Abstract
Purpose
Leaders in the oncology community are sounding a clarion call to promote “value” in cancer care decisions. Value in cancer care considers the clinical effectiveness, along with the costs, when selecting a treatment. To discuss possible solutions to the current obstacles to achieving value in the use of advanced technologies in oncology, the National Cancer Policy Forum of the National Academies of Sciences, Engineering, and Medicine held a workshop, “Appropriate Use of Advanced Technologies for Radiation Therapy and Surgery in Oncology” in July 2015. The present report summarizes the discussions related to radiation oncology.
Methods and Materials
The workshop convened stakeholders, including oncologists, researchers, payers, policymakers, and patients. Speakers presented on key themes, including the rationale for a value discussion on advanced technology use in radiation oncology, the generation of scientific evidence for value of advanced radiation technologies, the effect of both scientific evidence and “marketplace” (or economic) factors on the adoption of technologies, and newer approaches to improving value in the practice of radiation oncology. The presentations were followed by a panel discussion with dialogue among the stakeholders.
Results
Challenges to generating evidence for the value of advanced technologies include obtaining contemporary, prospective, randomized, and representative comparative effectiveness data. Proposed solutions include the use of prospective registry data; integrating radiation oncology treatment, outcomes, and quality benchmark data; and encouraging insurance coverage with evidence development. Challenges to improving value in practice include the slow adoption of higher value and the de-adoption of lower value treatments. The proposed solutions focused on engaging stakeholders in iterative, collaborative, and evidence-based efforts to define value and promote change in radiation oncology practice. Recent examples of ongoing or successful responses to the discussed challenges were provided.
Conclusions
Discussions of “value” have increased as a priority in the radiation oncology community. Practitioners in the radiation oncology community can play a critical role in promoting a value-oriented framework to approach radiation oncology treatment.
Introduction
Leaders in the oncology community are increasingly sounding a clarion call for promoting “value” in cancer care decisions—that is, to consider clinical effectiveness along with payer, societal, and patient costs when selecting a treatment (1–3). To better understand the current challenges to promoting value in the use of new device technologies in oncology, the National Cancer Policy Forum of the National Academies of Sciences, Engineering, and Medicine held a workshop, “Appropriate Use of Advanced Technologies for Radiation Therapy and Surgery in Oncology” in July 2015 (4).
Workshop participants included multiple stakeholders, including oncologists and representatives from research, payers, policymakers, and patients. At the workshop, speakers presented on the immense challenges posed by the need to measure effectiveness and the costs of radiation and surgical oncology treatment, in particular, as complex and expensive technologies continue to evolve. The presentations were followed by workshop dialogue sessions to elicit additional responses and potential solutions by stakeholders. Finally, a concluding session emphasized the increasing need to generate evidence for the value of emerging technologies and opportunities for practitioners in the oncology community to engage with other stake-holders to measure, improve, and promote value in everyday oncology practice. The present report reflects the content of the presentations and discussions at the workshop regarding the practice of radiation oncology.
Value Proposition in Oncology: A New Discussion Surrounding Medical Device Technologies
Choosing high-value cancer treatment involves identifying and selecting treatments that will provide the best possible clinical outcomes and minimize costs to payers, patients, and society (2, 5–11). The need for a value discussion in radiation oncology has been prompted by the rapid uptake of, and large capital investments involved in, the implementation of new medical device treatment technologies in cancer care. Despite ongoing discussions on the value of new cancer drugs (eg, targeted agents), less attention has been given to new cancer treatment technologies in the form of medical devices (8, 9). Moreover, discussions on the value of new drugs are not completely translatable to the value of new device technologies in radiation and surgery. The distinct uptake, approval, and pricing patterns of new technologies in devices versus new drugs distinguish their value-based examination. Specifically, the uptake of new technologies by practitioners is generally much more rapid than that for drugs, in part because of the less-stringent federal approval process for medical devices (which include new radiation and surgical technologies) (10, 11). Although the US Food and Drug Administration requires evidence from clinical efficacy trials to approve drugs, the requirements for devices differ. Efficacy trials are not necessarily required; rather, approval testing focuses on the technological characteristics and mechanisms of operation (12). Regarding pricing, the prices for drugs in the United States are set by the manufacturers. The prices for devices are also set by the manufacturers; however, another price is set by the payers for the procedure delivered using the device. The differences in the drug versus device life-cycle also influence the price. Thus, drugs typically begin under patent protection, and their prices tend to decline sharply after the patents expire and generics enter the market (13, 14). However, this does not occur for devices. Instead, devices are replaced over time after a large capital investment by providers because of depreciation or obsoleteness. The unique characteristics of technology uptake contribute to the knowledge gap in our understanding of the value of these new technologies. In particular, in radiation and surgical oncology, this knowledge gap is set to widen under the pressures of accelerated uptake, large capital investments, and constant pressure to replace devices with ever newer technologies.
Increasing Costs of New Technologies in Radiation Oncology
We need a value discussion specific to radiation oncology in part because of its overall trajectory of treatment costs, considered an outlier relative to the cost trajectory for other specialties (15). An important driver of the documented high growth rate in Medicare expenditures for radiation oncology has been the increasing use of advanced radiation technologies, accompanied by the increased costs of care to payers, society, and patients (16). These radiation costs still represent a fraction of the overall substantial costs of cancer care. Historically, the specialty of radiation oncology experienced major advancement during the past 2 decades, advancing from the era of “2-dimensional” radiation treatment to 3-dimensional (3D) conformal radiation planning and treatment, intensity modulated radiation treatment (IMRT), image guidance (and the alternative radiation treatment strategies that such technology affords, including hypofractionation), respiratory motion management, magnetic resonance linear accelerator, volumetric arc therapy, stereotactic radiosurgery or stereotactic ablative radiation therapy, and proton therapy. A previous workshop sponsored by the National Cancer Institute in 2013 highlighted the plethora of emerging technologies in radiation oncology and reviewed the associated challenges in implementing and evaluating new technologies and techniques (17).
New technologies and techniques in radiation oncology represent increasingly sophisticated means of tumor visualization, radiation beam targeting, treatment plan computing power, and treatment delivery. The goal of achieving a better therapeutic ratio—through increasingly conformal tumor targeting and minimizing normal tissue toxicity—is the scientific and clinical driver behind such advancements. However, the potentially greater costs of using these technologies have placed the use of advanced radiation technologies at the center of the discussions of value in cancer care. The 2013 National Cancer Institute–sponsored workshop did not have a primary focus on the value discussions surrounding emerging technologies in radiation oncology; thus, the reported workshop represents a logical next step in the dialogue among radiation oncologists and other stakeholders.
The rapid uptake and widespread diffusion of new radiation technologies inevitably present a unique source of financial strain for patients, who assume a portion of the overall economic burden. In the current value-based era of care, awareness of the implications of such financial strains on cancer patients and their families, who face high out-of-pocket expenses for cancer treatment, is increasing (18–21).
The estimates of overall out-of-pocket expenses after diagnosis vary; however, such expenses can be as high as $5000 to $18,000 (20–23). Until recently, longstanding cultural attitudes about cancer and resistance to considering cancer care costs have contributed to barriers toward achieving value in cancer care. For example, a strongly held belief among some patients and providers has been to pursue all available treatments, even those with little to no expected survival benefit, regardless of the financial consequences (24). Also, the societal expectation has been that costs for end-of-life cancer care would be unavoidably high. Today this is no longer the case. Not only have the costs of cancer care continued to increase, but the documented financial burdens for cancer survivors have included bankruptcy, foregoing spending on other necessary medications or food, declines in quality of life, and potentially worse health outcomes, including overall survival (18–20, 25–29). Accordingly, patients have now become key stakeholders in the discussion of how to incorporate the value proposition in radiation oncology, in particular, what constitutes the appropriate use of advanced technologies in the practice of radiation oncology (Fig. 1).
Fig. 1.
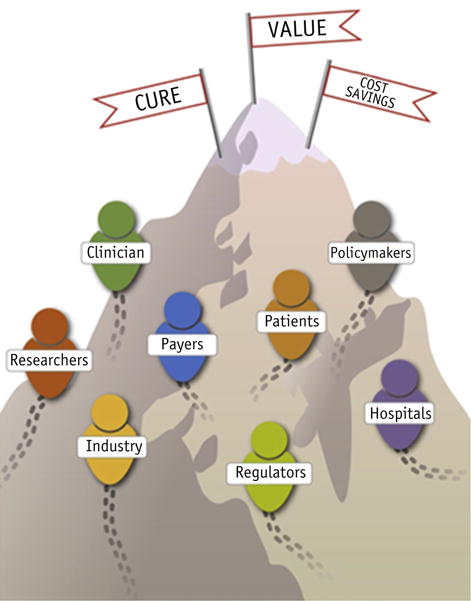
Stakeholders in the discussion of value in oncology.
Clinical Evidence, Evidentiary Gaps, and Future Horizons for Research
In this section of the workshop, the participants examined clinical research questions and the approaches currently used to address the evidentiary gaps regarding the comparative effectiveness and value of 2 illustrative technologies, IMRT and proton therapy. The uptake of these radiation treatment technologies and the ongoing barriers to developing the evidence to evaluate the effectiveness and value of these technologies were discussed.
Use of IMRT
The uptake of IMRT has steadily increased during the past decade but has shown signs of reaching a plateau analogous to the end segment of the proposed S-shaped uptake curve for new technologies (14, 30) (Fig. 2). This steady uptake pattern reflects the widely accepted dosimetric benefits of IMRT and the advent of routine reimbursement for IMRT. When used appropriately, IMRT allows for more conformal radiation treatment, with corresponding dosimetric benefits to tumors and normal tissues. The challenge in ongoing studies has been to prove whether these dosimetric benefits translate into improvements in clinically meaningful outcomes. Randomized data comparing the clinical outcomes for IMRT versus 2-dimensional or 3D therapy have been difficult to obtain, mostly because they require large numbers of patients and long follow-up times to demonstrate clinical benefit. Therefore, the rapid uptake of IMRT into the everyday practice of treating many disease sites was not necessarily based on comprehensive randomized data with long-term clinical outcomes such as cancer control or long-term organ function. Rather, this uptake often relied on consensus among practitioners on the dosimetric benefits achieved by IMRT. Nevertheless, several early randomized studies of IMRT were successfully conducted. For example, in 1 multicenter randomized trial of patients with breast cancer, a simple form of IMRT resulted in significant reductions in moist desquamation compared with a 2-dimensional standard wedge technique (31). For patients with head and neck cancer, IMRT was associated with a lower risk of xerostomia and improved overall quality of life throughout a 2-year follow-up period (32). However, equipoise is now difficult to achieve for new randomized studies seeking to accrue and evaluate the long-term outcomes of IMRT, in part, owing to its saturated uptake.
Fig. 2.
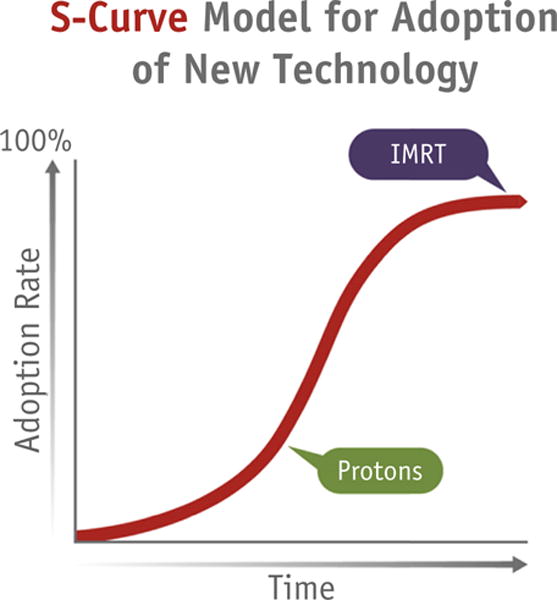
S-shaped curve representing the uptake of new technologies.
To overcome the challenges of a lack of equipoise, nonrandomized studies can provide secondary, single-institution, administrative, or other observational comparative effectiveness data—for example, the nonrandomized comparisons of patients with prostate cancer treated with IMRT versus 3D therapy have demonstrated reductions in gastrointestinal toxicity and hip fractures from IMRT, benefits generally accepted in clinical practice (33–35). Workshop participants underscored 3 persistent challenges to successful comparative effectiveness radiation oncology research. First, from a scientific perspective, a need exists to advance the study designs and platforms for data collection on the comparative effectiveness of newer radiation technologies outside the idealized randomized trial setting. Second, from the perspective of the community of stakeholders, a need exists for consensus on the approach to then judge the comparative effectiveness data and translate these findings into guidelines for appropriate use of technologies. Third, from the perspective of value assessment, an ongoing need exists to evaluate the costs of care from societal and patient standpoints. Ongoing comparative effectiveness research on IMRT is still necessary, including the effect of related advances such as optimized inverse planning, image-guided treatment planning and delivery, and complex quality assurance on its value.
Proton therapy
In contrast to IMRT, the uptake of proton radiation therapy appears only to have begun, with the curve of development recently increasing in a roughly exponential fashion—with <20 operating centers and ≥11 in development in the United States and 42 worldwide (Fig. 2). In countries with centralized allocation of health care resources, the rate of uptake has been substantially slower than that in the United States. For example, only 2 proton centers have been established in the United Kingdom. As a technology, proton therapy has the potential to produce incremental improvements in dosimetry compared with IMRT for numerous anatomic disease sites. However, the cost implications have been disproportionately challenging, with substantially greater installation and operational costs—approximately $20 to $30 million for a single gantry. With these higher costs, demonstrating the comparative effectiveness of proton therapy is also a priority for research.
Similar to IMRT, obtaining scientific data on the potential benefits of proton therapy has proved to be a persistent challenge, especially the sought-after level 1 evidence to quantify clinical benefits and identify the most appropriate indications for proton therapy. A few indications for proton treatment have been agreed on by consensus. For example, most oncologists consider pediatric cancer to be the best indication for proton therapy. This is because of the physical characteristics of particle beams, which allow the practitioner to reduce or eliminate an unnecessary exit dose to the normal tissues beyond the target volume. Children are uniquely sensitive to radiation, which has profound effects on their physical and intellectual growth and development and increases the risk of radiation-induced second cancer. Globally, the radiation oncology community supports pediatric cancer as an appropriate indication for proton therapy (36–40). For adults, the generally accepted indications are fewer, and most are for relatively rare conditions, such as high-dose radiation for skull base tumors, eye tumors, and spinal and sacral tumors. Little consensus has been reached for using proton therapy for more common types of cancer in adults. Evidence directly comparing the effectiveness of proton versus photon treatment is still evolving. The current evidence on the comparative effectiveness of protons is still dominated by retrospective and single-institution studies. Moreover, the aggregate follow-up time has been relatively short and the number of treated patients much lower relative to IMRT, generally because of the limited number of facilities with proton capabilities. Nevertheless, prospective studies, several randomized, have been proposed or are ongoing for common types of cancer, such as cancer of the prostate, breast, head and neck, lung, and central nervous system.
Evidentiary gaps in comparative effectiveness research on advanced radiation oncology technologies
Obtaining data on comparative effectiveness is critical for promoting the appropriate use of advanced radiation technologies. Although the importance of obtaining this evidence is well understood, no agreement has been reached regarding how to overcome the practical challenges of generating it. The randomized clinical trial has been the accepted reference standard for comparative data (41–43). In reality, however, both the accrual and the follow-up time for such trials can be long, which can compromise the application of the trial results, owing to shifts in indications or even outright outdating of existing technologies by the time the trials have been concluded. Furthermore, the need to evaluate long-term outcomes and late effects can be difficult to assess with rapidly changing technologies. Translating clinical trial results to actual clinical practice can be complicated by heterogeneity in patient characteristics (such as anatomy) (44), rendering technologies such as IMRT or proton therapy unlikely to be equally effective in all circumstances. Downstream clinical benefits could be further modified by other characteristics such as age, the presence of comorbid conditions, performance and functional status, life expectancy, and competing risks (45). Clinical trials alone might not provide sufficient data to fully explain how these modifying characteristics affect the comparative effectiveness of various forms of radiation therapy.
Thus, other study designs have been used to compare the effectiveness of new technologies in terms of disease outcomes and toxicity. For example, retrospective population-based and administrative data studies are popular nonrandomized approaches to address comparative effectiveness questions. The use of Surveillance, Epidemiology, and End Results (SEER)–Medicare data is a typical example. The detailed clinical data available in the SEER registry (eg, tumor type, disease stage, patient demographics, pathologic characteristics, and survival) are coupled with data on treatment, health care usage, and Medicare reimbursement as a measure of cost gleaned from the Medicare claims data. However, such retrospective studies can have several weaknesses (46). First among these weaknesses is the possibility of unmeasured confounding masking the independent contributions of meaningful differences in treatment effectiveness versus the effects of underlying characteristics of the physicians, facilities, or patients adopting the new technologies (47). Second, surrogate variables are often used as covariates or outcomes, because some clinical details might not be ascertainable from secondary data sets. Third, temporal factors are difficult to disentangle. The recent use of advanced technologies versus previous use of conventional treatment can contribute to unmeasured differences in patient, tumor, and quality of care factors according to the treatment era. Therefore, the strength of comparative effectiveness studies is in providing a body of consistent findings, with large administrative and population data providing complementary information to detailed single-institution or dosimetric studies and randomized trial data.
Future horizons in comparative effectiveness research
The discussion in this future horizons section sought to discuss solutions to address evidentiary gaps. Prospective registry studies represent a developing strategy to advance comparative effectiveness research in radiation oncology. In the current state, however, such studies have been limited by the existence of “siloed” radiation oncology data elements (Fig. 3). To compare the effectiveness of different technologies or treatment approaches, information must be gathered from the electronic medical record, treatment planning system, record and verify system, and even financial records to help assess the costs. Despite an abundance of potentially available information on treatment delivery, toxicity, and outcomes from these data sources, no well-established, disseminated system is yet available to integrate these elements. Even more challenging, in practicality, such data need to be extracted from a unique data structure (Digital Imaging and Communications in Medicine for radiation therapy [48]) from a variety of software options (eg, MOSAIQ and ARIA) from multiple vendors. Thus, the availability of “big data” alone does not address the need for data integration. Meaningful comparisons among treatments cannot be completed without addressing these data siloes, especially when the aim is to develop externally valid evidence representative of more than a single practice or institution.
Fig. 3.
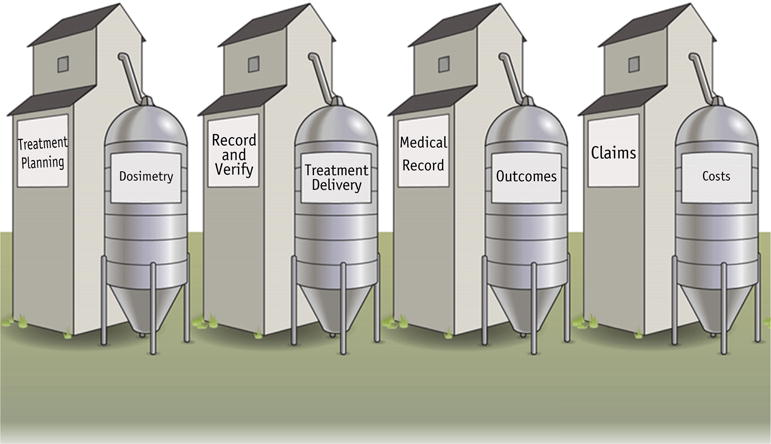
The challenge of “siloed” data elements.
At a national level, infrastructures are also needed to integrate radiation oncology data such that quality and outcomes can be benchmarked and evaluated in practice. An early example of such an initiative is the National Radiation Oncology Registry. The experience with the National Radiation Oncology Registry highlighted extraordinary real-life obstacles to operationalizing this concept, including achieving comprehensive inclusion of a large number of patients from different institutions and facilities into a single database, functional infrastructural integration of multiple electronic data sources, a user-friendly interface for data collection, and flexible ascertainment for evolving clinically meaningful and informative variables (49, 50). Relevant ongoing efforts to more broadly integrate oncology data include the statewide Michigan Radiation Oncology Quality Consortium effort; PCORnet (National Patient-Centered Clinical Research Network), which focuses on harnessing patient-centered outcomes data (51); MDEpiNET (Medical Device Epidemiology Network Initiative), which focuses on building a national medical device evaluation system; and CancerLinQ, a national effort to establish a learning oncology system (52). Although these examples show promise, they still represent relatively early efforts to integrate data in a truly seamless interface. If such efforts succeed, the primary benefit will be the development of an inclusive, “learning” health care system. Novel data fields could be actively ascertained as needs, indications, usage, and, even, early results as the use of new technologies evolve. Integrated prospective registry studies have the potential to address a knowledge gap that is distinct from (and complementary to) the answers that can be obtained from randomized clinical trials alone.
Another benefit of data captured outside the clinical trial setting is the potential to reflect actual community practice—moving beyond the understanding of efficacy to understanding true effectiveness. When advanced technology is disseminated into usual care, another important benchmark to ascertain is systematic quality assurance data collection across radiation oncology practice settings. The radiation oncology community of practitioners, including clinicians, radiation therapists, and medical physicists, is demonstrating an increasing awareness and investment in quality benchmarking (53). The scientific community certainly has a role in collecting data; professional societies can also participate by accreditation and evidence-based guidelines. Patients, too, as a community, can contribute their voice by providing patient-centered, patient-reported data (54).
Marketplace Factors and Technology Adoption
In reality, clinical considerations have not been the sole driver of technology usage. Workshop participants also discussed how “marketplace” factors interact with both payers and providers and how these interactions influence the use of advanced technology. The use of advanced radiation technology has inevitably affected expenditures in radiation oncology. IMRT is a prime example, given its uptake and saturation in daily radiation oncology practice. Medicare expenditures for IMRT have been estimated at about $200,000,000 in 2002 and ≤$800,000,000 in 2010, and those expenses were in large part responsible for the increasing cost of radiation therapy during that period (16). Costs are projected to plateau, in part, as payers influence the price of radiation treatment delivery, including IMRT delivery (55). Additionally, practitioners have sought to improve the efficiency of delivering IMRT and streamline computation costs to improve the efficiency gains and profit margins. The skill sets and team knowledge needed to use this technology have become increasingly refined and diffused and are now relatively accessible to most radiation oncology teams. More saturated usage and efficient service delivery might also positively contribute to stabilizing the projected cost trends associated with IMRT in the future.
The downside to marketplace influences on technology diffusion is the potential for financially motivated, rather than clinically appropriate, decision-making, which can lead to overusage of expensive technologies and increase health care spending without associated improvements in patient outcomes. Provider ownership with self-referral is 1 example, and links between provider ownership and the disproportionate adoption of IMRT have been documented in studies of men with prostate cancer (56, 57). The debate surrounding provider ownership in radiation oncology has raised awareness concerning the risk that reimbursement mechanisms can create perverse financial incentives for promoting potentially inappropriate adoption of expensive technology. Furthermore, although financial incentives can hasten the adoption of expensive technologies, they could also become barriers to the de-adoption of low-value practices. One example discussed was the uptake of hypofractionated breast radiation treatment, a lower-cost treatment option for appropriately selected patients with early-stage breast cancer compared with conventionally fractionated radiation (58–60). Despite established evidence that hypofractionated breast radiation therapy can provide excellent tumor control and an excellent toxicity profile, its uptake and the concomitant de-adoption of conventionally fractionated therapy in the United States has been surprisingly slow—increasing only from a usage rate of 11% in 2008 to 35% in 2013 within a cohort of appropriately selected patients in 1 population study (61). The lack of financial incentives to change practice in this case has been postulated as a contributing factor (62, 63). These examples illustrate the need in radiation oncology to promote incentives, not simply for adopting the newest treatments, but rather, for adopting high-value treatments and de-adopting lower value approaches.
Defining, Measuring, Promoting, and Improving Value
The goal of this workshop section was to introduce paradigms, approaches, and newer solutions for promoting and improving value in radiation oncology.
Defining value for radiation oncologists
Value in health care has been traditionally conceptualized as outcomes divided by costs, quantified over the entire cycle of care (4). A special consideration in the use of this basic conceptual model for radiation oncologists is in the definition of the “cycle of care” (Fig. 4). The acute cycle of care for radiation treatment typically lasts 2 to 7 weeks; however, this is not the only relevant care cycle. When assessing the potential harm/benefit tradeoff of radiation treatment, radiation oncologists usually consider the entire continuum of cancer survivorship, from the cancer diagnosis to death, with or without active cancer. The health benefits from radiation can persist for years or decades; therefore, radiation oncologists are interested in the benefits that last beyond the initial local remission, which can be assessed within weeks of treatment. In contrast, both acute and long-term toxic effects are significant risks to consider, and long-term toxicity affecting the cardiovascular or cerebrovascular systems, neurocognitive function, sexual function, and risk of second malignancies can also affect overall survival, patient functional status, and quality of life in the long term. A patient’s life expectancy also has a prominent role in affecting the relative risks and benefits. Therefore, this “cycle of care” makes quantifying the value of treatment exceptionally challenging in radiation oncology.
Fig. 4.
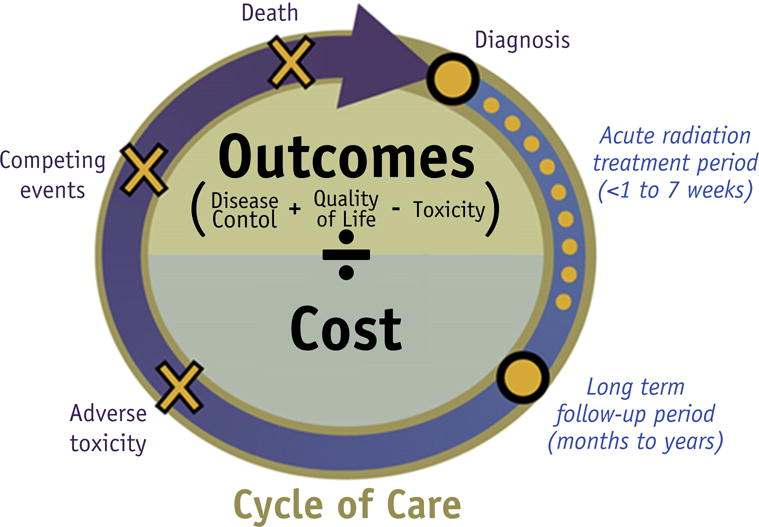
Value in care defined for the radiation oncologist.
Another challenge for comparing the value of radiation treatment with alternatives is the multiplicity of treatment choices and modalities. For example, treatment scenarios for comparison could include treatment versus no treatment (eg, definitive radiation vs active surveillance for prostate cancer), radiation versus another local treatment (eg, breast-conserving surgery with adjuvant radiation vs mastectomy alone for breast cancer), or radiation in lieu of additional systemic treatment (eg, shorter course chemotherapy plus consolidative radiation vs additional cycles of chemotherapy for Hodgkin lymphoma). The radiation treatment itself can also vary in terms of treatment schedule (eg, hypofractionation vs standard fractionation) and technique (eg, brachytherapy vs external beam radiation) or technology (eg, 3D vs IMRT). Nuanced examination of the multiple combinations of tradeoffs for short- and long-term disease outcomes, treatment toxicity, and the cost of these numerous options is difficult, often requiring an extraordinary size and breadth of the study sample to provide adequate statistical power for all the comparisons required for comprehensive comparative effectiveness studies.
Measuring value
Approaches to evaluate value in radiation oncology are evolving. Weighing the available published evidence on treatment outcomes is only an initial step in the evaluation process. Measures of value include simultaneous assessments of multiple tiers of clinical and health care value, health system value, and societal value (Fig. 5). In oncology, disease control (which includes both survival [overall and progression-free survival] and tumor control) is the most basic conceptualization of outcome. Beyond this, toxicity, quality of life, quality-adjusted life year, and functional status outcomes, including measures of time to functional recovery, treatment discontinuation, and morbidity (eg, the need for inpatient hospitalization or feeding tube placement), are also relevant. These considerations move beyond basic survival measures to address the sustainability of health in the long term. Simultaneously, health system value considers multiple tiers of system measures, including quality, structure, process, patient experience, and costs. The costs are also multifaceted, represented by the costs that are charged or reimbursed to the payer, costs to the health care provider, and costs to the patient. Concurrent consideration of the multiple tiers of societal value includes assessing the indirect costs of morbidity and mortality from disease and its treatment, as measured by lost wages and lost productivity. Together, these outcome and cost measures can then be translated into a cost-effectiveness metric of a new technology compared with an alternative technology (3). The cost-effectiveness of a new technology compared with an alternative technology (often the current standard of care) can be measured as either the incremental cost-effectiveness ratio or net benefits. The incremental cost-effectiveness ratio quantifies the additional costs required to achieve 1 unit of improvement in effectiveness from the new technology. Thus, a technology is considered to be cost-effective if the incremental cost-effectiveness ratio is less than the threshold value of societal willingness to pay (eg, $100,000 per quality-adjusted life year). The net benefit is calculated as follows: (societal willingness to pay multiplied by incremental effectiveness minus the incremental costs). Thus, a new technology is deemed more cost-effective if the net benefit is positive.
Fig. 5.
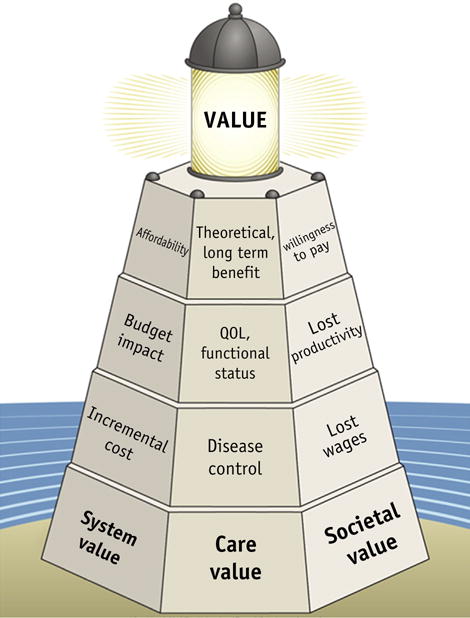
Health care value, system value, and societal value.
The evaluative process of assessing multiple tiers of care, system, and societal value is complex and evolving, given the need to consider the medical system and societal issues, along with clinical evidence to determine a net benefit. Involving stakeholders is an important component of this process (64, 65). The California Technology Assessment Forum was discussed as an innovative example of a program that uses a community forum approach as a solution for engaging stakeholders. Stake-holders, including practitioners, engage in the process of examining both the strength and the quality of the scientific evidence on the effectiveness of interventions to apply this evidence toward improving quality and value of care (64).
Additional points were discussed regarding solutions to the problem of defining and measuring the value of radiation oncology treatment technologies. Importantly, tradeoffs between multiple stakeholder perspectives still need to be reconciled to promote adoption of high-value technologies and dissuade the use of low-value technologies in daily practice. Practitioners in the radiation oncology community have a primary responsibility to uphold “care” value; however, practitioners cannot avoid acknowledging the multiple stakeholder perspectives on the value horizon. Although the concept of value to payers tends to reflect both comparative clinical effectiveness and budget impact, the concept of value for health care providers and manufacturers tends to reflect clinical effectiveness, additional theoretical or much longer term benefits, the intrinsic value of the availability of multiple treatment options, and return on investment. A proposed reconciliation of these views would be for payers to increase transparency, consistency, and focus on balancing long-term benefits and costs in their conception of value. Thus, providers and manufacturers would view affordability as a mutual and immediate imperative. Stakeholders would ideally seek a model in which payers, providers, and patients work together to establish a treatment’s value and to define the most appropriate clinical scenarios for the use of advanced technologies. This approach involves using iterative deliberations among stakeholders to reach the overarching goal of achieving evidence-based use of high-value technologies and dissuading the use of low-value technologies in daily practice (66). An illustration of this proposed solution exemplified is the Massachusetts Radiation Oncology Physicians Advisory Council and Blue Cross Blue Shield of Massachusetts collaborative effort to define standards for IMRT use in that state. The collaboration was associated with positive outcomes such as radiation treatment-related cost savings and improved efficiency in the insurance appeal process (66).
Improving value
However, the slow adoption of high-value treatments and de-adoption of low-value treatments tends to be a more common experience (61). This finding contrasts with the rapid uptake of new technology in radiation oncology (67). To address this problem, workshop participants discussed approaches to optimize adoption/de-adoption, emphasizing the shared responsibilities of multiple stakeholders. The radiation oncology community’s continued engagement in high-quality comparative effectiveness research is foundational to value-based treatment adoption/de-adoption in everyday radiation oncology practice. When the data are not yet available for newly evolving techniques, payers in particular can help to advance the science of evaluating new technologies. Although federal agencies continue to allot funds to support such science, resources are limited (68). However, the large—often massive—initial investment for new technologies that must be recouped by the institutions places practitioners in a development trap: developing evidence for comparative effectiveness with no reimbursement is difficult; however, the absence of evidence makes justifying reimbursement difficult. Yet an institution that invests in technology is compelled to provide supporting evidence for the technology’s indications and effectiveness. What begins to be lost in this cyclical trap is the impetus to generate unbiased data to advance the value perspective on new and developing technologies.
Can practitioners, institutions, and the scientific community partner with payers as stakeholders to accept a shared risk inherent in the venture to establish the value of new technologies? Such a venture requires generating best evidence in a setting of considerable pressure to avoid the inefficient use of health care dollars for treatments that might or might not be beneficial. Insurance coverage with evidence development was one solution discussed in this section for overcoming the economic development trap. This strategy would provide an opportunity for practicing radiation oncologists to engage proactively and collaboratively with payers. Quite simply, coverage denial threatens accrual to the very studies required to provide the evidence for or against the use of new technologies. Consistent payer reimbursement for treatment as a part of an evaluative study would support evidence development and help to justify either appropriate adoption or de-adoption of a new therapy (69). Clinicians and investigators engaging payers in the early stages of study design could help to enlist payer support (70).
Care pathways, treatment guidelines, and value initiatives from professional societies (eg, the “Choosing Wisely” campaign [71]) can serve as a vehicle to help disseminate evidence and therefore enable value-based choices. Another developing solution to improve value is to increase price transparency (72, 73). This strategy seeks to: (1) engage patients in the process of assessing a treatment’s value from a patient-centered perspective by helping patients understand the cost implications of treatment selection; and (2) reduce existing asymmetry in information—typically the provider and the patient do not have the same information about the components of care, especially on the cost and quality of treatment. Addressing this asymmetry could empower patients to seek high-value treatment choices. Level 1 data on the price transparency approach are eagerly awaited.
Increasingly, extrinsic payer and policy factors seek to create incentives or disincentives for practitioners as a solution to improve value in care (eg, through quality benchmark incentives and pay-for-performance and usage management and coverage policies). Moving from an encounter-based fee-for-service payment model to a value-based reimbursement model is still an evolutionary process. The main caution is to avoid unintended consequences of these forces—the possibility that structural changes could slow clinical care or impede access to beneficial treatments. The goal is to provide information and to share decision-making, with providers engaging in empowering patients to take an active role in the adoption or de-adoption of practices through their preferences. Collectively, such approaches can signal to both market and practitioners a need for different and better innovations.
Conclusions
Discussions of “value” have risen as a priority in the radiation oncology community, driven to the forefront by multiple forces. Practitioners in the radiation oncology community can have several critical roles in promoting value, by supporting the ongoing need to generate evidence for the comparative value of radiation treatment options, empowering patients by promoting a patient-centered approach in evaluating treatment value, and partnering with other stakeholders to adopt or de-adopt treatments using a value-oriented framework. The increasing costs of health care—and, in radiation oncology, the increasing expense of ever-advancing technologies—have prompted a societal imperative to examine value. The 2016 American Society for Radiation Oncology annual scientific meeting theme, “Enhancing Value, Improving Outcomes” (74) and other recent workshops on the value of radiation treatment (75, 76) have demonstrated that this value discussion is permeating the consciousness of the radiation oncology community.
Supplementary Material
Acknowledgments
This study was supported in part by the Cancer Prevention Research Institute of Texas (CPRIT grant RP140020 to G.L.S.), the Duncan Family Institute (to Y.T.S.), and grant K07-CA163616 from the National Cancer Institute (to J.E.B.). The authors are responsible for the content of the article, which does not necessarily represent the views of the National Academies of Sciences, Engineering, and Medicine.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Young RC. Value-based cancer care. N Engl J Med. 2015;373:2593–2595. doi: 10.1056/NEJMp1508387. [DOI] [PubMed] [Google Scholar]
- 2.Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology Statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Assessing and Improving Value in Cancer Care: Workshop Summary. Washington, DC: The National Academies Press; p. 2009. Available at: http://www.nationalacademies.org/hmd/Reports/2009/Assessing-Improving-Value-Cancer-Care.aspx. Accessed June 16, 2016. [PubMed] [Google Scholar]
- 4.National Academies of Sciences, Engineering, and Medicine Appropriate Use of Advanced Technologies for Radiation Therapy and Surgery: Workshop Summary. Washington, DC: The National Academies Press; 2016. Available at: http://www.nap.edu/21859. Accessed June 16, 2016. [PubMed] [Google Scholar]
- 5.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 6.Mohideen N, Kavanagh BD, Beyer D, et al. Radiation oncology: A perspective on health reform and value-based initiatives. J Oncol Pract. 2014;10:e212–e214. doi: 10.1200/JOP.2013.001337. [DOI] [PubMed] [Google Scholar]
- 7.Wallner PE, Steinberg ML. Feeding the beast” is not the road to value. Int J Radiat Oncol Biol Phys. 2015;93:13–15. doi: 10.1016/j.ijrobp.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Shih YC, Smieliauskas F, Geynisman DM, et al. Trends in the cost and use of targeted cancer therapies for the privately insured non-elderly: 2001 to 2011. J Clin Oncol. 2015;33:2190–2196. doi: 10.1200/JCO.2014.58.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih YC, Ganz PA, Aberle D, et al. Delivering high-quality and affordable care throughout the cancer care continuum. J Clin Oncol. 2013;31:4151–4157. doi: 10.1200/JCO.2013.51.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konski A. The war on cancer: Progress at what price? J Clin Oncol. 2011;29:1503–1504. doi: 10.1200/JCO.2010.34.2758. [DOI] [PubMed] [Google Scholar]
- 11.Wallner PE, Konski A. The impact of technology on health care cost and policy development. Semin Radiat Oncol. 2008;18:194–200. doi: 10.1016/j.semradonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. How to Study and Market Your Device. Available at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/. Accessed June 16, 2016.
- 13.Grabowski H, Vernon J. Longer patents for increased generic competition in the US: The Waxman-Hatch Act after one decade. Pharmacoeconomics. 1996;10(Suppl 2):110–123. doi: 10.2165/00019053-199600102-00017. [DOI] [PubMed] [Google Scholar]
- 14.US Congressional Budget Office. How Increased Competition from Generic Drugs has Affected Prices and Returns in the Pharmaceutical Industry. Washington, DC: US Government Printing Office; 1998. 1998. [Google Scholar]
- 15.Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole”. N Engl J Med. 2012;366:289–291. doi: 10.1056/NEJMp1113059. [DOI] [PubMed] [Google Scholar]
- 16.Shen X, Showalter TN, Mishra MV, et al. Radiation oncology services in the modern era: Evolving patterns of usage and payments in the office setting for Medicare patients from 2000 to 2010. J Oncol Pract. 2014;10:e201–e207. doi: 10.1200/JOP.2013.001270. [DOI] [PubMed] [Google Scholar]
- 17.Chetty IJ, Martel MK, Jaffray DA, et al. Technology for innovation in radiation oncology. Int J Radiat Oncol Biol Phys. 2015;93:485–492. doi: 10.1016/j.ijrobp.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer: The COST measure. Cancer. 2014;120:3245–3253. doi: 10.1002/cncr.28814. [DOI] [PubMed] [Google Scholar]
- 19.Jagsi R, Hawley ST, Abrahamse P, et al. Impact of adjuvant chemotherapy on long-term employment of survivors of early-stage breast cancer. Cancer. 2014;120:1854–1862. doi: 10.1002/cncr.28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagsi R, Pottow JA, Griffith KA, et al. Long-term financial burden of breast cancer: Experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol. 2014;32:1269–1276. doi: 10.1200/JCO.2013.53.0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard DS, Farr SL, Fang Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J Clin Oncol. 2011;29:2821–2826. doi: 10.1200/JCO.2010.33.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman DP, Joyce GF, Lawless G, et al. Benefit design and specialty drug use. Health Aff (Millwood) 2006;25:1319–1331. doi: 10.1377/hlthaff.25.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teckie S, McCloskey SA, Steinberg ML. Value: A framework for radiation oncology. J Clin Oncol. 2014;32:2864–2870. doi: 10.1200/JCO.2014.55.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710–3717. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsey S. Medical care and bankruptcy: The author replies. Health Aff (Millwood) 2013;32:1856. doi: 10.1377/hlthaff.2013.0800. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34:980–986. doi: 10.1200/JCO.2015.64.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkin EB, Bach PB. Cancer’s next frontier: Addressing high and increasing costs. JAMA. 2010;303:1086–1087. doi: 10.1001/jama.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers EM. Diffusion of Innovations. 4th. New York: The Free Press; 1995. [Google Scholar]
- 31.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 32.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michalski JM, Yan Y, Watkins-Bruner D, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87:932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 35.Bekelman JE, Mitra N, Efstathiou J, et al. Outcomes after intensity-modulated versus conformal radiotherapy in older men with non-metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:e325–e334. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016;17:287–298. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- 37.Allodji RS, Schwartz B, Veres C, et al. Risk of subsequent leukemia after a solid tumor in childhood: Impact of bone marrow radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2015;93:658–667. doi: 10.1016/j.ijrobp.2015.07.2270. [DOI] [PubMed] [Google Scholar]
- 38.Kahalley LS, Ris MD, Grosshans DR, et al. Comparing intelligence quotient change after treatment with proton versus photon radiation therapy for pediatric brain tumors. J Clin Oncol. 2016;34:1043–1049. doi: 10.1200/JCO.2015.62.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bekelman JE, Schultheiss T, Berrington De Gonzalez A. Subsequent malignancies after photon versus proton radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87:10–12. doi: 10.1016/j.ijrobp.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Chung CS, Yock TI, Nelson K, et al. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87:46–52. doi: 10.1016/j.ijrobp.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Henson KE, Jagsi R, Cutter D, et al. Inferring the effects of cancer treatment: Divergent results from Early Breast Cancer Trialists’ Collaborative Group meta-analyses of randomized trials and observational data from SEER registries. J Clin Oncol. 2016;34:803–809. doi: 10.1200/JCO.2015.62.0294. [DOI] [PubMed] [Google Scholar]
- 42.Lyman GH, Levine M. Epilogue: The peril and the promise of comparative effectiveness research in oncology. J Clin Oncol. 2012;30:4282. doi: 10.1200/JCO.2012.45.9800. [DOI] [PubMed] [Google Scholar]
- 43.Lyman GH, Levine M. Comparative effectiveness research in oncology: An overview. J Clin Oncol. 2012;30:4181–4184. doi: 10.1200/JCO.2012.45.9792. [DOI] [PubMed] [Google Scholar]
- 44.Yuan L, Ge Y, Lee WR, et al. Quantitative analysis of the factors which affect the interpatient organ-at-risk dose sparing variation in IMRT plans. Med Phys. 2012;39:6868–6878. doi: 10.1118/1.4757927. [DOI] [PubMed] [Google Scholar]
- 45.Smith GL, Smith BD. Radiation treatment in older patients: A framework for clinical decision making. J Clin Oncol. 2014;32:2669–2678. doi: 10.1200/JCO.2014.55.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagsi R, Bekelman JE, Chen A, et al. Considerations for observational research using large data sets in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90:11–24. doi: 10.1016/j.ijrobp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadley J, Yabroff KR, Barrett MJ, et al. Comparative effectiveness of prostate cancer treatments: Evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780–1793. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Law MYY, Liu B. Informatics in radiology: DICOM-RT and its utilization in radiation therapy. Radiographics. 2009;29:655–667. doi: 10.1148/rg.293075172. [DOI] [PubMed] [Google Scholar]
- 49.National Radiation Oncology Registry. Available at: http://www.roinstitute.org/What-We-Do/NROR/Index.aspx. Accessed June 16, 2016.
- 50.Efstathiou J, Bekelman J, Gabriel PE. Lessons learned from the National Radiation Oncology Registry (NROR) pilot. Int J Radiat Oncol Biol Phys. 2016 (in press) [Google Scholar]
- 51.PCORnet. The National Patient-Centered Clinical Research Network. Available at: http://www.pcornet.org/. Accessed June 16, 2016.
- 52.Medical Device Epidemiology Network. Available at: http://mdepinet.org/ Accessed June 16, 2016.
- 53.Benedict SH, El Naqa I, Klein EE. Introduction to big data in radiation oncology: Exploring opportunities for research, quality assessment, and clinical care. Int J Radiat Oncol Biol Phys. 2016;95:871–872. doi: 10.1016/j.ijrobp.2015.12.358. [DOI] [PubMed] [Google Scholar]
- 54.Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maroongroge S, Dosoretz AP, Kim SP, et al. Revisiting the sustainable growth rate “hole”: Sources of healthcare cost stabilization in 2010–2012. Int J Radiat Oncol Biol Phys. 2014;90:983–985. doi: 10.1016/j.ijrobp.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell JM. Urologists’ use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med. 2013;369:1629–1637. doi: 10.1056/NEJMsa1201141. [DOI] [PubMed] [Google Scholar]
- 57.Falit BP, Gross CP, Roberts KB. Integrated prostate cancer centers and over-utilization of IMRT: A close look at fee-for-service medicine in radiation oncology. Int J Radiat Oncol Biol Phys. 2010;76:1285–1288. doi: 10.1016/j.ijrobp.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 58.Group ST. Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) trial B of radiotherapy hypo-fractionation for treatment of early breast cancer: A randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Group ST. Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) trial A of radiotherapy hypo-fractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypo-fractionation for treatment of early breast cancer: 10-Year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 61.Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008-2013. JAMA. 2014;312:2542–2550. doi: 10.1001/jama.2014.16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jagsi R, Falchook AD, Hendrix LH, et al. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys. 2014;90:1001–1009. doi: 10.1016/j.ijrobp.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 63.Wang EH, Mougalian SS, Soulos PR, et al. Adoption of hypo-fractionated whole-breast irradiation for early-stage breast cancer: A National Cancer Data Base analysis. Int J Radiat Oncol Biol Phys. 2014;90:993–1000. doi: 10.1016/j.ijrobp.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 64.California Technology Assessment Forum. Available at: http://ctaf.org/ Accessed June 16, 2016.
- 65.Drummond MF, Schulpher MJ, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 3rd. New York: OU Press; 2005. [Google Scholar]
- 66.Steingisser L, Acker B, Berman S, et al. Bending the cost curve: A unique collaboration between radiation oncologists and Blue Cross Blue Shield of Massachusetts to optimize the use of advanced technology. J Oncol Pract. 2014;10:e321–e327. doi: 10.1200/JOP.2014.001473. [DOI] [PubMed] [Google Scholar]
- 67.Smith GL, Xu Y, Buchholz TA, et al. Brachytherapy for accelerated partial-breast irradiation: A rapidly emerging technology in breast cancer care. J Clin Oncol. 2011;10:157–165. doi: 10.1200/JCO.2009.27.0942. [DOI] [PubMed] [Google Scholar]
- 68.Steinberg M, McBride WH, Vlashi E, et al. National Institutes of Health funding in radiation oncology: A snapshot. Int J Radiat Oncol Biol Phys. 2013;86:234–240. doi: 10.1016/j.ijrobp.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bekelman JE, Hahn SM. Reference pricing with evidence development: A way forward for proton therapy. J Clin Oncol. 2014;32:1540–1542. doi: 10.1200/JCO.2014.55.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah A, Ricci KI, Efstathiou J. Beyond a moonshot: Insurance coverage for proton therapy. Lancet Oncol. 2016;17:559–561. doi: 10.1016/S1470-2045(16)00171-6. [DOI] [PubMed] [Google Scholar]
- 71.American Society for Radiation Oncology. Choosing Wisely List. 2014 Available at: https://www.astro.org/Patient-Care/Patient-Education/2014-Choosing-Wisely-List/. Accessed June 16, 2016.
- 72.Henrikson NB, Shankaran V. Improving price transparency in cancer care. J Oncol Pract. 2016;12:44–47. doi: 10.1200/JOP.2015.006171. [DOI] [PubMed] [Google Scholar]
- 73.Bullock AJ, Hofstatter EW, Yushak ML, et al. Understanding patients’ attitudes toward communication about the cost of cancer care. J Oncol Pract. 2012;8:e50–e58. doi: 10.1200/JOP.2011.000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.American Society for Radiation Oncology. ASTRO’s 58th annual meeting: Enhancing Value Improving Outcomes. Available at: https://www.astro.org/2016-Annual-Meeting.aspx. Accessed June 16, 2016.
- 75.Big Data Workshop: Exploring opportunities for radiation oncology in the era of big data. Available at: https://www.astro.org/Big-DataWorkshop.aspx. Accessed August 1, 2016.
- 76.The Global Health Movement in Radiation Oncology: A call to action in education, outreach, and advocacy. Available at: http://conferencecast.com/ASTRO/common/presentations.aspx/13/4/734. Accessed August 1, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


