Abstract
Background:
Questions remain regarding the true prevalence of cardiovascular events such as myocardial infarction (MI) among patients with Inflammatory Bowel Disease (IBD). Using the Nationwide Inpatient Sample (NIS), we aimed to compare the proportion of hospitalizations for acute MI among patients with IBD to that of the general population.
Methods:
This study used data from years 2000–2011 in NIS, the largest publicly available all-payer inpatient database in the US. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) discharge codes were used to identify adult patients with discharge diagnoses of IBD (Ulcerative Colitis or Crohn’s Disease), acute MI, and multiple comorbid risk factors for cardiovascular disease. The independent effect of a diagnosis of IBD on risk of acute MI was examined using a multivariable logistic regression model controlling for multiple confounders. Data were analyzed using SAS survey procedures and weighted to reflect national estimates.
Results:
We identified 567,438 hospitalizations among patients with IBD and 78,121,000 hospitalizations among the general population. Patients with IBD were less likely to be hospitalized for acute MI than patients in the general population (1.3% vs. 3.1%, p<0.001). In adjusted analyses, the odds of hospitalization for acute MI among patients with IBD were decreased when compared to the general population (OR 0.51, 95% CI 0.50 – 0.52).
Conclusions:
Despite prior reports of a potentially increased risk of acute MI among patients with IBD, in a nationwide inpatient database, lower rates of acute MI were demonstrated in the IBD population when compared to the general population.
Keywords: Cardiovascular disease, Coronary Artery Disease, Myocardial Infarction, Crohn’s Disease, Ulcerative Colitis
Introduction:
Patients with chronic inflammatory conditions such as rheumatoid arthritis (RA), ankylosing spondylitis, and systemic lupus erythematosus (SLE) are at increased risk for the development of endothelial dysfunction, atherosclerosis and ultimately atherothrombotic events.1–9 In particular, an inflammatory protein, C-reactive protein (CRP), has been identified as a predictor of cardiovascular risk given its association with increased morbidity and poor outcomes among patients with vascular disease as well as its potential role in atherogenesis.10–16 The Inflammatory Bowel Diseases (IBDs), including Crohn’s Disease (CD) and Ulcerative Colitis (UC) have been similarly associated with increased cardiovascular risks, including coronary artery disease (CAD) and thromboembolic events of the venous and arterial systems.17–20 Patients with IBD often have long periods of remission with intermittent relapses of intestinal inflammation, and these increased cardiovascular risks have been documented during periods of both IBD flare and remission.
The risk burden for cardiovascular disease in IBD is unique and perhaps less well-defined relative to that of the general population. Traditional risk factors such as hyperlipidemia, obesity, and diabetes mellitus are less common among patients with IBD,21 and thus the increased risk profile of cardiovascular disease in IBD is poorly defined.22 Additional potential risk factors for cardiovascular disease in IBD include elevated levels of inflammatory cytokines (tumor necrosis factor α, CRP, and interleukin-6) and hypercoagulable states.22 Prior meta-analyses have not consistently demonstrated an association between IBD and an increased risk for cardiovascular disease related mortality.19,23 Thus, the actual risk of cardiovascular disease, in particular myocardial infarction (MI), in IBD has become a growing area of clinical and scientific interest. In this context, we sought to compare the rate of acute MI among patients with IBD to that of the general United States (US) population using a nationwide sample of inpatient hospitalizations between 2000 and 2011.
Materials and Methods:
Data source:
We conducted a retrospective cross-sectional study using consecutive years of the Nationwide Inpatient Sample (NIS) database. NIS is the largest publicly available all-payer inpatient database in the United States. It is designed to be nationally representative, and for the years 2000 through 2011, NIS captured discharges from a 20% stratified probability sample of community hospitals in the United States.24 In 2011, NIS contained information from over 8 million discharges from 1049 hospitals across 46 states.25 The NIS contains information on demographic characteristics and insurance status, up to 15 diagnostic and procedure codes based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), hospital characteristics, and outcomes including hospital length of stay, inpatient death, and total costs. Due to the de-identified nature of the data, this study was determined exempt from review by the Partners Healthcare Institutional Review Board.
Study sample:
We examined the discharges of patients age 18 and older. Patients were determined to have IBD if they had presence of ICD-9-CM diagnosis codes indicating CD (555.xx) or UC (556.xx). To minimize misclassification of subgroups of IBD (CD or UC), we excluded patients with discharge codes for both CD and UC (n=3751).
Outcomes of Interest:
The primary outcome of interest, acute MI, was determined by the presence of ICD-9-CM codes 410.xx. We were also interested in the risk of developing coronary artery disease (CAD) among patients with IBD; however, we had concerns that existing CAD may be underdiagnosed/undercoded in patients admitted with known IBD but without cardiovascular complications at the time of a current admission. For this reason we chose to evaluate acute MI as the primary outcome and CAD as a secondary outcome. CAD was determined by presence of ICD-9-CM codes 414.xx.
Covariates:
Patient demographics included age, sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian or Pacific Islander, Native American, other, and unknown), primary insurance/payer for the admission, and year of admission. ICD-9-CM diagnosis codes were used to identify additional clinical factors known to be independent predictors or confounders of the relationships between IBD and acute MI/CAD including hypertension, diabetes, hyperlipidemia, obesity (defined as Body Mass Index [BMI] > 30 kg/m2), and tobacco use (Appendix 1). Additionally, ICD-9-CM diagnosis codes were used to identify other known inflammatory conditions, RA (714.xx) and SLE (710.0). We used the Deyo-modification of the Charlson Comorbidity Index as a marker of comorbid illness.26 When this comorbidity index was used in the evaluation of the primary outcome (acute MI), the index was further modified to remove acute MI and old MI from the scoring system.
Statistical Analysis:
All analyses were performed using survey procedures in SAS version 9.3 (SAS Institute, Cary, NC, USA) to account for the complex survey design. Data were weighted to reflect estimates of the national population including means and proportions presented herein. Chi-Square test and Student t-test were used to compare proportions and continuous variables (under the assumption of the Central Limit Theorem), respectively. First, a logistic regression model was constructed to estimate the unadjusted odds of a diagnosis of acute MI among patients with IBD compared to those patients without IBD. Based on clinical knowledge, a multivariable logistic regression model was then constructed to evaluate the adjusted association between IBD and acute MI, adjusting for the covariates identified above. All factors were included in the model due to their clinical relevance, with diabetes included in the Charlson Comorbidity Index. These analyses were then repeated to examine the relationship of IBD to the secondary outcome of diagnosis of CAD.
Given concerns that diagnoses of IBD might be underrepresented among patients hospitalized with acute MI, we also evaluated acute MI among two patient populations with other chronic inflammatory diseases (RA and SLE). A two tailed p-value of 0.05 was chosen as the threshold for statistical significance for all tests. Odds ratios (OR) and 95% confidence intervals (CI) are presented.
Results:
From 2000–2011, NIS included a total of 563,687 discharges for patients with IBD and 78,121,000 discharges for the general population, representing an estimated 2.77 million hospitalizations among patients with IBD and 382.79 million hospitalizations among the general population. Of the 563,687 discharges for patients with IBD, 359,098 (63.7%) had CD and 204,589 (36.3%) had UC. As noted above, 3,751 discharges recorded diagnosis codes pertaining to both CD and UC and were excluded from the analyses.
Baseline characteristics for patients with and without IBD are shown in Table 1. Compared to patients without IBD, patients with IBD tended to be younger (mean age 51.6 years vs. 57.0 years) and were less likely to have diagnosed comorbidities of hyperlipidemia, diabetes mellitus, hypertension, and obesity (all p-values <0.001).
Table 1.
Baseline Demographic and Clinical Characteristics among Hospitalized Patients in the Nationwide Inpatient Sample
| Patients with IBD (n=563,687) | Patients without IBD (n=78,121,000) | p-value | |
|---|---|---|---|
| Agea | 51.6 (0.15) | 57.0 (0.002) | <0.001 |
| Sex: | <0.001 | ||
| Female | 326153 (57.8%) | 47408967 (60.7%) | |
| Male | 237111 (42.1%) | 30623519 (39.2%) | |
| Race/ethnicity:b | <0.001 | ||
| Non-Hispanic White | 363699 (64.5%) | 42418256 (54.3%) | |
| Non-Hispanic Black | 41378 (7.3%) | 8446106 (10.8%) | |
| Hispanic | 21735 (3.8%) | 6549449 (8.3%) | |
| Asian or Pacific Islander | 3933 (0.7%) | 1382922 (1.7%) | |
| Native American | 1479 (0.3%) | 325294 (0.4%) | |
| Other | 8965 (1.6%) | 1737149 (2.2%) | |
| Unknown | 122498 (21.7%) | 17261824 (22.1%) | |
| Primary Insurance/Payer: | <0.001 | ||
| Medicare | 202096 (36.0%) | 34839556 (44.8%) | |
| Medicaid | 55608 (9.9%) | 11031686 (14.2%) | |
| Private Insurance | 258431 (45.9%) | 25246763 (32.3%) | |
| Self-Pay | 25757 (4.6%) | 3908146 (5.0%) | |
| No Charge | 2874 (0.5%) | 380445 (0.5%) | |
| Other | 17687 (3.1%) | 2544927 (3.2%) | |
| Hyperlipidemia | 71023 (12.6%) | 14350542 (18.4%) | <0.001 |
| Diabetes Mellitus | 69802 (12.4%) | 16061332 (20.6%) | <0.001 |
| Hypertension | 152018 (27.0%) | 27256308 (34.9%) | <0.001 |
| Tobacco Abuse | 68985 (12.2%) | 7961496 (10.2%) | <0.001 |
| Obese | 26545 (4.7%) | 5223999 (6.7%) | <0.001 |
| Modified Charlson Comorbidity Indexc | <0.001 | ||
| Index Score ≤ 1 | 476989 (84.6%) | 57175337 (73.2%) | |
| Index Score 2 | 49775 (8.8%) | 11261611 (14.4%) | |
| Index Score ≥ 3 | 36923 (6.6%) | 9684052 (12.4%) | |
Age is a continuous variable, presented as mean (Standard Deviation) Categorical variable presented as number with associated (%)
Race/ethnicity is a missing variable for 22% of patients in sample, listed as unknown
Charlson Comorbidity Index modified to remove acute MI and old MI from scoring
Overall, patients with IBD were less likely to have a diagnosis of acute MI compared to patients without IBD (1.3% vs 3.1%, p<0.001), with unadjusted OR 0.42, 95% CI 0.41 – 0.43. After adjusting for age, sex, race, hypertension, hyperlipidemia, obesity, tobacco abuse, modified Charlson Comorbidity Index, year of hospitalization, and primary insurance/payer, patients with IBD had a 0.51 fold odds of diagnosis of acute MI when compared to patients without IBD (adjusted OR 0.51, 95% CI 0.50 – 0.52, Table 2). The overall trends for acute MI per 100,000 hospitalizations, analyzed by hospitalization year, are seen in Figure 1.
Table 2.
Unadjusted and Adjusted Odds Ratios for the Relationship between Inflammatory Bowel Disease and Myocardial Infarction
| Odds Ratio (95% CI) | |
|---|---|
| Unadjusted Analysis | |
| Inflammatory Bowel Disease | 0.42 (0.41 – 0.43) |
| Adjusted Analysis (Mutivariable Model) | |
| Inflammatory Bowel Disease | 0.51 (0.50 – 0.52) |
| Age: | |
| Age 18–39 | 0.12 (0.12 – 0.12) |
| Age 40–60 | Reference |
| Age 60–80 | 1.69 (1.68 – 1.70) |
| Female Sex | 0.56 (0.56 – 0.56) |
| Race/ethnicity: | |
| Non-Hispanic White | Reference |
| Non-Hispanic Black | 0.79 (0.79 – 0.79) |
| Hispanic | 0.92 (0.92 – 0.93) |
| Asian or Pacific Islander | 1.12 (1.11 – 1.13) |
| Native American | 1.11 (0.99 – 1.04) |
| Other | 1.20 (1.19 – 1.21) |
| Unknown | 1.01 (1.01 – 1.02) |
| Hyperlipidemia | 2.19 (2.19 – 2.20) |
| Hypertension | 0.90 (0.90 – 0.91) |
| Obese | 1.07 (1.06 – 1.07) |
| Tobacco Abuse | 1.84 (1.83 – 1.85) |
| Modified Charlson Comorbidity Indexa: | |
| Index Score ≤ 1 | Reference |
| Index Score 2 | 1.11 (1.10 – 1.11) |
| Index Score ≥ 3 | 0.94 (0.94 – 0.95) |
| Primary Insurance/Payer: | |
| Medicare | Reference |
| Medicaid | 0.79 (0.79 – 0.80) |
| Private Insurance | 1.03 (1.02 – 1.03) |
| Self-Pay | 1.37 (1.36 – 1.38) |
| No Charge | 1.21 (1.19 – 1.24) |
| Other | 0.90 (0.80 – 0.91) |
| Year of Hospitalization: | |
| 2000 | Reference |
| 2001 | 0.95 (0.94 – 0.95) |
| 2002 | 0.91 (0.91 – 0.92) |
| 2003 | 0.88 (0.87 – 0.88) |
| 2004 | 0.80 (0.87 – 0.88) |
| 2005 | 0.74 (0.73 – 0.74) |
| 2006 | 0.73 (0.73 – 0.73) |
| 2007 | 0.68 (0.68 – 0.69) |
| 2008 | 0.68 (0.67 – 0.68) |
| 2009 | 0.66 (0.66 – 0.67) |
| 2010 | 0.64 (0.64 – 0.65) |
| 2011 | 0.62 (0.62 – 0.63) |
Charlson Comorbidity Index was modified to remove acute MI and old MI from scoring, as acute MI is the primary outcome being modeled
Figure 1.
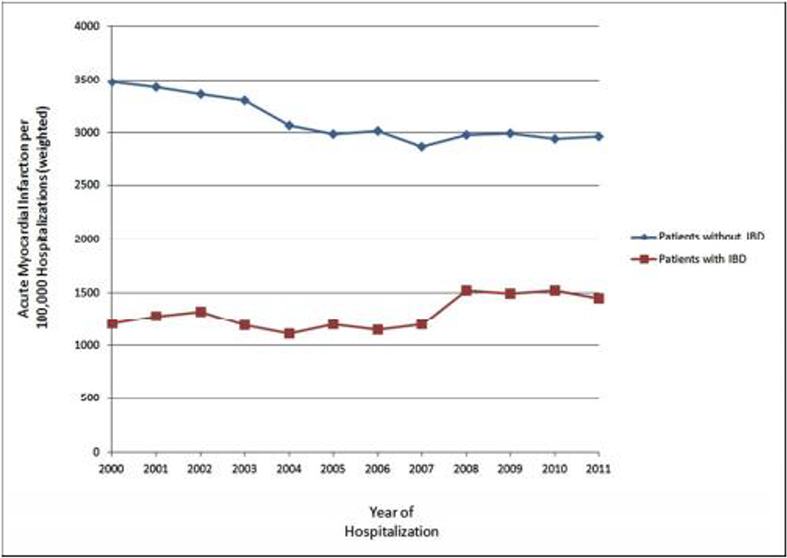
Number of Acute Myocardial Infarctions per 100,000 Hospitalizations among Patients with Inflammatory Bowel Disease compared to Patients without Inflammatory Bowel Disease 84×59mm (300 × 300 DPI)
When evaluating only patients with IBD, patients with UC were more likely to have a diagnosis of acute MI than patients with CD (1.8% vs. 1.1%, p<0.001). In an unadjusted analysis compared to patients without IBD, patients with UC (OR 0.57, 95% CI 0.55 – 0.59) and CD (OR 0.34, 95% CI 0.33 – 0.35) demonstrated decreased odds of being diagnosed with acute myocardial infarction. After adjusting for age, sex, race, hypertension, hyperlipidemia, obesity, tobacco abuse, modified Charlson Comorbidity Index, year of hospitalization, and primary insurance/payer, patients with both UC (OR 0.61, 95% CI 0.59 – 0.64) and CD (OR 0.44, 95% CI 0.43 – 0.46) continued to demonstrate significantly decreased odds of being diagnosed with acute myocardial infarction when compared to patients without IBD.
In evaluating the secondary outcome, 10.7% of patients with IBD had a discharge diagnosis of CAD compared to 18.8% of patients without IBD (p<0.001). In both an unadjusted analysis (OR 0.52, 95% CI 0.51 – 0.52) and an adjusted analysis controlling for age, sex, race, hypertension, hyperlipidemia, obesity, tobacco abuse, Charlson Comorbidity Index, year of hospitalization, and primary insurance/payer (OR 0.71, 95% CI 0.70 – 0.71) patients with IBD demonstrated lower odds of a discharge diagnosis of CAD when compared to patients without IBD (Table 3). When compared to patients without IBD, patients with UC demonstrated a decreased odds of diagnosis of CAD in both unadjusted (OR 0.65, 95% CI 0.65 – 0.66) and adjusted analyses controlling for age, sex, race, hypertension, hyperlipidemia, obesity, tobacco abuse, Charlson Comorbidity Index, year of hospitalization, and primary insurance/payer (OR 0.74, 95% CI 0.73 – 0.75). Patients with CD also demonstrated a decreased odds of diagnosis of CAD when compared to patients without IBD in both an unadjusted analysis (OR 0.44, 95% CI 0.44 – 0.45) and an adjusted analysis controlling for age, sex, race, hypertension, hyperlipidemia, obesity, tobacco abuse, Charlson Comorbidity Index, year of hospitalization, and primary insurance/payer (OR 0.71, 95% CI 0.70 – 0.72).
Table 3.
Unadjusted and Adjusted Odds Ratios for the Relationship between Inflammatory Bowel Disease and Coronary Artery Disease
| Odds Ratio (95% CI) | |
|---|---|
| Unadjusted Analysis | |
| Inflammatory Bowel Disease | 0.52 (0.51 – 0.52) |
| Adjusted Analysis (Mutivariable Model) | |
| Inflammatory Bowel Disease | 0.71 (0.70 – 0.71) |
| Age: | |
| Age 18–39 | 0.09 (0.09 – 0.09) |
| Age 40–60 | Reference |
| Age 60–80 | 2.09 (2.09 – 2.10) |
| Female Sex | 0.51 (0.51 – 0.51) |
| Race/ethnicity: | |
| Non-Hispanic White | Reference |
| Non-Hispanic Black | 0.75 (0.75 – 0.76) |
| Hispanic | 0.87 (0.87 – 0.88) |
| Asian or Pacific Islander | 0.78 (0.78 – 0.79) |
| Native American | 1.04 (1.03 – 1.05) |
| Other | 0.97 (0.96 – 0.97) |
| Unknown | 1.35 (1.35 – 1.35) |
| Hyperlipidemia | 3.25 (3.25 – 3.25) |
| Hypertension | 1.35 (1.35 – 1.35) |
| Obese | 1.10 (1.10 – 1.11) |
| Tobacco Abuse | 1.24 (1.24 – 1.25) |
| Charlson Comorbidity Index: | |
| Index Score ≤ 1 | Reference |
| Index Score 2 | 1.85 (1.85 – 1.86) |
| Index Score ≥ 3 | 1.94 (1.94 – 1.94) |
| Primary Insurance/Payer: | |
| Medicare | Reference |
| Medicaid | 0.73 (0.73 – 0.73) |
| Private Insurance | 0.70 (0.70 – 0.70) |
| Self-Pay | 0.67 (0.66 – 0.67) |
| No Charge | 0.64 (0.63 – 0.65) |
| Other | 0.64 (0.63 – 0.64) |
| Year of Hospitalization: | |
| 2000 | Reference |
| 2001 | 1.01 (1.01 – 1.02) |
| 2002 | 0.97 (0.97 – 0.98) |
| 2003 | 0.97 (0.97 – 0.98) |
| 2004 | 0.92 (0.92 – 0.92) |
| 2005 | 0.87 (0.87 – 0.87) |
| 2006 | 0.89 (0.89 – 0.89) |
| 2007 | 0.83 (0.82 – 0.83) |
| 2008 | 0.82 (0.82 – 0.82) |
| 2009 | 0.81 (0.81 – 0.82) |
| 2010 | 0.74 (0.74 – 0.75) |
| 2011 | 0.74 (0.73 – 0.74) |
Hospitalization for acute MI occurred more frequently among patients with RA (3.0%) and SLE (2.3%) than among patients with IBD (1.3%, p<0.001). Additionally, in both unadjusted and multivariable regression analysis controlling for age, sex, race, hypertension, hyperlipidemia, obesity, tobacco abuse, modified Charlson Comorbidity Index, year of hospitalization, and insurance/payer, patients with RA (OR 1.45, 95% CI 1.41 – 1.49) and SLE (OR 1.72, 95% CI 1.67 – 1.77) had significantly higher odds of discharge with a diagnosis of acute MI than patients with IBD (Table 4).
Table 4.
Unadjusted and Adjusted Odds Ratios for the Relationship between Rheumatoid Arthritis, Systemic Lupus Erythematosus, Inflammatory Bowel Disease and Myocardial Infarction
| Odds Ratio (95% CI) | |
|---|---|
| Unadjusted Analysis | |
| Inflammatory Bowel Disease | Reference |
| Systemic Lupus Erythematosus | 1.74 (1.69 – 1.80) |
| Rheumatoid Arthritis | 2.32 (2.26 – 2.38) |
| Adjusted Analysisa | |
| Inflammatory Bowel Disease | Reference |
| Systemic Lupus Erythematosus | 1.72 (1.67 – 1.77) |
| Rheumatoid Arthritis | 1.45 (1.41 – 1.49) |
Adjusted for age, sex, race, hypertension, hyperlipidemia, obesity, tobacco abuse, modified Charlson Comorbidity Index, year of hospitalization, and insurance/payer
Prior studies27 have raised concerns that women and younger patients with IBD may have greater risk of development of acute MI; thus, we performed subgroup analyses of these populations. In a comparison of diagnosis of acute MI stratified by sex, both women (1.0% vs. 2.2%, p<0.001) and men (1.7% vs. 4.5%, p<0.001) with IBD were less likely to have a diagnosis of acute MI than their respective populations without IBD. While patients with IBD who were diagnosed with acute MI had a lower mean age (68.4 years) than patients without IBD who were diagnosed with acute MI (69.3 years, p <0.001), when divided into age groups of greater than 50 years and less than 50 years, patients with IBD were less likely to have a diagnosis of acute MI than patients without IBD in both age groups (2.3% vs. 4.5% and 0.26% vs. 0.84%, respectively, p<0.001).
Discussion:
In this study we utilized a large, nationwide database to demonstrate a significant decrease in the odds of being discharged with a diagnosis of acute MI among patients with IBD compared to patients without IBD. In both a crude analysis and in a multivariable analysis adjusting for comorbidities associated with increased risk of cardiovascular disease and MI, we found that patients with IBD demonstrated significantly lower odds of being diagnosed with acute MI and CAD when compared to patients without IBD. The decreased odds of being diagnosed with acute MI or CAD were demonstrated in patients with both UC and CD when the IBD subtypes were evaluated independently.
The association between venous thromboembolic disease and IBD has been well established, as patients with IBD carry an estimated three-fold increased risk of venous thromboembolic events.20 However, the potential for IBD to act as a risk factor for development of CAD and the relationships between IBD and other cardiovascular outcomes such as mortality is less clear.19 While recent large database studies have demonstrated an increased risk of ischemic heart disease and MI among patients with IBD, particularly during times of increased disease activity,17,28 questions remain regarding the actual risk of MI in this population.29,30
Several mechanisms linking the pro-inflammatory state associated with active IBD and increased cardiovascular risk have been proposed, including derangements in the coagulation cascade,31 elevations in inflammatory mediators such as CRP32 and homocysteine,33–35 and alterations in endothelial, microvascular, and macrovascular function.36 As these factors would be suspected to carry the greatest risk during flares of disease, there remains the potential that the lack of clinical information regarding relapse/remission of disease and the cross sectional design of our study did not allow for an accurate comparison of odds of acute MI during the periods of greatest risk. However, the potential also remains that any increased risk associated with the pro-inflammatory state is offset by a lack of other risk factors such as obesity, hypertension, and hyperlipidemia, which were lower in the IBD population. The higher rate of tobacco abuse documented in patients with IBD may act in multiple ways among the patients with separate sub-types of IBD. While smoking is known to increase overall risk of cardiovascular disease,37 smoking among some patients with UC is associated with a milder disease course and thus less inflammatory burden overall.38–40 Alternatively, among patients with CD, smoking is associated with an increase in both disease activity and complications.41
In 2008, one of the first large studies evaluating the risk of ischemic heart disease in patients with IBD was published using a large registry database from Canada. Patients with IBD were found to have a significantly increased risk of ischemic heart disease when compared to non-IBD patients (IRR 1.26, 95% CI 1.11 – 1.44).42 Subsequently, a nationwide registry-based study of 4.6 million Danish patients, including 28,833 patients with IBD, revealed a modestly increased risk of ischemic heart disease in patients with IBD.28 This risk was particularly increased in the first year after diagnosis,28 perhaps indicating a different underlying pathophysiology of CAD in the IBD population. A historical cohort study of IBD patients undergoing cardiac catheterization found that patients with IBD developed CAD at a younger age, although their post-percutaneous coronary intervention outcomes were similar to those patients without IBD.27
One potential reason for the differences in findings between our study and prior database studies from Canada42 and Denmark28 relates to the nature of NIS as an inpatient hospitalization database. The Manitoba Health administrative database utilized by Bernstein, et al.42 contains information regarding both inpatient hospitalizations and outpatient physician visits. The Danish study cohort was derived from the Danish National Patient Register, which contains individual patient information about outpatient visits and inpatient hospitalizations.28 Similarly, a retrospective longitudinal cohort study from the US that demonstrated an increased risk for development of CAD among patients with IBD included both inpatient and outpatient evaluations.21 If there were an increased likelihood for more than one disease to be coded in an outpatient setting as opposed to an inpatient setting, this would not be reflected in NIS as it is an inpatient only database. Additionally, the mean ages of both the IBD and non-IBD patients in our study population were greater than the mean ages of those populations the prior database studies from Canada42 and Demark.28 This increased age would seem to favor an increased likelihood of acute MI in our population, given the mean ages of arterial thromboembolic disease demonstrated in the Manitoba Health administrative database.42 However, fundamental differences in the baseline demographics of these studies should be noted, as they are potentially indicative of the differences between an inpatient only population and a database that utilizes inpatient and outpatient visits.
Other studies have demonstrated risks of CAD among patients with IBD that are more similar to the population as a whole. A population-based study utilizing a large claims database from the US demonstrated an increased risk of MI only among a subgroup of women with IBD aged more than 40 years, after adjustment for many confounders including hyperlipidemia, diabetes, and hypertension.43 Similarly, a study from the United Kingdom (UK) evaluating 15,498 patients with UC and 9,829 patients with CD found an increased risk of acute MI among patients with IBD, however this risk was attenuated in a multivariable model.29 Meta-analyses have reported conflicting results, as cardiovascular disease (including ischemic heart disease) was increased in patients with IBD (OR 1.18, 95% CI 1.08 – 1.31) in one meta-analysis44 and not significantly increased in another meta-analysis (RR 1.23, 95% CI 0.94 – 1.62).19
In a study of cardiovascular morbidity among hospitalized patients with IBD that used a single year of data from NIS, Sridhar, et al. demonstrated lower odds of ischemic heart disease among patients with both UC and CD, after adjusting for hypertension, hyperlipidemia, and diabetes.30 Though we report a similar outcome of patients with IBD demonstrating decreased odds of being diagnosed with CAD, our analysis differs from the prior published study as we evaluated outcomes over a longer period of consecutive years. In addition, we attempted to control for several additional factors in our multivariable analysis, including the modified Charlson Comorbidity Index, and insurance/payer status. Given our concerns that CAD may potentially be underdiagnosed among inpatients with IBD, we also chose to evaluate acute MI as our primary outcome. In a comparison not used in the prior study, we also compared the rate of acute MI among inpatients with IBD to other chronic inflammatory conditions (RA and SLE).
Our study has several important results. The decrease in odds of being discharged with a diagnosis of acute MI among patients with IBD is an important finding, particularly as this was seen in both an unadjusted analysis and after adjustment for multiple potential confounders. While these results differ from two large studies from Canada42 and Denmark,17 they do mirror the results of database studies from the UK29 and the lack of overall difference demonstrated in at least one meta-analysis.19 Additionally, the lower frequency of diagnosis of acute MI among patients with IBD when compared to other chronic inflammatory conditions such as RA and SLE may indicate a difference in pathophysiology among these diseases.
This study does have multiple limitations. Notably, the NIS does not include information regarding an individual patient’s medication profile prior to admission or during the hospitalization. Thus, we were unable to evaluate the potential relationship between steroid use or other immunosuppressive therapy and risk of acute MI, an area where differing results have previously been reported.45,46 The NIS does not contain data regarding the severity of an individual patient’s IBD at the time of admission. In a large, nationwide Danish cohort study,17 the risk of MI was among patients with IBD was increased only during periods of flare and persistent disease activity and not during periods of disease remission. Additionally, the NIS does not contain data regarding the duration of IBD, and thus we were unable to evaluate the effect of prolonged inflammation on development of CAD or risk of acute MI.
With a large database such as NIS, there is potential for misclassification of both our exposure and outcome. We attempted to limit this by excluding any patient with an ICD-9-CM code for both UC and CD; however, this bias may still exist. While International Classification of Disease codes have been used in other studies examining the relationship between cardiovascular disease and IBD,17,18,21,27,28,30,42 the NIS does not contain personal identifiers, and thus we are unable to validate the ICD-9-CM diagnosis codes of individual patients using chart review. ICD-9-CM coding has demonstrated accuracy in IBD47,48 and other studies of gastrointestinal disorders,49 however this remains a concern in our population given mixed performance within studies of patients with IBD in the Veterans Affairs system.50,51 There also exists the potential for underdiagnosis of other risk factors, particularly tobacco use and obesity.52
Perhaps the most significant limitation of the use of administrative coding relates to the potential for an “undercoding” of IBD among patients that are experiencing acute MI. If a patient has an ICD-9-CM code for acute MI as a primary diagnosis, and an ICD-9-CM code for UC or CD is not included as a secondary diagnosis, this could lead to the appearance of fewer occurrences of acute MI among patients with IBD. To evaluate this limitation, we compared the frequency of acute MI among patients with IBD to two other chronic inflammatory conditions (RA and SLE). Although the clinical presentation and underlying disease processes differ among patients with RA and SLE, patients with these diseases share an underlying chronic inflammation that has been linked to increased cardiovascular risk.53 Patients with RA have demonstrated an increased risk of acute MI compared to the general population.54,55 Similarly patients with SLE have demonstrated an increased risk of acute MI,7 and in some cases acute MI can precede the diagnosis of SLE.56 In this evaluation, patients with IBD were less likely to have a diagnosis of acute MI than patients with RA or SLE. The differences in risk for arterial thromboemboli when comparing patients with IBD to patients with RA or SLE may be due to a difference in platelet dysfunction that leads to more venous thrombosis than arterial thrombosis among patients with IBD,29 though the biologic mechanisms underlying the different risk profiles associated with these chronic inflammatory conditions are not well understood.
In conclusion, using a large, nationwide inpatient database, we demonstrated decreased odds of diagnosis of acute MI among patients with IBD compared to patients without IBD. Additionally, patients with IBD were discharged with a diagnosis of acute MI less frequently than patients with other chronic inflammatory conditions such as RA and SLE. Although providers should be cognizant of cardiovascular risk factors among patients with IBD, the risk of acute MI may not be as significant as reported in other studies.
Supplementary Material
Acknowledgement of Support:
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Source of Funding:
Drs. Barnes and Winter are supported by the National Institutes of Health [T32 DK007533–29].
Footnotes
Conflicts of Interest: None
References:
- 1.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42(2):338–346. [DOI] [PubMed] [Google Scholar]
- 2.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am. J. Med 1992;93(5):513–519. [DOI] [PubMed] [Google Scholar]
- 3.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2005;52(3):722–732. [DOI] [PubMed] [Google Scholar]
- 4.Ajeganova S, Andersson MLE, Frostegård J, Hafström I. Disease factors in early rheumatoid arthritis are associated with differential risks for cardiovascular events and mortality depending on age at onset: A 10-year observational cohort study. J. Rheumatol 2013;40(12):1958–1966. [DOI] [PubMed] [Google Scholar]
- 5.Solomon DH, Reed GW, Kremer JM, et al. Disease Activity in Rheumatoid Arthritis and the Risk of Cardiovascular Events. Arthritis Rheumatol. 2015;67(6):1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungprasert P, Suksaranjit P, Spanuchart I, Leeaphorn N, Permpalung N. Risk of coronary artery disease in patients with idiopathic inflammatory myopathies: A systematic review and meta-analysis of observational studies. Semin. Arthritis Rheum 2014;44(1):63–67. [DOI] [PubMed] [Google Scholar]
- 7.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol 1997;145(5):408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 8.Frostegård J Atherosclerosis in patients with autoimmune disorders. Arterioscler. Thromb. Vasc. Biol 2005;25(9):1776–1785. [DOI] [PubMed] [Google Scholar]
- 9.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: A systematic review. Can. J. Cardiol 2011;27(2):174–182. [DOI] [PubMed] [Google Scholar]
- 10.Lagrand WK, Visser CA, Hermens WT, et al. C-Reactive Protein as a Cardiovascular Risk Factor: More than an Epiphenomenon? Circulation. 1999;100:96–102. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105(9):1135–1143. [DOI] [PubMed] [Google Scholar]
- 12.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: Risk marker or mediator in atherothrombosis? Hypertension. 2004;44(1):6–11. [DOI] [PubMed] [Google Scholar]
- 13.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113(17):2135–2150; discussion 2150. [PubMed] [Google Scholar]
- 14.Danesh J, Wheeler JG, Hirschfield GM, et al. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med 2004;350(14):1387–97. [DOI] [PubMed] [Google Scholar]
- 15.Padayachee L, Rodseth RN, Biccard BM. A meta-analysis of the utility of C-reactive protein in predicting early, intermediate-term and long term mortality and major adverse cardiac events in vascular surgical patients. Anaesthesia. 2009;64(4):416–424. [DOI] [PubMed] [Google Scholar]
- 16.Bibek S, Xie Y, Gao J, Wang Z, Wang J, Geng D. Role of Pre-procedural C-reactive Protein Level in the Prediction of Major Adverse Cardiac Events in Patients Undergoing Percutaneous Coronary Intervention: a Meta-analysisof Longitudinal Studies. Inflammation. 2014;38(1):159–169. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen SL, Ahlehoff O, Lindhardsen J, et al. Disease Activity in Inflammatory Bowel Disease Is Associated with Increased Risk of Myocardial Infarction, Stroke and Cardiovascular Death - A Danish Nationwide Cohort Study. PLoS One. 2013;8(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappelman MD, Horvarth-Puho E, Sandler RS, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: A population-based nationwide study. Gut. 2011;60:937–943. [DOI] [PubMed] [Google Scholar]
- 19.Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: A meta-analysis of observational studies. J. Crohn’s Colitis 2013;8(6):469–479. [DOI] [PubMed] [Google Scholar]
- 20.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375(9715):657–63. [DOI] [PubMed] [Google Scholar]
- 21.Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman D a. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am. J. Gastroenterol 2011;106(4):741–747. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi S, Narula N, Marshall JK, Farkouh M. Are Patients with Inflammatory Bowel Disease at Increased Risk of Coronary Artery Disease? Am. J. Med 2012;125(10):956–962. [DOI] [PubMed] [Google Scholar]
- 23.Dorn SD, Sandler RS. Inflammatory bowel disease is not a risk factor for cardiovascular disease mortality: results from a systematic review and meta-analysis. Am. J. Gastroenterol 2007;102(3):662–667. [DOI] [PubMed] [Google Scholar]
- 24.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff. Clin. Pract 5(3):143–51. [PubMed] [Google Scholar]
- 25.(HCUP) A for HR and QHC and UP. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2011. Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2011.pdf. Accessed December 28, 2015.
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal A, Atreja A, Kapadia S, Lopez R, Achkar J-P. Conventional risk factors and cardiovascular outcomes of patients with inflammatory bowel disease with confirmed coronary artery disease. Inflamm. Bowel Dis 2014;20(9):1593–601. [DOI] [PubMed] [Google Scholar]
- 28.Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut 2013;62:689–694. [DOI] [PubMed] [Google Scholar]
- 29.Osterman MT, Yang Y, Brensinger C, Forde K a., Lichtenstein GR, Lewis JD. No Increased Risk of Myocardial Infarction Among Patients With Ulcerative Colitis or Crohn’s Disease. Clin. Gastroenterol. Hepatol 2011;9(10):875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sridhar ARM, Parasa S, Navaneethan U, Crowell MD, Olden K. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J. Crohn’s Colitis 2011;5(4):287–294. [DOI] [PubMed] [Google Scholar]
- 31.Hatoum OA, Binion DG. The Vasculature and Inflammatory Bowel Disease. Inflamm. Bowel Dis 2005;11:304–313. [DOI] [PubMed] [Google Scholar]
- 32.Rungoe C, Nyboe Andersen N, Jess T. Inflammatory bowel disease and risk of coronary heart disease. Trends Cardiovasc. Med 2015;25(8):699–704. [DOI] [PubMed] [Google Scholar]
- 33.Romagnuolo J, Fedorak RN, Dias VC, Bamforth F, Teltscher M. Hyperhomocysteinemia and inflammatory bowel disease: prevalence and predictors in a cross-sectional study. Am. J. Gastroenterol 2001;96(7):2143–9. [DOI] [PubMed] [Google Scholar]
- 34.Koutroubakis IE, Dilaveraki E, Vlachonikolis IG, et al. Hyperhomocysteinemia in Greek patients with inflammatory bowel disease. Dig. Dis. Sci 2000;45(12):2347–51. [DOI] [PubMed] [Google Scholar]
- 35.Papa A, Danese S, Grillo A, et al. Hyperhomocysteinemia and prevalence of polymorphisms of homocysteine metabolism related enzymes in patients with inflammatory bowel disease. Dig. Liver Dis 2001;33(9):A120. [DOI] [PubMed] [Google Scholar]
- 36.Roifman I, Sun YC, Fedwick JP, et al. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol 2009;7(2):175–82. [DOI] [PubMed] [Google Scholar]
- 37.Morris PB, Ference BA, Jahangir E, et al. Cardiovascular Effects of Exposure to Cigarette Smoke and Electronic Cigarettes. J. Am. Coll. Cardiol 2015;66(12):1378–1391. [DOI] [PubMed] [Google Scholar]
- 38.Boyko EJ, Perera DR, Koepsell TD, Keane EM, Inui TS. Effects of cigarette smoking on the clinical course of ulcerative colitis. Scand. J. Gastroenterol 1988;23(9):1147–52. [DOI] [PubMed] [Google Scholar]
- 39.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin. Proc 2006;81(11):1462–71. [DOI] [PubMed] [Google Scholar]
- 40.Calabrese E, Yanai H, Shuster D, Rubin DT, Hanauer SB. Low-dose smoking resumption in ex-smokers with refractory ulcerative colitis. J. Crohns. Colitis 2012;6(7):756–62. [DOI] [PubMed] [Google Scholar]
- 41.Mahid SS, Minor KS, Stevens PL, Galandiuk S. The role of smoking in Crohn’s disease as defined by clinical variables. Dig. Dis. Sci 2007;52(11):2897–2903. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein CN, Wajda A, Blanchard JF. The Incidence of Arterial Thromboembolic Diseases in Inflammatory Bowel Disease: A Population-Based Study. Clin. Gastroenterol. Hepatol 2008;6(1):41–45. [DOI] [PubMed] [Google Scholar]
- 43.Ha C, Magowan S, Accortt NA, Chen J, Stone CD. Risk of arterial thrombotic events in inflammatory bowel disease. Am. J. Gastroenterol 2009;104(6):1445–1451. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Singh H, Loftus EV Jr., Pardi DS. Risk of Cerebrovascular Accidents and Ischemic Heart Disease in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol 2014;12(3):382–393. [DOI] [PubMed] [Google Scholar]
- 45.Zakroysky P, Thai W, Deaño RC, et al. Steroid Exposure, Acute Coronary Syndrome, and Inflammatory Bowel Disease: Insights into the Inflammatory Milieu. Am. J. Med 2015;128(3):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei L, MacDonald TM, Walker BR. Taking Glucocorticoids by Prescription Is Associated with Subsequent. Ann. Intern. Med 2004;141:764–771. [DOI] [PubMed] [Google Scholar]
- 47.Farrokhyar F, McHugh K, Jan Irvine E. Self-reported awareness and use of the International Classification of Diseases coding of inflammatory bowel disease services by Ontario physicians. Can. J. Gastroenterol 2002;16(8):519–526. [DOI] [PubMed] [Google Scholar]
- 48.Stepaniuk P, Bernstein CN, Nugent Z, Singh H. Characterization of inflammatory bowel disease in elderly hospitalized patients in a large central Canadian Health region. Can. J. Gastroenterol. Hepatol 2015;29(5):274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheurer DB, Hicks LS, Cook EF, Schnipper JL. Accuracy of ICD-9 coding for Clostridium difficile infections: a retrospective cohort. Epidemiol. Infect 2007;135(6):1010–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thirumurthi S, Chowdhury R, Richardson P, Abraham NS. Validation of ICD-9-CM diagnostic codes for inflammatory bowel disease among veterans. Dig. Dis. Sci 2010;55(9):2592–2598. [DOI] [PubMed] [Google Scholar]
- 51.Hou JK, Tan M, Stidham RW, et al. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn’s disease in the Veterans Affairs Health Care System. Dig. Dis. Sci 2014;59(10):2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al Kazzi ES, Lau B, Li T, Schneider EB, Makary MA, Hutfless S. Differences in the prevalence of obesity, smoking and alcohol in the United States Nationwide Inpatient Sample and the Behavioral Risk Factor Surveillance System. PLoS One. 2015;10(11):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Doornum S, Bohensky M, Tacey MA, Brand CA, Sundararajan V, Wicks IP. Increased 30-day and 1-year mortality rates and lower coronary revascularisation rates following acute myocardial infarction in patients with autoimmune rheumatic disease. Arthritis Res. Ther 2015;17(July 2005):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Södergren A, Stegmayr B, Lundberg V, Ohman M-L, Wållberg-Jonsson S. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann. Rheum. Dis 2007;66(2):263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–1307. [DOI] [PubMed] [Google Scholar]
- 56.Urowitz MB, Gladman DD, Anderson NM, et al. Cardiovascular events prior to or early after diagnosis of systemic lupus erythematosus in the systemic lupus international collaborating clinics cohort. Lupus Sci. Med 2016;3(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


