Abstract
OBJECTIVE
The purpose of this study is to determine the relationships between quantitative liver MRI measurements and liver biopsy findings in pediatric and young adult patients with nonalcoholic fatty liver disease (NAFLD).
MATERIALS AND METHODS
Data were obtained from a registry that prospectively enrolls pediatric and young adult patients with biopsy-confirmed NAFLD at our tertiary medical center with parent or guardian and subject informed consent, as appropriate. Patients enrolled between November 2007 and June 2016 with a quantitative liver MRI examination within 6 months of biopsy were included (n = 69). Liver stiffness (kilopascals), volume (milliliters), and fat fraction (percentage) were extracted from MRI records. Multiple linear regression was used to determine the relationships between NAFLD activity score and quantitative MRI measures, and between MRI liver stiffness and histopathologic scores (steatosis, lobular inflammation, portal inflammation, hepatocyte ballooning, and fibrosis). Histopathologic data were extracted from medical records, with severity graded by hepatopathologists using Nonalcoholic Steatohepatitis (NASH) Clinical Research Network criteria. Ordinal logistic regression was used to assess the relationship between categoric NAFLD severity (simple steatosis vs NASH vs NASH with significant fibrosis) and MRI measures.
RESULTS
The mean (± SD) patient age at the time of MRI was 14.3 ± 2.8 years (range, 8–21 years); 25 (36.2%) patients were female. Liver biopsy was performed within a mean of 64.4 days of the MRI examination. There was a positive correlation between histopathologic steatosis and MRI liver fat fraction (ρ = 0.57; p < 0.0001). MRI fat fraction was the only significant imaging predictor of NAFLD activity score (p = 0.017). Fibrosis score was the only significant histopathologic predictor of MRI liver stiffness (p = 0.001). MRI liver volume was the only imaging predictor of categoric NAFLD severity (odds ratio = 1.001; 95% CI, 1.000–1.002; p = 0.007).
CONCLUSION
There was significant positive correlation between histopathologic and MRI liver fat measurements in our cohort. MRI liver stiffness did not predict the severity of fatty liver disease in children and young adults.
Keywords: biopsy, children, MRI, nonalcoholic fatty liver disease, quantitative
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children and young adults in the United States and the developed world, and its frequency is increasing [1]. Although the exact frequency of NAFLD is unknown, it is estimated to range from 7.6% in the general pediatric population to 34.2% in the obese pediatric population [2]. A primary risk factor is obesity, although genetic and epigenetic factors also likely play important roles [3]. The prevalence of obesity in the adolescent population of the United States has nearly doubled between 1988–1994 and 2013–2014 (10.5% vs 20.6%), presumably due to a combination of adverse dietary and lifestyle changes [4].
Although most pediatric and young adult patients with NAFLD have simple or bland steatosis, a smaller percentage of individuals will develop liver inflammation and hepatocellular damage, including hepatocyte apoptosis and necrosis (nonalcoholic steatohepatitis [NASH]). A smaller but unknown percentage of these individuals with NASH will develop substantial liver fibrosis due to continuing liver injury with aberrant healing, with some eventually developing cirrhosis and end-stage liver disease. NAFLD is now the second leading indication for liver transplantation in adults [3, 5]. NAFLD also is strongly associated with other adverse health conditions in children, including hypertension, type 2 diabetes mellitus, and dyslipidemia; such diagnoses are considered features of metabolic syndrome in adults [6–8]. There are no approved medical therapies for treatment of NASH in children. Lifestyle modification remains the first-line approach but is commonly ineffective [9].
The current standard of care for diagnosis and monitoring of severity of NAFLD in both children and adults is percutaneous core needle liver biopsy [1, 3], which has drawbacks, including sampling error, relatively high cost, lack of patient and parent acceptance, and morbidity due to potential complications. An accurate noninvasive method for establishing the presence of NAFLD and determining the severity of disease (simple steatosis vs NASH vs NASH with significant fibrosis) could substantially improve current paradigms for diagnosis and monitoring of pediatric and young adult patients with NAFLD both in the clinical setting and in clinical trials. Although quantitative liver MRI, particularly MR elastography (MRE), has shown some promise for determining the presence of NAFLD and establishing disease severity in adults, there is a paucity of such data in children and young adults [10]. Two prior studies focused on the ability of MRE to detect more advanced fibrosis in children with NAFLD but did not address the relationship of multiparametric MRI measures to NAFLD severity [11, 12].
The purpose of our study was to establish the relationships between multiple quantitative liver MRI measurements and liver biopsy histopathologic findings in pediatric and young adult patients with NAFLD. Specifically, we sought to determine the relationships between a commonly used validated histopathologic NAFLD activity score [13] and quantitative MRI measures, including liver fat fraction, liver volume, and liver stiffness, and between MRI liver stiffness and various histopathologic findings. We also sought to evaluate the relationship between categoric histopathologic NAFLD severity (simple steatosis vs NASH vs NASH with significant fibrosis) and quantitative MRI measures.
Materials and Methods
This HIPAA-compliant study was approved by the institutional review board of Cincinnati Children’s Hospital Medical Center.
Patients
The study population was selected from a prospectively recruited consecutive cohort of 160 children and young adults up to 21 years old with biopsy-confirmed NAFLD enrolled into the NAFLD registry at Cincinnati Children’s Hospital Medical Center between November 2007 and June 2016. Informed consent for enrollment in our prospective registry was obtained from a parent or guardian with assent or consent obtained from all registry participants, as age appropriate. Registry enrollment criteria included biopsy confirmation of NAFLD and exclusion of other chronic liver diseases, including viral hepatitis B and C, autoimmune hepatitis, α1-antitrypsin deficiency, Wilson disease, hemochromatosis, and excess alcohol ingestion. From this cohort for our current study, we identified patients who had undergone a quantitative liver MRI examination within 6 months of the liver biopsy.
All patients with NAFLD included in our study underwent clinically indicated image-guided percutaneous core needle liver biopsy by our pediatric interventional radiology division. On the basis of the institutional standard of care, one or two 16-gauge core specimens (≥ 2 cm) were obtained to achieve a recommended minimum 2-cm length of core biopsy [13]. Per protocol at our institution, patients with NAFLD undergo liver biopsy if their liver enzyme levels remain persistently elevated after 3–6 months of lifestyle modification (i.e., lack of response to standard exercise and dietary recommendations) or if clinical features (e.g., laboratory values and comorbid conditions such as type 2 diabetes mellitus) indicate concern for advanced fatty liver disease.
Two investigators extracted the following data from pertinent clinical histopathology reports entered into our institution’s electronic medical record: fibrosis score based on NASH Clinical Research Network criteria [10] (0 is no fibrosis, 1 is portal or perisinusoidal fibrosis, 2 is periportal and perisinusoidal fibrosis, 3 is bridging fibrosis, and 4 is cirrhosis), steatosis score (0 is < 5%, 1 is 5–33%, 2 is > 33–66%, and 3 is > 66% of liver surface area involved by steatosis), lobular inflammation score (0 is no foci, 1 is < 2 foci/200× field, 2 is 2–4 foci/200× field, and 3 is > 4 foci/200× field), portal inflammation score (0 is none, 1 is mild, and 2 is more than mild), and hepatocyte ballooning score (0 is none, 1 is few but definite ballooned hepatocytes, and 2 is many prominent ballooned cells). Ballooning degeneration is indicative of apoptosis and is considered irreversible.
Histopathologic steatosis, lobular inflammation, and hepatocyte ballooning scores were then summated to create the NAFLD activity score (0–8), which is used to assess overall NAFLD necroinflammatory activity [13]. Fibrosis score (stage) is separate from, and not included in, the NAFLD activity score. All liver histopathologists at our institution were trained to use the NASH Clinical Research Network criteria for reporting NAFLD liver biopsies and use this system in routine clinical practice.
According to the institutional standard of care, pediatric and young adult patients with suspected or known NAFLD commonly undergo quantitative liver MRI to confirm the presence of liver fat, estimate the severity of steatosis, and measure liver stiffness. Quantitative liver MRI examinations were performed on one of two 1.5-T MRI scanner platforms (Ingenia, Philips Healthcare; or HDx, GE Healthcare) and provided the following data that were extracted from our institution’s electronic medical record, again by two investigators: liver fat fraction (percentage), liver shear stiffness (kilopascals), and liver volume (milliliters) (Fig. 1).
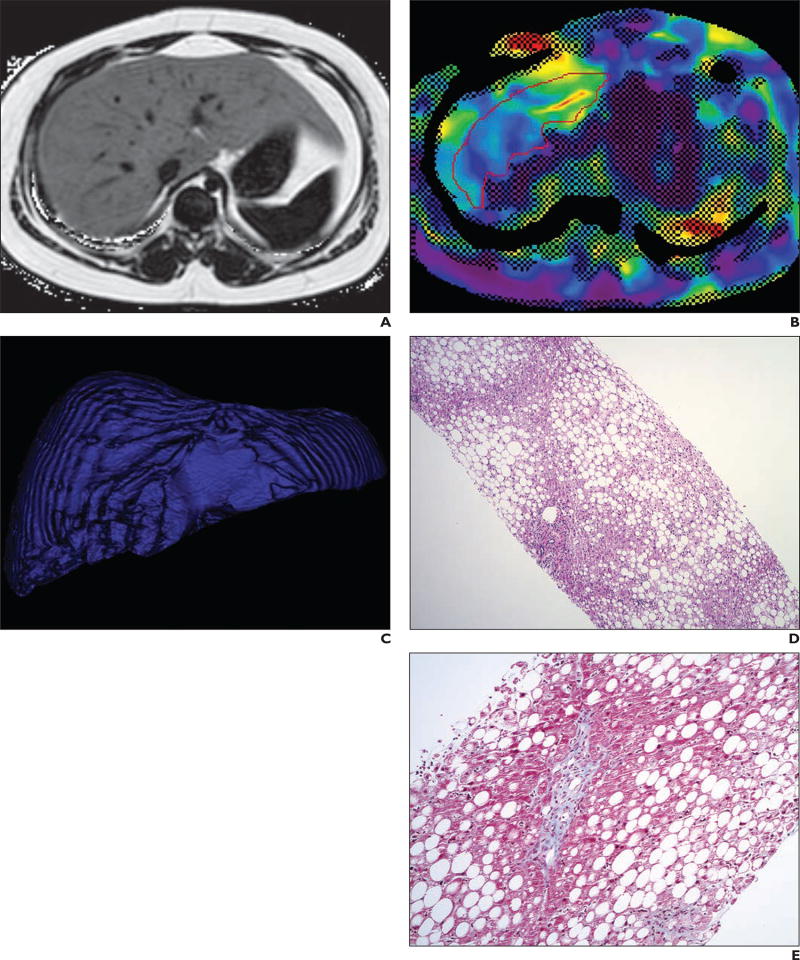
Fig. 1.
16-year-old boy with chronically elevated liver enzyme levels due to nonalcoholic steatohepatitis (histologic steatosis grade = 3, lobular inflammation score = 3, portal inflammation score = 2, fibrosis stage = 1, and nonalcoholic fatty liver disease activity score = 7).
A, Axial proton density fat fraction parametric MR image shows that liver has much higher signal intensity than spleen or skeletal muscle. Liver fat fraction measurement was 39%.
B, Axial MR elastography image shows mean liver stiffness of 2.1 kPa. Overall liver stiffness based on mean of four images through mid liver was 2.0 kPa.
C, Volume-rendered image of liver acquired from T2-weighted MRI series shows total liver volume of 2562.66 mL.
D, Histologic image (H and E, ×4) shows prominent macrovesicular steatosis involving greater than 70% of liver parenchyma, as well as mild-to-moderate portal inflammation and foci of lobular inflammation.
E, Histologic image (Masson trichrome, ×10) shows mild portal fibrosis, appearing blue.
Liver fat fraction was measured using one of two techniques. For all examinations performed on the HDx scanner and for examinations performed on the Ingenia scanner before July 2015, chemical-shift encoded imaging was used with parameters optimized to limit T1 bias and with normalization to a bag containing 20% lipid placed immediately adjacent to patients’ lateral abdominal wall. For examinations performed on the Ingenia scanner between July 2015 and June 2016, multiecho proton density fat fraction (mDixon Quant, Philips Healthcare) was used with the pulse sequence run as provided by the manufacturer [14]. Liver fat fraction measurements for both techniques used in our study were made by placing a single ROI of approximately 200–300 mm2 in the mid right hepatic lobe while avoiding large vessels and areas of artifact. For the chemical-shift encoded technique, a second ROI was placed in the lipid bag on the same slice as the hepatic ROI. The difference between the measured fat fraction in the lipid bag and the known value of 20% was applied as a correction factor to the measured fat fraction in the liver. Quantitative liver MRI examinations performed between January and December 2012 did not routinely include a liver fat fraction measurement (n = 14).
Liver shear stiffness was measured using MRE, which was performed using either a 2D gradient-recalled echo or 2D spin-echo echo-planar imaging pulse sequence [15, 16]. Four axial images through the mid liver were acquired and postprocessed using standard methods. ROIs were drawn manually, and liver stiffness was expressed as the mean of the mean stiffness values for each of the four images [17].
Whole liver volume was measured by manual segmentation of an axial T2-weighted fast spin-echo imaging series acquired with 5-mm slice thickness (no gap) (Vitrea, Vital Images). Liver fat fraction, stiffness, and volume measurements were made by one of four dedicated postprocessing technologists as a part of routine clinical care. Technologists were supervised by a clinical MRI physicist with more than 10 years’ experience in image analysis.
Statistical Analysis
Continuous data were summarized using means, SDs, and ranges, whereas categoric data were summarized as counts and percentages. Bivariate associations between quantitative liver MRI measurements and histopathologic scores were assessed using Spearman correlation coefficients (ρ). Spearman correlation was also used to assess the relationship between liver stiffness and liver fat fraction.
Multiple linear regression was used to assess the relationships between histopathologic NAFLD activity score and multiple MRI quantitative measurements (liver fat fraction [percentage], liver volume [milliliters], and liver stiffness [kilopascals]), adjusted for patient age and sex. Multiple linear regression also was used to assess the relationship between MRI-derived liver stiffness and histopathologic findings (steatosis, lobular inflammation, portal inflammation, hepatocyte ballooning, and fibrosis scores), adjusted for patient age and sex.
Next, subjects were placed into three cohorts categorized by histopathologic severity of NAFLD, according to the validated NAFLD activity score and severity of fibrosis [8]: the simple steatosis cohort had low histologic activity (NAFLD activity score = 0–4) and minimal or no fibrosis (fibrosis score ≤ 1), the NASH cohort had high histologic activity (NAFLD activity score ≥ 5) and minimal or no fibrosis (fibrosis score ≤ 1), and the NASH with significant fibrosis cohort had any degree of NAFLD activity (NAFLD activity score = 0–8) with significant fibrosis (fibrosis score ≥ 2). The nonparametric Kruskal-Wallis test was used to assess for differences in MRI quantitative measurements among cohorts, which were depicted using box plots. Ordinal (ordered) logistic regression was used to assess the association between categoric histopathologic severity of NAFLD and MRI quantitative measures; stepwise model section (significance levels for entering and staying in the model = 0.05) was used to arrive at the best model. Odds ratios with 95% CIs were used to indicate strength of association. ROC curves were created to assess the diagnostic performance of the final model for discriminating simple steatosis from NASH and NASH with significant fibrosis, and simple steatosis and NASH from NASH with significant fibrosis. AUC values were calculated.
A p < 0.05 was considered statistically significant for all inference testing. All analyses were performed using SAS (version 9.3, SAS Institute).
Results
There were 69 registry patients who underwent both quantitative liver MRI and liver biopsy within a 6-month period. The mean (± SD) patient age in the analysis cohort at the time of MRI was 14.3 ± 2.8 years (range, 8–21 years); 25 (36.2%) patients were female. The mean patient body mass index (weight in kilograms divided by the square of height in meters) was 36.5 ± 6.6 (range, 21.3–51.3). Means, SDs, and ranges for quantitative liver MRI measurements and histopathologic scores are provided in Table 1. Liver biopsy was performed within a mean of 64.4 ± 57.6 days of the MRI examination (range, 181 days before to 175 days after the MRI examination). Only three participants had the MRE performed after liver biopsy. All subjects were prescribed lifestyle modification (dietary changes and increased exercise to promote weight loss) from the time of NAFLD diagnosis, and only two subjects received medical therapy (vitamin E) between imaging and biopsy.
TABLE 1.
Quantitative Liver MRI Measurements and Liver Histopathologic Scores for 69 Pediatric and Young Adult Patients With Nonalcoholic Fatty Liver Disease (NAFLD)
| Variable | Mean | SD | Range |
|---|---|---|---|
|
| |||
| MRI quantitative measurements | |||
| Volume (mL) | 2379.0 | 696.9 | 812–4693 |
| Stiffness (kPa)a | 2.7 | 0.6 | 1.5–5.5 |
| Fat fraction (%)b | 14.5 | 10.3 | 0.7–39.0 |
| Histopathologic scores | |||
| Steatosis (0–3) | 2.0 | 0.9 | 0–3 |
| Lobular inflammation (0–3) | 1.2 | 0.7 | 0–3 |
| Portal inflammation (0–2) | 0.8 | 0.6 | 0–2 |
| Hepatocyte ballooning (0–2) | 0.7 | 0.6 | 0–2 |
| Fibrosis stage (0–4) | 1.0 | 1.1 | 0–4 |
| NAFLD score (0–8) | 3.8 | 1.6 | 0–7 |
Liver stiffness was measured in only 68 patients.
MRI examinations performed between January and December 2012 did not routinely include a liver fat fraction measurement; thus, fat fraction was measured in only 55 patients.
Bivariate Correlations
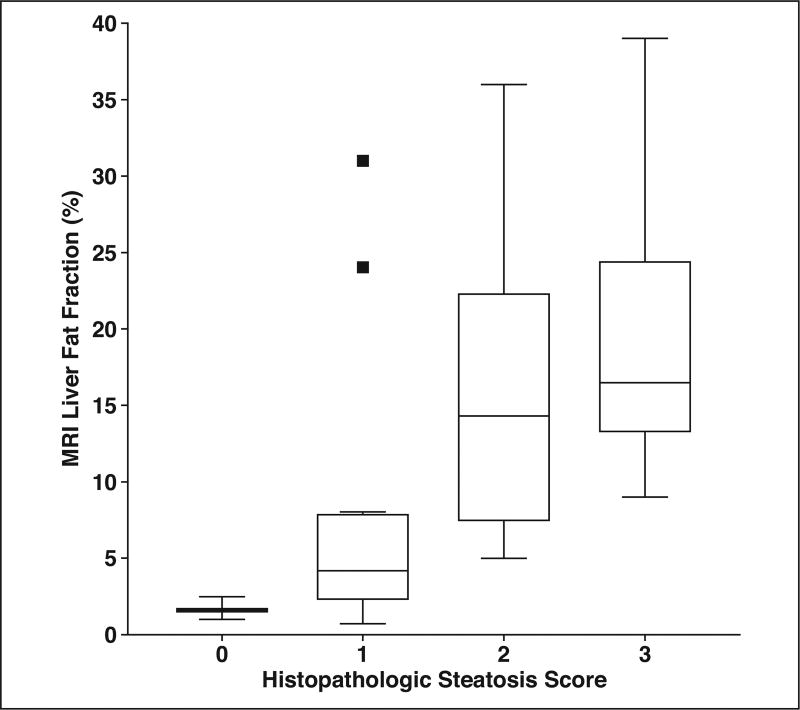
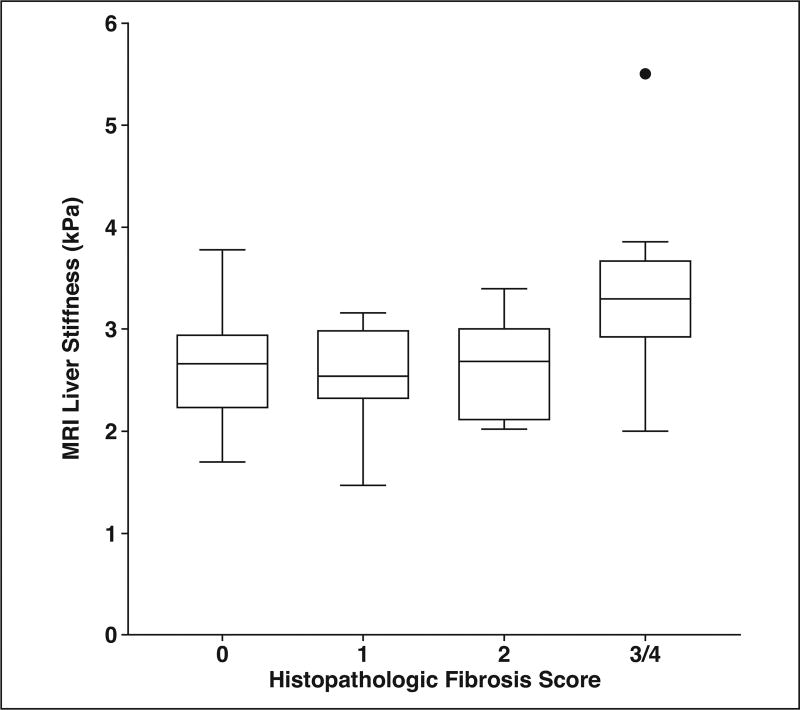
The relationships between quantitative liver MRI measurements and histopathologic scores are presented in Table 2. MRI liver volume was significantly positively correlated with histopathologic steatosis (ρ = 0.42; p = 0.0004), portal inflammation (ρ = 0.25; p = 0.04), fibrosis (ρ = 0.37; p = 0.002), and NAFLD activity score (ρ = 0.35; p = 0.003). MRI liver fat fraction was significantly correlated with histopathologic steatosis (ρ = 0.57; p < 0.0001), hepatocellular ballooning (ρ = 0.31; p = 0.02), fibrosis (ρ = 0.27; p = 0.046), and NAFLD activity score (ρ = 0.52; p < 0.0001). A box plot showing the relationship between histopathologic steatosis score and MRI liver fat fraction is presented in Figure 2. MRI liver stiffness was not significantly associated with any histopathologic score. A box plot showing the relationship between histopathologic fibrosis score and MRI liver stiffness is shown in Figure 3. There was a marginally significant negative correlation between liver fat fraction and liver stiffness (ρ = −0.24; p = 0.07).
TABLE 2.
Bivariate Correlations (ρ) Between Quantitative Liver MRI Measurements and Liver Histopathologic Scores for Pediatric and Young Adult Patients With Nonalcoholic Fatty Liver Disease
| Liver MRI Measurement | Volume (mL) (n = 69) | Stiffness (kPa) (n = 68) | Fat Fraction (%) (n = 55) |
|---|---|---|---|
|
| |||
| Steatosis (0–3) | |||
| ρ | 0.42 | −0.11 | 0.57 |
| p | 0.0004a | 0.36 | < 0.0001a |
| Lobular inflammation (0–3) | |||
| ρ | 0.15 | −0.0003 | 0.23 |
| p | 0.22 | 0.99 | 0.09 |
| Portal inflammation (0–2) | |||
| ρ | 0.25 | 0.09 | 0.21 |
| p | 0.04a | 0.48 | 0.13 |
| Hepatocyte ballooning (0–2) | |||
| ρ | 0.14 | 0.07 | 0.31 |
| p | 0.25 | 0.57 | 0.02a |
| Fibrosis score (0–4) | |||
| ρ | 0.37 | 0.22 | 0.27 |
| p | 0.002a | 0.08 | 0.046a |
| Nonalcoholic fatty liver disease activity score (0–8) | |||
| ρ | 0.35 | −0.02 | 0.52 |
| p | 0.003a | 0.87 | < 0.0001a |
Statistically significant.
Fig. 2.
Tukey box plot of relationship between histopathologic liver steatosis score (0–3) and MRI liver fat fraction. Squares represent statistical outliers, horizontal lines within boxes represent medians, and vertical lines and whiskers represent lowest and highest observations still within 1.5 interquartile ranges of lower and upper quartiles, respectively.
Fig. 3.
Tukey box plot of relationship between histopathologic liver fibrosis score (0–4) and liver stiffness determined by MR elastography. Circle represents statistical outlier, horizontal lines within boxes represent medians, and vertical lines and whiskers represent lowest and highest observations still within 1.5 interquartile ranges of lower and upper quartiles, respectively. Only one patient had fibrosis score of 4, and this patient reflects outlier seen in fibrosis score (3/4).
Relationships Between Nonalcoholic Fatty Liver Disease Activity Score and Quantitative Liver MRI Measurements
Using multiple linear regression to assess the relationships between histopathologic NAFLD activity score and MRI quantitative measurements, MRI liver fat fraction was the only imaging finding significantly associated with NAFLD activity score (increase in NAFLD activity score of 0.058 point per 1% increase in MRI liver fat fraction; p = 0.017). Liver volume (p = 0.36), liver stiffness (p = 0.90), age (p = 0.25), and sex (p = 0.65) were not significant predictor variables.
Predicting Categoric Nonalcoholic Fatty Liver Disease Histopathologic Severity Using MRI
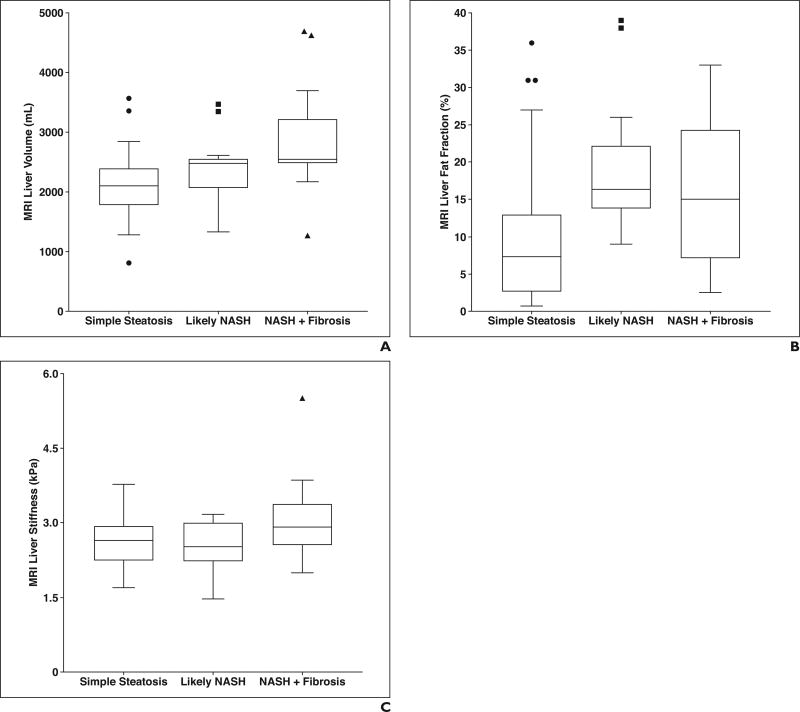
There were significant differences between the three NAFLD histopathologic severity cohorts (simple steatosis [n = 31], NASH [n = 18], NASH with significant fibrosis [n = 19]) in terms of MRI liver volume (p = 0.0005) and liver fat fraction (p = 0.004) using the Kruskal-Wallis test (Fig. 4). There were no significant differences between cohorts with regard to liver stiffness (p = 0.09) or age (p = 0.88).
Fig. 4.
Tukey box plots showing MRI values for each categoric nonalcoholic fatty liver disease (NAFLD) histopathologic severity cohort: simple steatosis (NAFLD activity score 0–4), likely nonalcoholic steatohepatitis (NASH; NAFLD activity score 5–8), and NASH with significant fibrosis (fibrosis score ≥ 2).
A–C, Box plots show liver volume (A), fat fraction (B), and stiffness (C) for each categoric NAFLD histopathologic severity cohort. There were significant differences between cohorts for liver volume (p = 0.0005; 32 vs 18 vs 19 subjects) and liver fat fraction (p = 0.004; 26 vs 16 vs 12 subjects). There was no difference in liver stiffness between cohorts (p = 0.09; 31 vs 18 vs 19 subjects). Circles, squares, and triangles represent statistical outliers, horizontal lines within boxes represent medians, and vertical lines and whiskers represent lowest and highest observations still within 1.5 interquartile ranges of lower and upper quartiles, respectively.
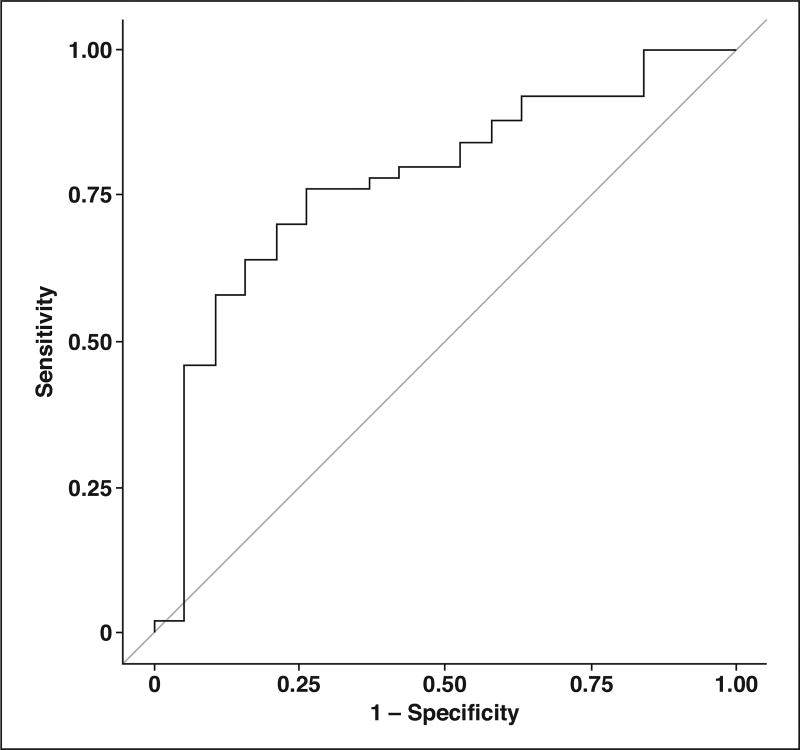
Ordinal logistic regression was used to assess the association between categoric NAFLD histopathologic severity and MRI quantitative measurements. Using stepwise model selection, the only significant MRI predictor variable was liver volume (odds ratio, 1.001; 95% CI, 1.000–1.002; p = 0.007). No other predictor variables made it into the final model. Using this model, the ROC AUC for discriminating simple steatosis from NASH and NASH with significant fibrosis on the basis of liver volume was 0.741. The ROC AUC for discriminating simple steatosis and NASH from NASH with significant fibrosis was 0.774 (Fig. 5).
Fig. 5.
ROC curve for discriminating simple steatosis and nonalcoholic steatohepatitis (NASH) from NASH with significant fibrosis based on MRI liver volume. AUC is 0.774.
Relationships Between MRI Liver Stiffness and Liver Histopathology
Using multiple linear regression to assess the relationship between MRI liver stiffness and histopathologic scoring, fibrosis score was significantly positively associated with liver stiffness (increase of 0.219 kPa per categoric increase in fibrosis score; p = 0.001); steatosis score was not significantly associated with liver stiffness (p = 0.20). Lobular inflammation (p = 0.59), portal inflammation (p = 0.58), hepatocyte ballooning (p = 0.57), age (p = 0.07), and sex (p = 0.72) were not statistically significant predictor variables for liver stiffness.
Interestingly, when MRI liver fat fraction, a more granular continuous variable, was substituted for histopathologic steatosis, a categoric variable, in the same model, the fat variable became significant (decrease of 0.019 kPa per 1% increase in MRI fat fraction; p = 0.019); histopathologic fibrosis remained significant (increase of 0.263 kPa per categoric increase in fibrosis score; p = 0.001). Lobular inflammation (p = 0.45), portal inflammation (p = 0.88), hepatocyte ballooning (p = 0.41), age (p = 0.16), and sex (p = 0.59) remained not significant.
Discussion
Our study has yielded several potentially important observations in a pediatric and young adult NAFLD population. First, we have confirmed that there is at least moderate bivariate correlation (ρ = 0.57) between histopathologic steatosis score and MRI liver fat fraction. Schwimmer et al. [12] also recently found a strong correlation between MRI-estimated liver proton density fat fraction and histologic steatosis stage in children (correlation coefficient = 0.73). Both our study and the study by Schwimmer et al. suggest that MRI is likely a viable alternative to liver biopsy for confirming the presence and estimating the severity of hepatic steatosis in the pediatric population. Lack of complete agreement between histopathologic findings and MRI with respect to the amount of fat in the liver in our study is likely multifactorial, due to a combination of biopsy-related sampling error, the categoric nature of histologic scoring, the fact that histologic scoring and MRI fat fraction are inherently different measures (percentage of hepatocytes with lipid content [histopathology] vs hepatic fat content [MRI]), and the fact that two different MRI methods for fat quantification were used during the study period. Importantly, our study shows that a significant correlation between MRI fat fraction and histologic scoring exists in a real-world clinical setting as well (clinical effectiveness as opposed to clinical efficacy [18]), given that data were derived from a prospectively enrolled clinical registry cohort.
Using multiple linear regression to assess the relationship between NAFLD activity score and quantitative liver MRI measurements, MRI fat fraction was the only imaging finding significantly associated with disease activity. Surprisingly, our study suggests that there is no statistically significant relationship between liver volume or liver stiffness and histopathologic NAFLD activity in our specific patient population. Although a study by Yin et al. [17] in adults showed that inflammation, like fibrosis, can cause liver stiffening at MRE (at least in patients with mild-to-moderate fibrosis), we failed to show increasing liver stiffness with increasing lobular or portal inflammation. These findings may be reflective of the fact that, in our population, very high levels of inflammatory activity and liver fibrosis were relatively uncommon or, if present, were confined predominantly to the portal areas [19].
Our results in a pediatric and young adult population with NAFLD are also discrepant from those of Chen et al. [10], who found that mean liver stiffness in adult patients (mean age, 51.5 years) with NAFLD with simple steatosis was significantly lower than that in patients with inflammation and no fibrosis and in patients with inflammation and fibrosis. Those authors concluded that, using a shear stiffness cutoff value of 2.74 kPa, MRE has a sensitivity of 94% and specificity of 73% for discriminating simple steatosis from NASH. A potential explanation for this discrepancy includes the fact that, in general, children have a greater predominance of portal inflammation compared with adults with NASH [19]. Furthermore, there was a generally low frequency of significant inflammation (both portal and lobular) in our study population, which is expected in this age group. The lack of predictive accuracy of quantitative MRI techniques (e.g., MRE) in our study suggests that MRI may not be usable to reliably detect milder degrees of hepatic inflammation in pediatric and young adult patients with NAFLD. A recent study by Schwimmer et al. [20] also found no consistent significant correlation (ρ = 0.05–0.27, depending on the reading center) between NAFLD activity score and MRE stiffness values, thus supporting our results.
By grouping patients instead into three categories on the basis of NAFLD activity and fibrosis stage (simple steatosis, NASH, and NASH with significant fibrosis), ordinal logistic regression using stepwise model selection yielded MRI liver volume as the only imaging predictor of categoric disease severity. This analysis, as well as a separate unadjusted Kruskal-Wallis test, showed that increasing liver volume is significantly associated with increasing fatty liver disease severity in our patient population. We suspect that the association between categoric disease severity and liver volume may be due to the combination of steatosis and inflammation causing liver enlargement, with fibrosis only causing liver shrinkage as the disease approaches end-stage cirrhotic liver disease, which is uncommon in children with NAFLD [7]. A bit surprisingly, neither MRI liver stiffness nor liver fat fraction was independently associated with categoric histopathologic severity on multivariate analysis. Because the ROC AUC values for discriminating varying levels of categoric NAFLD severity were less than 0.8, our results highlight that there is an unmet need in the pediatric and young adult NAFLD population for noninvasive imaging methods that can discriminate simple steatosis from histologically severe NAFLD with or without significant fibrosis.
Using multiple linear regression to assess the effect of five different histopathologic findings on MRI liver stiffness, only fibrosis score was significant. Neither the amount of steatosis, lobular inflammation, portal inflammation, nor degree of hepatocyte ballooning significantly affected liver stiffness. Interestingly, on replacing histopathologic steatosis score with MRI liver fat fraction in the same model, the fat variable became significant, in addition to histopathologic fibrosis score. This result is contrary to that of a recent study by Leitão et al. [21] suggesting that, on the basis of multivariable analysis, only fibrosis and not steatosis affects liver viscoelastic properties. We suspect that our observation is because we are investigating a young purely NALFD population without confounding conditions and that MRI fat fraction is much more granular than histopathologic steatosis staging. This observation (a negative correlation between liver fat fraction and liver stiffness) also adds to the list of liver parenchymal abnormalities, including fibrosis, inflammation, and congestion, which may affect measured liver stiffness at MRE [17, 22, 23].
Our study has limitations. First, although patients were prospectively enrolled in the registry from which this study population was drawn, the analysis was retrospective in design. A larger prospective study would be helpful to further validate our findings. Although 14 subjects were missing MRI fat fraction data, our study otherwise had very few missing data. Second, histopathologic data and severity scores were derived from the clinical record and thus reflect the observations of a varied group of pathologists. It is the current standard of care, however, for clinical hepatopathologists at our institution to use the validated NASH Clinical Research Network scoring system to grade and stage the severity of NAFLD. Furthermore, despite the use of clinical histopathologic data, our investigation shows numerous strong associations and highly significant results. Third, we allowed up to 6 months between the time of liver biopsy and quantitative liver MRI, which could affect the associations observed. That stated, on average, there were only 64 days between the biopsy and MRI, with the biopsy following the imaging study in all but three cases. Only two subjects received medical therapy (vitamin E) between imaging and biopsy, or vice versa. Moreover, the 6-month inclusion interval in our study is less than the interval between biopsy and MRI in multiple prior studies and is similar to a recent study by Schwimmer et al. [20] assessing the MRE findings as a biomarker in pediatric NAFLD. The time interval between imaging and biopsy is likely to have limited to no effect on fibrosis stage. Although fat and inflammation are more susceptible to change over such an interval, the biopsies were performed for clinical indications in patients with persistently elevated liver enzymes who were not responding to standard lifestyle recommendations, diminishing the potential for significant changes to occur in the time interval between MRE and liver biopsy.
A fourth limitation is that the reference standard used for our study—that is, core liver biopsy—can be affected by sampling error and may underestimate or overestimate the amounts of liver fat, inflammation, and fibrosis. However, at this time, there is no other acceptable reference standard to compare MRI against. Finally, our quantitative liver MRI protocol evolved over time in terms of both fat quantification technique and MRE sequence used. That said, multiple studies have documented excellent agreement between 2D gradient-recalled echo and spin-echo echo-planar imaging MRE techniques [15, 16]. Our results also should be generalizable across manufacturers and field strengths, because there is good evidence that MRI fat measurements are reproducible across vendors and field strengths [24], and because the vast majority of MRE technology emanates from a single entity (Redundant).
In conclusion, our study confirms that there is at least moderate correlation between histopathologic liver steatosis scoring and MRI-derived liver fat fraction in a pediatric and young adult population with NAFLD. Although increasing MRI liver fat fraction was shown to be significantly associated with increasing NAFLD activity score, there was no such association with liver volume or liver stiffness. Interestingly, MRI liver volume was the only significant predictor of categoric disease severity (simple steatosis vs NASH vs NASH with significant fibrosis) in this young cohort with biopsy-confirmed NAFLD. Of note, neither liver stiffness nor MRI-derived liver fat fraction was able to be used to discriminate simple steatosis from NASH from NASH with significant fibrosis at multivariate analysis. Furthermore, we have shown that, although liver stiffness is positively affected by histopathologic fibrosis and likely negatively affected by steatosis, neither lobular inflammation, portal inflammation, nor hepatocyte ballooning significantly affects this MRI measurement in our study population. Our study shows the need for continued development of noninvasive methods for determining severity of NAFLD, including the discrimination of simple steatosis from NASH with or without significant fibrosis, in the pediatric and young adult population.
Acknowledgments
J. R. Dillman has received grant funding unrelated to this study from Siemens Healthcare, Toshiba America Medical Systems, Guerbet LLC, and Bracco Diagnostics, Inc. A. T. Trout has received grant funding unrelated to this study from Siemens Healthcare and Toshiba America Medical Systems, has received royalties from Elsevier, and serves on the speaker’s bureau of Educational Symposia International. R. Kohli has received grant funding unrelated to this study from Raptor Pharmaceuticals, Shire Biopharma, Alexion Pharmaceuticals, and Intercept Pharmaceuticals. S. A. Xanthakos has received grant funding unrelated to this study from Ironwood Pharmaceuticals.
Supported by grants R01DK100314 to R. Kohli and R01DK100429 to S. A. Xanthakos from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Nobili V, Alkhouri N, Alisi A, et al. Nonalcoholic fatty liver disease: a challenge for pediatricians. JAMA Pediatr. 2015;169:170–176. doi: 10.1001/jamapediatrics.2014.2702. [DOI] [PubMed] [Google Scholar]
- 2.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J Pediatr Gastroenterol Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Mameli C, Zuccotti GV, Carnovale C, et al. An update on the assessment and management of metabolic syndrome, a growing medical emergency in paediatric populations. Pharmacol Res. 2017;119:99–117. doi: 10.1016/j.phrs.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Newton KP, Hou J, Crimmins NA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170:e161971. doi: 10.1001/jamapediatrics.2016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Zepeda A, Newton KP, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9:e112569. doi: 10.1371/journal.pone.0112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Lavine JE, Wilson LA, et al. In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed release improves liver enzymes but does not reduce disease activity scores. Gastroenterology. 2016;151:1141.e9–1154.e9. doi: 10.1053/j.gastro.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749–756. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xanthakos SA, Podberesky DJ, Serai SD, et al. Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr. 2014;164:186–188. doi: 10.1016/j.jpeds.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwimmer JB, Middleton MS, Behling C, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61:1887–1895. doi: 10.1002/hep.27666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34 doi: 10.1002/jmri.22580. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serai SD, Dillman JR, Trout AT. Spin-echo echo-planar imaging mr elastography versus gradient-echo MR elastography for assessment of liver stiffness in children and young adults suspected of having liver disease. Radiology. 2017;282:761–770. doi: 10.1148/radiol.2016160589. [DOI] [PubMed] [Google Scholar]
- 16.Wagner M, Besa C, Bou Ayache J, et al. Magnetic resonance elastography of the liver: qualitative and quantitative comparison of gradient echo and spin echo echoplanar imaging sequences. Invest Radiol. 2016;51:575–581. doi: 10.1097/RLI.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin M, Glaser KJ, Talwalkar JA, et al. Elastography: clinical performance in a series of 1377 consecutive examinations. Radiology. 2016;278:114–124. doi: 10.1148/radiol.2015142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. Criteria for distinguishing effectiveness from efficacy trials in systematic reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 19.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD—clinicopathologic correlations from the Nonalcoholic Steatohepatitis Clinical Research Network. Hepatology. 2009;49:809–820. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwimmer JB, Behling C, Angeles JE, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology. 2017 May 11; doi: 10.1002/hep.29241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitão HS, Doblas S, Garteiser P, et al. Hepatic fibrosis, inflammation, and steatosis: influence on the mr viscoelastic and diffusion parameters in patients with chronic liver disease. Radiology. 2017;273:98–107. doi: 10.1148/radiol.2016151570. [DOI] [PubMed] [Google Scholar]
- 22.DiPaola FW, Schumacher KR, Goldberg CS, Friedland-Little J, Parameswaran A, Dillman JR. Effect of Fontan operation on liver stiffness in children with single ventricle physiology. Eur Radiol. 2017;27:2434–2442. doi: 10.1007/s00330-016-4614-x. [DOI] [PubMed] [Google Scholar]
- 23.Yin M, Glaser KJ, Manduca A, et al. Distinguishing between hepatic inflammation and fibrosis with MR elastography. Radiology. 2017;284:694–705. doi: 10.1148/radiol.2017160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serai SD, Dillman JR, Trout AT. Proton density fat fraction measurements at 1.5- and 3-T hepatic MR imaging: same-day agreement among readers and across two imager manufacturers. Radiology. 2017;284:244–254. doi: 10.1148/radiol.2017161786. [DOI] [PubMed] [Google Scholar]