Abstract
A library of hybrid molecules was procured by the combination of triazine–indole adduct with morpholine/piperidine/pyrrolidine and pyrazole/pyrimidine/oxindole moieties. Enzyme immunoassays on cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) identified compound 6 having an IC50 value of 20 nM for COX-2 and 3000 nM for COX-1. The significant reduction in the formation of prostaglandin E2 in the lipopolysaccharide-treated (COX-2-activated) human whole blood, almost no change in the production of thromboxane B2 in the calcium ionophore-treated (COX-1-activated) sample of human whole blood, and the mechanistic studies on Swiss albino mice ensured that compound 6 is selective for COX-2. The association constant (Ka) of compound 6 with COX-2 was found to be of the order of 0.48 × 106 M–1. The diffusion spectroscopy experiments and relaxation time (T1) calculations of compound 6 in the presence of COX-2 assisted in identifying the site-specific interactions of 6 with the enzyme, and these results fall into nice correlation with the theoretical data obtained from molecular docking and quantitative structure–activity relationship studies. With maximum tolerable dose >2000 mg kg–1, compound 6 made 68 and 32% reduction in formalin-induced analgesia and carrageenan-induced inflammation in Swiss albino mice.
1. Introduction
Inflammation is a preeminent host defense to injury, infectious agents, and autoimmune responses.1 However, the chronic inflammation leads to the emergence of various diseases such as rheumatoid arthritis, neurodegenerative disorder, and cancer and cardiovascular diseases.2 The most important markers of inflammation include cytokine receptors, nitric oxide synthase (NOS), nuclear factor kappa-B, chemokines, tumor necrosis factor alpha, interferons, and proinflammatory enzymes cyclooxygenase-2 (COX-2) and lipoxygenase.3 Among these mediators, cyclooxygenases or prostaglandin endoperoxide synthases, taking part in arachidonic acid (AA) metabolism for the formation of inflammation causing prostaglandins,4 are the decisive players. Of the two isoforms of cyclooxygenases, cyclooxygenase-1 (COX-1) and COX-2 are almost identical in structure but have distinct biological functions.5 The inducible enzyme COX-2 mediates the synthesis of proinflammatory prostaglandins and thromboxanes,6 and it becomes the major therapeutic target for the treatment of inflammatory diseases. On the other hand, housekeeping enzyme COX-1 helps in homeostasis and blood clotting. As far as the treatment of inflammatory diseases is concerned, it has mostly been dependent on the use of nonsteroidal anti-inflammatory drugs including COX-1/2 nonselective inhibitors such as aspirin, ibuprofen, indomethacin, and diclofenac and selective COX-2 inhibitors (COXIBS) such as celecoxib.7 However, the severe side effects including gastrointestinal lesions, renal injury, and cardiovascular diseases associated with the clinical usage of these therapeutic agents8 have necessitated the search for new chemical entities with higher efficacy and low/no side effects.
The pattern of exhibiting diverse biological activities by the heterocyclic moieties has imparted them paramount importance in constituting the skeleton of several medicinally significant compounds.9 Specifically, the aza-heterocycles such as indole, pyrimidine, pyrazole, morpholine, piperidine, pyrrolidine, and triazine are serving as templates of a number of clinically used anti-inflammatory, antifungal, antileukemic, and neuroprotective agents10 and antiamoebic, anticancer, antileishmanial, antimalarial, antiviral, antitubercular, carbonic anhydrase inhibitor, cathepsin B inhibitor, and antimicrobial agents.11−14 In the context of these reports, it was envisaged that a hybrid molecule obtained by the combination of two/three aza-heterocycles may prove more efficacious than those drugs, which are made up of the individual aza-heterocycle moieties. Therefore, in continuation to the efforts for the development of indole–pyrazole/indolinone–chrysin-based conjugates as selective COX-2 inhibitors (A–D Figure 1),15 it was further planned to replace the chrysin moiety of the molecules with the substituted triazine. Hence, the replacement of the red colored part of molecules A–D with the blue colored component (consisting of indole, triazine, morpholine, piperidine, pyrrolidine, pyrimidine, and pyrazole heterocycles) led to the design of compounds 2–22 (Figure 1). These compounds were synthesized and screened for anti-inflammatory activity by using enzyme immunoassays and animal models. COX-2 as the cellular target of the molecules was confirmed by the in vivo mechanistic experiments supported by the physicochemical and the molecular modeling studies.
Figure 1.

Previously reported molecules A–D and the library of newly designed compounds 2–22.
2. Results and Discussion
2.1. Chemistry
The reaction of morpholine and cyanuric chloride provided di- and monosubstituted products 1a and 1b (Scheme 1). Taken in acetonitrile (ACN) in the presence of NaH, compound 1a was treated with indole-3-carboxaldehyde at room temperature (rt) and compound 2 was procured. Treatment of compound 2 with 1-(2,6-dichlorophenyl)-1,3-dihydroindol-2-one(indolinone)/1,3-dihydroindol-2-one(oxindole)/N,N-dimethylbarbituric acid/1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one under microwave irradiation in the presence of piperidine afforded compounds 3–6 (Scheme 1).
Scheme 1. Synthesis of Compounds 2–6.
Reagents and conditions: (a) Et3N, acetone, 4 h, 0 °C to rt; (b) NaH, ACN, 3 h, rt; and (c) MWI, CHCl3, piperidine, 2 h, 100 °C.
Similar to the reaction of 1a, treatment of compound 1b with indole-3-carboxaldehyde provided compound 7. Reaction of compound 7 with 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one/N,N-dimethyl barbituric acid/oxindole/indolinone under microwave irradiation in the presence of piperidine afforded compounds 8–12, respectively. Likewise, compounds 13–17 were prepared when compound 7 was reacted with 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one/N,N-dimethyl barbituric acid/oxindole/indolinone in the presence of pyrrolidine under microwave irradiation (Scheme 2). Interestingly, the incorporation of a heterocycle moiety at the CHO group of 7 and replacement of its Cl with piperidine/pyrrolidine occurred in one pot. However, stepwise reaction of 7, first with piperidine/pyrrolidine and then with the heterocycle moiety or vice versa, was also successful.
Scheme 2. Synthesis of Compounds 7–17.
Reagents and conditions: (a) NaH, ACN, 3 h, rt and (b) microwave irradiation (MWI), CHCl3, piperidine/pyrrolidine, 2 h, 100 °C.
Compounds 18–20 were obtained by heating compound 7 (1 mmol) with 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one/N,N-dimethyl barbituric acid/oxindole (1 mmol) at 155–160 °C for 25–30 min (Scheme 3).
Scheme 3. Synthesis of Compounds 18–20.
Reaction of piperidine (2 mmol) with cyanuric chloride (1 mmol) provided product 23 (Scheme 4). Treatment of compound 23 with indole-3-carboxaldehyde resulted into the formation of compound 21. Compound 21 on further reaction with 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one under microwave irradiation in the presence of piperidine provided compound 22 (Scheme 4).
Scheme 4. Synthesis of Compounds 21–22.
Interestingly, the treatment of cyanuric chloride with indole-3-carboxaldehyde provided compound 24 (Scheme 5). However, because of the high melting point (>300 °C) and insolubility of compound 24 in most of the polar and nonpolar solvents, we were not able to make further derivatization of this compound.
Scheme 5. Synthesis of Compound 24.
Reagents and conditions: (i) NaH, ACN, 0 °C, 0.5 h.
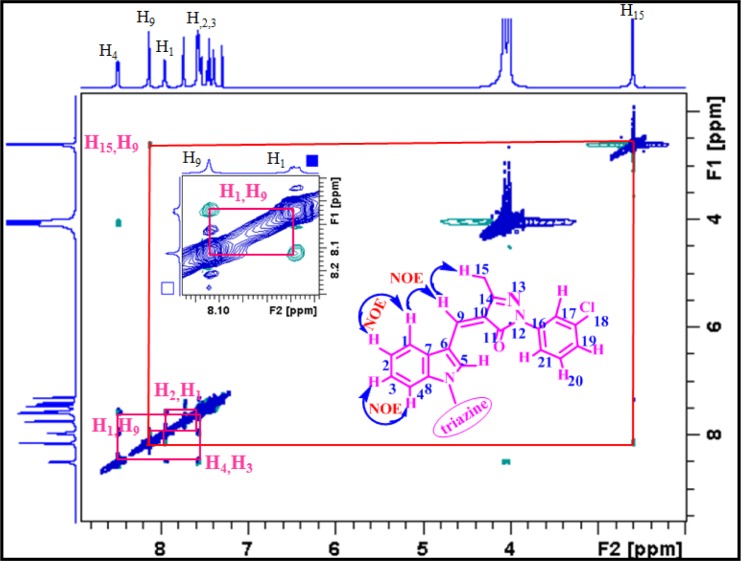
All the compounds were characterized by using NMR, IR, and high-resolution mass spectral techniques. NMR spectral data have unambiguously assigned Z-configuration at the double bond across C9–C10 (Figure 2). On the basis of various 1D and 2D NMR experiments including 1H, 13C, nuclear Overhauser enhancement spectroscopy (NOESY), correlation spectroscopy, heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond correlation (HMBC) (Figures 2 and 3), all the proton and carbon resonance frequencies of compound 6 were assigned. Characteristically, the peak at δ 8.12 is assigned to olefinic proton (H-9). The NOESY spectrum of compound 6 clearly showed the presence of NOE between H-9 and H-15 as well as between H-9 and H-1 (Figures 3 and S33), which confirmed the Z-configuration at the bridged C=C (C9–C10). Likewise, the H and C resonances of other compounds and their geometry across the bridged C=C bond were assigned.
Figure 2.

Overlay of HSQC (red contours) and HMBC (blue contours) NMR spectrum of compound 6.
Figure 3.
1H–1H NOESY NMR spectrum of compound 6. Inset: expansion of a part of the spectrum.
2.2. Biological Studies
2.2.1. In Vitro COX inhibitory Activity
By using the protocol available with the assay kits,16 COX-1 and COX-2 inhibitory activities of compounds 2–22 were checked. The enzyme inhibition assay was based on the quantification of the prostaglandins produced by the COX in AA metabolism, in the absence and presence of the test compounds. All the compounds were tested in triplicate at 10–4, 10–5, 10–6, 10–7, and 10–8 M concentration, and the 50% inhibitory concentration (IC50) for each of the compounds was calculated. The difference in the IC50 of the three enzyme assays was <3%, and the average of the three is reported in Table 1.
Table 1. IC50 (μM) of Compounds 2–22 against COX-1 and COX-2.

IC50 (COX-1)/IC50 (COX-2).
Appreciable inhibition of the COX-2 activity was observed in the presence of compound 2, and its IC50 was calculated 0.03 μM. Desirably, it was observed that compound 2 is selective for COX-2 over COX-1 and that the selectivity index was higher than that seen in the case of diclofenac and less than that of celecoxib. The presence of two morpholine moieties in compound 2 seems enviable for COX-2 inhibition because the analogue compounds 7, 8, 13, and 21 exhibit higher IC50 than that of compound 2. Compounds 2, 7, 8, 13, and 21 were further derivatized by incorporating a heterocycle moiety at their CHO group.
Compounds 3–6 (obtained by the derivatization of compound 2) exhibited wide spectrum of their COX-2 inhibitory profile. Compound 6 has an IC50 of 0.02 μM for COX-2, which is 2-fold higher than that of celecoxib and is comparable to the IC50 of diclofenac. The selectivity index 150 of compound 6 for COX-2 was much better than that of diclofenac and indomethacin. Compound 3 with an IC50 of 0.4 μM was also found to exhibit appreciable inhibition of COX-2, whereas compound 4 exhibited an IC50 of 1 μM for COX-2. The poor solubility of compound 5 in the assay medium did not allow its screening in the enzyme immunoassays.
Modification of compound 8 to compounds 9–12 also resulted into a significant variation in their IC50 for COX-2. Compounds 10, 11, and 12 exhibited IC50 values of 0.08, 0.6, and 0.05 μM, respectively, for COX-2. The selectivity index > 200 for compound 12 was appreciably higher than for diclofenac and indomethacin. However, the incorporation of a heterocycle moiety at the CHO group of compound 13 resulted into relatively less potent compounds 13–17. The derivatization of compound 7 into compounds 18–20 improved their COX-2 inhibitory profile. Conversion of compound 21 to compound 22 made 70-fold increases in its IC50 for COX-2 (Table 1).
Apparently, the compounds 6, 18, 12, 17, and 22 obtained by the incorporation of 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one on compounds 2, 7, 8, 13, and 21, respectively, resulted into better inhibition of COX-2. Moreover, in addition to the contribution of the pyrazole moiety, the presence of two morpholine units in compound 6 also seems responsible for the higher efficacy of this compound. The role of a morpholine moiety was also apparent from the comparison of IC50 values of compound 3 with that of compounds 9 and 14. However, for compounds 4, 10, 15, and 20, compound 10 with morpholine and piperidine moieties along with oxindole exhibited better IC50 (0.08 μM) for COX-2. In this group, compound 4 with two morpholine units along with oxindole has an IC50 value of 1 μM. Conspicuously, the presence of a pyrrolidine moiety in compounds 14, 15, and 17 increased the IC50 in comparison to that of compounds 3, 9, 4, 10, 6, and 12. Therefore, the analysis of the data given in Table 1 indicated that the morpholine/piperidine/pyrrolidine moiety contributes for the COX-2 inhibition in association with the heterocycle unit present on C-3 of indole. Hence, the prevalence of significant structure–activity relationship in these compounds may not put them into the category of PAINS assay.17 Overall, the presence of two morpholine units and a pyrazole-bearing indole moiety on the triazine template was optimized for the COX-2 inhibitory activity. On the basis of the preliminary investigations, compounds 2, 3, 4, 6, 10, 12, 17, 18, and 22 were further screened over the animal models for their analgesic and anti-inflammatory activity.
2.2.2. Human Whole Blood COX-1 and COX-2 Assay
Calcium ionophore (A23187)-stimulated production of thromboxane B2 (TXB2) in whole blood platelets was used to measure the activity of COX-1, whereas prostaglandin E2 (PGE2) production in lipopolysaccharide (LPS)-stimulated whole blood was used to assess the COX-2 activity.18 In both the cases, enzyme-linked immunosorbent assays were performed.16,19 These assays measured the amount of TXB2 and PGE2 in the serum that was produced in the presence and absence of the compound.
A23187-stimulation of human whole blood resulted in the increase in TXB2 production compared with the control blood sample (Table 2). Addition of 1 μM of compounds 6, 10, 12, and 17 to the blood sample did not affect ionophore-stimulated TXB2 production, indicating that compounds 6, 10, 12, and 17 exhibited almost negligible inhibition of COX-1. On the other hand, LPS stimulation of human whole blood increased the PGE2 production compared with the control blood sample (Table 2, Figure 4), and the addition of 1 μM of compounds 6, 10, 12, and 17 significantly decreased the PGE2 production, hence indicating that compounds 6, 10, 12, and 17 inhibited COX-2.
Table 2. Results of Enzyme Immunoassays Showing the Role of Compounds 6, 10, 12, and 17 (Final Concn 1 μM) in Inhibition of Calcium Ionophore-Stimulated TXB2 and LPS-Stimulated PGE2 in Whole Blood.
| TXB2 (ng/mL) |
PGE2 (ng/mL) |
|||
|---|---|---|---|---|
| –calcium ionophore | +calcium ionophore | –LPS | +LPS | |
| control | 0.16 | 2.00 | 0.145 | 1.98 |
| indomethacin | 1.05 | 1.35 | ||
| 6 | 1.85 | 0.91 | ||
| 10 | 1.55 | 1.02 | ||
| 12 | 1.75 | 0.95 | ||
| 17 | 1.70 | 1.00 | ||
Figure 4.
Graphical representation of the data given in Table 2. (A) TXB2 production in the presence of compounds is the same as in the control experiment, indicating that compounds did not inhibit COX-1. (B) PGE2 production in the presence of compounds is significantly lower than the control experiment indicating the inhibition of COX-2.
2.2.3. In Vivo Biological Studies
In vivo biological experiments were performed with the Swiss albino mice of either sex weighing 25–30 g. The study design was duly approved by the Institutional Animal Ethical Committee (IAEC). The formalin-induced hyperalgesia and carrageenan-induced paw inflammation models were used to study the analgesic and anti-inflammatory activities, respectively, of the compounds 2, 3, 4, 6, 10, 12, 17, 18, and 22.20 For analgesic activity, 11 groups of animals comprising five animals in each group were used. Group 1 was administered vehicle, group 2 diclofenac (10 mg kg–1), and groups 3–11 received compounds 2, 3, 4, 6, 10, 12, 17, 18, and 22, respectively, at the dose of 10 mg kg–1. All the compounds were administered intraperitoneally (i.p.) 30 min before formalin injection. For studying if COX, LOX, and nitric oxide pathways are the potential targets of the compounds, three groups including five animals per group were used. These groups received substance P (COX and LOX pathway stimulator), l-arginine (NO donor), and l-NAME (NOS inhibitor) 30 min before the most potent compound 6 was given and then were injected formalin after 30 min. To check if voltage-gated sodium channels and calcium influx processes are targeted by the compounds, the animals were pretreated with veratrine and A23187, respectively. For anti-inflammatory activity, three groups were used. The first group served as control, the second group was treated with standard drug indomethacin (10 mg kg–1), and the third received the most potent compound 6, followed by carrageenan.
2.2.3.1. Formalin-Induced Analgesia
Administration of diclofenac and compounds 2, 3, 4, 6, 10, 12, 17, 18, and 22 significantly decreased the number of flinching in the inflammatory phase as compared to the control group (Figure 5, Table 3). The effect of compounds 6, 12, and 17 was relatively higher in the series, although the difference in the analgesic effect of the various other analogues was statistically nonsignificant. Compounds 6, 12, and 17 were found to decrease formalin-induced analgesia by 68.24, 74.25, and 69%, respectively. The analgesic effect of compound 6 was significantly attenuated on pretreatment with substance P, whereas pretreatment with nitric oxide donor, l-arginine, or NOS inhibitor l-NAME did not alter the analgesic effect of compound 6 (Figure 6). Because substance P is known to stimulate COX-2 and LOX pathways15b and compound 6 did not inhibit 5-LOX activity (as determined by the enzyme immunoassay, IC50 > 100 μM), the analgesic effect of this compound was probably due to the inhibition of the COX-2 pathway. Because the analgesic effect of compound 6 was not altered on l-arginine or l-NAME pretreatment, the nitric oxide pathway may not be involved in the observed effect (Figure 6). Moreover, TXA2 stimulator A23187 pretreatment did not alter the analgesic effect of compound 6, which indicated that compound 6 does not affect COX-1.
Figure 5.

Graph showing change in formalin-induced hyperalgesia in the presence of compounds 2, 3, 4, 6, 10, 12, 17, 18, and 22. The values are given as mean ± SD, * is p < 0.05 vs control.
Table 3. Analgesic Activity of Compounds 2, 3, 4, 6, 10, 12, 17, 18, and 22.
| compd | % inhibition of algesia (analgesic effect) |
|---|---|
| 2 | 57.41 |
| 3 | 55.56 |
| 4 | 61.34 |
| 6 | 68.24 |
| 10 | 54.85 |
| 12 | 74.25 |
| 17 | 69.02 |
| 18 | 58.95 |
| 22 | 61.94 |
Figure 6.

Study of the mechanism of analgesic effect of compound 6 by using substance P, l-arginine, l-NAME, veratrine, and calcium ionophore. All values are given as mean ± SD, # is p < 0.05 vs compound 6.
2.2.3.2. Anti-Inflammatory Studies
A marked increase in the paw thickness of the control animals was observed on carrageenan injection, and it was reached maximum by 30–45 min of injection. Treatment of the animals with indomethacin and compounds 6 and 12 was found to decrease the paw thickness significantly as compared to the untreated control group (Figure 7). In our experiments, decrease of 18% (30 min) and 14% (60 min) in paw thickness at peak response time (30–60 min) was noticed in the presence of compound 6, whereas the presence of indomethacin decreased inflammation by 30% (30 min) and 25% (60 min) (Figure 7).
Figure 7.

Effect of compounds 6 and 12 on carrageenan-induced paw inflammation in mice. The values are taken as mean ± SEM. *p < 0.05 vs control group.
2.2.3.3. Acute Toxicity Studies
OECD guidelines for the acute oral toxicity of 14 days duration were followed for checking the toxicity of compound 6.21 Briefly, four groups comprising three animals in each group were studied. Vehicle was given to the first group, whereas second, third, and fourth groups were treated with compound 6 at 50, 300, and 2000 mg kg–1 dose. As per the protocol of acute toxicity studies, there was no mortality or any gross behavioral impairment after the 14th day. The tissue histology revealed no significant lesions except for some degree of congestion especially in the renal photomicrograph, and also the mesangium appeared shrunken and increased capsular space was seen in comparison to the control group (Figure 8). Hence, desirably, compound 6 did not exhibit toxicity even at a dose of 2000 mg kg–1.
Figure 8.

Histology of liver: (A) control and (B) compound 6 treated. Histology of kidney: (C) control and (D) compound 6 treated. Histology of myocardium: (E) control and (F) compound 6 treated. Each image was 20× magnified.
2.3. In Vivo Pharmacokinetic Studies
The in vivo pharmacokinetic studies of compound 6 were carried out by using a male wistar rat (250–300 g). The compound was suspended in 0.1% critical micelle concentration (CMC) and administered i.p. to the rats at a dose of 10 mg kg–1. The animals were anesthetized with ketamine (50 mg kg–1 i.p.). The blood samples were withdrawn from the jugular vein at an interval of 30 and 60 min and 2, 3, 4, 6, 8, 11, and 24 h of compound administration. The concentration of compound in the serum was determined using liquid chromatography–mass spectrometry (LC–MS). The different pharmacokinetic parameters were determined (Table 4, Figure 9) following non compartmental analysis in PK solver software. Compound 6 exhibited half-life 5.5 h and Cmax 58.5 μg mL–1.
Table 4. Pharmacokinetic Studies of Compound 6.
| parameter | unit | value |
|---|---|---|
| lambda_z | 1/min | 0.002110056 |
| t1/2 | Min | 328.497015 |
| Tmax | Min | 180 |
| Cmax | μg/mL | 58.5 |
| AUC 0–t | μg/mL·min | 11 421.9 |
| MRT 0–inf_obs | Min | 489.609039 |
| Vz/F_obs | (mg)/(μg/mL) | 0.379638448 |
| Cl/F_obs | (mg)/(μg/mL)/min | 0.000801058 |
Figure 9.

Pharmacokinetic study of compound 6.
2.4. Isothermal Titration Calorimetric (ITC) and UV–Vis Experiments
Because the biological results were in favor of COX-2 as the cellular target of compound 6, the binding affinity of the compound with the enzyme was checked with ITC experiments. Isothermal titration calorimetry measures the magnitude of the two thermodynamic terms: the enthalpy (ΔH) and entropy (ΔS) change in a single experiment and the combination of these two parameters defines the binding affinity (binding constant, K) between the two chemical entities. The solution of enzyme in phosphate buffer at pH 7.4 was put in the sample cell, and the solution of compound 6 (in the syringe) was injected stepwise (2 μL of 50 μM) after an interval of 120 s. For one experiment, 19 consecutive additions of the compound were made, and Ka, ΔH, and ΔS were measured. The heat change Q involved in the active cell during the interaction of the compound and the enzyme is given by eq 1
| 1 |
Here, Mt denotes the total concentration of the enzyme, n represents the binding sites of the enzyme, Vo is the cell volume, and ΔH is the molar heat of ligand binding.
For the ith injection of the compound with volume dVi to the cell-containing enzyme, the enthalpy change ΔH(i) is given by eq 2 and the values of different parameters are given in Table 5.
| 2 |
Table 5. Isothermal Calorimetric Data of Compound 6 during Its Interaction with COX-1 and COX-2.
The negative values of free energy (ΔG = −33.41 kJ/mol) and enthalpy (ΔH = −40.52 kJ/mol) indicated that the binding of compound 6 with COX-2 is spontaneous and exothermic.
UV–vis spectral studies with the solution of compound 6 and COX-2 were also performed, and the binding constant of the compound with COX-2 was calculated using the Benesi–Hildebrand equation.
where Af is the absorbance of the free host, Aobs is the absorbance observed, Afc is the absorbance at saturation, K is the binding constant, and L is the ligand concentration. Compound 6 showed significant interaction with COX-2 with a binding constant, Ka 1.92 × 105 M–1.
2.5. Docking Studies and Molecular Dynamics (MD) Simulation
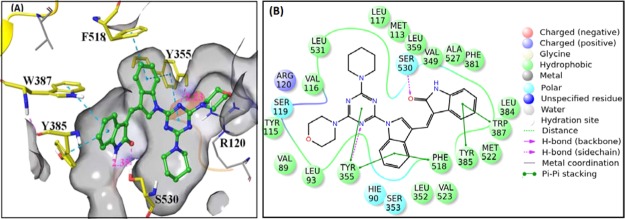
Molecular docking of the compounds in the enzyme active site was performed so that the nature of interactions between the compounds and the active site amino acid residues of the enzyme is explored. A 2.4 Å resolution structure of ovine COX-2 in complex with AA (PDB ID 1CVU)22 and a 3.0 Å resolution structure of COX-1 in complex with AA (PDB ID 1DIY)23 were taken from the protein data bank (www.rcsb.org). After refinement, these proteins were used for performing molecular docking and MD experiments. Flexibly docking mode of Schrodinger software package24 was used for molecular docking, and the nature of interactions of compounds with the surrounding amino acids was parameterized by using the binding modes and binding affinities in the active site of COX-2. Docking procedure was validated by docking AA in the active site of COX-2, and its root-mean-square deviation (rmsd) from the native AA was 1.18 Å (Figure S152). Compound 6 exhibited two H-bond interactions through its carbonyl oxygen and one of the three nitrogens of triazine with S530 (2.25 Å) and R120 (2.79 Å)—the amino acids which play major role during the catalytic phase of COX-2 (Figure 10a). It also displayed π–π interactions with Y355 and cation−π interaction with R513 (Figure 10a,b). Compounds 3, 4, 8, 10, 11, 12, 13, 17, 18, 19, and 22 also showed well-docked poses in the COX-2 active site (Figures S153–S198, Supporting Information) exhibiting H-bond and hydrophobic interactions. However, compounds 9 and 14 did not dock in the active site pocket of COX-2.
Figure 10.
(a) Crystal coordinates of compound 6 in association with the COX-2 active site (PDB ID 1CVU): (A) 3D view and (B) 2D view. (b) Hydrophobic interactions of compound 6 with the active site residues of COX-2.
To elucidate the importance of protein flexibility in the ligand binding site and to observe the dynamics of protein–ligand interactions, the energy-minimized docked complex of compound 6 with COX-2 was subjected to MD simulations in an aqueous solution environment for 50 ns. The overall stability of the system under simulation was evaluated using the rmsd of the backbone atoms. It was found that the rmsd of the protein backbone is significantly stable over the course of the MD simulation (Figure 11). The oxygen atom of morpholine and the nitrogen atom of the triazine ring of compound 6 effectively interact with the surrounding water molecules, which in turn are involved in the formation of hydrogen bonds with E524 and R513 (Figure 12). The stacked bar charts were normalized over the course of the trajectory. V523 formed strong hydrophobic interactions with compound 6, which were conserved along the simulations. Compound 6 formed hydrogen bonds and hydrophobic interactions with Y355 and L352 for more than 50% of the simulation time (Figure 13). N87 also contributed in H-bonding and water bridges with the ligand. Moreover, H90, P86, Y385, F518, and A527 exhibited polar and nonpolar interactions for <50% of the MD time.
Figure 11.

Rmsd of backbone atoms during evolution of trajectory of Cα (blue), side chain of protein (brown), and atoms of ligand 6 (pink) are shown.
Figure 12.

Interactions of compound 6 with the protein residues during evolution of trajectory (0.00–50 ns) that occur more than 10%.
Figure 13.

Interaction analysis between protein and compound 6 throughout the simulation over the period 0.00–50 ns. The stacked bar charts were normalized throughout the trajectory.
Compound 10 also showed well-docked pose in the COX-2 active site. H-bonding interactions through one of the nitrogens of triazine with phenolic −OH of Y355 side chain (2.23 Å) and another through its carbonyl oxygen with S530 (2.35 Å) were observed (Figure 14A). It also exhibited π–π interactions between 1,3,5-triazine ring and Y355 and between the aromatic ring of oxindole part and W387 and Y385 of COX-2 (Figure 14A,B). The aromatic ring of indole was also involved in π–π interactions with Y355 and F518 of COX-2.
Figure 14.
(A) Crystal coordinates of compound 10 and COX-2 active site (PDB ID 1CVU). (B) Interactions of compound 10 with the amino acids.
2.6. NMR Experiments for Enzyme–Ligand Interactions
To corroborate the results of docking studies, solution-phase NMR experiments of compound 10 in the absence and presence of COX-2 were performed. 1H chemical shift and spin–lattice relaxation time (T1) of various protons were measured by 1D 1H NMR and inversion recovery NMR experiments. The 1H NMR spectrum of 10 (12 mM) was recorded in 0.5 mL of DMSO-d6 at 25 °C (blue trace, Figure 15). Addition of 5 μL of COX-2 to the solution of compound 10 resulted in significant upfield shift of aromatic protons H-5, H-9, H-4, and H-16 (Figure 15), whereas no visible change in the CH2 protons of morpholine and piperidine ring was observed. These observations indicated that compound 10 interacts with COX-2. The protons H-5, H-9, H-4, and H-16 are probably under the shielding effect of hydrophobic residues of COX-2. The binding of compound with the enzyme was also confirmed by T1 experiments where spin–lattice relaxation time (T1) of various protons of compound 10 was measured (Figure 16). Characteristically, supporting the chemical shifts data, the relaxation time of H-5, H-9, H-1, H-4, and H-16 protons was considerably decreased in the presence of COX-2. Hence, the results of T1 measurement experiments indicated that an aromatic ring of indole and oxindole part of molecule 10 interacts with COX-2. All these protons were found to interact with the amino acid residues during the docking of compound 10 in the active site of COX-2 (Figure 17). Probably, these protons come under the shielding effect of the hydrophobic residues of the enzyme, and this was evident from the upfield shift of their resonance frequencies in the presence of the enzyme. Because of the less solubility of compound 6 in comparison to the solubility of 10 for NMR experiments, we did not perform 1H NMR and T1 experiments with 6.
Figure 15.
Part of 1H NMR spectrum of compound 10 (blue trace) and compound 10 in the presence of COX-2 (red trace).
Figure 16.

1H T1 relaxation times of compound 10 (12 mM) in the absence (red dots) and presence (blue dots) of COX-2.
Figure 17.

Compound 10 was positioned in the interacting pocket of COX-2 (PDB ID 1CVU). Arrows indicate the protons interacting with the amino acid residues. The same protons undergo change in the chemical shift and T1 in the presence of COX-2.
2.7. 3D-Quantitative Structure–Activity Relationship Study Model
Using partial least square (PLS) factor of two, the field-based 3D-quantitative structure–activity relationship (QSAR) model was generated by correlating the activity with steric, electrostatic, hydrophobic, hydrogen bond donor (HBD), and hydrogen bond acceptor (HBA) factors (Figures 18 and S205). Leave-one-out cross-validation and non-cross-validation analysis gave R2CV and R2 0.66 and 0.96, respectively. The standard error of the estimate was 0.18, and the F ratio was 102.0 (Tables 6 and S3). It was observed that the steric, electrostatic, hydrophobic, HBA, and HBD fields contribute 0.46, 0.093, 0.209, 0.227, and 0.00, respectively, for the activity of the compound. The green region in Figure 18a represents that bulky substituents are favorable for the activity, whereas the negative effect of the steric substituents is represented by the yellow region. The diagrammatic representation of the electrostatic factor is shown in Figure 18b where the blue contours indicate electropositive groups that may increase the activity of the compound and the red region displays electronegative groups. Desirably, the electropositive region of compounds 6, 12, and 17 represented by Ph and CH3 groups falls in the blue region, whereas the electronegative part of nitrogens is placed in the red contours of the map. In compounds 16 and 19, the electronegative oxygen part of the molecules lies in the unfavorable blue region, which might be leading to the decrease in their activity. The placement of Cl-phenyl/2,6-dichlorophenyl ring of compounds 3, 6, 9, 12, 18, and 22 in the yellow contours might be responsible for the higher activity of these molecules (Figure 18c). The docked complex of compound 6 with COX-2 (Figure 10b) also shows the hydrophobic interaction through Cl-phenyl ring and hence complies to the comparative molecular field analysis (CoMFA) model of the compound. The red color in Figure 18d favors the HBA group/s, whereas the presence of HBA group/s in the magenta contours is disfavored and may lead to reduced activity. In most of our compounds, the nitrogen atom of triazine ring and carbonyl oxygen is involved in hydrogen bonding with R120, S530, Y385, and Y355. Representing HBD contribution, the blue-violet region in Figure 18e favors the activity of the compound, whereas green color disfavors the activity. Because no HBD group is present in the blue contours, the contribution of HBD in the present CoMFA model is 0.00.
Figure 18.

QSAR contour maps showing contribution of various descriptors: (a) steric factor—yellow represents negative saturation and green represents positive saturation; (b) electrostatic factor—blue indicates positive effect and red represents negative saturation; (c) hydrophobic contours—white region represents negative and yellow represents positive saturation; (d) HBA—magenta region shows negative effect and maroon indicates positive saturation; (e) HBD—green represents negative saturation and blue-violet represents positive saturation. The molecules with better activity are shown in the form of a tube and inactive molecules in the form of thin wires.
Table 6. PLS-Based Statistical Parameters for the Selected 3D-QSAR Modela.
| PLS factors | SD | R2 | R2CV | R2 scramble | stability | F | P | rmse | Q2 | Pearson-R |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.4160 | 0.7322 | 0.5459 | 0.3537 | 0.955 | 32.8 | 9.49 × 10–5 | 0.38 | 0.4999 | 0.7701 |
| 2 | 0.1899 | 0.9688 | 0.6599 | 0.6149 | 0.798 | 102 | 7.92 × 10–8 | 0.27 | 0.7452 | 0.9163 |
SD, standard deviation of regression; R, squared value of R2 for the regression; F, variance ratio. Large value of F indicates more statistical regression; P, significance level of variance ratio. Smaller value indicates a greater degree of confidence; rmse, root-mean-square error; Q, squared value of Q2 for the predicted activities; Pearson-R, Pearson-R value for the correlation between the predicted and observed activity for the test set.
Barring the HBD descriptor that was contributing equally in all the compounds, the collective role of steric and hydrophobic parameters is shown in Figure 19. The large green contour present at the center of Cl-phenyl ring and 2,6-dichlorophenyl ring shows steric contribution to the activity of compounds 3, 6, 9, 10, 11, 17, and 22, whereas in compound 14, 2,6-dichlorophenyl ring did not fall in the green region. For the contribution of hydrophobicity, the favorable yellow region was found around the Cl-phenyl ring and 2,6-dichlorophenyl ring of compounds 3, 4, 6, 9, 10, 12, 17, and 22. Moreover, the hydrophobic contours partially overlap with sterically favorable region. Therefore, the COX-2 inhibitory activity of compounds 3, 4, 6, 9, 10, 12, 17, and 22 seems to be influenced by the synergistic effect of steric and hydrophobic parameters.
Figure 19.
Steric (A) and hydrophobic (B) contour fields generated around all compounds.
Significant fitness between the experimental activity and the predicted activity of the training and test set was observed in the QSAR model (Table S3) (Figures 20 and 21). A common pharmacophore model was generated by aligning all the active and inactive ligands to the pharmacophore (Figure 22).
Figure 20.
Scatter plot of observed versus predicted activity for training and test set compounds.
Figure 21.
Plot between observed and predicted COX-2 inhibitory activity of training set (a) and test set molecules (b) using a field-based 3D-QSAR model.
Figure 22.

Alignment of all ligands (active and inactive) to the pharmacophore.
3. Conclusions
With the help of in vitro and in vivo experiments on a series of compounds, we were able to identify compound 6 as a new lead for anti-inflammatory drugs. Compound 6 exhibited IC50 20 nM and Ka 4.83 × 105 M–1 for COX-2. The hydrophobic interactions of 6 with the enzyme were apparent from the change in chemical shifts and T1 of aromatic protons. Significant analgesic and anti-inflammatory activity of compound 6 with minimum toxicity risk was observed over Swiss albino mice. We observed a close correlation between the results of solution-phase NMR experiments and the molecular modeling studies, which could be helpful for the further refinement of the structure of the molecule.
4. Experimental Section
4.1. General
Melting points of the solid compounds were determined in capillaries. Using CDCl3 and/or dimethyl sulfoxide (DMSO)-d6 as the solvent and tetramethylsilane as the internal reference, 1H and 13C NMR spectra were recorded on Bruker 500 and 125 MHz NMR spectrometers. The chemical shifts are in parts per million, and coupling constants are given in hertz. For representing the multiplicity of signals in 1H NMR, s was used for singlet, d for doublet, dd for double-doublet, t for triplet, and m for multiplet. A Bruker micrOTOF Q II mass spectrometer was used for recording mass spectra. All the compounds were procured as single geometrical isomer (Z-isomer) except in the case of compounds 9 and 14 where inseparable E- and Z-isomers were obtained in a 2:1 ratio. The purity of the compounds (P %) was assessed by the q1H NMR method (absolute q1H NMR with internal calibration),25 and it was >98%.
where MW is the molecular weight; P is the purity of the internal calibrant; mIC represents the amount of internal calibrant; ms is the amount of sample (compound); Int is the integral; and n is the number of protons for an NMR signal. IC is the internal calibrant, and t is the target analyte or compound.
4.1.1. Synthesis of Compounds 1a and 1b
Cyanuric chloride (1 g, 5.43 mmol) was taken in acetone (40 mL) at 0 °C, and morpholine (0.94 g, 10.86 mmol) was added dropwise followed by the addition of triethylamine (1.09 g, 10.86 mmol). Then, the reaction mixture was stirred at 25–28 °C for 1 h. The reaction was quenched with water and extracted with ethyl acetate (4 × 25 mL). The organic layer was separated, dried over Na2SO4, and concentrated in vacuum to procure crude product that was column-chromatographed using ethyl acetate and hexane as eluents to procure products 1a and 1b.
4.1.2. Synthesis of Compounds 3–6: General Procedure
Compounds 3–6 were prepared through Knoevenagel condensation of compound 2 (1 mmol) with active methylene compounds including 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one; N,N-dimethylbarbituric acid; and oxindole and indolinone (1 mmol) in the presence of piperidine in CHCl3 at 100 °C for 2 h under microwave irradiation. The completion of the reaction was monitored with thin-layer chromatography (TLC). After completion of the reaction, the reaction mixture was quenched with water and extracted with CHCl3 (4 × 25 mL). The organic layer was separated, dried over Na2SO4, and concentrated in vacuum to procure crude product. The crude product was further purified by diethyl ether or by recrystallization in chloroform: methanol (2:8) to obtain compounds 3–6 with yield 80–85%.
4.1.3. Synthesis of Compounds 9–12: General Procedure
Compounds 9–12 were prepared through Knoevenagel condensation of compound 7 (1 mmol) with active methylene compounds including 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one; N,N-dimethylbarbituric acid; and oxindole and indolinone (1 mmol) in the presence of piperidine in CHCl3 at 100 °C for 2 h under microwave irradiation. After completion of the reaction (TLC), the reaction mixture was quenched with water and extracted with CHCl3 (4 × 25 mL). The chloroform layers were collected, dried over Na2SO4, and concentrated in vacuum to procure crude product that was purified by washing with ether or by recrystallization in chloroform: methanol (2:8) to obtain compounds 9–12 with yield 70–85%.
4.1.4. Synthesis of Compounds 13–17: General Procedure
Compounds 13–17 were prepared by the reaction of compound 7 (1 mmol) with active methylene compounds including 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one; N,N-dimethylbarbituric acid; and oxindole and indolinone (1 mmol) in the presence of pyrrolidine in CHCl3 at 100 °C for 2 h under microwave irradiation. After the reaction is completed (TLC), the reaction mixture was quenched with water and extracted with CHCl3 (4 × 25 mL). The chloroform part was dried over Na2SO4 and concentrated in vacuum to procure crude product. The crude product was further purified by washing with ether or by recrystallization in chloroform: methanol (2:8) to obtain compounds 13–17 with yield 70–85%.
4.1.5. Synthesis of Compounds 18–20: General Procedure
A finely ground mixture of 7 (1 mmol) and 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one/1,3-dimethyl barbituric acid/oxindole (1 mmol) was heated at 155–160 °C for 25–30 min. After completion of the reaction (TLC), the crude reaction mass was washed with diethyl ether to procure pure compounds 18–20.
4.1.5.1. 2-Chloro-4,6-dimorpholino-1,3,5-triazine (1a)
Compound 1a was procured as per the procedure given above. Colorless solid, yield 65%, mp 175–176 °C. 1H NMR (500 MHz, CDCl3): δ 3.70–3.80 (m, 16H, 8 × CH2). 13C NMR (125 MHz, CDCl3): δ 43.86 (−ve, CH2), 66.53 (−ve, CH2), 66.71 (−ve, CH2), 164.48 (ab, ArC), 169.69 (ab, ArC). HRMS (microTOF-QII, MS, ESI): calcd for C11H16N5O2Cl ([M + H]+), 286.1065; found, 286.1049.
4.1.5.2. 2,4-Dichloro-6-morpholino-1,3,5-triazine (1b)
Compound 1b was procured as per the procedure given above. Colorless solid, yield 30%, mp 161–162 °C. 1H NMR (500 MHz, CDCl3): δ 3.76 (t, 4H, J = 9.82 Hz, 2 × CH2), 3.90 (t, 4H, J = 9.82 Hz, 2 × CH2). 13C NMR (125 MHz, CDCl3): δ 44.47 (−ve, CH2), 66.38 (−ve, CH2), 164.10 (ab, ArC), 170.44 (ab, ArC). HRMS (ESI): calcd for C7H8N4OCl2 ([M + H]+), 235.0147; found, 235.0123.
4.1.5.3. 1-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-1H-indole-3-carbaldehyde (2)
NaH (1.2 mmol) was washed 3–4 times with dry hexane for the removal of paraffin coating, and then it was suspended in dry ACN (20 mL). Indole-3-carboxaldehyde (1 mmol) was added to NaH suspension in ACN, and the reaction mixture was stirred at 0 °C for 5–10 min until the whole reactant gets dissolved. Then, compound 1a (1.2 mmol) was added with continuous stirring. On completion of the reaction (TLC), the reaction was quenched by adding ice cold water. The reaction mixture was extracted with ethyl acetate. After drying over anhydrous Na2SO4, ethyl acetate was removed under vacuum. The residue was column-chromatographed by using ethyl acetate–hexane as eluents to procure pure product 2. Colorless solid, yield 76%, mp 244–245 °C. IR (KBr): 3135, 2961, 2902, 2860, 1730, 1684, 1571 cm–1. 1H NMR (500 MHz, CDCl3): δ 3.82–3.95 (m, 16H, 8 × CH2), 7.41–7.44 (m, 2H, ArH), 8.35 (d, 1H, J = 7.18 Hz, ArH), 8.61 (d, 1H, J = 7.99 Hz, ArH), 8.84 (s, 1H, CH), 10.16 (s, 1H, CHO). 13C NMR (125 MHz, CDCl3): δ 43.93 (−ve, CH2), 66.73 (−ve, CH2), 116.30 (+ve, ArCH), 120.74 (ab, ArC), 122.02 (+ve, ArCH), 124.10 (+ve, ArCH), 125.29 (+ve, ArCH), 127.01 (ab, ArC), 136.33 (ab, ArC), 136.82 (+ve, ArCH), 162.74 (ab, ArC), 165.13 (ab, ArC), 185.82 (C=O). HRMS (ESI): calcd for C20H22N6O3 ([M + H]+), 395.1826; found, 395.1819.
4.1.5.4. (Z)-1-(2,6-Dichlorophenyl)-3-((1-(4,6-dimorpholino-1,3,5-triazin-2-yl)1H-indolyl)methylene)indolin-2-one (3)
According to the general procedure given above, compound 3 was procured by the reaction of compound 2 and 1-(2,6-dichlorophenyl)indolin-2-one. Yellow solid, yield 80%, mp > 300 °C. IR (KBr): 3174, 3052, 1607, 1570, 1407 cm–1. 1H NMR (500 MHz, CDCl3): δ 3.79–3.96 (m, 16H, 8 × CH2), 6.46 (d, 1H, J = 7.63 Hz, ArH), 7.17 (t, 1H, J = 7.60 Hz, ArH), 7.23 (t, 1H, J = 7.60 Hz, ArH), 7.39–7.42 (m, 3H, ArH), 7.54–7.57 (m, 2H, ArH), 7.77 (d, 1H, J = 7.23 Hz, ArH), 7.93–7.94 (m, 1H, ArH), 8.03 (s, 1H, bridged H), 8.65–8.67 (m, 1H, ArH), 10.23 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 43.98 (−ve, CH2), 66.80 (−ve, CH2), 108.85 (+ve, ArCH), 113.83 (ab, C), 116.61 (+ve, ArCH), 117.88 (+ve, ArCH), 118.72 (+ve, ArCH), 121.68 (ab, ArC), 122.28 (+ve, ArCH), 122.88 (+ve, ArCH), 124.19 (+ve, ArCH), 126.40 (+ve, ArCH), 127.85 (+ve, ArCH), 128.89 (ab, ArC), 129.02 (+ve, ArCH), 129.13 (+ve, ArCH), 130.42 (+ve, ArCH), 131.25 (ab, ArC), 133.76 (+ve, ArCH), 135.46 (ab, ArC), 136.06 (ab, ArC), 139.75 (ab, ArC), 163.01 (ab, ArC), 165.27 (C=O). HRMS (ESI): calcd for C34H29N7O3Cl2 ([M + H]+), 654.1781; found, 654.1768.
4.1.5.5. (Z)-3-((1-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)indolin-2-one (4)
As per the general procedure given above, compound 4 was synthesized by using 2 and indolin-2-one. Yellow solid, yield 82%, mp > 300 °C. IR (KBr): 3430, 3127, 3064, 1697, 1605, 1410 cm–1. 1H NMR (500 MHz, CDCl3): δ 4.01–4.07 (m, 16H, 8 × CH2), 7.00 (t, 1H, J = 7.60 Hz, ArH), 7.14 (d, 1H, J = 7.97 Hz, ArH), 7.35 (t, 1H, J = 8.64 Hz, ArH), 7.48 (t, 1H, J = 7.60 Hz, ArH), 7.54 (t, 1H, J = 7.60 Hz, ArH), 7.72–7.77 (m, 2H, ArH), 8.07 (s, 1H, NH), 8.41 (d, 1H, J = 8.41 Hz, ArH), 8.69 (s, 1H, bridged H), 9.73 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 45.72 (−ve, CH2), 65.97 (−ve, CH2) 110.93 (ab, ArC), 111.91 (+ve, ArH), 113.19 (ab, ArC), 115.46 (ab, ArC), 116.36 (+ve, ArH), 117.72 (ab, ArC), 118.39 (ab, ArC), 120.83 (+ve, ArH), 121.52 (ab, ArC), 123.28 (+ve, ArH), 125.23 (+ve, ArH), 126.45 (+ve, ArH), 127.12 (ab, ArC), 127.70 (+ve, ArH), 130.08 (+ve, ArH), 130.17 (ab, ArC), 130.50 (+ve, ArH), 135.33 (ab, ArC), 140.10 (ab, ArC), 157.13 (ab, ArC), 159.63 (ab, ArC), 160.60 (ab, ArC), 160.94 (ab, ArC), 161.02 (ab, ArC), 161.28 (ab, ArC), 161.36 (ab, ArC), 161.62 (ab, ArC), 161.70 (C=O), 172.31 (ab, ArC). HRMS (ESI): calcd for C28H27N7O3 ([M + H]+), 510.2248; found, 510.2234.
4.1.5.6. 1,3-Dimethyl-5-((1-(4,6-dimorpholino-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)pyrimidine-2,4,6(1,3,5H)-trione (5)
In accordance to the general procedure given above, compound 5 was synthesized by using 2 and 1,3-dimethyl barbituric acid. Yellow solid, yield 80%, mp > 300 °C. IR (KBr): 3194, 2983, 2840, 1662, 1572, 1496 cm–1. 1H NMR (500 MHz, CDCl3): δ 3.45 (s, 3H, CH3), 3.46 (s, 3H, CH3), 3.84–4.01 (m, 16H, 8 × CH2), 7.42–7.45 (m, 2H, ArH), 7.96–7.98 (m, 1H, ArH), 8.63–8.64 (m, 1H, ArH), 8.99 (s, 1H, bridged H), 10.43 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 28.19 (+ve, CH3), 28.92 (+ve, CH3), 43.93 (−ve, CH2), 44.12 (−ve, CH2), 66.76 (−ve, CH2), 112.24 (ab, ArC), 113.84 (ab, ArC), 116.69 (+ve, ArCH), 118.31 (+ve, ArCH), 123.88 (+ve, ArCH), 124.95 (+ve, ArCH), 131.76 (ab, ArC), 135.64 (ab, ArC), 139.01 (+ve, ArCH), 146.25 (+ve, ArCH), 151.65 (ab, ArC), 161.50 (ab, ArC), 162.91 (C=O), 163.37 (C=O). HRMS (ESI): calcd for C26H28N8O5 ([M + Na]+), 555.2074; found, 555.2131.
4.1.5.7. (4Z)-1-(3-Chlorophenyl)-3-methyl-4-((1-(4,6-dimorpholino-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)-1H-pyrazol-5(4H)-one (6)
Using the general procedure given above, compound 6 was synthesized from 2 and 1-(3-chlorophenyl)-3-methyl-1H-pyrazol-5(4H)-one. Yellow solid, yield 85%, mp > 300 °C. IR (KBr): 3158, 3040, 2894, 1681, 1593, 1569, 1390 cm–1. 1H NMR (500 MHz, CDCl3): δ 2.59 (s, 3H, CH3), 4.00–4.06 (m, 16H, 8 × CH2), 7.37–7.38 (m, 1H, ArH), 7.43 (t, 1H, J = 8.14 Hz, ArH), 7.52–7.56 (m, 3H, ArH), 7.73 (s, 1H, ArH), 7.93–7.95 (m, 1H, ArH), 8.12 (s, 1H, bridged H), 8.47–8.49 (m, 1H, ArH), 10.55 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 12.35 (+ve, CH3), 44.89 (−ve, CH2), 66.31 (−ve, CH2), 110.95 (ab, ArC), 113.22 (ab, ArC), 115.49 (ab, ArC), 116.31 (ab, ArC), 116.72 (+ve, ArCH), 117.75 (ab, ArC), 118.38 (+ve, ArCH), 120.59 (+ve, ArCH), 121.35 (ab, ArC), 122.93 (+ve, ArCH), 125.46 (+ve, ArCH), 126.48 (+ve, ArCH), 127.89 (+ve, ArCH), 130.09 (ab, ArC), 130.35 (+ve, ArCH), 134.98 (ab, ArC), 135.67 (ab, ArC), 136.70 (ab, ArC), 139.49 (+ve, ArCH), 140.44 (+ve, ArCH), 153.05 (ab, ArC), 162.46 (C=O). HRMS (ESI): calcd for C30H29N8O3Cl ([M + H]+), 585.2123; found, 585.2156.
4.1.5.8. 1-(4-Chloro-6-morpholino-1,3,5-triazin-2-yl)-1H-indole-3-carbaldehyde (7)
After washing 3–4 times with dry hexane, NaH (1.22 mmol) was suspended in dry ACN (20 mL). To this suspension, 1 mmol indole-3-carboxaldehyde was added and stirred at 0 °C for 5–10 min until the whole reactant was dissolved. Compound 1b (1.2 mmol) was added to the above reaction mixture, and it was stirred continuously. On completion of the reaction (TLC), ice cold water was added and washed with ethyl acetate. The combined organic layers were dried over anhydrous Na2SO4 and concentrated under vacuum. The residue was column-chromatographed by using ethyl acetate–hexane as eluents to procure pure product 7. White solid, yield 65%, mp 235–236 °C. IR (KBr): 3146, 2959, 2899, 2858, 1678, 1573, 1460 cm–1. 1H NMR (500 MHz, CDCl3): δ3.83 (t, 2H, J = 4.76 Hz, CH2), 3.88 (t, 2H, J = 4.39 Hz, CH2), 3.98–4.02 (m, 4H, 2 × CH2), 7.41–7.46 (m, 2H, ArH), 8.34 (d, 1H, J = 7.63 Hz, ArH), 8.57 (d, 1H, J = 8.12 Hz, ArH), 8.78 (s, 1H, CH), 10.15 (s, 1H, CHO). 13C NMR (125 MHz, CDCl3): δ 44.4 (−ve, CH2), 44.5 (−ve, CH2), 66.2 (−ve, CH2), 66.5 (−ve, CH2), 116.5 (+ve, ArCH), 122.1 (+ve, ArCH), 122.3 (+ve, ArCH), 124.9 (+ve, ArCH), 126.0 (+ve, ArCH), 127.1 (ab, ArC), 136.0 (ab, ArC), 136.2 (+ve, ArCH), 162.6 (ab, ArC), 164.6 (ab, ArC), 170.5 (ab, ArC), 185.7 (C=O). HRMS (ESI): calcd for C16H14N5O2Cl ([M + H]+) 344.0908; found, 344.0938.
4.1.5.9. 1-(4-Morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)-1H-indole-3-carbaldehyde (8)
Compound 8 was prepared by the nucleophilic substitution of chlorine of compound 7 (1 mmol) with piperidine by performing the reaction in CHCl3 at 100 °C for 0.5 h under microwave irradiation. After completion of the reaction (TLC), water was added to the reaction mixture and washed with CHCl3 (4 × 25 mL). The combined organic layers were dried over Na2SO4 and concentrated in vacuum to procure crude product. The crude product was further purified by washing with ether to obtain pure compound 8. Creamish white solid, yield 75%, mp 236–237 °C. IR (KBr): 3143, 2939, 2854, 1667, 1591, 1572, 1444, 1408 cm–1. 1H NMR (500 MHz, CDCl3): δ 1.68–1.75 (m, 6H, 3 × CH2), 3.82–3.92 (m, 12H, 6 × CH2), 7.36–7.44 (m, 2H, ArH), 8.34 (d, 1H, J = 7.37 Hz, ArH), 8.64 (d, 1H, J = 8.19 Hz, ArH), 8.86 (s, 1H, CH), 10.16 (s, 1H, CHO). 13C NMR (125 MHz, CDCl3): δ 24.7 (−ve, CH2), 25.7 (−ve, CH2), 43.9 (−ve, CH2), 44.5 (−ve, CH2), 44.8 (−ve, CH2), 66.7 (−ve, CH2), 116.3 (+ve, ArCH), 120.5 (ab, ArC), 121.9 (+ve, ArCH), 123.9 (+ve, ArCH), 125.1 (+ve, ArCH), 126.9 (ab, ArC), 136.4 (ab, ArC), 137.0 (+ve, ArCH), 162.7 (ab, ArC), 165.3 (ab, ArC), 185.8 (C=O). HRMS (ESI): calcd for C21H24N6O2 ([M + H]+), 393.2033; found, 393.2022.
4.1.5.10. (Z)-1-(2,6-Dichlorophenyl)-3-((1-(4-morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)indolin-2-one (9)
Compound 9 was synthesized as two inseparable Z- and E-isomers (1:2, 1H NMR) according to the general procedure given above using compound 7 and 1-(2,6-dichlorophenyl)indolin-2-one. Yellow solid, yield 73%, mp > 300 °C. IR (KBr): 3170, 3051, 2933, 2852, 1717, 1608, 1570, 1445, 1406, 1361 cm–1. 1H NMR (500 MHz, CDCl3): δ 1.70–1.75 (m, 9H, 3 × CH2(maj) + 3 × CH2(min)), 3.78–3.94 (m, 18H, 6 × CH2(maj) + 6 × CH2(min)), 6.45–6.47 (m, 1H, ArHmin), 6.48–6.49 (m, 1H, ArHmaj), 7.00 (t, 1H, J = 7.70 Hz, ArHmaj), 7.17 (t, 1H, J = 7.7 Hz, ArHmin), 7.22 (t, 2H, J = 7.7 Hz, ArHmaj + ArHmin), 7.35–7.38 (m, 1H, ArHmaj), 7.39–7.49 (m, 4H, 2 × ArHmaj + 2 × ArHmin), 7.54–7.57 (m, 4H, 2 × ArHmaj + 2 × ArHmin), 7.76 (d, 1H, J = 7.4 Hz, ArHmin), 7.82 (d, 1H, J = 8.08 Hz, ArHmaj), 7.92–7.94 (m, 1H, ArHmin), 8.04 (s, 1H, bridged Hmin), 8.13 (d, 1H, J = 8.08 Hz, ArHmaj), 8.21 (s, 1H, bridged Hmaj), 8.71 (d, 2H, J = 8.08 Hz, ArHmaj + ArHmin), 9.11 (s, 1H, CHmaj), 10.23 (s, 1H, CHmin). 13C NMR (125 MHz, CDCl3): δ 24.8 (−ve, CH2), 25.8 (−ve, CH2), 43.8 (−ve, CH2), 44.5 (−ve, CH2), 44.8 (−ve, CH2), 66.8 (−ve, CH2), 108.8 (+ve, ArCH), 109.1 (+ve, ArCH), 113.5 (ab, ArC), 114.5 (ab, ArC), 116.5 (+ve, ArCH), 116.6 (+ve, ArCH), 117.7 (+ve, ArCH), 118.6 (+ve, ArCH), 119.8 (+ve, ArCH), 121.3 (ab, ArC), 122.2 (+ve, ArCH), 122.28 (ab, ArC), 122.7 (+ve, ArCH), 122.9 (+ve, ArCH), 123.1 (+ve, ArCH), 123.8 (ab, ArC), 124.0 (+ve, ArCH), 124.6 (+ve, ArCH), 125.0 (+ve, ArCH), 127.7 (+ve, ArCH), 128.8 (+ve, ArCH), 128.9 (+ve, ArCH), 128.97 (+ve, ArCH), 129.0 (+ve, ArCH), 129.2 (+ve, ArCH), 130.3 (+ve, ArCH), 130.4 (ab, ArC), 130.6 (+ve, ArCH), 130.8 (ab, ArC), 131.1 (ab, ArC), 131.3 (ab, ArC), 133.9 (+ve, ArCH), 135.5 (ab, ArC), 135.6 (ab, ArC), 135.9 (ab, ArC), 136.0 (ab, ArC), 139.7 (ab, ArC), 141.7 (ab, ArC), 162.7 (ab, ArC), 163.0 (ab, ArC), 164.7 (ab, ArC), 164.8 (ab, ArC), 165.3 (ab, ArC), 165.4 (C=O), 167.5 (C=O). HRMS (ESI): calcd for C35H31N7O2Cl2 ([M + H]+), 652.1989; found, 652.1955.
4.1.5.11. (Z)-3-((1-(4-Morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)indolin-2-one (10)
As per the general procedure, compound 10 was synthesized using 7 and indolin-2-one. Yellow solid, yield 69%, mp 294–296 °C. IR (KBr): 3001, 2936, 2850, 1697, 1605, 1570, 1447, 1408 cm–1. 1H NMR (500 MHz, CDCl3): δ 1.82 (m, 6H, 3 × CH2), 3.93–4.04 (m, 12H, 6 × CH2), 7.00 (t, 1H, J = 6.7 Hz, ArH), 7.09 (d, 1H, J = 7.81 Hz, ArH), 7.33 (t, 1H, J = 7.81 Hz, ArH), 7.46–7.47 (m, 1H, ArH), 7.51–7.53 (m, 1H, ArH), 7.73 (d, 1H, J = 7.81 Hz, ArH), 7.82 (d, 1H, J = 7.81 Hz, ArH), 8.05 (s, 1H, bridged H), 8.45 (d, 1H, J = 7.81 Hz, ArH), 8.75 (s, 1H, NH), 9.69 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 23.4 (−ve, CH2), 25.4 (−ve, CH2), 46.0 (−ve, CH2), 47.5 (−ve, CH2), 65.9 (−ve, CH2) 111.2 (ab, ArC), 111.5 (+ve, ArCH), 113.4 (ab, ArC), 115.7 (ab, ArC), 116.5 (+ve, ArCH), 118.0 (ab, ArC), 120.6 (+ve, ArCH), 121.6 (ab, ArC), 123.0 (+ve, ArCH), 123.2 (+ve, ArCH), 125.0 (+ve, ArCH), 126.3 (+ve, ArCH), 127.8 (+ve, ArCH), 129.6 (+ve, ArCH), 130.3 (+ve, ArCH), 135.3 (ab, ArC), 140.2 (ab, ArC), 154.9 (ab, ArC), 156.3 (C=O), 172.2 (ab, ArC). HRMS (ESI): calcd for C29H29N7O2 ([M + H]+), 508.2455; found, 508.2434.
4.1.5.12. 1,3-Dimethyl-5-((1-(4-morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)pyrimidine-2,4,6(1,3,5H)-trione (11)
Compound 11 was synthesized according to the general procedure given above using 7 and 1,3-dimethyl barbituric acid. Yellow solid, yield 81%, mp > 300 °C. IR (KBr): 3193, 2924, 2849, 1662, 1595, 1496, 1450, 1388 cm–1. 1H NMR (500 MHz, CDCl3): δ 1.74–1.75 (m, 6H, 3 × CH2), 3.45 (s, 3H, CH3), 3.46 (s, 3H, CH3), 3.83–3.97 (m, 12H, 6 × CH2), 7.41–7.43 (m, 2H, ArH), 7.96–7.97 (m, 1H, ArH), 8.65–8.67 (m, 1H, ArH), 8.99 (s, 1H, bridged H), 10.45 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 24.7 (−ve, CH2), 25.7 (−ve, CH2), 28.1 (+ve, CH3), 28.9 (+ve, CH3), 43.9 (−ve, CH2), 44.5 (−ve, CH2), 44.9 (−ve, CH2), 66.8 (−ve, CH2), 111.9 (ab, ArC), 113.6 (ab, ArC), 116.7 (+ve, ArCH), 118.1 (+ve, ArCH), 123.7 (+ve, ArCH), 124.8 (+ve, ArCH), 131.7 (ab, ArC), 135.7 (ab, ArC), 139.3 (+ve, ArCH), 146.2 (+ve, ArCH), 151.7 (ab, ArC), 161.5 (ab, ArC), 162.9 (ab, ArC), 163.4 (ab, ArC), 164.7 (C=O), 165.3 (C=O). HRMS (ESI): calcd for C27H30N8O4 ([M + H]+), 531.2462; found, 531.2494.
4.1.5.13. (Z)-1-(3-Chlorophenyl)-3-methyl-4-((1-(4-morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)-1H-pyrazol-5(4H)-one (12)
As per the procedure given above, compound 12 was synthesized using 7 and 1-(3-chlorophenyl)-3-methyl-1H-pyrazol-5(4H)-one. Orange solid, yield 75%, mp > 300 °C. IR (KBr): 3156, 2922, 2851, 1682, 1592, 1570, 1500, 1445, 1401, 1288 cm–1. 1H NMR (500 MHz, CDCl3): δ 1.72–1.76 (m, 6H, 3 × CH2), 2.40 (s, 3H, CH3), 3.86 (m, 12H, 6 × CH2), 7.14 (d, 1H, J = 6.82 Hz, ArH), 7.34 (t, 1H, J = 7.96 Hz, ArH), 7.38–7.42 (m, 2H, ArH), 7.68 (s, 1H, bridged H), 7.79 (d, 1H, J = 6.82 Hz, ArH), 7.94 (d, 1H, J = 7.96 Hz, ArH), 8.28 (s, 1H, ArH), 8.67 (d, 1H, J = 7.96 Hz, ArH), 10.83 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 13.1 (+ve, CH3), 24.8 (−ve, CH2), 25.8 (−ve, CH2), 43.9 (−ve, CH2), 44.7 (−ve, CH2), 66.8 (−ve, CH2), 113.8 (ab, ArC), 116.1 (+ve, ArCH), 116.9 (+ve, ArCH), 117.6 (+ve, ArCH), 118.4 (+ve, ArCH), 122.6 (ab, ArC), 123.3 (+ve, ArCH), 123.9 (+ve, ArCH), 124.6 (+ve, ArCH), 129.6 (+ve, ArCH), 130.5 (ab, ArC), 134.3 (ab, ArC), 134.8 (+ve, ArCH), 135.7 (ab, ArC), 138.2 (+ve, ArCH), 140.0 (ab, ArC), 150.6 (ab, ArC), 162.7 (ab, ArC), 162.9 (C=O), 164.7 (ab, ArC), 165.2 (ab, ArC). HRMS (ESI): calcd for C31H31N8O2Cl ([M + H]+), 583.2331; found, 583.2308.
4.1.5.14. 1-(4-Morpholino-6-(pyrrolidin-1-yl)-1,3,5-triazin-2-yl)-1H-indole-3-carbaldehyde (13)
Compound 13 was prepared by the nucleophilic substitution of the chlorine atom of compound 7 (1 mmol) with pyrrolidine by performing the reaction in CHCl3 at 100 °C for 0.5 h under microwave irradiation. After completion of the reaction (TLC), water was added and extracted with CHCl3 (4 × 25 mL). The combined organic layers were dried over Na2SO4 and concentrated in vacuum to procure crude product. The crude product was further purified by washing with ether to obtain pure compound 13. Creamish white solid, yield 71%, mp 215–216 °C. IR (KBr): 3137, 2958, 2863, 1663, 1572, 1510, 1446, 1409 cm–1. 1H NMR (500 MHz, CDCl3): δ 2.01–2.07 (m, 4H, 2 × CH2), 3.61–3.64 (m, 2H, CH2), 3.75–3.93 (m, 12H, 6 × CH2), 7.36–7.43 (m, 2H, ArH), 8.34 (d, 1H, J = 7.61 Hz, ArH), 8.74 (d, 1H, J = 7.61 Hz, ArH), 8.88 (s, 1H, CH), 10.15 (s, 1H, CHO). 13C NMR (125 MHz, CDCl3): δ 25.1 (−ve, CH2), 25.3 (−ve, CH2), 43.9 (−ve, CH2), 46.3 (−ve, CH2), 46.5 (−ve, CH2), 66.7 (−ve, CH2), 116.6 (+ve, ArCH), 120.5 (ab, ArC), 121.8 (+ve, ArCH), 123.9 (+ve, ArCH), 125.1 (+ve, ArCH), 127.0 (ab, ArC), 136.5 (ab, ArC), 136.8 (+ve, ArCH), 162.3 (ab, ArC), 163.5 (ab, ArC), 164.9 (ab, ArC), 185.8 (C=O). HRMS (ESI): calcd for C20H22N6O2 ([M + H]+), 379.1877; found, 379.1890.
4.1.5.15. (Z)-1-(2,6-Dichlorophenyl)-3-((1-(4-morpholino-6-(pyrrolidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)indolin-2-one (14)
Compound 14 was synthesized as inseparable E- and Z-isomers (2:1, 1H NMR) according to the general procedure given above using compound 7 and 1-(2,6-dichlorophenyl)indolin-2-one. Yellow solid, yield 72%, mp 290–292 °C. IR (KBr): 3165, 3049, 2961, 2857, 1713, 1607, 1568, 1511, 1446, 1404, 1361 cm–1. 1H NMR (500 MHz, CDCl3): δ 1.99–2.08 (m, 6H, 2 × CH2(maj) + 2 × CH2(min)), 3.61–3.66 (m, 3H, CH2(maj) + CH2(min)), 3.78–3.95 (m, 15H, 5 × CH2(maj) + 5 × CH2(min)), 6.45–6.49 (m, 2H, ArHmaj + ArHmin), 7.01 (t, 1H, J = 8.05 Hz, ArHmaj), 7.15–7.18 (m, 1H, ArHmin), 7.21–7.24 (m, 2H, ArHmaj + ArHmin), 7.35–7.38 (m, 1H, ArHmaj), 7.39–7.45 (m, 4H, 2 × ArHmaj + 2 × ArHmin), 7.54–7.57 (m, 4H, 2 × ArHmaj + 2 × ArHmin), 7.76 (d, 1H, J = 7.3 Hz, ArHmin), 7.82 (d, 1H, J = 7.82 Hz, ArHmaj), 7.92–7.94 (m, 1H, ArHmin), 8.04 (s, 1H, bridged Hmin), 8.14 (d, 1H, J = 7.82 Hz, ArHmaj), 8.21 (s, 1H, bridged Hmaj), 8.79–8.81 (m, 2H, ArHmaj + ArHmin), 9.13 (s, 1H, CHmaj), 10.23 (s, 1H, CHmin). 13C NMR (125 MHz, CDCl3): δ 25.2 (−ve, CH2), 25.3 (−ve, CH2), 43.9 (−ve, CH2), 46.2 (−ve, CH2), 46.5 (−ve, CH2), 66.8 (−ve, CH2), 108.7 (+ve, ArCH), 109.1 (+ve, ArCH), 113.5 (ab, ArC), 114.5 (ab, ArC), 116.8 (+ve, ArCH), 117.0 (+ve, ArCH), 117.6 (+ve, ArCH), 118.6 (+ve, ArCH), 119.7 (+ve, ArCH), 121.3 (ab, ArC), 122.2 (+ve, ArCH), 122.3 (ab, ArC), 122.7 (+ve, ArCH), 122.9 (+ve, ArCH), 123.1 (+ve, ArCH), 123.8 (ab, ArC), 124.0 (+ve, ArCH), 124.6 (+ve, ArCH), 125.1 (ab, ArC), 126.5 (+ve, ArCH), 127.7 (+ve, ArCH), 128.8 (+ve, ArCH), 128.9 (+ve, ArCH), 129.0 (+ve, ArCH), 129.3 (+ve, ArCH), 130.3 (+ve, ArCH), 130.4 (ab, ArC), 130.5 (+ve, ArCH), 130.9 (ab, ArC), 131.2 (ab, ArC), 131.3 (ab, ArC), 133.8 (+ve, ArCH), 135.6 (ab, ArC), 135.7 (ab, ArC), 136.1 (ab, ArC), 139.7 (ab, ArC), 141.8 (ab, ArC), 162.3 (ab, ArC), 162.5 (ab, ArC), 163.6 (ab, ArC), 163.7 (ab, ArC), 165.0 (ab, ArC), 165.2 (C=O), 167.5 (C=O). HRMS (ESI): calcd for C34H29N7O2Cl2 ([M + H]+), 638.1832; found, 638.1864.
4.1.5.16. (Z)-3-((1-(4-Morpholino-6-(pyrrolidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)indolin-2-one (15)
Compound 15 was synthesized according to the general procedure by using 7 and indolin-2-one. Yellow solid, yield 70%, mp 260–262 °C. IR (KBr): 3054, 2958, 2868, 1692, 1606, 1567, 1447, 1407 cm–1. 1H NMR (500 MHz, CDCl3): δ 2.01–2.05 (m, 4H, 2 × CH2), 3.62–3.65 (m, 2H, CH2), 3.77–3.84 (m, 6H, 3 × CH2), 3.94–3.97 (m, 4H, 2 × CH2), 6.90–6.91 (m, 1H, ArH), 7.09 (t, 1H, J = 7.86 Hz, ArH), 7.21–7.24 (m, 2H, ArH), 7.38–7.42 (m, 2H, ArH), 7.65 (d, 1H, J = 8.65 Hz, ArH), 7.82 (s, 1H, NH), 7.93 (s, 1H, bridged H), 8.82 (d, 1H, J = 7.35 Hz, ArH), 10.35 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 25.2 (−ve, CH2), 25.4 (−ve, CH2), 43.8 (−ve, CH2), 45.0 (−ve, CH2), 46.2 (−ve, CH2), 46.5 (−ve, CH2), 66.8 (−ve, CH2), 109.2 (+ve, ArCH), 113.6 (ab, ArC), 116.8 (ab, ArC), 117.0 (+ve, ArH), 117.6 (+ve, ArH), 118.6 (+ve, ArH), 121.5 (+ve, ArH), 122.1 (ab, ArC), 122.7 (+ve, ArH), 124.1 (+ve, ArH), 125.7 (ab, ArC), 126.0 (+ve, ArH), 127.7 (+ve, ArH), 129.0 (ab, C), 133.6 (+ve, ArH), 135.6 (ab, ArC), 138.6 (ab, ArC), 162.5 (ab, ArC), 163.7 (ab, ArC), 165.0 (C=O), 168.3 (ab, ArC). HRMS (ESI): calcd for C28H27N7O2 ([M + H]+), 494.2299; found, 494.2309.
4.1.5.17. 1,3-Dimethyl-5-((1-(4-morpholino-6-(pyrrolidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)pyrimidine-2,4,6(1,3,5H)-trione (16)
As per the procedure given above, compound 16 was procured using 7 and 1,3-dimethyl barbituric acid. Yellow solid, yield 82%, mp 253–254 °C. IR (KBr): 3192, 2963, 2868, 1663, 1570, 1501, 1448, 1388 cm–1. 1H NMR (500 MHz, CDCl3): δ 2.02–2.09 (m, 4H, 2 × CH2), 3.46 (s, 3H, CH3), 3.46 (s, 3H, CH3), 3.63–3.65 (m, 2H, CH2), 3.79–3.82 (m, 6H, 3 × CH2), 3.91–3.96 (m, 4H, 2 × CH2), 7.41–7.43 (m, 2H, ArH), 7.96–7.97 (m, 1H, ArH), 8.77–8.78 (m, 1H, ArH), 9.00 (s, 1H, bridged H), 10.47 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 25.2 (−ve, CH2), 25.3 (−ve, CH2), 28.1 (+ve, CH3), 28.9 (+ve, CH3), 43.8 (−ve, CH2), 46.2 (−ve, CH2), 46.3 (−ve, CH2), 46.5 (−ve, CH2), 66.8 (−ve, CH2), 111.9 (ab, ArC), 113.6 (ab, ArC), 117.1 (+ve, ArCH), 118.1 (+ve, ArCH), 123.7 (+ve, ArCH), 124.8 (+ve, ArCH), 131.7 (ab, ArC), 135.8 (ab, ArC), 139.1 (+ve, ArCH), 146.4 (+ve, ArCH), 151.7 (ab, ArC), 161.5 (ab, ArC), 162.4 (ab, ArC), 163.4 (ab, ArC), 163.5 (C=O), 164.9 (C=O). HRMS (ESI): calcd for C26H28N8O4 ([M + H]+), 517.2306; found, 517.2286.
4.1.5.18. (Z)-1-(3-Chlorophenyl)-3-methyl-4-((1-(4-morpholino-6-(pyrrolidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)-1H-pyrazol-5(4H)-one (17)
In accordance to the general procedure, compound 17 was synthesized using 7 and 1-(3-chlorophenyl)-3-methyl-1H-pyrazol-5(4H)-one. Orange solid, yield 76%, mp 280–282 °C. IR (KBr): 3151, 2956, 2864, 1682, 1592, 1571, 1525, 1446, 1390 cm–1. 1H NMR (500 MHz, CDCl3): δ 2.01–2.09 (m, 4H, 2 × CH2), 2.41 (s, 3H, CH3), 3.60–3.63 (m, 2H, CH2), 3.81–4.06 (m, 10H, 5 × CH2), 7.14 (d, 1H, J = 8.19 Hz, ArH), 7.34 (t, 1H, J = 8.19 Hz, ArH), 7.39–7.42 (m, 2H, ArH), 7.70 (s, 1H, bridged H), 7.79 (d, 1H, J = 6.83 Hz, ArH), 7.95 (d, 1H, J = 6.83 Hz, ArH), 8.27 (s, 1H, ArH), 8.78 (d, 1H, J = 6.83 Hz, ArH), 10.84 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 13.2 (+ve, CH3), 25.2 (−ve, CH2), 25.3 (−ve, CH2), 43.8 (−ve, CH2), 46.3 (−ve, CH2), 46.5 (−ve, CH2), 66.9 (−ve, CH2), 113.8 (ab, ArC), 116.2 (+ve, ArCH), 117.2 (+ve, ArCH), 117.5 (+ve, ArCH), 118.5 (+ve, ArCH), 122.6 (ab, ArC), 123.3 (+ve, ArCH), 123.9 (+ve, ArCH), 124.6 (+ve, ArCH), 129.5 (+ve, ArCH), 130.5 (ab, ArC), 134.3 (ab, ArC), 134.8 (+ve, ArCH), 135.8 (ab, ArC), 138.0 (+ve, ArCH), 140.0 (ab, ArC), 150.6 (ab, ArC), 162.3 (ab, ArC), 163.0 (C=O), 163.6 (ab, ArC), 164.8 (ab, ArC). HRMS (ESI): calcd for C30H29N8O2Cl ([M + H]+), 569.2174; found, 569.2145.
4.1.5.19. (Z)-4-((1-(4-Chloro-6-morpholino-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)-1-(3-chlorophenyl)-3-methyl-1H-pyrazol-5(4H)-one (18)
As per the general procedure, compound 18 was synthesized using 7 and 1-(3-chlorophenyl)-3-methyl-1H-pyrazol-5(4H)-one. Orange solid, yield 77%, mp 225–227 °C. IR (KBr): 3152, 2960, 2917, 2858, 1678, 1594, 1572, 1494, 1448, 1389 cm–1. 1H NMR (500 MHz, CDCl3): δ 2.35 (s, 3H, CH3), 3.79–3.81 (m, 2H, CH2), 3.89–3.91 (m, 4H, 2 × CH2), 3.92–4.05 (m, 2H, CH2), 7.16 (d, 1H, J = 8.18 Hz, ArH), 7.33–7.42 (m, 3H, ArH), 7.47 (s, 1H, bridged H), 7.66 (d, 1H, J = 7 Hz, ArH), 7.92–7.93 (m, 1H, ArH), 8.20 (s, 1H, ArH), 8.64 (d, 1H, J = 8.18 Hz, ArH), 10.51 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 13.0 (+ve, CH3), 44.2 (−ve, CH2), 44.5 (−ve, CH2), 66.4 (−ve, CH2), 66.5 (−ve, CH2), 115.2 (ab, ArC), 116.2 (+ve, ArCH), 117.3 (+ve, ArCH), 117.7 (+ve, ArCH), 118.4 (+ve, ArCH), 124.1 (+ve, ArCH), 124.2 (+ve, ArCH), 125.3 (+ve, ArCH), 126.8 (ab, ArC), 129.7 (+ve, ArCH), 130.1 (ab, ArC), 130.4 (ab, ArC), 133.5 (+ve, ArCH), 134.4 (ab, ArC), 135.3 (ab, ArC), 136.2 (+ve, ArCH), 139.7 (ab, ArC), 150.4 (ab, ArC), 162.5 (ab, ArC), 164.2 (C=O), 170.7 (ab, ArC). HRMS (ESI): calcd for C26H21N7O2Cl2 ([M + H]+), 534.1206; found, 534.1184.
4.1.5.20. 5-((1-(4-Chloro-6-morpholino-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)-1,3-dimethylpyrimidine-2,4,6(1,3,5H)-trione (19)
Compound 19 was synthesized using 7 and 1,3-dimethyl barbituric acid. Yellow solid, yield 75%, mp 268–270 °C. IR (KBr): 3186, 2962, 2851, 1726, 1669, 1577, 1499, 1300 cm–1. 1H NMR (500 MHz, CDCl3): δ 3.46 (s, 6H, 2 × CH3), 3.84 (t, 2H, J = 4.95 Hz, CH2), 3.90 (t, 2H, J = 4.71 Hz, CH2), 4.00 (t, 2H, J = 4.95 Hz, CH2), 4.06 (t, 2H, J = 4.95 Hz, CH2), 7.45–7.47 (m, 2H, ArH), 7.93–7.95 (m, 1H, ArH), 8.65–8.67 (m, 1H, ArH), 8.91 (s, 1H, bridged H), 10.28 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 28.3 (+ve, CH3), 28.9 (+ve, CH3), 44.4 (−ve, CH2), 44.6 (−ve, CH2), 66.4 (−ve, CH2), 66.5 (−ve, CH2), 113.9 (ab, ArC), 115.0 (ab, ArC), 117.0 (+ve, ArCH), 118.4 (+ve, ArCH), 124.5 (+ve, ArCH), 125.6 (+ve, ArCH), 131.7 (ab, ArC), 135.3 (ab, ArC), 137.4 (+ve, ArCH), 145.4 (+ve, ArCH), 151.4 (ab, ArC), 161.3 (ab, ArC), 162.8 (ab, ArC), 163.0 (C=O), 164.5 (C=O), 170.8 (ab, ArC). HRMS (ESI): calcd for C22H20N7O4Cl ([M + H]+), 482.1338; found, 482.1365.
4.1.5.21. (Z)-3-((1-(4-Chloro-6-morpholino-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)indolin-2-one (20)
Compound 20 was synthesized using 7 and indolin-2-one. Yellow solid, yield 68%, mp 294–296 °C. IR (KBr): 3160, 3072, 2862, 1696, 1608, 1565, 1502, 1451 cm–1. 1H NMR (500 MHz, CDCl3): δ 3.73–3.96 (m, 8H, 4 × CH2), 6.87 (d, 1H, J = 7.78 Hz, ArH), 7.03 (t, 1H, J = 7.75 Hz, ArH), 7.23 (t, 1H, J = 6.45 Hz, ArH), 7.44–7.48 (m, 2H, ArH), 7.97–7.99 (m, 1H, ArH), 8.06–8.08 (m, 1H, ArH), 8.28–8.31 (m, 1H, ArH), 8.60 (s, 1H, bridged H), 10.20 (s, 1H, CH), 10.69 (s, 1H, NH). 13C NMR (125 MHz, CDCl3): δ 44.5 (−ve, CH2), 44.7 (−ve, CH2), 66.0 (−ve, CH2), 66.1 (−ve, CH2), 109.8 (+ve, ArCH), 115.7 (ab, ArC), 117.0 (+ve, ArH), 119.7 (+ve, ArH), 120.4 (+ve, ArH), 121.3 (+ve, ArH), 124.3 (+ve, ArH), 125.1 (ab, ArC), 125.5 (+ve, ArH), 125.6 (+ve, ArH), 129.0 (+ve, ArH), 131.5 (ab, C), 131.6 (+ve, ArH), 134.9 (ab, ArC), 140.7 (ab, ArC), 162.5 (ab, ArC), 164.5 (C=O), 168.1 (ab, ArC), 169.8 (ab, ArC). HRMS (ESI): calcd for C24H19N6O2Cl ([M + H]+), 459.1330; found, 459.1293.
4.1.5.22. 1-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-yl)-1H-indole-3-carbaldehyde (21)
NaH (1.2 mmol) was washed 3–4 times with dry hexane and then suspended in dry ACN (20 mL). To the NaH suspension in dry ACN, 1 mmol indole-3-carboxaldehyde was added and stirred at 0 °C for 5–10 min. Then, compound 23 (1.2 mmol) was added, and the reaction mixture was stirred continuously. On completion of the reaction (TLC), ice cold water was added and washed with ethyl acetate (4 × 25 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuum. The residue was column-chromatographed by using ethyl acetate–hexane as eluents to procure product 21. Creamish white solid, yield 62%, mp 210–212 °C. IR (KBr): 3130, 3000, 2928, 2851, 2209, 1584, 1513, 1446, 1406, 1287 cm–1. 1H NMR (500 MHz, CDCl3): δ 1.67–1.74 (m, 12H, 6 × CH2), 3.88 (m, 8H, 4 × CH2), 7.35–7.42 (m, 2H, ArH), 8.34 (d, 1H, J = 7.5 Hz, ArH), 8.68 (d, 1H, J = 8.4 Hz, ArH), 8.89 (s, 1H, CH), 10.16 (s, 1H, CHO). 13C NMR (125 MHz, CDCl3): δ 24.8 (−ve, CH2), 25.8 (−ve, CH2), 44.5 (−ve, CH2), 116.4 (+ve, ArCH), 120.3 (+ve, ArCH), 121.8 (+ve, ArCH), 123.8 (+ve, ArCH), 125.0 (+ve, ArCH), 126.9 (ab, ArC), 136.4 (ab, ArC), 137.2 (+ve, ArCH), 162.7 (ab, ArC), 164.8 (ab, ArC), 185.9 (C=O). HRMS (ESI): calcd for C22H26N6O ([M + H]+), 391.2240; found, 391.2262.
4.1.5.23. (Z)-1-(3-Chlorophenyl)-4-((1-(4,6-di(piperidin-1-yl)-1,3,5-triazin-2-yl)-1H-indol-3-yl)methylene)-3-methyl-1H-pyrazol-5(4H)-one (22)
Compound 22 was prepared through Knoevenagel condensation of compound 21 (1 mmol) with 1-(3-chlorophenyl)-3-methyl-2-pyrazolin-5-one (1 mmol) in the presence of piperidine in CHCl3 at 100 °C for 2 h under microwave irradiation. After completion of the reaction (TLC), water was added to the reaction mixture and extracted with CHCl3 (4 × 25 mL). The combined organic layers were dried over Na2SO4 and concentrated in vacuum to procure crude product that was further purified by recrystallization from chloroform/methanol (2:8) to obtain pure compound 22. Orange solid, yield 71%, mp 270–272 °C. IR (KBr): 2934, 2850, 2209, 1688, 1592, 1571, 1499, 1445, 1404, 1286 cm–1. 1H NMR (500 MHz, CDCl3): δ 1.79 (s, 12H, 6 × CH2), 2.54 (s, 3H, CH3), 3.94 (s, 8H, 4 × CH2), 7.20–7.21 (m, 1H, ArH), 7.31–7.41 (m, 2H, ArH), 7.48–7.55 (m, 2H, ArH), 7.75–7.77 (m, 1H, ArH), 7.84–7.91 (m, 1H, ArH), 8.02–8.06 (m, 1H, bridged H), 8.45–8.46 (m, 1H, ArH), 10.50 (s, 1H, CH). 13C NMR (125 MHz, CDCl3): δ 12.5 (+ve, CH3), 23.4 (−ve, CH2), 25.3 (−ve, CH2), 47.5 (−ve, CH2), 111.0 (ab, ArC), 113.2 (ab, ArC), 115.5 (ab, ArC), 116.2 (ab, ArC), 116.7 (+ve, ArCH), 117.7 (ab, ArC), 118.3 (+ve, ArCH), 119.9 (+ve, ArCH), 122.2 (+ve, ArCH), 125.2 (+ve, ArCH), 126.2 (+ve, ArCH), 127.2 (ab, ArC), 130.0 (+ve, ArCH), 130.2 (+ve, ArCH), 130.5 (ab, ArC), 134.6 (ab, ArC), 135.4 (ab, ArC), 137.7 (+ve, ArCH), 138.3 (+ve, ArCH), 154.7 (ab, ArC), 162.7 (C=O). HRMS (ESI): calcd for C32H33N8OCl ([M + H]+), 581.2538; found, 581.2509.
4.1.5.24. 2-Chloro-4,6-di(piperidin-1-yl)-1,3,5-triazine (23)
Piperidine (0.92 g, 10.86 mmol) was added dropwise to the stirred solution of cyanuric chloride (1 g, 5.43 mmol) in acetone (40 mL) at 0 °C, followed by the addition of triethylamine (1.09 g, 10.86 mmol). Then, the reaction mass was stirred at rt for 1 h. The reaction was quenched with water and washed with ethyl acetate (4 × 25 mL). The combined organic layers were dried over Na2SO4 and concentrated under vacuum to procure crude product. The crude product was further purified by washing with diethyl ether to obtain pure compound 23. White solid, yield 62%, mp 95–96 °C. 1H NMR (500 MHz, CDCl3): δ 1.58–1.59 (m, 8H, 4 × CH2), 1.65–1.66 (m, 8H, 4 × CH2), 3.73 (t, 8H, J = 5.28 Hz, 4 × CH2). 13C NMR (125 MHz, CDCl3): δ 24.6 (−ve, CH2), 25.7 (−ve, CH2), 44.4 (−ve, CH2), 164.2 (ab, ArC), 169.5 (ab, ArC). HRMS (MS, ESI): calcd for C13H20N5Cl ([M + H]+), 282.1480; found, 282.1508.
4.1.6. Synthesis of Compound 24
NaH (1.2 mmol) was washed with dry hexane and suspended in dry ACN (20 mL) to which indole-3-carboxaldehyde (1 mmol) was added at 0 °C. After stirring for 5–10 min, 1,3,5-triazine (1 mmol) was added to the reaction mixture. On completion of the reaction (TLC), ice cold water was added. The solid product was separated, which was filtered and dried under vacuum to procure pure product 24. White solid, yield 45%, mp > 300 °C. 1H NMR (500 MHz, CDCl3 + TFA): δ 7.58–7.61 (m, 3H, ArH), 7.65–7.68 (m, 3H, ArH), 8.39 (d, 3H, J = 7.66 Hz, ArH), 8.76 (d, 3H, J = 8.39 Hz, ArH), 9.11 (s, 3H, ArH), 10.16 (s, 3H, CHO). 13C NMR (125 MHz, CDCl3 + TFA): δ 110.8 (ab, C), 113.0 (ab, C), 115.3 (ab, C), 116.2 (+ve, ArCH), 117.6 (ab, C), 122.3 (ab, C), 122.9 (+ve, ArCH), 126.5 (ab, C), 126.5 (+ve, ArCH), 127.3 (+ve, ArCH), 136.0 (ab, C), 138.4 (+ve, ArCH), 189.9 (C=O). HRMS (APCI): calcd for C30H18N6O3 ([M + H]+), 511.1513; found, 511.1521.
4.2. Docking Procedure
Schrodinger software package was used for performing the molecular docking of the compounds in the COX-2 (PDB ID 1CVU) and COX-1 (PDB ID 1DIY) active site by following the procedure reported in the previous paper.20
4.2.1. Field-Based 3D-QSAR Model
Field-based QSAR tool of Schrodinger 2015-4 was used for the 3D-QSAR analysis by following the reported procedure.20
4.3. Procedure for MD Simulation
The docked complex of compound 6 with COX-2 was optimized using MD simulation on DESMOND module in the Schrodinger Maestro 10.1 version with OPLS-2005 force field in explicit solvent with the TIP3P water model. The docked complex was placed in TIP3P water molecules adequately, the dimensions of each orthorhombic water box were 10 Å × 10 Å × 10 Å, and the systems were neutralized by adding Na+ counterions to balance the net charges of the systems, and then 0.15 M NaCl was added. The generated solvent model for the docked complex contained about 59 176 atoms. Before MD simulations, the systems were minimized and pre-equilibrated using the default relaxation protocol executed in DESMOND. NVT MD simulations were performed at 10 K for 100 ps with restraints on heavy atoms. Then, the system was simulated for another 12 ps at 10 K with the same settings. This was followed by NPT equilibration at 10 K for 12 ps. Then, the system was simulated for 12 ps at 300 K with restraints on heavy atoms. Finally, restraints on heavy atoms were removed, and the system was simulated for 24 ps at 300 K with a thermostat relaxation time of 1 ps and a barostat relaxation time of 2 ps. After minimization and equilibration, MD simulations were performed at 300 K for 50 ns with the Martyna–Tobias–Klein method. Data were collected every 1.5 ps during the MD runs.
4.4. Procedure for COX-1 and COX-2 Enzyme Immunoassay
COX-1/2 inhibitory immunoassays were performed by following the already reported procedure.20
4.5. Human Whole Blood Assay
The human whole blood assay was performed by using the procedure given in the previous report.20
4.6. Analgesic and Anti-Inflammatory Activity
Prior permission was sought from the Institutional Animal Ethical Committee of Guru Nanak Dev University, Amritsar, for using animals. The analgesic and anti-inflammatory activity of the compounds was determined by using male/female Swiss albino mice weighing 25–35 g. The animals were given free supply of food and water and kept at 22 ± 2 °C under 12 h light/dark cycle. A total of 12 groups of animals with five animals in each group and a previously described15b,19 procedure were used for screening the analgesic and anti-inflammatory activity of the compounds.
4.6.1. Mechanistic Studies
Three groups of mice, five in each group, were used to explore the mode of working of the compound.20 Substance P, l-arginine, and NOS inhibitor, l-NAME pretreatment, were given 30 min before administering compound 6.
4.6.2. Acute Toxicity Studies
Acute toxicity studies were performed with four groups of animals, three animals in each group. After 4 h of fasting, the first group of animals was given vehicle and served as the control group; the second, third, and the fourth groups were given compound 6 at doses of 50, 300, and 2000 mg kg–1, respectively. During the first 4 h, the animals were observed continuously and periodically during the next 24 h. After 14 days, one animal each from the first and third group was sacrificed, and the histological studies were performed using H and E staining.
4.7. In Vivo Pharmacokinetic Studies
The in vivo pharmacokinetic properties were performed by using a male Wistar rat (250–300 g). A dose of 10 mg kg–1 of 0.1% CMC suspension of the compound was administered i.p. to the rats. The animals were anesthetized with ketamine (50 mg kg–1 i.p.). The blood samples from the jugular vein were withdrawn in heparinized tubes at an interval of 30 and 60 min and 2, 3, 4, 6, 8, 11, and 24 h of compound administration. The samples were centrifuged at 8000 rpm for 10 min at −4 °C, and the clear serum was separated and stored at −20 °C until analyzed. The concentration of compound in the serum was determined using LC–MS after preparing the samples by the protein precipitation method. Plasma sample (100 μL) was taken in 1.5 mL tube and vortexed for 3 min. ACN (300 μL) with internal standard was added to the above tube and vortexed for 5 min. The tube was centrifuged at 4 °C, 16 000 rpm for 40 min. The compound with initial concentration 3 mg mL–1 followed by serial dilution was used for obtaining the standard curve. LC–MS was performed with a Dionex ultimate 3000 HPLC system attached to a Bruker MicroTof QII mass spectrometer. A 50 mm, 5 μm PRP C18 column was used for high-performance liquid chromatography (HPLC), and the gradient mobile phase consisted of water and acetonitrile (each containing 0.1% formic acid). The initial composition was 20% acetonitrile and linearly increased to 100% in 30 min. The column eluent was introduced to the ESI source of mass spectrometer operating in +ve mode. The different pharmacokinetic parameters such as t1/2 (min), area under curve (AUC), Cmax (μg/mL), tmax(min), mean residence time (min), and clearance (Cl) (mg)/(μg/mL)/min were determined following noncompartmental analysis in PK solver software.26
4.8. NMR Studies for Protein–Ligand Interactions
NMR experiments were performed on a Bruker AVANCE 500 NMR spectrometer at 298.2 K. The diffusion measurements were carried out using “ledbpgppr2s” pulse program. The gradient pulse length (P30) and the diffusion time (D20) were kept fixed after optimizing the parameters. The values for δ and Δ are 1.5 and 50 ms, respectively. A longitudinal eddy current delay (D21) of 5 ms was used. The sample temperature was kept constant at 298 ± 0.1 K.
The pseudo-2D data were processed. The diffusion coefficients were obtained with the help of the T1/T2 relaxation module of Topspin as described in the diffusion manual of this software, whereas the fitting-type “intensity” was used.
4.8.1. 1H NMR T1 Relaxation Time Measurements
1H NMR T1 relaxation time measurements were performed on a Bruker AVANCE 500 NMR spectrometer at 298.2 K. The longitudinal relaxation time (T1) was determined by 180°–90° inversion recovery pulse sequence. Sixteen values of delay time (τ) were applied, and 16 scans for each τ value were recorded. The preacquisition delay (D1) was set to 2 × T1 (5 s) of the longest relaxation time. The value of the longitudinal relaxation time was obtained with the help of T1/T2 relaxation module of Topspin as described in the manual of this software, whereas the fitting function “invec” and fitting type ‘‘area” were used.
4.9. UV–Visible Spectral Studies
Enzyme (3 μL) was diluted to 100 μL in Tris-HCl buffer, and 10 μM solution of the compound was prepared in HPLC-grade DMSO and Tris-HCl buffer (1:9) (pH 7.4). Incremental addition of the enzyme solution to the compound solution (10 μM) was made, and spectra were recorded.
4.10. ITC Experiments
Solution (50 μM) of compound 6 in HPLC-grade ethanol and phosphate buffer (1:99) (pH 7.4) was prepared. Enzyme (10 μL) was diluted in 1 mL of phosphate buffer. The enzyme solution was taken in the sample cell to which 19 additions of compound 6, each of 2 μL, were made at equal intervals of 120 s. The experiments were performed in triplicate, and the difference between the values was <1%. The control experiments were performed with the same titrant (solution of compound 6) with buffer in the cell. Also, the successive buffer additions to the enzyme solution were carried out. The background heat of the control experiments was subtracted from the main experiment. To eliminate heat of mixing and heat of dilution, the total heat change Q involved in the cell volume Vo at fractional saturation Q after the ith injection was determined. The binding parameters were read with origin 7.0 software of MicroCal, and a single binding site model was used to fit the data.
Acknowledgments
Financial assistance from SERB-DST, New Delhi, and CSIR, New Delhi, is gratefully acknowledged. S.K. thanks CSIR, New Delhi, for SRF, and P.K. thanks SERB-DST for JRF. UGC, New Delhi, is gratefully acknowledged for grant to GNDU under University with Potential for Excellence Programme.
Glossary
Abbreviation
- IC50
concentration for 50% inhibition
- DMSO
dimethyl sulfoxide
- ACN
acetonitrile
- DMF
dimethylformamide
- TFA
trifluoro acetic acid
- SEM
standard error mean
- i.p.
intraperitoneally
- MWI
microwave irradiation
- QSAR
quantitative structure–activity relationship
- CoMFA
comparative molecular field analysis
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00445.
The authors declare no competing financial interest.
Supplementary Material
References
- a Ward P. A.Fundamentals of Inflammation; Serhan C. N., Ward P. A., Gilroy D. W., Eds.; Cambridge University Press: Cambridge, 2010; p 1–16. [Google Scholar]; b Medzhitov R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- a Abou-Raya A.; Abou-Raya S. Inflammation: A Pivotal Link between Autoimmune Disease and Artherosclerosis. Autoimmun. Rev. 2006, 5, 331–337. 10.1016/j.autrev.2005.12.006. [DOI] [PubMed] [Google Scholar]; b Lucas S.-M.; Rothwell N. J.; Gibson R. M. The role of inflammation in CNS Injury and Disease. Br. J. Pharmacol. 2006, 147, S232–S240. 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Marnett L. J. Inflammation and Cancer: Chemical Approaches to Mechanisms, Imaging, and Treatment. J. Org. Chem. 2012, 77, 5224–5238. 10.1021/jo300214d. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Coussens L. M.; Werb Z. Inflammation and Cancer. Nature 2002, 420, 860–867. 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ridker P. M.; Cushman M.; Stampfer M. J.; Tracy R. P.; Hennekens C. H. Inflammation, Aspirin and The Risk of Cardiovascular disease in Apparently Healthy man. N. Engl. J. Med. 1997, 336, 973–979. 10.1056/nejm199704033361401. [DOI] [PubMed] [Google Scholar]
- a Ballantyne C. M.; Nambi V. Markers of Inflammation and their clinical significance. Atherosclerosis Suppl. 2005, 6, 21–29. 10.1016/j.atherosclerosissup.2005.02.005. [DOI] [PubMed] [Google Scholar]; b Ridker P. M.; Hennekens C. H.; Buring J. E.; Rifai N. C-Reactive Protein and Other Markers of Inflammation in the prediction of Cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. 10.1056/nejm200003233421202. [DOI] [PubMed] [Google Scholar]; c Stanisic M.; Lyngstadaas S. P.; Pripp A. H.; Aasen A. O.; Lindegaard K.-F.; Ivanovic J.; Ilstad E.; Konglund A.; Sandell T.; Ellingsen O.; Sæhle T. Chemokines as markers of local inflammation and angiogenesis in patients with chronic subdural hematoma: a prospective study. Acta Neurochir. 2012, 154, 113–120. 10.1007/s00701-011-1203-2. [DOI] [PubMed] [Google Scholar]; d Teixeira B. C.; Lopes A. L.; Macedo R. C. O.; Correa C. S.; Ramis T. R.; Ribeiro J. L.; Reischak-Oliveira A. Inflammatory markers, endothelial function and cardiovascular risk. J. Vasc. Bras. 2014, 13, 108–115. 10.1590/jvb.2014.054. [DOI] [Google Scholar]; e Barnes P. J.; Karin M. Nuclear Factor- KB – A Pivotal Transcription Factor in Chronic Inflammatory Diseases. N. Engl. J. Med. 1997, 336, 1066–1071. 10.1056/nejm199704103361506. [DOI] [PubMed] [Google Scholar]; f Lawrence T. The Nuclear Factor NF-KB Pathway in Inflammation. Cold Spring Harb Perspect Biol. 2009, 1, a001651. 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Szmitko P. E.; Wang C.-H.; Weisel R. D.; de Almeida J. R.; Anderson T. J.; Verma S. New Markers of Inflammation and Endothelial Cell Activation. Circulation 2003, 108, 1917–1923. 10.1161/01.cir.0000089190.95415.9f. [DOI] [PubMed] [Google Scholar]; h Libby P.; Ridker P. M. Novel Inflammatory Markers of Coronary Risk. Circulation 1999, 100, 1148–1150. 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]; i Lubrano V.; Balzan S. Consolidated and Emerging Inflammatory Markers in coronary artery disease. World J. Exp. Med. 2015, 5, 21–32. 10.5493/wjem.v5.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Suzuki H.; Kayama Y.; Sakamoto M.; Iuchi H.; Shimizu I.; Yoshino T.; Katoh D.; Nagoshi T.; Tojo K.; Minamino T.; Yoshimura M.; Utsunomiya K. Arachidonate 12/15- Lipoxygenase–Induced Inflammation and Oxidative Stress Are Involved in the Development of Diabetic Cardiomyopathy. Diabetes 2015, 64, 618–630. 10.2337/db13-1896. [DOI] [PubMed] [Google Scholar]
- a Rocca B.; FitzGerald G. A. Cyclooxygenases and Prostaglandins: shaping up the immune response. Int. Immunopharmacol. 2002, 2, 603–630. 10.1016/s1567-5769(01)00204-1. [DOI] [PubMed] [Google Scholar]; b Needleman P.; Truk J.; Jakschik B. A.; Morrison A. R.; Lefkowith J. B. Arachidonic acid metabolism. Annu. Rev. Biochem. 1986, 55, 69–102. 10.1146/annurev.biochem.55.1.69. [DOI] [PubMed] [Google Scholar]; c Smith W. L.; Garavito R. M.; DeWitt D. L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996, 52, 33157–33160. 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]; d Dubois R. N.; Abramson S. B.; Crofford L.; Gupta R. A.; Simon L. S.; Van De Putte L. B. A.; Lipsky P. E. Cyclooxygenase in biology and disease. Faseb. J. 1998, 12, 1063–1073. 10.1096/fasebj.12.12.1063. [DOI] [PubMed] [Google Scholar]; e Marnett L. J.; Rowlinson S. W.; Goodwin D. C.; Kalgutkar A. S.; Lanzo C. A. Arachidonic acid oxygenation by COX-1 and COX-2. J. Biol. Chem. 1999, 274, 22903–22906. 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]; f Garavito R. M.; DeWitt D. L. The cyclooxygenase isoforms: structural insights into the conversion of arachidonic acid to prostaglandins. Biochim. Biophys. Acta 1999, 1441, 278–287. 10.1016/s1388-1981(99)00147-x. [DOI] [PubMed] [Google Scholar]
- a Vane J. R.; Bakhle Y. S.; Botting R. M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]; b Pairet M.; Engelhardt G. Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: possible physiological and therapeutic implications. Fundam. Clin. Pharmacol. 1996, 10, 1–15. 10.1111/j.1472-8206.1996.tb00144.x. [DOI] [PubMed] [Google Scholar]; c Rouzer C. A.; Marnett L. J. Cycooxygenases: structural and functional insights. J. Lipid Res. 2009, S29–S34. 10.1194/jlr.r800042-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zidar N.; Odar K.; Glavac D.; Jerse M.; Zupanc T.; Stajer D. Cyclooxygenase in normal human tissues- is COX-1 really a constitutive isoform, and COX-2 an inducible form. J. Cell. Mol. Med. 2009, 13, 3753–3763. 10.1111/j.1582-4934.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Simmons D. L.; Botting R. M.; Hla T. Cyclooxygenase Isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]; c Ricciotti E.; FitzGerald G. A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. 10.1161/atvbaha.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Gilroy D. W.; Colville-Nash P. R. New insights into role of COX-2 in inflammation. J. Mol. Med. 2000, 78, 121–129. 10.1007/s001090000094. [DOI] [PubMed] [Google Scholar]; e Tilley S. L.; Coffman T. M.; Koller B. H. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Invest. 2001, 108, 15–23. 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Patrono C.; Baigent C. Low-dose aspirin, coxibs, and other NSAIDS: a clinical mosaic emerges. Mol. Interv. 2009, 9, 31–39. 10.1124/mi.9.1.8. [DOI] [PubMed] [Google Scholar]; g Skoutakis V. A.; Carter C. A.; Mickle T. R.; Smith V. H.; Arkin C. R.; Alissandratos J.; Petty D. E. Review of diclofenac and evaluation of its place in therapy as a nonsteroidal anti-inflammatory agent. Drug Intell. Clin. Pharm. 1988, 22, 850–859. 10.1177/106002808802201102. [DOI] [PubMed] [Google Scholar]
- a Brunton L.; Chabner B.; Knollman B.. Anti-Inflammatory, Antipyretic and Analgesic Agents. In The Pharmacological Basis of Therapeutics, 12th ed.; Goodman L. S., Gilman A., Eds.; McGraw-Hill Inc: New York, 2011; Chapter 34, pp 959–1003. [Google Scholar]; b Shi S.; Klotz U. Clinical use and pharmacological properties of selective COX-2 inhibitors. Eur. J. Clin. Pharmacol. 2008, 64, 233–252. 10.1007/s00228-007-0400-7. [DOI] [PubMed] [Google Scholar]; c Chan F. K. L.; Hung L. C. T.; Suen B. Y.; Wu J. C. Y.; Lee K. C.; Leung V. K. S.; Hui A. J.; To K. F.; Leung W. L.; Wong V. W. S.; Chung S. C. S.; Sung J. J. Y. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N. Engl. J. Med. 2002, 347, 2104–2110. 10.1056/nejmoa021907. [DOI] [PubMed] [Google Scholar]; d Moore R. A.; Derry S.; Phillips C. J.; McQuay H. J. Nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 selective inhibitors (coxibs) and gastrointestinal harm: review of clinical trials and clinical practice. BMC Muscoskel. Disord. 2006, 7, 79. 10.1186/1471-2474-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Capone M. L.; Tacconelli S.; Sciulli M. G.; Patrignani P. Clinical pharmacology of selective COX-2 inhibitors. Int. J. Immunopathol. Pharmacol. 2003, 16, 49–58. [PubMed] [Google Scholar]; f Dogné J.-M.; Supuran C. T.; Pratico D. Adverse cardiovascular effects of the coxibs. J. Med. Chem. 2005, 48, 2251–2256. 10.1021/jm0402059. [DOI] [PubMed] [Google Scholar]; g Kawahito Y. Clinical implications of cyclooxygenase-2 inhibitors. Inflamm. Regen. 2007, 27, 552–558. 10.2492/inflammregen.27.552. [DOI] [Google Scholar]
- a White W. B.; West C. R.; Borer J. S.; Gorelick P. B.; Lavange L.; Pan S. X.; Weiner E.; Verburg K. M. Risk of cardiovascular events in patients receiving celecoxib: A meta-analysis of randomized clinical trials. Am. J. Cardiol. 2007, 99, 91–98. 10.1016/j.amjcard.2006.07.069. [DOI] [PubMed] [Google Scholar]; b Brueggemann L. I.; Mackie A. R.; Mani B. K.; Cribbs L. L.; Byron K. L. Differential effects of selective cyclooxygenase-2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol. Pharmacol. 2009, 76, 1053–1061. 10.1124/mol.109.057844. [DOI] [PMC free article] [PubMed] [Google Scholar]; c FitzGerald G. A. Cardiovascular pharmacology of nonselective nonsteroidal anti-inflammatory drugs and coxibs: clinical considerations. Am. J. Cardiol. 2002, 89, 26–32. 10.1016/s0002-9149(02)02234-8. [DOI] [PubMed] [Google Scholar]; d Solomon S. D.; McMurray J. J. V.; Pfeffer M. A.; Wittes J.; Fowler R.; Finn P.; Anderson W. F.; Hawk E.; Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005, 352, 1071–1080. 10.1056/nejmoa050405. [DOI] [PubMed] [Google Scholar]; e Cairns J. A. The coxibs and traditional nonsteroidal anti-inflammatory drugs: A current perspective on cardiovascular risks. Can. J. Cardiol. 2007, 23, 125–131. 10.1016/s0828-282x(07)70732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Li J. J.Heterocyclic Chemistry in Drug Discovery; John Wiley and Sons Inc: Hoboken, New Jersey, 2013. [Google Scholar]; b Biswal S.; Sahoo U.; Sethy S.; Kumar H. K. S.; Banerjee M. Indole: The Molecule of Diverse Biological Activities. Asian J. Pharm. Clin. Res. 2012, 5, 1–6. [Google Scholar]; c Kumar J. R. Review of Imidazole Heterocyclic Ring containing Compounds with their Biological Activity. Pharmacophore 2010, 1, 167–177. [Google Scholar]; d Dua R.; Shrivastava S.; Sonwane S. K.; Srivastava S. K. Pharmacological Significance of Synthetic Heterocycles Scaffold: A Review. Adv. Biol. Res. 2011, 5, 120–144. [Google Scholar]
- a Abdel-Megeed A. M.; Abdel-Rahman H. M.; Alkaramany G.-E. S.; El-Glendy M. A. Design, synthesis and molecular modeling study of acylated 1,2,4-triazole-3-acetates with potential anti-inflammatory activity. Eur. J. Med. Chem. 2009, 44, 117–123. 10.1016/j.ejmech.2008.03.017. [DOI] [PubMed] [Google Scholar]; b Banerjee A. G.; Das N.; Shengule S. A.; Srivastava R. S.; Shrivastava S. K. Synthesis, characterization, evaluation and molecular dynamics studies of 5, 6-diphenyl-1,2,4-triazine-3-(2H)-one derivatives bearing 5esubstituted 1,3,4-oxadiazole as potential anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2015, 101, 81–95. 10.1016/j.ejmech.2015.06.020. [DOI] [PubMed] [Google Scholar]; c Dianzani C.; Collino M.; Gallicchio M.; Fantozzi R.; Samaritani S.; Signore G.; Menicagli R. Evaluation of in-vitro anti-inflammatory activity of some 2-alkyl-4,6-dimethoxy-1,3,5 triazines. J. Pharm. Pharmacol. 2006, 58, 219–226. 10.1211/jpp.58.2.0009. [DOI] [PubMed] [Google Scholar]; d Shah D. R.; Modh R. P.; Chikhalia K. H. Privileged s-triazines: structure and pharmacological applications. Future Med. Chem. 2014, 6, 463–477. 10.4155/fmc.13.212. [DOI] [PubMed] [Google Scholar]; e Zhou C.-H.; Wang Y. Recent Researches in Triazole Compounds as Medicinal Drugs. Curr. Med. Chem. 2012, 19, 239–280. 10.2174/092986712803414213. [DOI] [PubMed] [Google Scholar]; f Singla P.; Luxami V.; Paul K. Triazine as a promising scaffold for its versatile biological behavior. Eur. J. Med. Chem. 2015, 102, 39–57. 10.1016/j.ejmech.2015.07.037. [DOI] [PubMed] [Google Scholar]
- Triazine based heterocycles:; a Avupati V. R.; Yejella R. P.; Parala V. R.; Killari K. N.; Papasani V. M. R.; Cheepurupalli P.; Gavalapu V. R.; Boddeda B. Synthesis, characterization and in vitro biological evaluation of some novel 1,3,5-triazine–Schiff base conjugates as potential antimycobacterial agents. Bioorg. Med. Chem. Lett. 2013, 13, 5968–5970. 10.1016/j.bmcl.2013.08.063. [DOI] [PubMed] [Google Scholar]; b Pastorin G.; Federico S.; Paoletta S.; Corradino M.; Cateni F.; Cacciari B.; Klotz K.-N.; Gao Z.-G.; Jacobson K. A.; Spalluto G.; Moro S. Synthesis and pharmacological characterization of a new series of 5,7-disubstituted-[1,2,4]triazolo[1,5-a][1,3,5]triazine derivatives as adenosine receptor antagonists: A preliminary inspection of ligand–receptor recognition process. Bioorg. Med. Chem. 2010, 18, 2524–2536. 10.1016/j.bmc.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wani M. Y.; Bhat A. R.; Azam A.; Choi I.; Athar F. Probing the antiamoebic and cytotoxicity potency of novel tetrazole and triazine Derivatives. Eur. J. Med. Chem. 2012, 48, 313–320. 10.1016/j.ejmech.2011.12.033. [DOI] [PubMed] [Google Scholar]; d Popowycz F.; Schneider C.; DeBonis S.; Skoufias D. A.; Kozielski F.; Galmarini C. M.; Joseph B. Synthesis and antiproliferative evaluation of pyrazolo[1,5-a]-1,3,5-triazine myoseverin derivatives. Bioorg. Med. Chem. 2009, 17, 3471–3478. 10.1016/j.bmc.2009.03.007. [DOI] [PubMed] [Google Scholar]; e Sunduru N.; Agarwal A.; Katiyar S. B.; Nishi; Goyal N.; Gupta S.; Chauhan P. M. S. Synthesis of 2,4,6-trisubstituted pyrimidine and triazine heterocycles as antileishmanial agents. Bioorg. Med. Chem. 2006, 14, 7706–7715. 10.1016/j.bmc.2006.08.009. [DOI] [PubMed] [Google Scholar]; f Vilaivan T.; Saesaengseerung N.; Jarprung D.; Kamchonwongpaisan S.; Sirawaraporn W.; Yuthavong Y. Synthesis of Solution-Phase Combinatorial Library of 4,6-Diamino-1,2-dihydro-1,3,5-triazine and Identification of New Leads Against A16V+S108T Mutant Dihydrofolate Reductase of Plasmodium falciparum. Bioorg. Med. Chem. 2003, 11, 217–224. 10.1016/s0968-0896(02)00344-9. [DOI] [PubMed] [Google Scholar]; g Zhou C.; Min J.; Liu Z.; Young A.; Deshazer H.; Gao T.; Chang Y.-T.; Kallenbach N. R. Synthesis and biological evaluation of novel 1,3,5-triazine derivatives as antimicrobial agents. Bioorg. Med. Chem. Lett. 2008, 18, 1308–1311. 10.1016/j.bmcl.2008.01.031. [DOI] [PubMed] [Google Scholar]; h Krečmerová M.; Masojídková M.; Holý A. Acyclic nucleoside phosphonates with 5-azacytosine base moiety substituted in C-6 position. Bioorg. Med. Chem. 2010, 18, 387–395. 10.1016/j.bmc.2009.10.044. [DOI] [PubMed] [Google Scholar]; i Sunduru N.; Gupta L.; Chaturvedi V.; Dwivedi R.; Sinha S.; Chauhan P. M. S. Discovery of new 1, 3, 5-triazine scaffolds with potent activity against Mycobacterium tuberculosis H37Rv. Eur. J. Med. Chem. 2010, 45, 3335–3345. 10.1016/j.ejmech.2010.04.017. [DOI] [PubMed] [Google Scholar]; j Garaj V.; Puccetti L.; Fasolis G.; Winum J.-Y.; Montero J.-L.; Scozzafava A.; Vullo D.; Innocenti A.; Supuran C. T. Carbonic anhydrase inhibitors: Novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg. Med. Chem. Lett. 2005, 15, 3102–3108. 10.1016/j.bmcl.2005.04.056. [DOI] [PubMed] [Google Scholar]; k Sosič I.; Mirković B.; Turk S.; Štefane B.; Kos J.; Gobec S. Discovery and kinetic evaluation of 6-substituted 4-benzylthio-1,3,5-triazin-2(1H)-ones as inhibitors of cathepsin B. Eur. J. Med. Chem. 2011, 46, 4648–4656. 10.1016/j.ejmech.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Indole based heterocycles:; a Sharma V.; Kumar P.; Pathak D. Biological Importance of the Indole Nucleus in Recent Years: A Comprehensive Review. J. Heterocycl. Chem. 2010, 47, 491–502. 10.1002/jhet.349. [DOI] [Google Scholar]; b Kaushik N.; Kaushik N.; Attri P.; Kumar N.; Kim C. H.; Verma A.; Choi E. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. 10.3390/molecules18066620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrazole based heterocycles:; a Chauhan A.; Sharma P. K.; Kaushik N. Pyrazole: A Versatile Moiety. Int. J. Tech. Res. 2011, 3, 11–17. [Google Scholar]; b Shaveta; Singh A.; Kaur M.; Sharma S.; Bhatti R.; Singh P. Rational design, synthesis and evaluation of chromone-indole and chromone-pyrazole based conjugates: Identification of a lead for anti-inflammatory drug. Eur. J. Med. Chem. 2014, 77, 185–192. 10.1016/j.ejmech.2014.03.003. [DOI] [PubMed] [Google Scholar]; c Küçükgüzel Ş. G.; Senkardes S. Recent advances in bioactive pyrazoles. Eur. J. Med. Chem. 2015, 97, 786–815. 10.1016/j.ejmech.2014.11.059. [DOI] [PubMed] [Google Scholar]
- a Khanum S. A.; Begum B. A.; Girish V.; Khanum N. F. Synthesis and Evaluation of Benzophenone-N-ethyl Morpholine Ethers as Anti-inflammatory Agents. Int. J. Biomed. Sci. 2010, 6, 60–65. [PMC free article] [PubMed] [Google Scholar]; b Murhekar M. M.; Padghan P. D.; Mhaske S. S.; Khadsan R. E. Synthesis and Antimicrobial activity of new series of s-triazines and its derivatives Synthesis and Antimicrobial activity of new series of s-triazines and its derivatives. Der Pharma Chem. 2011, 3, 243–246. [Google Scholar]; c Panneerselvam P.; Priya M.; Kumar N.; Saravanan G. Synthesis and Pharmacological Evaluation of Schiff Bases of 4-(2-Aminophenyl)-Morpholines. Ind. J. Pharm. Sci. 2009, 71, 428–432. 10.4103/0250-474x.57292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Singh P.; Shaveta; Sharma S.; Bhatti R. Rationally Designed Hybrid Molecules with Appreciable COX-2 Inhibitory and Antinociceptive Activities. Bioorg. Med. Chem. Lett. 2014, 24, 77–82. 10.1016/j.bmcl.2013.11.080. [DOI] [PubMed] [Google Scholar]; b Singh P.; Kaur J.; Singh G.; Bhatti R. Tri-block Conjugates: Identification of a Highly Potent Anti-inflammatory Agent. J. Med. Chem. 2015, 58, 5989–6001. 10.1021/acs.jmedchem.5b00952. [DOI] [PubMed] [Google Scholar]
- COX Inhibitor Screening Assay Kit, Item No. 560131. Cayman Chemical Co. [Google Scholar]
- a Baell J. B.; Ferrins L.; Falk H.; Nikolakopoulos G. PAINS: Relevance to Tool Compound Discovery and Fragment-Based Screening. Aust. J. Chem. 2013, 66, 1483–1494. 10.1071/ch13551. [DOI] [Google Scholar]; b Baell J. B.; Holloway G. A. New Substructure Filters for Removal of Pan Assay Interference compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53, 2719–2740. 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]; c Doman T. N.; McGovern S. L.; Witherbee B. J.; Kasten T. P.; Kurumbail R.; Stallings W. C.; Connolly D. T.; Shoichet B. K. Molecular Docking and High-Throughput Screening for Novel Inhibitors of Protein Tyrosine Phosphatase-I<SUB>B</SUB>. J. Med. Chem. 2002, 45, 2213–2221. 10.1021/jm010548w. [DOI] [PubMed] [Google Scholar]; d Rajamaki S.; Innitzer A.; Falciani C.; Tintori C.; Christ F.; Witvrouw M.; Debyser Z.; Massa S.; Botta M. Exploration of Novel Thiobarbituric acid, Rhodamine- and Thiohydantoin-Based HIV-1 Integrase Inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 3615–3618. 10.1016/j.bmcl.2009.04.132. [DOI] [PubMed] [Google Scholar]; e Baell J.; Walters M. A. Chemical Con Artists Foil Drug Discovery. Nature 2014, 513, 481–483. 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- a Warner T. D.; Giuliano F.; Vojnovic I.; Bukasa A.; Mitchell J. A.; Vane J. R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 7563–7568. 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Laufer S.; Greim C.; Luik S.; Ayoub S. S.; Dehner F. Human whole blood assay for rapid and routine testing of non-steroidal anti-inflammatory drugs (NSAIDs) on cyclo-oxygenase-2 activity. Inflammopharmacology 2008, 16, 155–161. 10.1007/s10787-008-8007-x. [DOI] [PubMed] [Google Scholar]
- TXB2 Express ELISA kit-Monoclonal, Item No. 10004023. Cayman Chemical Co. [Google Scholar]
- Singh P.; Kaur S.; Kaur J.; Singh G.; Bhatti R. Rational Design of Small Peptides for Optimal Inhibition of Cyclooxygenase-2: Development of a Highly Effective Anti-inflammatory Agent. J. Med. Chem. 2016, 59, 3920–3934. 10.1021/acs.jmedchem.6b00134. [DOI] [PubMed] [Google Scholar]
- Acute Oral Toxicity—Acute Toxic Class Method. OECD Guidelines for Testing of Chemicals; Guideline 423; OECD: Paris, 2001.
- Kiefer J. R.; Pawlitz J. L.; Moreland K. L.; Stegeman R. A.; Hood W. F.; Gierse J. K.; Stevens A. M.; Goodwin D. C.; Rowlinson S. W.; Marnett L. J.; Stallings W. C.; Kurumbail R. G. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature 2000, 405, 97–101. 10.1038/35011103. [DOI] [PubMed] [Google Scholar]
- Malkowski M. G.; Ginell S. L.; Smith W. L.; Garavito R. M. The productive conformation of arachidonic acid bound to prostaglandin synthase. Science 2000, 289, 1933–1937. 10.1126/science.289.5486.1933. [DOI] [PubMed] [Google Scholar]
- a Greenwood J. R.; Calkins D.; Sullivan A. P.; Shelley J. C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aided Mol. Des. 2010, 24, 591–604. 10.1007/s10822-010-9349-1. [DOI] [PubMed] [Google Scholar]; b Shelley J. C.; Cholleti A.; Frye L. L.; Greenwood J. R.; Timlin M. R.; Uchimaya M. Epik: a software program for pKa prediction and protonation state generation for drug-like molecules. J. Comput.-Aided Mol. Des. 2007, 21, 681–691. 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]; c Shivakumar D.; Williams J.; Wu Y.; Damm W.; Shelley J.; Sherman W. Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J. Chem. Theory Comput. 2010, 6, 1509–1519. 10.1021/ct900587b. [DOI] [PubMed] [Google Scholar]; d Jorgensen W. L.; Maxwell D. S.; Tirado-Rives J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. 10.1021/ja9621760. [DOI] [Google Scholar]; e Jorgensen W. L.; Tirado-Rives J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. 10.1021/ja00214a001. [DOI] [PubMed] [Google Scholar]
- Pauli G. F.; Chen S.-N.; Simmler C.; Lankin D. C.; Gödecke T.; Jaki B. U.; Friesen J. B.; McAlpine J. B.; Napolitano J. G. Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay. J. Med. Chem. 2014, 57, 9220–9231. 10.1021/jm500734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Huo M.; Zhou J.; Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Meth. Progr. Biomed. 2010, 99, 306–314. 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.