Abstract
Objectives
In pancreatic ductal adenocarcinoma (PDAC) patients, increased expression of proinflammatory neurotrophic growth factors (e.g. nerve growth factor (NGF)) correlates with a poorer prognosis, perineural invasion (PNI) and, with regard to NGF, pain severity. We hypothesized that NGF sequestration would reduce inflammation and disease in the KPC mouse model of PDAC.
Methods
Following biweekly injections of NGF antibody or control IgG, beginning at 4 or 8 wk of age, inflammation and disease stage were assessed using histological, protein expression, and qPCR analyses.
Results
In the 8 wk anti-NGF group, indicators of neurogenic inflammation in the dorsal root ganglia ([DRG], substance P and CGRP) and spinal cord (GFAP) were significantly reduced. In the 4 wk anti-NGF group, TRPA1 mRNA in DRG and spinal p-ERK protein were elevated, but GFAP expression was unaffected. In the 8 wk anti-NGF group, there was a 40% reduction in the proportion of mice with microscopic PNI and no macrometastases were observed.
Conclusions
Anti-NGF treatment beginning at 4 wk may increase inflammation and negatively impact disease. Treatment starting at 8 wk (after disease onset), however, reduces neural inflammation, neural invasion, and metastasis. These data indicate that NGF impacts PDAC progression and metastasis in a temporally dependent manner.
Keywords: nerve growth factor, pancreatic ductal adenocarcinoma, inflammation, perineural invasion, metastasis
INTRODUCTION
Nearly all patients (80–100%) with pancreatic ductal adenocarcinoma (PDAC) exhibit neural inflammation (neuritis), pain and tumor cell invasion into the perineurium and/or endoneurium of nerve fibers (PNI).1–5 Increased expression of members of the transforming, hepatocyte and endothelial growth factor families has been postulated as a driver of PNI, promoting “acinar to ductal metaplasia” and inflammatory desmoplasia, features seen in early stage neoplasia.6–11 More recently however, ligands in the neurotrophin and glial cell line-derived neurotrophic factor (GDNF) families and their cognate receptors have been implicated in proliferation and invasiveness of PDAC. Many of these growth factors and their receptors exhibit increased immunoreactivity in intra-pancreatic nerves and pancreatic tissues.12–21 Up regulation of these factors and/or receptors in patients has been correlated with a poor prognosis, extensive neural invasion, and, in the case of nerve growth factor (NGF), severe pain.22–25
The increased production/concentration of neurotrophic factors by tumor cells and intra-pancreatic nerve fibers raises the question as to whether suppression of growth factor signaling might inhibit the development and progression of pancreatic disease. Nerve growth factor (NGF) is a prime candidate for this role because of its strong neurotropic effects on peripheral neurons [reviewed in26,27]. NGF not only promotes neuronal survival but is also a key modulator of neurogenic inflammation and pain through its up regulation of inflammatory peptides (e.g. calcitonin gene related peptide (CGRP) and substance P (SP)) and its sensitizing effects on sensory neuron firing properties. Several completed and ongoing clinical trials have utilized NGF sequestration as a strategy to block pain signaling.28–34 Preclinically, NGF sequestration has ameliorated pain behaviors in a variety of animal models with the most relevant being pancreatitis post-surgical pain, and metastatic cancer pain.35–37
Although the role of NGF in cancer pain has been documented, there is limited information available regarding how NGF signaling impacts cancer progression. Studies of human and murine PDAC cell lines indicate that NGF can promote proliferation, migration, and invasiveness of tumor cells.20,38–40 Other in vitro and xenograft experiments show that NGF antibody (anti-NGF) treatment or siRNA-mediated knockdown of NGF reduces cell proliferation and inhibits growth of breast, prostate, and oral squamous carcinomas.25,41,42 However, there are no studies that directly examine how suppression of NGF signaling affects PDAC in an in vivo transgenic model.
Genetically engineered mouse models (GEMMs) of PDAC that express the most common human mutation associated with the disease (KrasG12D) provide an important physiologically relevant tool to investigate the role of growth factor signaling. These GEMMs share many of the pathological features of human PDAC including temporal progression of precursor lesions (pancreatic intraepithelial neoplasias, PanINs) to primary and metastatic tumors. With disease progression, intra-pancreatic nerve fibers exhibit hypertrophy, and mice exhibit pain-related behaviors that correlate with a significant up regulation of NGF and its receptor TrkA.43 Interestingly, during initial acinar to ductal metaplasia and early PanIN development, the peripheral nervous system exhibits signs of injury that may be linked to an influx of pancreatic lineage cells and up-regulation of neural inflammatory markers.44 These data are in line with other studies reporting that dissemination of pancreas lineage cells precedes tumor formation.45,46 Because increased NGF/TrkA expression is correlated with greater inflammation, cell proliferation, invasion and poorer prognosis in both humans and xenograft models, we explored the hypothesis that NGF sequestration could reduce neural inflammation and impede PDAC development in a physiologically relevant GEMM.
2. MATERIALS AND METHODS
2. 1 Animals
The KPC mouse model of PDAC was used for all experiments.44 In this model the Pft1a/p48 promoter drives expression of a mutant Kras allele (LSL-KrasG12D) and one allele of the p53 tumor suppressor gene is deleted in a Cre-dependent manner. Some KPC mice also expressed the fluorescent reporter protein tdTomato in a Cre-dependent manner. Mice were group-housed in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Division of Laboratory Animal Resources at the University of Pittsburgh. They were maintained in a 12-h light/dark cycle and temperature-controlled environment with ad libitum access to water and food. Mice were cared for and used in these studies following guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Anti-NGF Treatment
Mice were randomly assigned to receive biweekly intraperitoneal injections of anti-NGF (200µg/kg, Catalog # AF-556-NA, R&D systems, Minneapolis, Minn) or immunoglobulin G (IgG, 200µg/kg; R&D Systems) beginning at either age 4 or 8 wk of age. Unless mice succumbed to disease prematurely (n = 3), animals were euthanized via an overdose of inhaled isoflurane, perfused transcardially with saline at ≥16 weeks of age and tissue collected for analyses.
2.3 Antibody Immunolabeling
Mice were euthanized with inhaled isoflurane and perfused with saline. Superior cervical ganglia (SCG) and dorsal root ganglia (DRG) were removed, post-fixed for 30 min in 4% paraformaldeyhyde (PFA) and cryoprotected in 25% (wt/vol) sucrose in 0.1 M PB at 4°C. Pancreata were post-fixed overnight in 4% PFA with 15% (vol/vol) picric acid prior to cryoprotection. SCG and pancreata were embedded in Tissue-Tek OCT compound (Sakura Finetek USA, Torrance, Calif), sectioned at 14 and 30 µm respectively and mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, Pa). Sections were incubated in 10mM citrate buffer, pH 6.0 for 3–8 min at 95°C followed by 20 min at room temperature, then washed, blocked with 5% (vol/vol) normal horse serum in 0.1 M PB containing 0.25% Triton X-100 and incubated overnight in primary antibody. Antibodies used were: anti-tyrosine hydroxylase (1:500, AB152, Millipore, Temecula, Calif) or anti-CGRP (1:500, C8198, Sigma-Aldrich, St. Louis, Mo). Floating thoracic spinal cord sections (30 µm) were incubated overnight in anti-GFAP (1:500, 3670S clone GA5, Cell Signaling Technology, Danvers, Mass.) or anti-NeuN (1:500, MAB377 clone A60, Millipore). Following incubation with primary antibody, sections were washed and immunoreactivity detected using dye-conjugated secondary antibodies (1:500, Jackson ImmunoResearch Laboratories, West Grove, Pa). Sections were photographed using LAS software version 4.7 (Leica Microsystems, Buffalo Grove, Ill) and a Leica DM 4000B microscope and fluorescent intensity measured using Image J version 1.49 (NIH, Bethesda, Md).
2.4 Western Immunoblot Analysis
Total protein was extracted on ice by homogenization in 50 mM Tris·HCl lysis buffer (pH 7.4) containing 0.5% SDS and protease inhibitors (Cell Signaling Technology). Protein concentration was determined via bicinchoninic acid assay (Fisher Scientific) and aliquots (40µg) separated on 12% SDS/PAGE gels were transferred to PDVF membranes using the Transblot system (Bio-Rad, Hercules, Calif). Nonspecific binding was blocked using 5% (wt/vol) BSA, membranes were incubated overnight in primary antibodies directed against p-ERK (p44/42) or GFAP (1:1,000; Cell Signaling Technology) and protein bands detected using HRP-conjugated secondary antibodies (1:5,000). Membranes were washed and then probed with GAPDH or total ERK antibodies as loading controls. Densitometry readings were performed using SuperSignal Chemilumescent Detection reagents (Fisher Scientific), a LAS3000 imager (Fujifilm, Stamford, Conn), and Image J software. Protein levels were normalized to the IgG treated groups.
2.5 Semi-quantitative Real-time PCR
RNA from thoracic level DRG (T9–12) was isolated using the RNeasy mini kit (Qiagen, Germantown, Md), treated with DNase (Invitrogen, Pittsburgh, Pa) and reverse-transcribed using Superscript II reverse transcriptase (Invitrogen). SYBR Green PCR amplification was performed using a BioRad CFX connect real time system. After amplification, a dissociation curve was plotted against melting temperature to ensure amplification of a single product. All samples were run in duplicate and control reactions run (e.g., RT carried out without template). The relative fluorescence of SYBR Green bound to double-stranded DNA was compared with a passive reference for each cycle. Threshold cycle (Ct) values were used as a measure of initial template concentration. Fold changes in RNA levels were calculated by the ΔΔCt method using GAPDH as a reference standard: Ct values from duplicate samples were averaged and then subtracted from the reference standard, yielding ΔCt. Primer sequences are available upon request.
2.6 Histopathologic Analysis
Animals were examined for the presence of gross metastatic tumors at the time of dissection. The pancreas was then dissected en bloc with stomach and spleen and the number of additional organs involved was noted (observed range: 0–3). Hematoxylin and eosin stained sections of de-identified pancreas were used to define the disease stage for each animal (independently by Drs. Singhi and Hartman). Histopathology of lesions and fibro-inflammatory stroma were quantified by measuring the percentage of total analyzed surface area occupied. For each case, 10–15 fields of view were analyzed. Lesions were classified as low-grade (PanIN-1/2), high-grade (PanIN-3) or PDAC (tumor) based on the 2015 classification guidelines.47
2.7 Statistical Analysis
All numerical data were compared using two-way ANOVA followed by Sidak’s test for multiple comparisons. Categorical data (disease stage) were compared using a cumulative link model. Ordinal data (number of metastatic sites) were analyzed using Poisson’s regression.
3. RESULTS
3.1 Anti-NGF Reduces Markers Associated With Neurogenic Inflammation and PDAC Progression
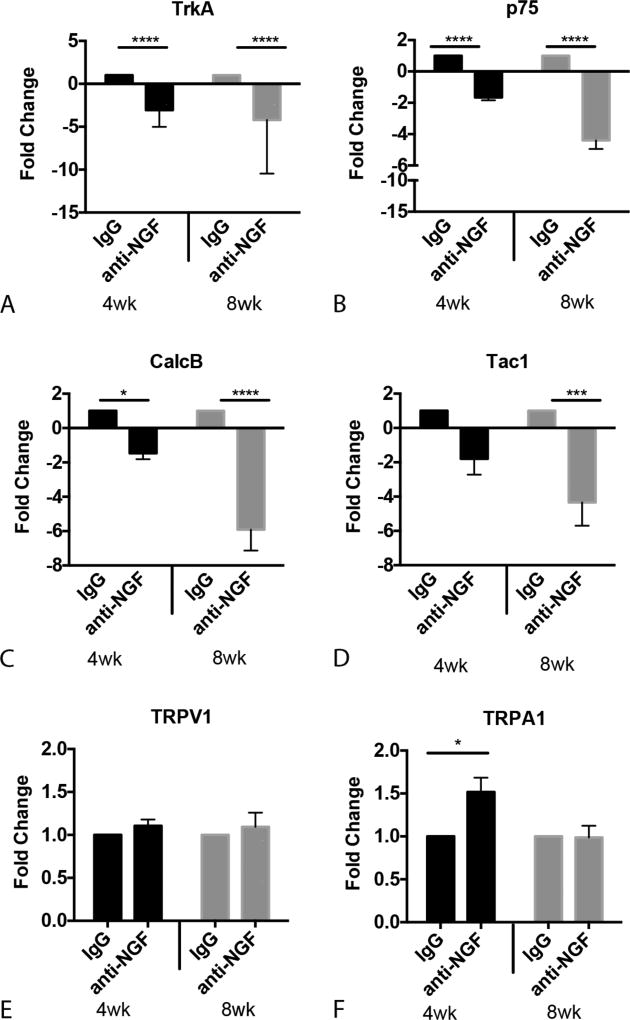
In the absence of intervention, PDAC progression and behavioral hypersensitivity in the KPC GEMM is associated with increased expression of growth factors, growth factor receptors, and neuropeptides associated with neurogenic inflammation (NI).43,44 To determine if suppression of NGF signaling alters these PDAC-induced changes we used RT-PCR, protein immunoblotting and tissue immunolabeling to assess relative expression in anti-NGF and IgG treated KPC mice. We first examined mRNAs encoding the NGF receptors, TrkA and p75.26,48 Both were significantly decreased in DRG of anti-NGF treated mice compared to IgG-treated mice, which was expected if sequestration of NGF ligand by antibody injection was effective. Specifically, there was a main effect of anti-NGF on TrKA mRNA regardless of age at onset of intervention [drug: F(1,30) = 78.65, P <0.0001, Fig. 1A]. Anti-NGF also reduced p75 mRNA, with a greater reduction in the group that began treatment at 8 wk of age [time×treatment: F(1,30) = 18.94, P = 0.0001, Fig. 1B]. The decrease in NGF receptors was accompanied by a reduction in mRNAs that encode proinflammatory peptides. For example, Tac1 (tachykinin 1), which encodes SP was decreased in DRG from KPC mice that began treatment at 8 wk of age as compared to IgG treated mice [time: F(1,26) = 18.90, P = 0.0002, Fig. 1C]. Compared to IgG treated mice, CalcB mRNA, which encodes CGRP, was decreased in mice that started anti-NGF at 4 wk of age but was down-regulated to a greater extent in mice that started treatment at 8 wk [time × treatment: F(1,34) = 13.48, P =0.0008, Fig. 1D]. Anti-NGF treatment did not affect mRNA encoding transient receptor potential cation channel subfamily V member 1 (TRPV1, a channel shown to be important for inflammatory pain49) (Fig. 1E), but a significant increase in DRG mRNA encoding the related TRPA1 channel was seen in mice beginning anti-NGF treatment at 4 wk of age was measured [time × treatment: F(1,30) = 4.536, P =0.041, Fig. 1F].
FIGURE 1.
Anti-NGF treatment alters levels of mRNAs encoding genes related to nociception and neurogenic inflammation in DRG T9–T12. A, TrkA and (B) p75 mRNA levels are significantly reduced following anti-NGF treatment regardless of age at onset of intervention. C, Substance P (Tac1) mRNA is significantly decreased in ganglia from mice treated with anti-NGF beginning at 8 wk of age. D, CGRP (CalcB) is significantly down regulated by anti-NGF. E) TRPV1 mRNA levels are unchanged by anti-NGF treatment. F, TRPA1 mRNA is significantly increased in thoracic DRG in the 4 wk anti-NGF group. Fold change data were compared by two-way ANOVA followed by Sidak post-hoc test. *P < 0.05, ***P < 0.001, ****P < 0.0001 n = 6–10/group.
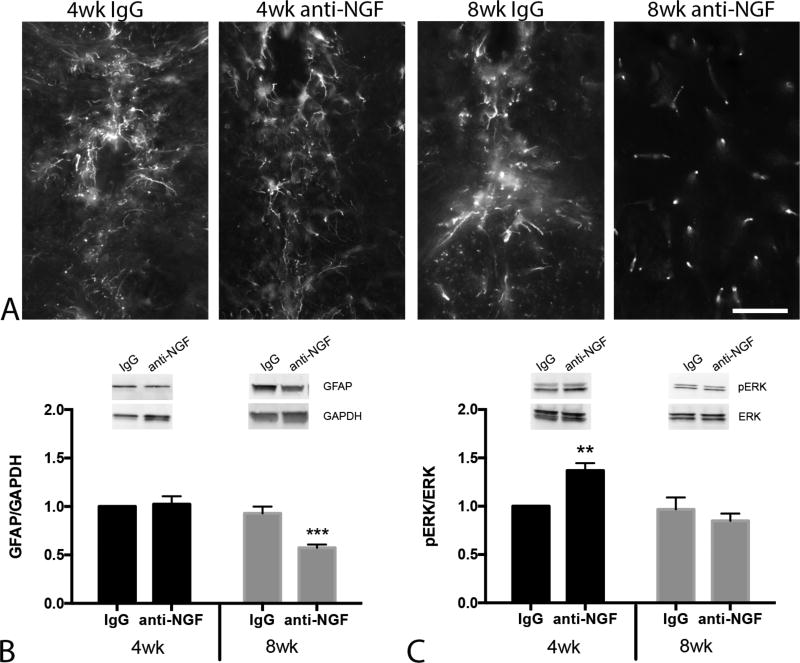
Perineural invasion in PDAC is associated with local nerve injury, neuritis and accompanied by increased spinal glial activation in both human and mouse.5,44 To determine if anti-NGF impacts PDAC associated glial activation, levels of glial fibrillary acidic protein (GFAP) were assessed in the thoracic spinal cord, at the vertebral levels that innervate the pancreas.50 Staining of thoracic spinal cord sections suggested a reduction in GFAP immunoreactivity and a lack of ‘activated morphology’ (as signified by the punctate nature of the staining) in the 8wk treatment group (Fig. 2A). The effects of anti-NGF of GFAP expression were quantified by immunoblotting of total protein from spinal cord, which showed a reduction of GFAP level in anti-NGF mice compared to IgG injected mice that was dependent on the age of treatment onset [time × treatment: F(1,36) = 9.71, P = 0.004]. KPC mice that began treatment at 4 wk exhibited no effect of anti-NGF on GFAP level whereas mice treated at 8 wk of age exhibited significantly lower GFAP immunoreactivity compared to all other groups (Fig. 2B).
FIGURE 2.
Anti-NGF treatment reduces levels of the inflammatory marker GFAP in the thoracic spinal cord. A, High power micrographs show astrocyte morphology and GFAP immunoreactivity in the central canal region of the thoracic spinal cord across experimental groups. Note the lack of ‘activated’ astrocyte morphology in 8 wk treatment group. B, Western blots confirm reduced GFAP expression in 8 wk treatment group. Inset: Representative blot compares relative GFAP expression in IgG and anti-NGF treated mice. Band densities were normalized to GAPDH level. C, Mice that began anti-NGF at 4 wk of age exhibited significantly higher p-ERK expression. Inset: Representative western blot shows pERK expression (normalized to total ERK) in thoracic spinal cord. Immunoblot data were analyzed by two-way ANOVA followed by Sidak’s test for multiple comparisons. **P < 0.01, ***P < 0.001 n = 7–11/group. Scale bar in A =50µm.
Anti-NGF injections were also found to have an effect on spinal levels of phosphorylated ERK (p-ERK), an indicator of inflammation and pain51 that is elevated at the PanIN stage of PDAC development.5,44 Spinal p-ERK was increased in mice that began anti-NGF injections at 4 wk of age compared to all other groups, while there was no difference observed in the 8 wk anti-NGF group compared to either IgG treated group [time × treatment interaction: F(1,36) = 8.51, P = 0.006, Fig. 2C]. The differential increase in TRPA1 mRNA and p-ERK protein suggest that timing of anti-NGF intervention is a significant factor: at 4 wk, when animals are relatively healthy, anti-NGF may drive neural inflammation whereas intervention at 8 wk, when PanINs are present, inhibits neural inflammation.
3.2 Anti-NGF Does Not Directly Impact Primary Disease
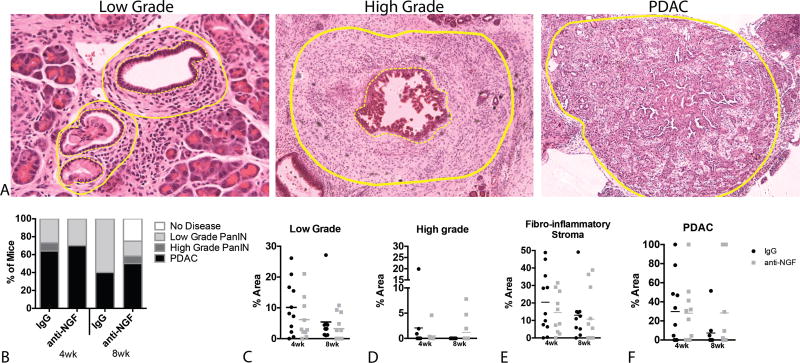
To determine the effects of anti-NGF treatment on the initial occurrence of PDAC, sections of pancreas tissue were scored in a blinded manner to classify animals based on the most severe lesion present: normal, low grade PanIN, high grade PanIN, or PDAC (Fig. 3A). Specifically, the percent area occupied by low or high grade PanINs, surrounding fibro-inflammatory stroma, and tumors were measured in 10–15 fields of view from each animal. Using a cumulative link model to analyze the distributions of diagnoses, no significant differences were detected (Fig. 3B). Similar to human cancers, every case in the mouse was different and this variability prevented the detection of significant differences in any of the measured parameters (Fig. 3C–F). However, it is worth noting that 25% of the 8 wk anti-NGF exhibited no pancreatic disease whereas all mice in the other groups exhibited abnormal pancreata, suggesting that suppression of NGF signaling may slow the development of pancreatic disease.
FIGURE 3.
Anti-NGF does not alter the development of primary pancreatic disease. A, Hemotoxylin and eosin staining of sections of pancreas from low- and high-grade PanIN lesions and PDAC tumor. Fibro-inflammatory stroma is demarcated between dotted and straight lines. B, The distribution of diagnoses was similar across treatment groups when compared using a cumulative link model. C–F, Charts indicate the percent area occupied by lesion, fibro-inflammatory stroma and PDAC tumor in pancreata of KPC mice. No significant difference in area is found in comparison of all groups using a two-way ANOVA. n = 10–12/group.
3.3 Anti-NGF Therapy Reduces Tumor Cell Mobility and Metastases
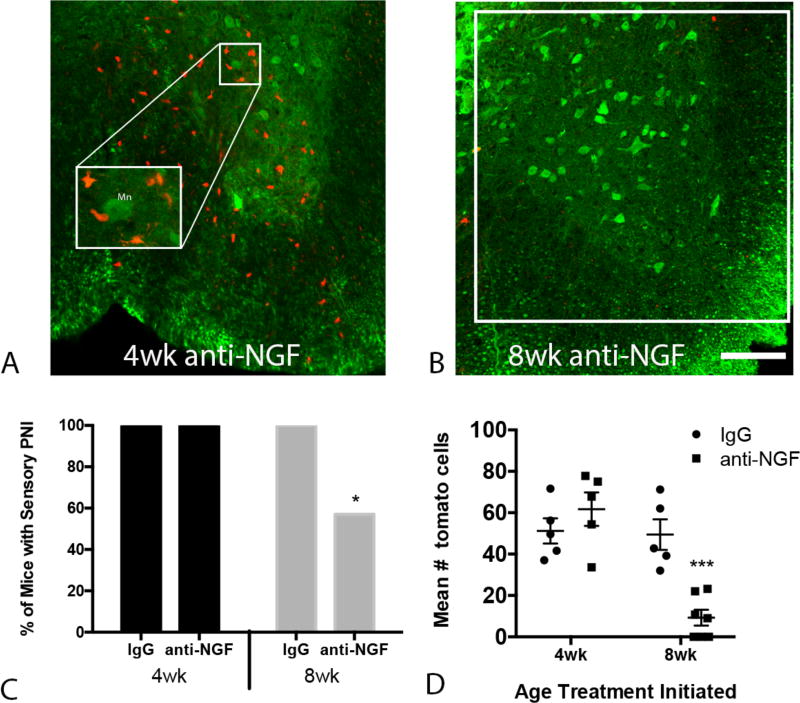
To assess the effect of anti-NGF on PNI we crossed a Cre-dependent tdTomato allele into the KPC GEMM. TdTomato expression enabled tracking of pancreas-lineage cells that disseminated from the pancreas to the thoracic spinal cord (Fig. 4A,B). Pancreatic cell invasion of the thoracic spinal cord was present in 100% of mice that began treatment (anti-NGF or IgG) at 4 wk of age, whereas there was a significant reduction in the proportion of mice exhibiting PNI in the 8 wk anti-NGF group [Logistic Regression, P =0.045, Fig. 4C]. In order to assess the extent of PNI, the average number of tdTomato+ cells in a 0.25mm2 region of interest (indicated in Fig. 4B) was determined in five random sections of spinal cord per mouse. There was a significant reduction in the extent of PNI in the 8 wk anti-NGF group relative to the 4 wk and IgG treatment groups [time × treatment: F(1,18) = 16.57, P = 0.0007, Fig. 4D].
FIGURE 4.
Anti-NGF inhibits perineural migration of pancreatic lineage cells into the thoracic spinal cord. Representative micrographs of the ventral thoracic spinal cord of KPC mice treated with anti-NGF starting at 4 wk (A) or at 8wk of age (B). Pancreas-lineage cells are identified by tdTomato expression (red); neurons are immunolabeled with anti-NeuN (green). C, The proportion of mice exhibiting perineural invasion is reduced in the 8 wk anti-NGF group (logistic regression analysis, P t6 = 0.045). D, The number of tdTomato positive cells (sum of 5 tissue sections per mouse) is reduced in mice that received anti-NGF treatment beginning at 8 wk (two-way ANOVA followed by Sidak’s post-hoc test). Cells within a 0.25mm2 region of interest (indicated in panel B) were counted. *P < 0.05, ***P < 0.001, n = 5–8/group. Scale bar = 100µm. Mn = motor neuron
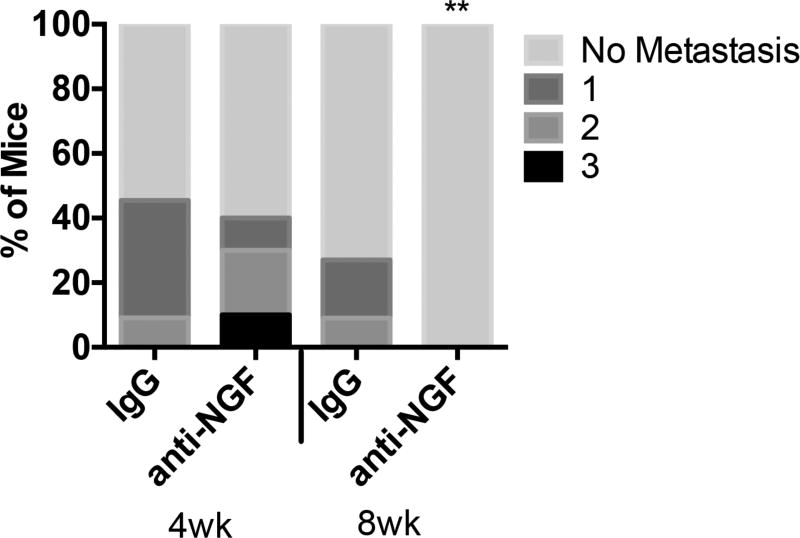
In vitro and xenograft studies suggest growth factor signaling stimulates the invasive and migratory capabilities of cancer cells.20,38–40 A prediction from these observations is that suppression of growth factor signaling in a GEMM should reduce metastases. We found that similar to other studies,52,53 grossly detectable metastases were present in 30.2% (13/43) of untreated KPC mice. Importantly, anti-NGF treatment affected this critical disease feature in a manner dependent on the age of treatment onset. In the 4 wk group, the same number of mice exhibited metastasis regardless of treatment (Fig. 5). However, mice that began anti-NGF treatment at 8 wk of age exhibited a complete absence of grossly detectable metastatic tumors [Poisson regression, P = 0.007]. That later intervention reduces metastases suggests that anti-NGF treatment could be used as a potential neoadjuvant to restrict tumor cells to the pancreas, providing a greater chance for successful surgical intervention.
FIGURE 5.
Anti-NGF inhibits metastases. Mice were examined for the presence of gross metastatic tumors at the time of euthanasia and dissection. The number of extrapancreatic sites involved (0–3) was compared by a two-way Poisson regression analysis. Anti-NGF treatment beginning at 8 wk of age was associated with a complete absence of grossly detectable metastatic tumors, **P <0.01, n = 10–12/group.
4. DISCUSSION
Inflammation and tissue injury are key features of PDAC that extend beyond the pancreas to the nervous system. Perineural invasion and increased levels of neurotrophic factors correlate with poorer prognosis.22–25 In mice, neural inflammation and PNI occur early in the disease process and are accompanied by an up regulation of NGF.43,44 In the current study, we present evidence that suppressing the rise in NGF can inhibit neural inflammation and restrict the dissemination of pancreas cells. If the extent of dissemination predicts the incidence of recurrence following pancreatic resection, the ability to inhibit cell migration from the diseased pancreas could significantly enhance the potential for successful treatment.
Our data show the effects of anti-NGF therapy depend on the time at which treatment begins. Intervention initiated at 4 wk (when mice are healthy) had no influence on PNI or spinal GFAP expression when assayed at 16 wk of age. However, the percent of mice with involvement of multiple (>1) additional organs was higher if anti-NGF treatment was begun at 4 wk, suggesting that sequestration of NGF prior to the appearance of disease may worsen the outcome. Mechanistically, this may relate to an increase in neurogenic inflammation, as suggested by the increased levels of TRPA1 and p-ERK in this group. This change in inflammation could be due to the long-term suppression of NGF signaling (i.e., starting at 4 wk), that may generate an imbalance in growth factor signaling that promotes the development and aggressiveness of the disease.21,54,55 NGF sequestration may also impact other cell types in the tumor microenvironment. NGF is essential for the survival and differentiation of sympathetic neurons,56 and anti-NGF treatment significantly reduced transcription of tyrosine hydroxylase (TH) in the postganglionic sympathetic neurons of the superior cervical ganglia (SCG) [F(1,25) = 23.81; P < 0.001, Supplementary Fig. 1]. Interestingly, both p75 and trkA are also expressed by immune cells.57 Reduction of NGF could therefore impact neural-immune communication and in so doing impede immune surveillance of tumor progression.12,20,21,54,55,58–67 Significant differences in the cellular microenvironment on which anti-NGF is acting (healthy at 4wk vs. pathological at 8wk) could also contribute to the time-dependent effects we observed.
In contrast to mice that began treatment at 4 wk, mice treated with anti-NGF beginning at 8 wk, when PanINs are typically present, exhibited significant differences in disease parameters. In 8 wk mice we observed a significant decrease in SP and CGRP in primary afferents as well as a reduction in GFAP, a marker of spinal astrocyte activation and inflammation. Secondly, anti-NGF starting at 8 wk correlated with a significant reduction in the migration of tdTomato-expressing cells to the spinal cord. The ability of pancreas-derived cells to migrate from the pancreas is proposed to reflect cells undergoing an epithelial to mesenchymal transition (EMT), during which invasive and stem-like properties are acquired.68,69 Early EMT and dissemination of pancreas-derived cells (preceding tumor formation) have previously been documented in PDAC GEMMs.44,45 Anti-NGF mediated inhibition of pancreatic cell invasion of the cord therefore suggests a reduction in metastatic potential, spinal inflammation and cancer-related pain. Finally, animals treated with anti-NGF starting at 8 wk exhibited a significant decrease in gross metastasis, with none of the treated mice exhibiting tumors outside of the pancreas. In humans, the absence of pancreatic cell dissemination, and ultimately metastasis, is the difference between a prognosis in which resection can produce long-term survival versus one in which surgery is unlikely to prevent recurrence.
Whether the primary effect of anti-NGF treatment is on sensory afferents, sympathetic efferents or both, remains to be determined. Early sensory denervation is sufficient to slow or block tumorigenesis in an animal model of PDAC,44,70 but sympathetic denervation was sufficient to reduce tumor burden in a model of prostate cancer.71–74 If sympathetic neurons have a prominent role in PDAC progression, a prediction is that anti-NGF would have a more robust effect than that seen in prior studies where only sensory neurons were depleted using neonatal capsaicin treatment44,70 Our results show this does not occur, at least in the treatment paradigm employed (starting treatment at either 4 or 8 wk). Although not killing sensory or sympathetic neurons,33 anti-NGF did decrease TH immunoreactivity in the SCG as well as reduced mRNAs encoding TrKA and p75, in DRG, which confirms anti-NGF bioactivity. Even so, this treatment was not sufficient for total denervation of the pancreas since TH and CGRP immunoreactivity were still present in nerve fibers within the pancreata of treated animals (Supplementary Fig. 2).
That ablation (surgical or chemical) of adult sensory and/or sympathetic innervation of the pancreas is a useful strategy is suggested by studies demonstrating the efficacy of celiac plexus block or ganglion neurolysis for palliative pain management in PDAC patients with unresectable PDAC.75–78 In one of the first double-blind placebo controlled study, patients with unresectable PDAC received either placebo or 50% ethanol-induced celiac plexus block.79,80 Patients that received an ethanol-induced splanchnicectomy survived significantly longer than saline treated patients (median 9.15 vs. 6.75 months). An initial interpretation of this study was that patients lived longer because sensory depletion and resulting reduction in pain led to an improved quality of life. Unfortunately, subsequent studies failed to detect similar effects on survival, although all concluded that celiac block/neurolysis significantly improved quality of life.76,78 In the context of the present study, results indicate that the time at which an anti-neural intervention is carried out is critical. Thus, neuron ablation or anti-NGF treatment must be performed within a window where inhibition of aberrant tumor-nerve interactions is effective. The challenge is to identify when this window of opportunity appears in each individual human patient.
Supplementary Material
SUPPLEMENTARY FIGURE 1. Effectiveness of NGF sequestration is evident by reduction in tyrosine hydroxylase expression in sympathetic ganglia. A, Representative micrographs show normal level of TH immunoreactivity in superior cervical ganglia (SCG) from IgG treated mice and reduction of staining in SCG of anti-NGF treated mice. B, Quantification of fluorescence intensity of TH immunoreactivity presented as mean ± SEM. Data were compared by two-way ANOVA and Sidak’s post-hoc test. **P <0.01, n = 6–8/group. Scale bar = 100 µm.
SUPPLEMENTARY FIGURE 2. NGF sequestration does not eliminate neural innervation of the pancreas. Micrographs show the presence of TH- and CGRP-positive nerve fibers within pancreata of mice treated with either IgG or anti-NGF. Scale bar = 100 µm.
Acknowledgments
Supported by: NCI CA177857 (BMD); The Hirshberg Foundation (JLS)
We are grateful for the skilled technical expertise of Christopher Sullivan and the consultation and assistance provided by Dr. Marsha Ritter Jones.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26:322–325. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bapat AA, Hostetter G, Von Hoff DD, et al. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 3.Marchesi F, Piemonti L, Mantovani A, et al. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor. Rev. 2010;21:77–82. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Schorn S, Demir IE, Haller B, et al. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma - A systematic review and meta-analysis. Surg Oncol. 2017;26:105–115. doi: 10.1016/j.suronc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Imoto A, Mitsunaga S, Inagaki M, et al. Neural invasion induces cachexia via astrocytic activation of neural route in pancreatic cancer. Int J Cancer. 2012;131:2795–2807. doi: 10.1002/ijc.27594. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L, Qin H, Li PY, et al. Response gene to complement-32 enhances metastatic phenotype by mediating transforming growth factor beta-induced epithelial-mesenchymal transition in human pancreatic cancer cell line BxPC-3. J Exp Clin Cancer. Res. 2012;31:29. doi: 10.1186/1756-9966-31-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Renzo MF, Poulsom R, Olivero M, et al. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer. Res. 1995;55:1129–1138. [PubMed] [Google Scholar]
- 8.Liang QL, Wang BR, Chen GQ, et al. Clinical significance of vascular endothelial growth factor and connexin43 for predicting pancreatic cancer clinicopathologic parameters. Med Oncol. 2010;27:1164–1170. doi: 10.1007/s12032-009-9354-1. [DOI] [PubMed] [Google Scholar]
- 9.Lohr M, Schmidt C, Ringel J, et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer. Res. 2001;61:550–555. [PubMed] [Google Scholar]
- 10.Bockman DE, Buchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107:219–230. doi: 10.1016/0016-5085(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 11.Kitaura Y, Chikazawa N, Tasaka T, et al. Transforming growth factor beta1 contributes to the invasiveness of pancreatic ductal adenocarcinoma cells through the regulation of CD24 expression. Pancreas. 2011;40:1034–1042. doi: 10.1097/MPA.0b013e31821ea286. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Okada Y, Sato M, et al. Expression of glial cell line-derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery. 2005;138:788–794. doi: 10.1016/j.surg.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Ketterer K, Rao S, Friess H, et al. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer. Res. 2003;9:5127–5136. [PubMed] [Google Scholar]
- 14.Schneider MB, Standop J, Ulrich A, et al. Expression of nerve growth factors in pancreatic neural tissue and pancreatic cancer. J Histochem Cytochem. 2001;49:1205–1210. doi: 10.1177/002215540104901002. [DOI] [PubMed] [Google Scholar]
- 15.Scharfmann R, Tazi A, Polak M, et al. Expression of functional nerve growth factor receptors in pancreatic beta-cell lines and fetal rat islets in primary culture. Diabetes. 1993;42:1829–1836. doi: 10.2337/diab.42.12.1829. [DOI] [PubMed] [Google Scholar]
- 16.Ohta T, Numata M, Tsukioka Y, et al. Neurotrophin-3 expression in human pancreatic cancers. J Pathol. 1997;181:405–412. doi: 10.1002/(SICI)1096-9896(199704)181:4<405::AID-PATH786>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Vidaltamayo R, Mery CM, Angeles-Angeles A, et al. Expression of nerve growth factor in human pancreatic beta cells. Growth Factors. 2003;21:103–107. doi: 10.1080/08977190310001629566. [DOI] [PubMed] [Google Scholar]
- 18.Miknyoczki SJ, Lang D, Huang L, et al. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–427. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto Y, Kitajima Y, Edakuni G, et al. Expression of Trk tyrosine kinase receptor is a biologic marker for cell proliferation and perineural invasion of human pancreatic ductal adenocarcinoma. Oncol. Rep. 2001;8:477–484. doi: 10.3892/or.8.3.477. [DOI] [PubMed] [Google Scholar]
- 20.Ceyhan GO, Schafer KH, Kerscher AG, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg. 2010;251:923–931. doi: 10.1097/SLA.0b013e3181d974d4. [DOI] [PubMed] [Google Scholar]
- 21.Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann of Surg. 2006;244:274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang C, Zhang Y, Ma Q, et al. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J Gastroenterol Hepatol. 2006;21:850–858. doi: 10.1111/j.1440-1746.2006.04074.x. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Jiang Y, Jiang Y, et al. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroenterol Hepatol. 2008;23:1852–1859. doi: 10.1111/j.1440-1746.2008.05579.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Z, Friess H, diMola FF, et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419–2428. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y, Dang D, Zhang J, et al. Nerve Growth Factor Links Oral Cancer Progression, Pain, and Cachexia. Mol Cancer Ther. 2011;10:1667–1676. doi: 10.1158/1535-7163.MCT-11-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45:252–261. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Jankowski MP, Koerber HR. Neurotrophic Factors and Nociceptor Sensitization. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton, FL: CRC Press/Taylor & Francis; 2010. [PubMed] [Google Scholar]
- 28.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J. Med. 2010;363:1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Te AE. A study to investigate tanezumab in patients with interstitial cystitis/painful bladder syndrome. Curr Urol. Rep. 2011;12:245–246. doi: 10.1007/s11934-011-0194-0. [DOI] [PubMed] [Google Scholar]
- 30.Katz N, Borenstein DG, Birbara C, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152:2248–2258. doi: 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Evans RJ, Moldwin RM, Cossons N, et al. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185:1716–1721. doi: 10.1016/j.juro.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 32.Tiseo PJ, Kivitz AJ, Ervin JE, et al. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155:1245–1252. doi: 10.1016/j.pain.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Marcek J, Okerberg C, Liu CN, et al. Anti-NGF monoclonal antibody muMab 911 does not deplete neurons in the superior cervical ganglia of young or old adult rats. J Chem Neuroanat. 2016;76:133–141. doi: 10.1016/j.jchemneu.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Mullard A. Drug developers reboot anti-NGF pain programmes. Nat Rev Drug Discov. 2015;14:297–298. doi: 10.1038/nrd4612. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Colak T, Shenoy M, et al. Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis. Gastroenterology. 2011;141:370–377. doi: 10.1053/j.gastro.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banik RK, Subieta AR, Wu C, et al. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Andrade JM, Ghilardi JR, Castañeda-Corral G, et al. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152:2564–2574. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Z, Kleeff J, Kayed H, et al. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Mol Carcinog. 2002;35:138–147. doi: 10.1002/mc.10083. [DOI] [PubMed] [Google Scholar]
- 39.Okada Y, Eibl G, Guha S, et al. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285–292. doi: 10.1023/b:clin.0000046131.24625.54. [DOI] [PubMed] [Google Scholar]
- 40.Zhu ZW, Friess H, Wang L, et al. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin Cancer. Res. 2001;7:105–112. [PubMed] [Google Scholar]
- 41.Warrington RJ, Lewis KE. Natural antibodies against nerve growth factor inhibit in vitro prostate cancer cell metastasis. Cancer Immunol Immunother. 2011;60:187–195. doi: 10.1007/s00262-010-0934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adriaenssens E, Vanhecke E, Saule P, et al. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer. Res. 2008;68:346–351. doi: 10.1158/0008-5472.CAN-07-1183. [DOI] [PubMed] [Google Scholar]
- 43.Stopczynski RE, Normolle DP, Hartman DJ, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer. Res. 2014;74:1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saloman JL, Albers KM, Li D, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016;113:3078–3083. doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148:21–23. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Basturk O, Hong SM, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitzman PH, Perrone TN, LeMaster AM, et al. Level of p75 receptor expression in sensory ganglia is modulated by NGF level in the target tissue. J Neurobiol. 1998;35:258–270. doi: 10.1002/(sici)1097-4695(19980605)35:3<258::aid-neu3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 49.Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Fasanella KE, Christianson JA, Chanthaphavong RS, et al. Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol. 2008;509:42–52. doi: 10.1002/cne.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji RR, Gereau RWt, Malcangio M, et al. MAP kinase and pain. Brain. Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bardeesy N, Aguirre AJ, Chu GC, et al. Both p16Ink4a and the p19Arf-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bardeesy N, Cheng K-h, Berger JH, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes. Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K, Demir IE, D'Haese JG, et al. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis. 2014;35:103–113. doi: 10.1093/carcin/bgt312. [DOI] [PubMed] [Google Scholar]
- 55.Zeng Q, Cheng Y, Zhu Q, et al. The relationship between overexpression of glial cell-derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J Int. Med Res. 2008;36:656–664. doi: 10.1177/147323000803600406. [DOI] [PubMed] [Google Scholar]
- 56.Raynaud B, Faucon-Biguet N, Vidal S, et al. Regulation of neurotransmitter metabolic enzymes and tyrosine hydroxylase mRNA level by nerve growth factor in cultured sympathetic neurones. Development. 1988:102361–368. doi: 10.1242/dev.102.2.361. [DOI] [PubMed] [Google Scholar]
- 57.Minnone G, De Benedetti F, Bracci-Laudiero L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int J. Mol Sci. 2017;18:pii: E1028. doi: 10.3390/ijms18051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gil Z, Cavel O, Kelly K, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng LX, Chi YH, Wang XX, et al. Neurotrophic artemin promotes motility and invasiveness of MIA PaCa-2 pancreatic cancer cells. Asian Pac J Cancer Prev. 2012;13:1793–1797. doi: 10.7314/apjcp.2012.13.5.1793. [DOI] [PubMed] [Google Scholar]
- 60.Funahashi H, Takeyama H, Sawai H, et al. Alteration of integrin expression by glial cell line-derived neurotrophic factor (GDNF) in human pancreatic cancer cells. Pancreas. 2003;27:190–196. doi: 10.1097/00006676-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 61.Okada Y, Eibl G, Duffy JP, et al. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery. 2003;134:293–299. doi: 10.1067/msy.2003.239. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi H, Funahashi H, Sawai H, et al. Glial cell line-derived neurotrophic factor enhances nuclear factor-kappaB activity and invasive potential in human pancreatic cancer cells. Pancreas. 2004;29:22–27. doi: 10.1097/00006676-200407000-00051. [DOI] [PubMed] [Google Scholar]
- 63.Cavel O, Shomron O, Shabtay A, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer. Res. 2012;72:5733–5743. doi: 10.1158/0008-5472.CAN-12-0764. [DOI] [PubMed] [Google Scholar]
- 64.Okada Y, Takeyama H, Sato M, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto-oncogene with reference to glial-cell-line-derived neurotrophic factor (GDNF) Int J Cancer. 1999;81:67–73. doi: 10.1002/(sici)1097-0215(19990331)81:1<67::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 65.Funahashi H, Okada Y, Sawai H, et al. The role of glial cell line-derived neurotrophic factor (GDNF) and integrins for invasion and metastasis in human pancreatic cancer cells. J Surg Oncol. 2005;91:77–83. doi: 10.1002/jso.20277. [DOI] [PubMed] [Google Scholar]
- 66.Liu H, Li X, Xu Q, et al. Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 2012;1826:112–120. doi: 10.1016/j.bbcan.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 67.Veit C, Genze F, Menke A, et al. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer. Res. 2004;64:5291–5300. doi: 10.1158/0008-5472.CAN-04-1112. [DOI] [PubMed] [Google Scholar]
- 68.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 70.Sinha S, Fu YY, Grimont A, et al. PanIN neuroendocrine cells promote tumorigenesis via neuronal crosstalk. Cancer. Res. 2017;77:1868–1879. doi: 10.1158/0008-5472.CAN-16-0899-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 72.Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl. Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horvathova L, Padova A, Tillinger A, et al. Sympathectomy reduces tumor weight and affects expression of tumor-related genes in melanoma tissue in the mouse. Stress. 2016;19:528–534. doi: 10.1080/10253890.2016.1213808. [DOI] [PubMed] [Google Scholar]
- 74.Lackovicova L, Banovska L, Bundzikova J, et al. Chemical sympathectomy suppresses fibrosarcoma development and improves survival of tumor-bearing rats. Neoplasma. 2011;58:424–429. doi: 10.4149/neo_2011_05_424. [DOI] [PubMed] [Google Scholar]
- 75.Fujii-Lau LL, Bamlet WR, Eldrige JS, et al. Impact of celiac neurolysis on survival in patients with pancreatic cancer. Gastrointest Endosc. 2015;82:46–56.e42. doi: 10.1016/j.gie.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lavu H, Lengel HB, Sell NM, et al. A prospective, randomized, double-blind, placebo controlled trial on the efficacy of ethanol celiac plexus neurolysis in patients with operable pancreatic and periampullary adenocarcinoma. J Am Coll Surg. 2015;220:497–508. doi: 10.1016/j.jamcollsurg.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh TK, Lee WJ, Woo SM, et al. Impact of Celiac Plexus Neurolysis on Survival in Patients with Unresectable Pancreatic Cancer: A Retrospective, Propensity Score Matching Analysis. Pain Physician. 2017;20:E357–E365. [PubMed] [Google Scholar]
- 78.Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092–1099. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 79.Lillemoe KD, Cameron JL, Kaufman HS, et al. Chemical splanchnicectomy in patients. Ann Surg. 1993;217:447–455. doi: 10.1097/00000658-199305010-00004. discussion 456–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Staats PS, Hekmat H, Sauter P, et al. The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: a double-blind, randomized, placebo-controlled study. Pain. Med. 2001;2:28–34. doi: 10.1046/j.1526-4637.2001.002001028.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE 1. Effectiveness of NGF sequestration is evident by reduction in tyrosine hydroxylase expression in sympathetic ganglia. A, Representative micrographs show normal level of TH immunoreactivity in superior cervical ganglia (SCG) from IgG treated mice and reduction of staining in SCG of anti-NGF treated mice. B, Quantification of fluorescence intensity of TH immunoreactivity presented as mean ± SEM. Data were compared by two-way ANOVA and Sidak’s post-hoc test. **P <0.01, n = 6–8/group. Scale bar = 100 µm.
SUPPLEMENTARY FIGURE 2. NGF sequestration does not eliminate neural innervation of the pancreas. Micrographs show the presence of TH- and CGRP-positive nerve fibers within pancreata of mice treated with either IgG or anti-NGF. Scale bar = 100 µm.