema N. Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLoS One. 2012;7(11):e48599. doi: 10.1371/journal.pone.0048599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maior Mde L, Guerra RL, Cailleaux-Cezar M, Golub JE, Conde MB. Time from symptom onset to the initiation of treatment of pulmonary tuberculosis in a city with a high incidence of the disease. J Bras Pneumol. 2012;38(2):202–209. doi: 10.1590/s1806-37132012000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conde MB, Melo FA, Marques AM, Cardoso NC, Pinheiro VG, Dalcin P de T. III Brazilian Thoracic Association Guidelines on tuberculosis. J Bras Pneumol. 2009;35(10):1018–1048. doi: 10.1590/S1806-37132009001000011. [DOI] [PubMed] [Google Scholar]
- 11.Telles MA, Menezes A, Trajman A. Bottlenecks and recommendations for the incorporation of new technologies in the tuberculosis laboratory network in Brazil. J Bras Pneumol. 2012;38(6):766–770. doi: 10.1590/S1806-37132012000600013. [DOI] [PubMed] [Google Scholar]
- 12.Furini AA, Pedro Hda S, Rodrigues JF, Montenegro LM, Machado RL, Franco C. Detection of Mycobacterium tuberculosis complex by nested polymerase chain reaction in pulmonary and extrapulmonary specimens. J Bras Pneumol. 2013;39(6):711–718. doi: 10.1590/S1806-37132013000600010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barreto LB, Lourenço MC, Rolla VC, Veloso VG, Huf G. Use of amplified Mycobacterium tuberculosis direct test in respiratory samples from HIV-infected patients in Brazil. J Bras Pneumol. 2014;40(2):148–154. doi: 10.1590/S1806-37132014000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amicosante M, D'Ambrosio L, Munoz M, Mello FCQ, Tebruegge M, Chegou NN. Current use and acceptability of novel diagnostic tests for active tuberculosis a worldwide survey. J Bras Pneumol. 2017;43(5):380–392. doi: 10.1590/s1806-37562017000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durovni B, Saraceni V, Van Den Hof S, Trajman A, Cordeiro-Santos M, Cavalcante S. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil a stepped-wedge cluster-randomized trial. PLoS Med. 2014;11(12):e1001766. doi: 10.1371/journal.pmed.1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva Antunes R, Pinto M, Trajman A. Patient cost for the diagnosis of tuberculosis in Brazil comparison of Xpert MTB/RIF and smear microscopy. Int J Tuberc Lung Dis. 2014;18(5):547–551. doi: 10.5588/ijtld.13.0637. [DOI] [PubMed] [Google Scholar]
- 17.Pinto M, Trajman A, Steffen R, Entringer AP. Cost analysis of nucleic acid amplification for diagnosing pulmonary tuberculosis, within the context of the Brazilian Unified Health Care System. J Bras Pneumol. 2015;41(6):536–538. doi: 10.1590/s1806-37562015000004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brasil. Ministério da Saúde. Biblioteca Virtual em Saúde . Portaria MS no. 48 de 10 de setembro de 2013. Brasília: o Ministério; http://www.bvsms.saude.gov.br/bvs/saudelegis/sctie/2013/prt0048_10_09_2013.html [Google Scholar]
- 19.Brasil. Ministério da Saúde. Secretaria de Vigilância à Saúde . Recomendações sobre o diagnóstico da tuberculose por meio do teste rápido molecular para tuberculose: nota informativa no. 9. Brasília: Ministério da Saúde; 2014. [Google Scholar]
- 20.Bahia. Secretaria Estadual de Saúde. Superintendência de Vigilância em Saúde. Sistema de Informação de Agravos de Notificação . Casos de tuberculose notificados no SINAN. Salvador: a Secretaria; http://www3.saude.ba.gov.br/cgi/deftohtm.exe?sinan/tube.def [Google Scholar]
- 21.Oliveira EXG, Silveira JC, Jr, Souza-Santos R, Pina MF, Portugal JL. Santos S, Souza-Santos R. Sistemas de informações geográficas e análise espacial na saúde pública. Brasília: Ministério da Saúde, Fundação Oswaldo Cruz; 2017. Análise de dados espaciais. [Google Scholar]
- 22.Ngabonziza JC, Ssengooba W, Mutua F, Torrea G, Dushime A, Gasana M. Diagnostic performance of smear microscopy and incremental yield of Xpert in detection of pulmonary tuberculosis in Rwanda. BMC Infect Dis. 2016;16(1):660–660. doi: 10.1186/s12879-016-2009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowan J, Michel C, Manhiça I, Monivo C, Saize D, Creswell J. Implementing rapid testing for tuberculosis in Mozambique. Bull World Health Organ. 2015;93(2):125–130. doi: 10.2471/BLT.14.138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auld SC, Moore BK, Kyle RP, Eng B, Nong K, Pevzner ES. Mixed impact of Xpert() MTB/RIF on tuberculosis diagnosis in Cambodia. Public Health Action. 2016;6(2):129–135. doi: 10.5588/pha.16.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ardizzoni E, Fajardo E, Saranchuk P, Casenghi M, Page AL, Varaine F. Implementing the Xpert MTB/RIF Diagnostic Test for Tuberculosis and Rifampicin Resistance Outcomes and Lessons Learned in 18 Countries PLoS. One. 2015;10(12):e0144656. doi: 10.1371/journal.pone.0144656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opota O, Senn L, Prod'hom G, Mazza-Stalder J, Tissot F, Greub G. Added value of molecular assay Xpert MTB/RIF compared to sputum smear microscopy to assess the risk of tuberculosis transmission in a low-prevalence country. Clin Microbiol Infect. 2016;22(7):613–619. doi: 10.1016/j.cmi.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Blakemore R, Nabeta P, Davidow AL, Vadwai V, Tahirli R, Munsamy V. A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. Am J Respir Crit Care Med. 2011;184(9):1076–1084. doi: 10.1164/rccm.201103-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde . Rede de Teste Rápido para Tuberculose no Brasil -- Primeiro ano de implantação. Brasília: Ministério da Saúde; 2015. [Google Scholar]
- 29.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;(1):CD009593–CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trajman A, Durovni B, Saraceni V, Cordeiro-Santos M, Cobelens F, Van den Hof S. High positive predictive value of Xpert in a low rifampicin resistance prevalence setting. Eur Respir J. 2014;44(6):1711–1713. doi: 10.1183/09031936.00115514. [DOI] [PubMed] [Google Scholar]


